ABSTRACT
Off-treatment HBsAg reversion occurs in a considerable number of chronic hepatitis B(CHB) patients after IFN(interferon)-induced HBsAg clearance. HBV vaccination protects the general population against HBV infection. However, it remains unclear whether HBV vaccination could prevent off-treatment HBsAg reversion in CHB patients with HBsAg clearance. CHB patients (n = 199) with HBsAg clearance were included in the current study, comprising spontaneous HBsAg clearance group (n = 51), NA (nucleoside/nucleotide analogues)-induced group (n = 36) and IFN-induced group (n = 112). Log-rank test was performed to compare the cumulative incidences of HBsAg reversion between groups. Cox regression model was used to identify the factors associated with off-treatment HBsAg reversion. The 5-year cumulative incidence of HBsAg reversion in IFN-induced group was significantly higher than that in NA-induced group or spontaneous group (27.6% vs. 3.3% vs. 8.1%, both p < .05). In IFN-induced group, 66.7% of CHB patients received HBV vaccination. The cumulative incidence of HBsAg reversion in individuals with strong responses to HBV vaccination (HBsAb level >100mIU/ml) was significantly lower than that in those with weak responses to HBV vaccination (HBsAb level ≤100mIU/ml) or without HBV vaccination in IFN-induced group (7.7% vs. 58.5% vs. 31.9%, both p < .05). Multivariate Cox regression analysis confirmed strong responses to HBV vaccination were independently associated with a lower cumulative incidence of HBsAg reversion after IFN-induced HBsAg clearance (HR = 0.246, 95%CI: 0.066–0.907, p = .035). HBV vaccination has potential to prevent off-treatment HBsAg reversion in CHB patients after IFN-induced HBsAg clearance via a sufficiently high level of HBsAb, helping clinicians optimize the clinical management of such patients.
KEYWORDS: Chronic hepatitis B, functional cure, HBsAg clearance, HBV vaccination, interferon, HBsAg reversion
Introduction
Chronic hepatitis B virus (HBV) infection remains a severe threat to global public health, leading to a high rate of morbidity and mortality.1 Especially in the Asian-Pacific region, it is the predominant etiology of liver cirrhosis and hepatocellular carcinoma (HCC).2–4 Functional cure, defined as hepatitis B surface antigen (HBsAg) clearance with or without hepatitis B surface antibody (HBsAb) appearance, is regarded as the ideal endpoint of antiviral treatment in patients with chronic hepatitis B (CHB), which is associated with a favorable long-term prognosis.5–7 It is well known that the functional cure can be achieved spontaneously or by antiviral agents.8 Interferon (IFN) treatment has remarkable advantages of higher rates of hepatitis B e antigen (HBeAg) seroconversion and HBsAg clearance and reduced risk of HCC development over NA (nucleoside/nucleotide analogues).9–11 However, HBsAg reversion occurs in a considerable proportion of patients after IFN-induced HBsAg clearance.12,13 Pan et al. reported that the incidences of HBsAg reversion at the end of the 96-week follow-up were 24.83% or 23.08% in CHB patients treated with IFN monotherapy or a combination of IFN and NA.13
As we all know, HBV vaccination has made a great contribution to preventing maternal-neonatal transmission and eradicating HBV infection across the world.3 It could clear the circulating HBsAg in the HBsAb-mediated way, providing long-lasting protection against HBV infection in a generally healthy population.14 Several previous studies have shown that the patients with HBsAb levels >100 mIU/ml at the end of IFN-based therapy were less likely to have HBsAg reversion than those with HBsAb levels ≤100 mIU/ml,12,13,15,16 suggesting HBsAb level is one of the influencing factors for off-treatment HBsAg reversion. So, HBV vaccination has the potential to reduce off-treatment HBsAg reversion after HBsAg clearance by stimulating the generation of HBsAb in theory. Nevertheless, the data on whether HBV vaccination could prevent HBsAg reversion in CHB patients with functional cures have not been available to date.
The present study aimed to explore the potential effect of HBV vaccination on HBsAg reversion in a long-term follow-up retrospective cohort of CHB patients with functional cure, hoping to help clinicians optimize the clinical management of such a population.
Patients and methods
Study design and patients
This is a retrospective cohort study. All CHB patients achieving HBsAg clearance spontaneously or by antiviral agents were retrospectively screened via our hospital information system from January 2010 to December 2021. The general enrollment criteria were: (1) patients aged 18–80 years old; (2) serum HBsAg positive for >6 months; (3) with functional cure, defined as HBsAg clearance with or without HBsAb appearance; (4) follow-up time ≥24 weeks. The general exclusion criteria were: (1) co-infection with another virus, e.g. hepatitis A virus, hepatitis C virus, hepatitis D virus or human immunodeficiency virus; (2) concomitant with other chronic liver diseases, e.g. autoimmune hepatitis and alcoholic hepatitis; (3) with HCC or other malignant tumors before HBsAg clearance; (4) under immunosuppressive therapy or chemotherapy during the follow-up period; (5) remained under ongoing antiviral treatment; (6) lack of key data for analysis, e.g. the course of antivirals; (7) with positive HBeAg at the end of treatment.
Based on the method of HBsAg clearance, the patients eligible for our retrospective analysis were classified into three groups: spontaneous HBsAg clearance group, NA-induced group and IFN-induced group. Patients in the spontaneous group never received any antiviral agents, including NA and IFN. Patients in the NA-induced group only took nucleoside/nucleotide analogues and were never exposed to IFN. Patients in the IFN-induced group received standard interferon or pegylated interferon (PEG-IFN) treatment with or without NA for ≥24 weeks.
The study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine according to the Declaration of Helsinki (No.2019–30). Written informed consent was waived by the Committee considering the retrospective design.
HBV vaccination procedure
According to Chinese Guideline of Prevention and Treatment for Chronic Hepatitis B,17 these CHB patients after acquiring HBsAg clearance were recommended to receive three intramuscular injections of recombinant HBV vaccine containing 20 μg HBsAg at months 0, 1, and 6, respectively. As for individuals who did not respond to a three-dose immunization series, additional doses of recombinant HBV vaccine would be administered.
Data collection
All participants were followed up every 12 or 24 weeks. Clinical data collected in our study included age, sex, blood routine examination, liver function, renal function, alpha-fetoprotein (AFP), HBsAg, HBsAb, HBeAg, hepatitis B e antibody (HBeAb) and serum HBV DNA level. Additionally, the medications used (IFN or NA), their dosage, and start and cessation dates, were all recorded. Data on HBV vaccination were collected via the medical records and/or telephone calls, comprising patients’ vaccination status (vaccinated or unvaccinated), the time point of initiation of vaccination, the total doses of vaccines and the antibody response to vaccination.
Laboratory testing for HBV markers
HBV serological biomarkers (HBsAg, HBsAb, HBeAg, and HBeAb) were determined using automated chemiluminescent microparticle immunoassays (CMIA) (Abbott, Chicago, IL, USA). HBsAg level >0.05 IU/ml, HBsAb level >10 mIU/ml, HBeAg level >1.0 S/CO and HBeAb level <1.0 S/CO were defined as positive, respectively. Serum HBV DNA was measured by using CobasTaqMan96 real-time quantitative PCR detection reagent (Roche, Pleasanton, CA, USA). Undetectable HBV DNA was defined as HBV DNA <20 IU/mL.
Study outcomes and definition of events
The outcome of this study was off-treatment HBsAg reversion in CHB patients after IFN-induced HBsAg clearance. HBsAg reversion was defined as HBsAg level >0.05 IU/ml after HBsAg clearance.
The follow-up time of the spontaneous group referred to the period from the first detection of HBsAg clearance to the first detection of HBsAg reversion or the last visit to hospitals in the absence of HBsAg reversion. Follow-up time of IFN-induced or NA-induced group referred to the period from cessation of IFN or NA to the first detection of HBsAg reversion or the last visit to hospital in the absence of HBsAg reversion. Duration of IFN consolidation treatment referred to the period from the first detection of HBsAg clearance to the cessation of IFN.
Simultaneous administration of IFN and HBV vaccine was defined as initiating injection of vaccine between HBsAg clearance and cessation of IFN treatment. Sequential administration of IFN and HBV vaccine was defined as initiating injection of vaccine after cessation of IFN treatment.
The individual who had a HBsAb level of ≥10 mIU/ml after HBV vaccination was defined as responder. The individual who had a HBsAb level of <10 mIU/ml after HBV vaccination was defined as non-responder. Weak response to HBV vaccination referred to the highest HBsAb level ≤100 mIU/ml after the injection of HBV vaccine (including the non-responder). Strong response to HBV vaccination referred to the highest HBsAb level >100 mIU/ml after the injection of HBV vaccine.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range [IQR]) according to data distribution. Categorical variables were shown as number (percentage). Student’s t-test or Mann–Whitney test was used to compare continuous variables between two groups. One-way ANOVA or Kruskal–Wallis test were used to compare continuous variables among three groups, as appropriate. Differences between categorical variables were determined using the chi-squared test or Fisher’s exact test. Log-rank test was performed to compare the cumulative incidences of HBsAg reversion between two groups. Cox regression model was used to identify the factors associated with the off-treatment HBsAg reversion in CHB patients with IFN-induced HBsAg clearance. Continuous variables in the Cox regression model were converted to dichotomous variables based on median values. The method of selecting and eliminating variables in the Cox regression model was performed by stepwise method with a P-value of significance level for entry = 0.10 and for stay = 0.05. All statistical analyses were conducted with SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) and figures were plotted using GraphPad Prism (version 8.3 for Windows, San Diego, California, USA). Statistical significance was achieved when a two-tailed p-value was < .05.
Results
Clinical characteristics of CHB patients with HBsAg clearance
A total of 199 CHB patients achieving HBsAg clearance were eligible for analysis, comprising spontaneous HBsAg clearance group (n = 51), NA-induced group (n = 36) and IFN-induced group (n = 112). The details of the screening and enrollment process of all participants were illustrated in Figure 1.
Figure 1.
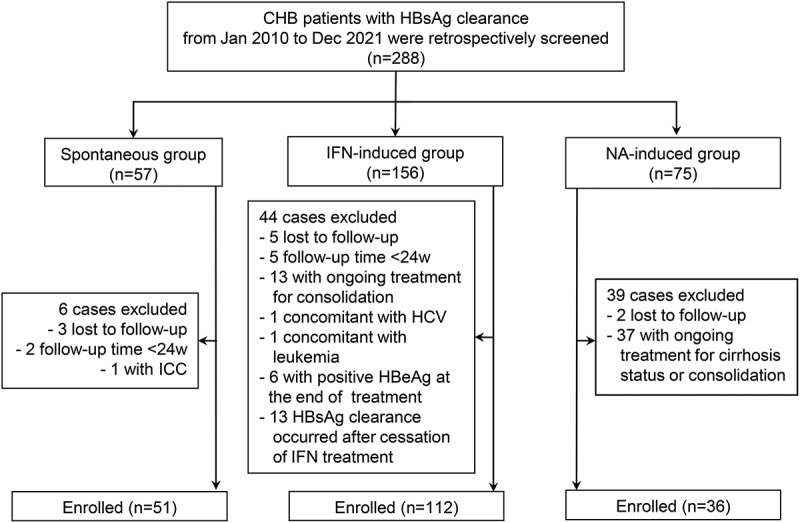
Flow chart of patient enrollment. Abbreviations: CHB, chronic hepatitis B; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; ICC, intrahepatic cholangiocarcinoma; IFN, interferon; NA, nucleoside/nucleotide analogues; w, weeks.
At the time of HBsAg clearance, the IFN-induced group was younger and had a higher proportion of male patients than the NA-induced or spontaneous group (both p < .05) (Table 1). The spontaneous group had the highest rate of HBeAb positivity compared to the other two groups (p = .002) (Table 1). No significant differences were observed in HBsAb positivity, the distribution of diverse HBsAb levels, HBeAg positivity and undetectable HBV DNA rates among the three groups at the time of HBsAg clearance.
Table 1.
Clinical characteristics of CHB patients with HBsAg clearance.
| Characteristics | IFN-induced group(n = 112) | NA-induced group(n = 36) | Spontaneous group(n = 51) | P†-value |
|---|---|---|---|---|
| At the time of HBsAg clearance | ||||
| Age, years, median (IQR) | 36.6 (32.0–42.7) | 42.8 (36.3–54.1) | 49.7 (40.4–57.0) | <.001 |
| Male sex, n (%) | 100 (89.3%) | 23 (63.9%) | 41 (80.4%) | .002 |
| HBsAg negativity, n (%) | 112 (100%) | 36 (100%) | 51 (100%) | 1.000 |
| HBsAb positivity, n (%) | 34 (31.2%) | 11 (32.4%) | 19 (37.3%) | .746 |
| HBsAb level, mIU/ml, n(%) | .433 | |||
| < 10 mIU/ml | 75 (68.8%) | 23 (67.6%) | 32 (62.7%) | |
| 10–100 mIU/ml | 24 (22.0%) | 6 (17.6%) | 15 (29.4%) | |
| 100–1000 mIU/ml | 10 (9.2%) | 5 (14.7%) | 3 (5.9%) | |
| > 1000 mIU/ml | 0 (0.0%) | 0 (0.0%) | 1 (2.0%) | |
| HBeAg positivity, n (%) | 6 (5.5%) | 2 (5.9%) | 0 (0.0%) | .179 |
| HBeAb positivity, n (%) | 77 (70.6%) | 23 (67.6%) | 48 (94.1%) | .002 |
| Undetectable HBV DNA, n (%) | 112 (100%) | 36 (100%) | 51 (100%) | 1.000 |
| At the end of follow-up | ||||
| Duration of treatment, weeks# or years*, median (IQR) | 62.0# (48.0–107.5) | 10.0* (4.3–12.0) | <0.001¶ | |
| Follow-up time, weeks, median (IQR) | 131.5 (51.8–215.8) | 113.5 (66.0–229.5) | 217.0 (101.0–355.0) | 0.004 |
| HBsAg reversion, n (%) | 24 (21.4%) | 1 (2.8%) | 3 (5.9%) | 0.003 |
Abbreviations: HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon; IQR, interquartile range; NA, nucleoside/nucleotide analogues.
Note: HBsAg level > 0.05 IU/ml, HBsAb level >10 mIU/ml, HBeAg level >1.0 S/CO and HBeAb level <1.0 S/CO were defined as positive, respectively. HBsAg reversion was defined as HBsAg level > 0.05 IU/ml after HBsAg clearance.
†Differences of clinical characteristics among the three groups were examined using Kruskal–Wallis test for continuous variables and chi-square test or Fisher’s exact test for categorical variables.
¶Differences of duration of treatment between the the former two groups were determined using Mann-Whitney U test.
#Denotes weeks. * Denotes years.
At the end of follow-up, HBsAg reversion occurred in 24 (21.4%) patients from the IFN-induced group, 1 (2.8%) patient from the NA-induced group, and 3 (5.9%) patients from the spontaneous group (p = .003) (Table 1). The duration of treatment in the IFN-induced group was much shorter than that in the NA-induced group (p < .001) (Table 1).
Cumulative incidences of HBsAg reversion in different methods of HBsAg clearance
After a median follow-up time of 131.5 weeks, 113.5 weeks, or 217.0 weeks respectively, the 5-year cumulative incidences of HBsAg reversion were 27.6% in the IFN-induced group, 3.3% in the NA-induced group, and 8.1% in the spontaneous group (Figure 2). The peak of HBsAg reversion in the IFN-induced group occurred within 96 weeks after cessation of IFN treatment, during which 21 of 24 reversion cases were observed. The Log-rank test demonstrated that the 5-year cumulative incidence of HBsAg reversion in the IFN-induced group was significantly higher than that in the NA-induced group (27.6% vs. 3.3%, Log-rank p = .015) or the spontaneous group (27.6% vs. 8.1%, Log-rank p = .008) (Figure 2), whereas there was no significant difference in 5-year cumulative incidence of HBsAg reversion between the latter two groups (3.3% vs. 8.1%, Log-rank p = .718).
Figure 2.

Cumulative incidences of HBsAg reversion according to the different HBsAg clearance methods. Abbreviations: HBsAg, hepatitis B surface antigen; IFN, interferon; NA, nucleoside/nucleotide analogues.
Notes: * Represents p < .05, ** Represents p < .01, *** Represents p < .001, ns represents no significance.
HBV vaccination in IFN-induced group
After four patients without available data on HBV vaccination were excluded from the IFN-induced group, 108 patients were included in the final analysis to investigate the potential effect of HBV vaccination on off-treatment HBsAg reversion. Two-thirds (66.7%) of these patients received HBV vaccines (Figure 3a). As illustrated in Figure 3b, 68.1% (49/72) of vaccinated individuals were administered with IFN and HBV vaccine sequentially, while the remaining 31.9% (23/72) received simultaneous administration of both them. The proportion of individuals injected with 1 dose, 2 doses or ≥3 doses of HBV vaccines was 8.3% (6/72), 23.6% (17/72) or 68.1% (49/72), respectively (Figure 3c). Among 72 vaccinated patients, the HBsAb levels were classified into four grades: <10 mIU/ml (n = 4, 5.6%), 10–100 mIU/ml (n = 19, 26.4%), 100–1000 mIU/ml (n = 34, 47.2%), and >1000 mIU/ml (n = 15, 20.8%) (Figure 3d). As prespecified, non-responders (<10 mIU/ml) or responders (≥10 mIU/ml) to HBV vaccination accounted for 5.6% (4/72) or 94.4% (68/72), respectively (Figure 3e). 31.9% (23/72) of patients showed weak response to HBV vaccination (HBsAb level ≤100 mIU/ml) and 68.1% (49/72) showed strong response (HBsAb level >100 mIU/ml) (Figure 3f).
Figure 3.

HBV vaccination in IFN-induced group. (a) The proportion of the patients with or without HBV vaccination. (b) The proportion of the patients with sequential administration vs. simultaneous administration of IFN and HBV vaccine. (c) The proportion of the patients receiving 1 dose, 2 doses or ≥ 3 doses of HBV vaccine. (d) The proportion of the patients with diverse HBsAb levels following HBV vaccination. (e) The proportion of responders or non-responders to HBV vaccination. (f) The proportion of the patients with weak or strong response to HBV vaccination. Abbreviations: HBV, hepatitis B virus; IFN, interferon.
Notes: Simultaneous administration of IFN and HBV vaccine was defined as initiating injection of vaccine between HBsAg clearance and cessation of IFN treatment. Sequential administration was defined as initiating injection of vaccine after cessation of IFN treatment. The individual who had a HBsAb level of ≥10 mIU/ml or <10 mIU/ml after HBV vaccination was defined as responder or non-responder, respectively. Weak or strong response to HBV vaccination refers to the highest HBsAb level ≤100 mIU/ml or >100 mIU/ml after injection of vaccine, respectively.
Cumulative incidences of HBsAg reversion according to HBV vaccination in IFN-induced group
Clinical characteristics of the patients with or without HBV vaccination in the IFN-induced group were described in Table 2. The two groups did not differ significantly in age, sex, the distribution of HBsAb levels, HBeAg positivity, HBeAb positivity and undetectable HBV DNA rates both at the time of HBsAg clearance and the end of interferon treatment (All p > .05). It also showed no significant difference in duration of IFN treatment and follow-up time between the two groups (Both p > .05). However, the patients with HBV vaccination had a higher percentage of combined therapeutic regimen (IFN+NA) (83.3% vs. 61.1%, p = .011) and a longer median duration of IFN consolidation treatment (26.0 vs. 17.5 weeks, p = .008) than those without vaccination.
Table 2.
Clinical characteristics of the patients with or without HBV vaccination in IFN-induced HBsAg clearance group.
| Characteristics | Without HBV vaccination (n = 36) | With HBV vaccination (n = 72) | p*-value |
|---|---|---|---|
| Status at the time of HBsAg clearance | |||
| Age, years, median (IQR) | 36.5 (31.0–42.8) | 37.0 (33.0–43.8) | .559 |
| Male sex, n (%) | 32 (88.9%) | 64 (88.9%) | 1.000 |
| HBsAb level, mIU/ml, n (%) | .435 | ||
| < 10 mIU/ml | 21 (61.8%) | 50 (70.4%) | |
| 10–100 mIU/ml | 8 (23.5%) | 16 (22.5%) | |
| 100–1000 mIU/ml | 5(14.7%) | 5 (7.0%) | |
| > 1000 mIU/ml | 0 (0.0%) | 0 (0.0%) | |
| HBeAg positivity, n (%) | 2 (5.9%) | 4 (5.6%) | 1.000 |
| HBeAb positivity, n (%) | 24 (70.6%) | 50 (70.4%) | .986 |
| Undetectable HBV DNA, n (%) | 36 (100%) | 72 (100%) | 1.000 |
| Status at the end of IFN treatment | |||
| HBsAb level, mIU/ml, n (%) | .594 | ||
| < 10 mIU/ml | 11 (30.6%) | 17 (23.6%) | |
| 10–100 mIU/ml | 11 (30.6%) | 20 (27.8%) | |
| 100–1000 mIU/ml | 11 (30.6%) | 31(43.1%) | |
| > 1000 mIU/ml | 3 (8.3%) | 4 (5.6%) | |
| HBeAg positivity, n (%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| HBeAb positivity, n (%) | 25 (69.4%) | 52 (72.2%) | .764 |
| Undetectable HBV DNA, n (%) | 36 (100%) | 72(100%) | 1.000 |
| Therapeutic regimen, n (%) | .011 | ||
| IFN+NA | 22 (61.1%) | 60 (83.3%) | |
| IFN only | 14 (38.9%) | 12 (16.7%) | |
| Duration of IFN treatment, weeks, median (IQR) | 68.0 (48.0–115.3) | 61.0 (48.0–103.3) | .646 |
| Duration of IFN consolidation treatment, weeks, median (IQR) | 17.5 (4.5–31.0) | 26.0 (16.0–35.8) | .008 |
| Follow-up time, weeks, median (IQR) | 132.0 (64.5–208.8) | 116.5 (48.3–206.8) | .616 |
Abbreviations: HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon; IQR, interquartile range; NA, nucleoside/nucleotide analogues.
Note: HBsAg level > 0.05 IU/ml, HBsAb level >10 mIU/ml, HBeAg level >1.0 S/CO and HBeAb level <1.0 S/CO were defined as positive, respectively.
*Differences of clinical characteristics between the two groups were determined using Mann-Whitney U test for continuous variables and chi-square test or Fisher’s exact test for categorical variables.
There was no significant difference in 5-year cumulative incidences of HBsAg reversion between patients with or without HBV vaccination in the IFN-induced group (27.7% vs. 31.9%, Log-rank p = .753) (Figure 4a). The cumulative incidence of HBsAg reversion in responders to HBV vaccination was much lower than that in non-responders (24.8% vs. 75.0%, Log-rank p < .001), but did not differ significantly from that in patients without HBV vaccination (24.8% vs. 31.9%, Log-rank p = .459), suggesting the conventional cutoff value of protective HBsAb level (≥10 mIU/ml) is not sufficient for patients after IFN-induced HBsAg clearance in preventing off-treatment HBsAg reversion (Figure 4b).
Figure 4.

Cumulative incidences of HBsAg reversion in IFN-induced group according to HBV vaccination. (a) The patients without HBV vaccination vs. with HBV vaccination. (b) The patients without HBV vaccination vs. responders to vaccination vs. non-responders to vaccination in the entire IFN-induced group. (c) The patients without HBV vaccination vs. with weak response to vaccination vs. with strong response to vaccination in the entire IFN-induced group. (d) The patients without HBV vaccination vs. with weak response to vaccination vs. with strong response to vaccination in individuals receiving sequential administration of IFN and HBV vaccine. Abbreviations: HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon.
Notes: The individual who had a HBsAb level of ≥10 mIU/ml or <10 mIU/ml after HBV vaccination was defined as responder or non-responder, respectively. Weak or strong response to HBV vaccination refers to the highest HBsAb level ≤100 mIU/ml or >100 mIU/ml after injection of vaccine, respectively. Sequential administration was defined as initiating injection of vaccine after cessation of IFN treatment. * represents p < .05, ** represents p < .01, *** represents p < .001, ns represents no significance.
Fortunately, patients with strong response to vaccination had a much lower 5-year cumulative incidence of HBsAg reversion in contrast to those without HBV vaccination (7.7% vs. 31.9%, Log-rank p = .023) or those with weak response to HBV vaccination (7.7% vs. 58.5%, Log-rank p < .001) (Figure 4c). In contrast, patients with weak response to vaccination had a higher 5-year cumulative incidence of HBsAg reversion than those without HBV vaccination (58.5% vs. 31.9%, Log-rank p = .039).
To rule out the effect of IFN on the increase of HBsAb after HBsAg clearance, the patients receiving simultaneous administration of IFN and HBV vaccines were removed from the next analysis. In individuals receiving sequential administration of the two agents, patients with strong response to vaccination had a much lower 5-year cumulative incidence of HBsAg reversion compared to those without HBV vaccination (3.1% vs. 31.9%, Log-rank p = .012) or those with weak response to HBV vaccination (3.1% vs. 50.8%, Log-rank p = .001), which was similar to the results in the entire IFN-induced group (Figure 4d). There was no significant difference in the cumulative incidences of HBsAg reversion between the latter two groups in such a population (31.9% vs. 50.8%, Log-rank p = .244).
Cox regression analysis of factors associated with HBsAg reversion in IFN-induced group
Univariate Cox regression analysis demonstrated that strong response to HBV vaccination was identified as the only protective factor for off-treatment HBsAg reversion (HR = 0.246, 95%CI: 0.066–0.907, p = .035), while weak response to HBV vaccination was a risk factor for off-treatment HBsAg reversion (HR = 2.448, 95%CI: 1.029–5.823, p = .043) (Table 3). Other factors, including age, sex, therapeutic regimens, duration of IFN treatment and duration of IFN consolidation treatment, showed no significant influence on HBsAg reversion (All p > .05). Multivariate Cox regression analysis further corroborated that strong response to HBV vaccination was independently associated with a lower risk of off-treatment HBsAg reversion after IFN-induced HBsAg clearance (HR = 0.246, 95%CI: 0.066–0.907, p = .035), instead of therapeutic regimens or duration of IFN consolidation treatment (Table 3).
Table 3.
Cox regression analysis of factors associated with the off-treatment HBsAg reversion in IFN-induced HBsAg clearance group.
| Characteristics | Univariate Cox regression |
Multivariate Cox regression |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age, years | ||||
| >37 vs. ≤37 | 0.506 (0.216–1.183) | .116 | - | - |
| Sex | ||||
| Male vs. female | 0.801 (0.238–2.695) | .720 | - | - |
| Therapeutic regimen | ||||
| IFN+NA vs. IFN only | 1.335 (0.498–3.581) | .566 | - | - |
| Duration of IFN treatment, weeks | ||||
| >62 vs. ≤62 | 1.294 (0.573–2.922) | .535 | - | - |
| Duration of IFN consolidation treatment, weeks | ||||
| >24 vs. ≤24 | 0.518 (0.222–1.212) | .129 | - | - |
| HBV vaccination | ||||
| Vaccinated vs. unvaccinated | 0.876 (0.383–2.002) | .753 | - | - |
| Weak response to vaccination vs. unvaccinated | 2.448 (1.029–5.823) | .043 | 2.448 (1.029–5.823) | .043 |
| Strong response to vaccination vs. unvaccinated | 0.246 (0.066–0.907) | .035 | 0.246 (0.066–0.907) | .035 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IFN, interferon; NA, nucleoside/nucleotide analogues.
Note: HBsAg reversion was defined as HBsAg level >0.05 IU/ml after HBsAg clearance. Weak or strong response to HBV vaccination refers to the highest HBsAb level ≤100 mIU/ml or > 100 mIU/ml after injection of vaccine, respectively. Continuous variables in the Cox regression model were converted to dichotomous variables based on median values.
Cumulative incidences of HBsAg reversion according to HBV vaccination in NA-induced and spontaneous group
Among all individuals with available data on HBV vaccination, 44.4% (16/36) or 4.1% (2/49) of patients received vaccination in NA-induced or spontaneous group, respectively (Supplementary Figure S1). In NA-induced group, there was no significant difference in cumulative incidences of HBsAg reversion between patients with or without HBV vaccination (0% vs. 5.9%, Log-rank p = .382) (Supplementary Figure S2a). In spontaneous group, the cumulative incidences of HBsAg reversion also did not differ significantly between patients with or without HBV vaccination (0% vs. 8.8%, Log-rank p = .671) (Supplementary Figure S2b).
Discussion
HBsAg reversion occurs in a part of CHB patients achieving functional cure spontaneously or by antivirals (NA or IFN).12,13,18 The IFN-induced HBsAg clearance group appeared to have a higher cumulative incidence of HBsAg reversion than the other two groups.12,13,18 Several potential predictors for off-treatment HBsAg reversion have been reported in patients with IFN-induced HBsAg clearance, which included HBsAb level, quantitative hepatitis B core-related antigen (HBcrAg), quantitative hepatitis B core antibody (HBcAb), HBV RNA and duration of IFN consolidation treatment.12–18–21 However, it is unclear whether HBV vaccination could prevent off-treatment HBsAg reversion in such a population. Here, a retrospective cohort of 199 CHB patients achieving functional cure was established and consisted of three groups: spontaneous group, NA-induced group and IFN-induced group. The cumulative incidences of HBsAg reversion were compared among the three groups and the potential effect of HBV vaccination on off-treatment HBsAg reversion was investigated.
HBsAg clearance serves as the hallmark of functional cure and is recommended as the ideal endpoint of antiviral treatment by the guidelines of CHB management, due to its association with a better clinical outcome compared to those with persistent HBsAg positivity.4–7 A meta-analysis of 28 studies suggested that HBsAg clearance could notably delay the disease progression and decrease the HCC incidence in CHB patients.22 Nevertheless, HCC still inevitably occurs in a part of CHB patients achieving functional cure, and its relevant risk factors consist of the presence of cirrhosis, male sex, and age ≥50 years at the time of HBsAg clearance.22 Therefore, it is of great importance to achieve a functional cure as early as possible before the development of liver cirrhosis. In the present study, we found that the IFN-induced HBsAg clearance group was younger at the time of HBsAg clearance than the other two groups and had a much shorter duration of antiviral treatment, which means IFN-treated CHB patients achieve functional cure earlier and sooner and avoid the long course of NA treatment.
Regrettably, our results revealed that the IFN-induced HBsAg clearance group had the highest 5-year cumulative incidence of HBsAg reversion among the three groups. The 5-year cumulative incidence of HBsAg reversion was 27.6% in the IFN-induced group, 3.3% in the NA-induced group and 8.1% in the spontaneous group, respectively, which were similar to other studies. Yip et al. performed a large-scale study, including 3,563 patients with spontaneous HBsAg clearance and 475 with NA-induced clearance, and reported that the 5-year cumulative incidence of confirmed HBsAg clearance was 88.1% vs. 92.2% in spontaneous or NA-induced group (p = .964). That is to say, the cumulative incidences of HBsAg reversion were 11.9% vs. 7.8% in the two groups, respectively.18 Other studies presented an HBsAg reversion incidence of 8.2% or 12.79% in HBeAg-positive or HBeAg-negative CHB patients with IFN-based therapy during the 48-week follow-up after treatment cessation.16,21 24.83% or 23.08% of CHB patients treated with IFN monotherapy or a combination of IFN and NA underwent HBsAg reversions at the end of the 96-week follow-up.13 The cumulative incidences of HBsAg reversion in our study were 11.9% for 48 weeks and 21.0% for 96 weeks, which were very close to the results from other studies. With the follow-up time increasing, the cumulative incidences of HBsAg reversion in this present study were 22.3%, 22.3% and 27.6% for 144, 192 and 240 weeks, respectively. Thus, the follow-up time for the determination of HBsAg reversion should be extended to 96 weeks due to its reversion peak occurring within 96 weeks.
To reduce the relatively high incidence of HBsAg reversion after cessation of IFN treatment, preventative measures need to be explored urgently. It has been proven that extending the duration of IFN consolidation treatment contributed to preventing HBsAg reversion.21 The favorable role of HBsAb in maintaining a sustained functional cure of CHB was also validated by other studies.13,15,16 Nevertheless, there were no reports on whether HBV vaccination could prevent off-treatment HBsAg reversion in CHB patients after IFN-induced HBsAg clearance. Our study showed that patients with strong response to HBV vaccination had a much lower 5-year cumulative incidence of HBsAg reversion in contrast to those without HBV vaccination or those with weak response to HBV vaccination. Furthermore, multivariate Cox regression analysis confirmed that strong response to HBV vaccination was independently associated with a lower HBsAg reversion risk, instead of therapeutic regimens or duration of IFN consolidation treatment. In line with other reports, therapeutic regimens (IFN only versus the combination of IFN and NA) did not have a significant impact on HBsAg reversion.15,21 But there is an inconsistency in the role of duration of IFN consolidation treatment between our results and prior studies.21 The possible reasons for this inconsistency are attributed to the differences in follow-up time, statistical regression model, sample size and heterogeneity of participants.
With regard to the underlying mechanism behind the protective role of strong response to HBV vaccination, it could be partly explained in terms of immunology. HBsAg clearance reflects an effective immune control against HBV replication, and the appearance of HBsAb indicates the restoration of humoral immunity, which was evidenced by the stable proportion and activation of total and memory B cells in CHB patients maintaining sustained response from a recent study.12 Strong response to HBV vaccination after achieving HBsAg clearance indicates a potent and durable restoration of humoral immunity predicting a pretty low incidence of HBsAg reversion, while weak response to HBV vaccination indicates a fragile and unstable restoration of humoral immunity leading to an unacceptably high incidence of HBsAg reversion. Hence, we have reasons to speculate that the higher the HBsAb level is, the better the restoration of humoral immunity is, and the lower the HBsAg reversion incidence will be.
Combining our data with other studies,15,16,18,21 a new decision-making roadmap has been proposed to evaluate the risk of HBsAg reversion in CHB patients achieving functional cure by different methods and to illustrate the application of HBV vaccine in reducing HBsAg reversion (Figure 5). Patients with spontaneous HBsAg clearance or those with NA-induced HBsAg clearance after a sufficient duration of consolidation treatment should be considered as the population at low risk of HBsAg reversion regardless of any levels of HBsAb. Patients with IFN-induced HBsAg clearance who have finished a sufficient duration of IFN consolidation treatment and obtained an HBsAb level >100 mIU/ml at the end of treatment should be considered as at low risk of HBsAg reversion. Patients with IFN-induced HBsAg clearance who have an HBsAb level ≤100 mIU/ml at the end of treatment should be regarded as at high risk of HBsAg reversion and be suggested to receive HBV vaccination sequentially to raise HBsAb level as high as possible. After HBV vaccination, those who show strong response to vaccination (HBsAb level increasing to >100 mIU/ml) are categorized into a subgroup at low risk of HBsAg reversion. Those who show weak response to vaccination (HBsAb level remaining ≤100 mIU/ml) are still at high risk of HBsAg reversion, among whom additional doses of the HBV vaccine are needed to reach the target of HBsAb level >100 mIU/ml. Bianchi et al. reported that 96.2% of non-responders to the booster dose of HBV vaccine developed a positive titer of HBsAb following two additional doses of vaccine,23 providing a feasible and effective strategy for the management of non-responders. The optimal application method of HBV vaccines in reducing HBsAg reversion, e.g. the best total number of doses or appropriate single dosage at each vaccination, deserves further exploration. Individuals at low risk of HBsAg reversion are recommended to receive regular monitoring every 6 months. In contrast, those at high risk of HBsAg reversion are strongly recommended to take close monitoring every 3 months within 2 years after cessation of IFN treatment for detecting HBsAg reversion as early as possible.
Figure 5.

A new decision-making roadmap proposed to evaluate the risk of HBsAg reversion in CHB patients achieving HBsAg clearance by the different methods and to illustrate the application of HBV vaccine in reducing HBsAg reversion. Abbreviations: CHB, chronic hepatitis B; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon; NA, nucleoside/nucleotide analogues.
In our study, some patients with HBsAg clearance could not reach the target of HBsAb level >100 mIU/ml even after receiving additional doses of HBsAg vaccine, with an unacceptably high incidence of HBsAg reversion. Effective measures are needed for these patients to prevent HBsAg reversion. Previous studies using conventional HBsAg vaccines in chronic HBV carriers showed that there was no significant difference in achieving HBV DNA clearance, HBeAg seroconversion or HBsAg loss between vaccinated and unvaccinated groups.24–26 Nowadays, various novel HBV therapeutic vaccines have been developed for functional cure of CHB, including protein (HBsAg/HBcAg/hepatitis B X protein), DNA and viral vector-based vaccines.27,28 A number of clinical trials using these novel vaccines have been conducted to evaluate their efficacy in the treatment of CHB patients,27–32 such as GS-4774 (NCT02174276), NASVAC (NCT10374308), and YIC (ChiCTR-TRC-11003189). In a phase II, multicenter, randomized, controlled open-label study, GS-4774, a yeast-derived vaccine containing HBsAg, HBcAg and hepatitis B X, did not significantly reduce levels of HBsAg in CHB patients, although it showed a strong immune stimulatory effect on HBV-specific CD8+ T cells.29 A phase III, controlled open-label and randomized clinical trial was performed to evaluate the efficacy of a novel intranasal therapeutic vaccine containing HBsAg and HBcAg (NASVAC) versus Peg-IFN in naïve CHB patients, demonstrating that sustained control of HBV DNA and HBeAg clearance were significantly more frequent in NASVAC group compared to Peg-IFN group.30 These novel therapeutic vaccines may be an effectively alternative option for those who showed weak responses to conventional HBsAg vaccines, which is an interesting direction for future studies.
Our study demonstrated conventional recombinant HBsAg vaccines could prevent off-treatment HBsAg reversion in patients with HBsAg clearance, while clinical trials using the novel therapeutic vaccines achieved little success in inducing functional cure among CHB patients to date. The different outcomes may result from different study subjects: the patients achieving HBsAg clearance versus patients with ongoing chronic HBV infection. Dysfunctional HBV-specific immune cells were recovered to some extent in the patients achieving HBsAg clearance,12 and yielded a good antibody response following HBV vaccination in most of these patients in our study. In contrast, driven by repetitive antigen stimulation over long periods, the patients with chronic HBV infection have both B and T cell dysfunction, which may result in underproduction of HBsAb and reduced HBsAg neutralization.27 These novel HBV therapeutic vaccines are unable to induce HBV-specific immune responses of adequate magnitude for functional cure in established chronic HBV infection.27 Thus, new strategies are required to improve the efficacy of novel therapeutic vaccines in chronic HBV infection in future.
It must be acknowledged that there are some limitations in this retrospective study. First, due to a fairly low rate of HBsAg clearance and the dilemma of obtaining a large number of samples for analysis, we provided a relatively small sample size in our single center. Second, other factors before initiation of antivirals, including the duration of HBV infection, HBsAg level, HBV DNA level and HBV genotypes, were unable to be collected in all participants for analysis. Third, in addition to humoral immunity, HBV-specific CD8+ T cells play a critical role in controlling HBV infection.33 Regrettably, our study lacked data related to the role of cellular immunity in HBsAg reversion, which will be investigated in the future. Fourth, data on HBV vaccination status collected by phone calls in some participants may suffer from recall bias, which is a common methodological limitation of retrospective studies and could affect the accuracy of data. Lastly, the optimal application method of HBV vaccines in preventing HBsAg reversion needs further exploration in different dosages and time interval. However, the present study is the first one to investigate the role of HBV vaccination in preventing off-treatment HBsAg reversion although it is preliminary and exploratory. A prospective, large-scale and multicenter study is required to further validate our findings.
In conclusion, HBV vaccination has the potential to prevent off-treatment HBsAg reversion in CHB patients after IFN-induced HBsAg clearance via a sufficiently high level of HBsAb, which will help clinicians optimize the clinical management of such a population.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions from all of the patients, and the clinical and research staff in the Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 82070604, No. 81770587), Key Projects in the National Science & Technology Pillar Program during the Thirteenth Five-year Plan Period (2017ZX10203201-008, 2018ZX09201016-003-001, and 2017ZX10202202-005-004), the Shanghai Municipal Key Clinical Specialty (shslczdzk01103), and the Shanghai Ruijin Hospital Three-Year Plan of the Clinical Skills and Innovations (2018CR005).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2161254.
References
- 1.Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen D-S, Van Damme P, Abbas Z, Abdulla M, Abou Rached A, Adda D, Aho I, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–10. doi: 10.1016/s2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 2.Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, Chan HLY, Ng SC.. The changing epidemiology of liver diseases in the Asia–Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. Asia–pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–70. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao JH, Jeng WJ, Ning Q, Su TH, Tseng TC, Ueno Y, Yuen MF.. APASL guidance on stopping nucleos(t)ide analogues in chronic hepatitis B patients. Hepatol Int. 2021;15(4):833–51. doi: 10.1007/s12072-021-10223-5. [DOI] [PubMed] [Google Scholar]
- 6.EASL . Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr., Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song A, Lin X, Chen X. Functional cure for chronic hepatitis B: accessibility, durability, and prognosis. Virol J. 2021;18(1):114. doi: 10.1186/s12985-021-01589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, et al. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–44.e110. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Ren P, Cao Z, Mo R, Liu Y, Chen L, Li Z, Zhou T, Lu J, Liu Y, Guo Q, et al. Interferon-based treatment is superior to nucleos(t)ide analog in reducing HBV-related hepatocellular carcinoma for chronic hepatitis B patients at high risk. Expert Opin Biol Ther. 2018;18(10):1085–94. doi: 10.1080/14712598.2018.1518423. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence: Clinical Guidelines . Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young people and adults. London: National Institute for Health and Care Excellence (UK) Copyright © National Clinical Guideline Centre; 2013. [PubMed] [Google Scholar]
- 12.Huang D, Wu D, Wang P, Wang Y, Yuan W, Hu D, Hu J, Wang Y, Tao R, Xiao F, et al. End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after Peg-IFN-based therapy in patients with CHB. J Hepatol. 2022;77(1):42–54. doi: 10.1016/j.jhep.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Pan CQ, Li MH, Yi W, Zhang L, Lu Y, Hao HX, Wan G, Cao WH, Wang XY, Ran CP, et al. Outcome of Chinese patients with hepatitis B at 96 weeks after functional cure with IFN versus combination regimens. Liver Int. 2021;41(7):1498–508. doi: 10.1111/liv.14801. [DOI] [PubMed] [Google Scholar]
- 14.Brunskole Hummel I, Zitzmann A, Erl M, Wenzel JJ, Jilg W. Characteristics of immune memory 10–15 years after primary hepatitis B vaccination. Vaccine. 2016;34(5):636–42. doi: 10.1016/j.vaccine.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Liu Y, Lu J, Cao Z, Jin Y, Ma L, Geng N, Ren S, Zheng Y, Shen C, et al. Durability of interferon-induced hepatitis B surface antigen seroclearance. Clin Gastroenterol Hepatol. 2020;18(2):514–6.e512. doi: 10.1016/j.cgh.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Li MH, Yi W, Zhang L, Lu Y, Lu HH, Shen G, Wu SL, Hao HX, Gao YJ, Chang M, et al. Predictors of sustained functional cure in hepatitis B envelope antigen-negative patients achieving hepatitis B surface antigen seroclearance with interferon-alpha–based therapy. J Viral Hepat. 2019;26(Suppl 1):32–41. doi: 10.1111/jvh.13151. [DOI] [PubMed] [Google Scholar]
- 17.Hou J, Wang G, Wang F, Cheng J, Ren H, Zhuang H, Sun J, Li L, Li J, Meng Q, et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J Clin Transl Hepatol. 2017;5(4):297–318. doi: 10.14218/jcth.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip TC, Wong GL, Wong VW, Tse YK, Lui GC, Lam KL, Chan HL. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol. 2017;68(1):63–72. doi: 10.1016/j.jhep.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Wang X, Lin X, Shen C, Chen X. Quantitative of serum hepatitis B core antibody is a potential predictor of recurrence after interferon-induced hepatitis B surface antigen clearance. J Microbiol Immunol Infect. 2021;54(2):238–44. doi: 10.1016/j.jmii.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Chen X, Wu Y, Cao Z, Wang L, Huang H, Chen X, Lu F. Serum HBV RNA is a potential predictor of hepatitis B surface antigen reversion. Hepatol Commun. 2018;2(10):1168–71. doi: 10.1002/hep4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Sun F, Bi X, Lin Y, Yang L, Lu Y, Zhang L, Wan G, Yi W, Zhao L, et al. Consolidation treatment needed for sustained HBsAg-negative response induced by interferon-alpha in HBeAg positive chronic hepatitis B patients. Virol Sin. 2022;37(3):390–97. doi: 10.1016/j.virs.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Wang XW, Chen L, Hu P, Ren H, Hu HD. Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 2016;43(12):1253–61. doi: 10.1111/apt.13634. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi FP, Gallone MS, Gallone MF, Larocca AMV, Vimercati L, Quarto M, Tafuri S. HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-Hepatitis B vaccine: an Italian study among medical students. J Viral Hepat. 2019;26(1):136–44. doi: 10.1111/jvh.13001. [DOI] [PubMed] [Google Scholar]
- 24.Dikici B, Bosnak M, Ucmak H, Dagli A, Ece A, Haspolat K. Failure of therapeutic vaccination using hepatitis B surface antigen vaccine in the immunotolerant phase of children with chronic hepatitis B infection. J Gastroenterol Hepatol. 2003;18(2):218–22. doi: 10.1046/j.1440-1746.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 25.Dikici B, Kalayci AG, Ozgenc F, Bosnak M, Davutoglu M, Ece A, Ozkan T, Ozeke T, Yagci RV, Haspolat K. Therapeutic vaccination in the immunotolerant phase of children with chronic hepatitis B infection. Pediatr Infect Dis J. 2003;22(4):345–49. doi: 10.1097/01.inf.0000059443.49414.8b. [DOI] [PubMed] [Google Scholar]
- 26.Yalcin K, Acar M, Degertekin H. Specific hepatitis B vaccine therapy in inactive HBsAg carriers: a randomized controlled trial. Infection. 2003;31(4):221–25. doi: 10.1007/s15010-003-3187-1. [DOI] [PubMed] [Google Scholar]
- 27.Cargill T, Barnes E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin Exp Immunol. 2021;205(2):106–18. doi: 10.1111/cei.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fung S, Choi HSJ, Gehring A, Janssen HLA. Getting to HBV cure: the promising paths forward. Hepatology. 2022;76(1):233–50. doi: 10.1002/hep.32314. [DOI] [PubMed] [Google Scholar]
- 29.Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, Yoshida EM, Trinh H, Rodell TC, Laccabue D, et al. Combined GS-4774 and tenofovir therapy can improve HBV-specific T-cell responses in patients with chronic hepatitis. Gastroenterology. 2019;157(1):227–41.e227. doi: 10.1053/j.gastro.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Al Mahtab M, Akbar SMF, Aguilar JC, Guillen G, Penton E, Tuero A, Yoshida O, Hiasa Y, Onji M, Wong V. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One. 2018;13(8):e0201236. doi: 10.1371/journal.pone.0201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou C, Li C, Gong GZ, Wang S, Zhang JM, Xu DZ, Guo LM, Ren H, Xu M, Xie Q, et al. Analysis of immunological mechanisms exerted by HBsAg-HBIG therapeutic vaccine combined with Adefovir in chronic hepatitis B patients. Hum Vaccin Immunother. 2017;13(9):1989–96. doi: 10.1080/21645515.2017.1335840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, Zhang JM, Xu M, Wang HF, Huang WX, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS One. 2008;3(7):e2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8 + T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol. 2010;184(1):287–95. doi: 10.4049/jimmunol.0902761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


