Abstract
INTRODUCTION
Cancer-related cognitive impairment (CRCI) is prevalent in cancer survivors, and impairments affect daily living tasks and overall wellbeing. This review aimed to identify and evaluate published randomized controlled trials (RCTs) of interventions to manage CRCI in adult populations, to analyze their effectiveness and to investigate the quality of the studies.
EVIDENCE ACQUISITION
Seven databases were searched (Medline, Scopus, CINAHL, AMED, PsychINFO, OTseeker, and the Cochrane Database of Systematic Reviews), including years 2005-2020, for randomized controlled trials (RCTs) investigating interventions to address cognition for adults with cancer. The final search was conducted in February 2021. The quality of studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for RCTs. Meta-analysis used comprehensive meta-analysis software. The study protocol was registered with PROSPERO (registration N. CRD42017076868).
EVIDENCE SYNTHESIS
A total of 45 studies involving 4727 participants examined interventions for CRCI and met selection criteria. Categories of interventions included cognitive training-based intervention (N.=15), cognitive behavior therapy (CBT) (N.=4), physical activity (N.=16) and other supportive therapies (N.=10). Meta-analysis indicated beneficial overall effects for all categories of interventions: cognitive training (standardized mean difference [SMD]=0.41, 95% CI: 0.28-0.53, I2=88.87%); CBT (SMD=0.30, 95% CI: 0.14-0.46, I2=44.86%); physical activity (SMD=0.27, 95% CI: 0.20-0.35, I2=37.67%); and supportive therapies (SMD=0.27, 95% CI: 0.16-0.39, I2=64.94%). Studies used self-report cognitive outcome measures and neurocognitive testing, or a mixture.
CONCLUSIONS
Findings suggest that effective intervention for CRCI exist, and cognitive training is consistently supported as an effective intervention; however, a high level of heterogeneity was found. CRCI research is currently dominated by breast cancer survivors, and quality research is also needed to address the broader population of cancer survivors who experience CRCI.
Key words: Neoplasms, Cognition, Systematic review
Introduction
Up to 75% of cancer survivors face cancer-related cognitive impairment (CRCI).1 CRCI refers to impairments in cognitive functioning that are the direct result of cancer, or cancer treatment. Currently, there is a lack of consensus about the definition, assessment, and impact of CRCI,2 and the condition present in various ways, and there are differences in how cognition is measured.3 Allied health professional have an important role to play in supporting adults with cancer4 and have much to offer cancer survivors experiencing CRCI.5-8 However, allied health practice for cancer survivors with CRCI is not well developed. This review used the Occupational Performance Model of Australia (OPMA) definition of cognition to understand CRCI. The OPMA reports cognitive performance to be “the operations and interactions of mental processes used during task performance.”9 Therefore, cancer-related cognitive change can affect many of these mental processes, including language, learning and memory, complex attention (e.g. sustained attention, divided attention, selective attention, processing speed), and executive function.
CRCI affects areas of daily life including self-care, social activity, work, and productivity.10 However, the impacts vary between individuals. Previous studies have demonstrated that impaired cognitive functioning can increase job stress, cause anxiety in seeking employment,10 and can impact job performance and work output.11 It has also been reported that feelings of embarrassment and low self-esteem can influence the way in which cancer survivors experiencing CRCI participate in social activities, and interact within social relationships.12 Developing effective and accessible interventions for CRCI is therefore essential in allowing cancer survivors to return to every-day life and the best possible quality of life. However, health professionals do not have clear evidence upon which to base decisions when selecting an intervention to assist cancer survivors with CRCI or designing a comprehensive rehabilitation program.
The development of interventions to address CRCI is based on the premise that cognitive functioning can be improved, by practicing components of cognitive skills to improve occupational performance. Cognitive training has been found to improve performance on the specific tasks involved in the training (and those skills that are assessed), but there is less support that this can extend to improving performance on related tasks or everyday activities.13, 14 Neuroplasticity, or the capacity of neural networks to change and reorganize, is the theoretical basis of such interventions where participants engage in intensive targeted cognitive activities.14 Fatigue, anxiety, depression and sleep difficulties are also common cancer-related symptoms which may impact on cognitive functioning, making interventions such as cognitive behavioral therapy, exercise and meditation effective in improving cognition alongside improving self-efficacy and compensatory strategies.15
Many interventions have been considered for the management and prevention of CRCI. These can be grouped into four main areas.16 These include: 1) cognitive training or rehabilitation, where cognitive skills are retrained and graded through practice, using computerized and functional activities;17 2) cognitive Behavior Therapy (CBT) that uses behaviorally oriented programs to retrain, and compensate for lost cognitive abilities;17 3) exercise and physical activity may be used as a means of reducing the effects of CRCI, and improving cognition in cancer survivors as a primary, or secondary outcome;18 and 4) interventions including yoga, tai chi, meditative, or mindfulness programs, which are often evaluated in breast cancer survivors.19 Prescription medications may be used to improve cognition and to increase alertness, and supplements including various herbal medicines, are also used.20
However, the prevention and management of CRCI is not always discussed with cancer survivors and referral to services for management or support for cognitive concerns is limited.2 To develop an effective cancer rehabilitation program to support survivors with CRCI through short-term, and long-term recovery, a comprehensive analysis of available cognitive interventions and their effectiveness is needed.2 Previous reviews have included people with brain cancers which could have a more direct impact on cognitive functioning,21 have only included self-reported outcome measures of cognitive functioning,17 or must have completed systemic treatment such as chemotherapy or hormone therapy.16 The year range of studies identified in these reviews included 2007-2014,21 2012-2014,16 or 2012-2019.17 Therefore, this review aimed to consolidate the current evidence that examined non-pharmacological interventions such as cognitive training and supportive therapists to address CRCI in adult populations at any time in the treatment process, to identify the outcome measures used (whether self-reported or performance based evaluations of cognitive functioning) and to investigate the quality of the studies included.
Evidence acquisition
This study was a systematic review of randomized control trials (RCTs) that meet the criteria listed below. The study protocol is registered with PROSPERO (registration N. CRD42017076868).
Study criteria
Studies were included regardless of the type or stage of systemic medical cancer treatment, and studies were not excluded based on the type or stage of cancer treatment participants were undergoing at the time of the study. Using the PICO format, the population for the review was cancer survivors with cognitive impairment following cancer treatment, interventions were any interventions aimed at improving cognitive functioning, comparison was either wait-list or control, and outcomes were self-reported, or performance based cognitive measures.
Inclusion criteria
Inclusion criteria were:
participants within the RCT who had a primary cancer diagnosis and were at any stage of treatment;
a RCT that included the effect of a single intervention used to optimize cognitive functioning where a cognitive impairment was of concern. Quasi-randomization included;
cancer survivors defined as anyone post-cancer diagnosis;
adult participants within the RCT (18 years and over) of any gender;
any non-pharmacological intervention provided by a health professional with the aim to reduce cognitive impairment;
Use of any type of cognitive outcome measure;
cognitive function measured as a primary or secondary outcome;
control comparisons that could be usual care or an alternative or sham intervention;
pilot studies and studies conducted from 2005-2020, in English.
Exclusion criteria
Among the exclusion criteria:
cancer diagnosis of participants occurred prior to adulthood. The relationship between cognitive function and illness in childhood is fundamentally different to that of adulthood as the developing brain is particularly vulnerable to change.22 Primary diagnosis of participants with cancers of the brain and spinal cord (including head and neck cancer). Cancers of this kind are likely to have a direct or indirect effect on the brain and therefore cognitive functioning has the potential to be impacted via a different mechanism to CRCI;23
study participants had undergone, or were undergoing, treatment that directly targets the brain or spinal cord (e.g. cranial radiation or chemotherapy directly delivered to the brain or spine);
pharmacological interventions and other supplements available over the counter;
combined interventions where data on the outcomes could not be identified separately for each intervention;
functional MRI studies as these are diagnostic methods rather than interventions;
studies where participants were their own controls;
studies in languages other than English;
study protocols, reviews, and feasibility studies.
Outcome measures and interventions
Outcome measures included either performance-based cognitive functioning via valid neuropsychological testing, or self-reported measures completed by participants, or a mixture of these. Studies using a quality of life (QoL) measure were included if they provided a score for the cognitive domain separately to overall QoL. Studies included in this review examined interventions where cognitive function was a primary or secondary outcome measure. Interventions were defined as any treatment, distinct from systemic cancer treatment, that could improve cognitive functioning and did not involve the medical prescription of medications or use of herbal supplements.
Search methods
An electronic search of seven databases was completed in consultation with an academic liaison librarian at the University of Sydney to identify relevant articles in the literature published between 2005 and 2020. Limiting this search to the last 15 years allowed for the most up-to-date interventions and technologies to be included in the study, while capturing most studies in previous reviews.2 Databases included in this search were Medline, Scopus, CINAHL, AMED, PsychINFO, OTseeker, and the Cochrane Database of Systematic Reviews, accessed via the University of XXX library. Search strategies varied by database; however, search terms included “cancer” OR “neoplasms,” “cognitive” OR “cognitive dysfunction,” “cogniti*,” “neurocognition,” “neurocognitive,” “cognitive rehabilitation,” “chemobrain,” “chemo brain,” “chemofog,” “chemo fog.” Searches were limited to the English language and randomized control trials. In databases where restriction by study type was not possible, key terms “RCT” and “trial” were added to search terms. The studies included in the previous reviews identified above were also cross referenced to determine if they met the criteria for this review.
Study selection
Articles identified were exported to Endnote for screening and removal of duplicates. Individual studies from any reviews were included if relevant. One researcher (KM) completed the initial title screen of articles and relevant studies were entered into a Microsoft Excel (Windows; Redmond, WA, USA) spreadsheet for further screening. Two researchers (KM and LM) then independently screened abstracts of the remaining articles against the inclusion and exclusion criteria. Any disagreements were resolved via discussion. Reasons for exclusion were recorded and documented in a PRISMA flow chart. Following this, full-text articles were screened independently for inclusion by the two researchers.
Data extraction and quality analysis
Eligible studies were grouped based on their consideration of cognition as a primary or secondary outcome. Only outcome measures providing information about cognitive functioning were included in the analysis of effectiveness. Data were then extracted from all studies using an adaption of the Cochrane Data Extraction and Assessment Template.24 Information was extracted regarding the characteristics of the study, the intervention being trialed, and the results of the study. The intervention was categorized according to the label used by the study authors. For instance, if a study identified CBT as the intervention, the study was allocated to the CBT group. Identified interventions were evaluated using an Excel spreadsheet developed by the researchers. The spreadsheet was populated according to the level of training of those administering the intervention, intensity, duration, follow-up, cognitive outcome measures used, and accessibility to cancer survivors was defined as high if it was freely available in the community, did not need a referral or training. Accessibility was rated as moderate if some access to a health professional was needed, or low if there were specific eligibility criteria or a prescribed program from a healthcare professional was required.
Quality assessments were completed on each of the studies using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for RCTs.25 This tool consists of 13 items assessing risk of bias within RCTs on which the assessor checked “yes,” “no,” or “unclear.” Where no reference was made in the article to methods indicated by the appraisal instrument an “unclear” rating was given. Again, KM and LM independently rated the studies against these criteria and any differences were resolved via discussion. A quality score for each of the studies was calculated as the percentage of “yes” marks received out of the total applicable items.
Data synthesis
Interventions were categorized into two main groups by the authors based on the characteristics of the interventions. The mean outcome scores of both control and intervention groups for each study were entered into the Comprehensive Meta-Analysis software (https://www.meta-analysis.com/) for analysis of effect size, both for individual studies and for the group of interventions. Meta-analysis data analysis included the standardized mean difference, Hedges g, 95% confidence intervals, P value and I2 statistics.26 To determine long term gains, for RCTs where outcomes were assessed at numerous timepoints, mean scores were only entered for the final timepoint to demonstrate the overall effect of the intervention at completion of the study. As this review was focused on published data, authors were not contacted to provide further data that was unpublished. Where studies used multiple cognitive outcome measures and published these scores, results were pooled for each study to obtain summary effect size data for each study. To determine if the results for studies with low risk of bias differed from the results of studies with a high risk of bias, a separate analysis was done to compare studies with a quality score of greater than 70% with those with a quality score of less than 70%.
Evidence synthesis
Study selection
The total number of studies identified via electronic search was 2253 (Figure 1). Of the 294 studies included following the abstract screen, 15 abstracts needed to be discussed by the researchers to reach consensus. A total of 45 studies met the inclusion criteria following the screening process. Of the included studies, 35 examined cognitions as a primary outcome, while the remaining 10 included cognitive function as a secondary measure. The PRISMA flow diagram (Figure 1) outlines the full selection process, including reasons for exclusion of studies.
Figure 1.
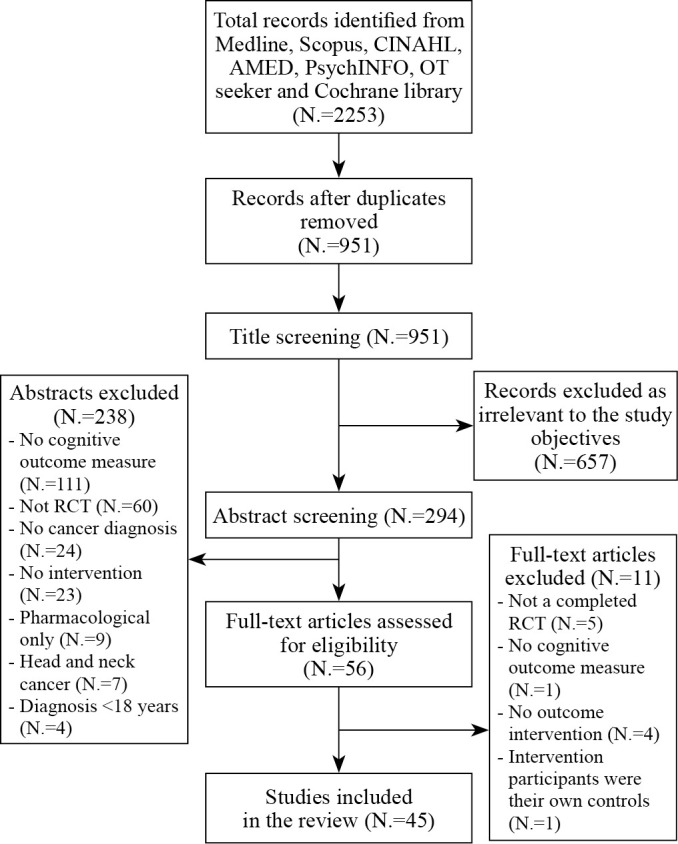
—PRISMA flow diagram.
Study characteristics
A total of 4727 participants from 15 countries were included across the 45 studies. Most studies (22) were completed in the USA, and middle-aged populations represented a high proportion of participants. Of the population groups, 31 studies (67.4%) were specific to breast cancer survivors only. The remaining studies either looked at a select group of cancers (including breast cancer) or were open to all cancer types. Finally, 29 (63%) of included articles had been published in the five years prior to the search (2015 and later). Full characteristics of each study are presented in Supplementary Digital Material 1: Supplementary Table I.
Intervention characteristics
Of the included studies, a wide range of interventions were identified, with different time schedules. Dos Santos,27 Freeman et al.28 and Von Ah et al.29 included two intervention groups with controls, and Peterson et al.30 included three intervention groups with an alternative intervention control. Ferguson et al.,31 Johns et al.,32 Larkey et al.,33 May et al.,34 McDougall et al.,35 Myers et al.,36 Schmidt et al.,37 Steindorf et al.38 and Vadiraja et al.39 also compared their intervention group with an alternative intervention (or a sham intervention), without a no-intervention control. The interventions within studies included various forms of physical activity (N.=16), supportive therapies (N.=10), cognitive retraining or rehabilitation (N.=15), and interventions identified as cognitive behavior therapy by authors (N.=4). Physical activity and supportive therapies included aerobic and resistance exercise, interval training, walking, use of a treadmill, exercise bikes and speed-feedback therapy, conducted individually or in a group, yoga and Tai Chi based practices, support group, psychosocial, and behavioral interventions, mindfulness or meditation interventions and acupuncture (Supplementary Table I).
Of the interventions where the level of training required for administration was classified as “low” (no specific training in cognition), all studies were within the supportive therapies category. These included trained yoga instructors, meditation specialists, and acupuncturists. However, this category also scored the highest regarding accessibility of the intervention to the general population. Most studies (60.8%) obtained high scores for duration of intervention (over six weeks in duration). However, the duration and intensity of interventions varied considerably from four to 24 weeks.
Common outcome measures
A total of 51 outcome measures were used across the studies. Supplementary Digital Material 2: Supplementary Table II summarizes the tools that were used.
The most commonly used tools were the Functional Assessment of Cancer Therapy- Cognition (FACT-Cog)40 and the European Organization for Research and Treatment of Cancer- Quality of Life C30 (EORTCQoL C30).41 There was more variation in performance-based objective measures. Of the 35 studies that included objective or performance-based assessment, 29 used a combination of multiple measures or a test battery, with the most commonly used objective measurement tools used being the trail making test, components of the Wechsler Adult Intelligence Scale (WAIS),42 and the Controlled Oral Word Association test (COWA).43 Studies within the cognitive training category tended to favor the use of neuropsychological testing. Across these 15 studies, three studies did not use a self-report measure. CBT based studies used a relatively equal combination of both subjective and objective measures, with six tests of each completed across the four studies. Finally, studies within the physical activity and supportive therapies group were the most likely to use self-report measures alone, with 10 of the 27 not including any performance measure of cognition.
Risk of bias within studies
Within the critical appraisal of the included studies (Supplementary Digital Material 3: Supplementary Table III), the studies with the highest percentage scores reported on methods allowing for blinding of participants and researchers or alternative interventions where participants may not be able to determine if they were in the intervention group or not. Key issues relating to the appraisal of the studies was a lack of reported concealed allocation of participants, and lack of information related to blinding of participants and outcome assessors. In studies such as these most participants would have known they were receiving an intervention or not (in the case of usual care), and researchers providing interventions would have been aware of who was receiving the intervention. Some outcomes were measured using online assessments that would be free of interference from study staff. No obvious relationships were observed between critical appraisal scores and study results. Studies may have incorporated these items in their studies but failed to report them in the article.
Meta-analysis
Of the included studies, 49 interventions were described within the 46 studies that provided sufficient data to be included in the meta-analyses. Meta-analyses were conducted for studies within the categories of intervention identified above using a random effects model. Figures indicate if mixed, or self-report or performance-based outcomes were used to calculate the effect size for each study.
Cognitive training interventions
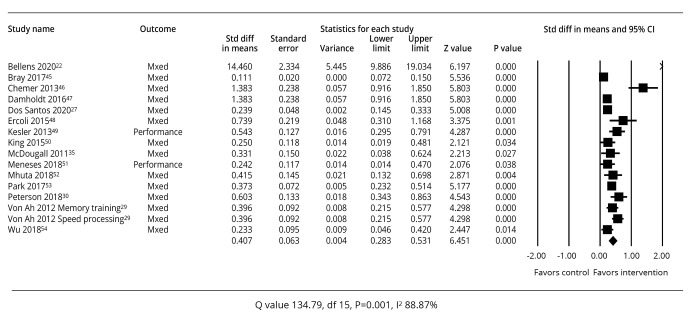
A summary of the study outcomes related to cognitive training interventions is provided in Figure 2.22, 27, 29, 30, 35, 44-53 All the studies of cognitive training (N.=16) had a P value <0.05 for their effect sizes in relation to cognitive performance which favored the interventions.
Figure 2.
—Meta-analysis of effect sizes for studies of cognitive training.22, 27, 29, 30, 35, 44-53
The summary effect sizes for all 16 studies indicated a standardized mean difference of 0.407 (95% CI: 0.283-0.531), P=0.001. Between-study heterogeneity in the cognitive training analysis indicated that the studies did not share a common effect size (Q=134.79, P=0.001, I2=88.87%).26 Seven of the studies were focused on breast cancer survivors. When analyzed separately, these studies had a standardized mean difference of 0.455 (95% CI: 0.244-0.665) and between-study heterogeneity of Q=54.995, P=0.0001, I2=87.271%.
Cognitive behavioral therapy
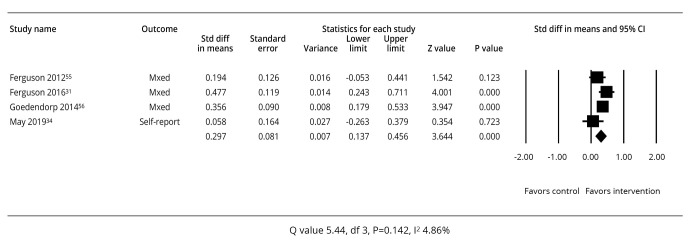
Of the four studies using cognitive behavioral therapy (CBT) interventions there was a mixed effect on cognitive performance (Figure 3).31, 34, 54, 55
Figure 3.
—Meta-analysis of effect sizes for studies of cognitive behavioral therapy.31, 34, 54, 55
Only the Ferguson et al.31 and Goedendorp et al.55 studies had a positive mean effect size on cognition (P≤0.05) which favored the intervention. The summary effect sizes for all four studies indicated a standardized mean difference of 0.297 (95% CI: 0.137-0.456), P=0.001. Between-study heterogeneity in the cognitive training analysis indicated that the studies had a high level of heterogeneity (Q=5.44, P=0.142, I2=44.86%).26 Two studies were focused on breast cancer survivors.31, 54 When analyzed separately these studies had a standardized mean difference of 0.338 (95% CI: 0.061-0.616), P=0.17, and between study heterogeneity of Q=2.665, P=0.103, I2=62.483%.
Physical activity
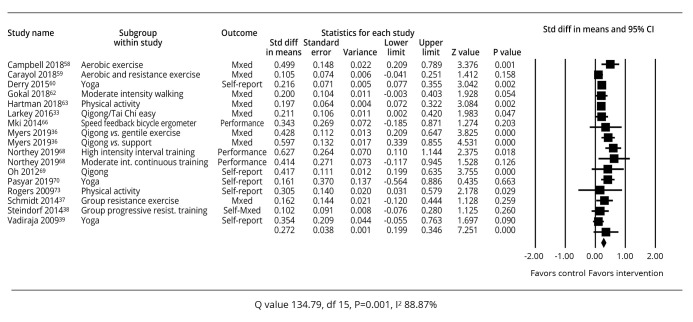
A summary of the study outcomes related to studies specifying physical activity is shown in Figure 4.33, 36-39, 56-65
Figure 4.
—Meta-analysis of effect sizes for studies of physical activity.33, 36-39, 56-65
Across all 16 studies included there were some inconsistencies in effect size results. Eight studies demonstrated a P value of >0.05 indicating an insignificant difference in the intervention and control groups. These were Carayol57 for aerobic and resistance exercise, Gokal59 for moderate intensity walking, Miki61 for speed feedback ergometer, Northey62 for moderate intensity continuous training, Schmidt37 for group resistance exercise, Steindorf38 for group progressive resistance training and Vadiraja et al.39 for yoga.
However, similar interventions had more positive effect sizes such as Campbell56 and Peterson30 for aerobic exercise, Derry et al.58 for yoga, Hartman60 and Rogers65 for physical activity, and Northey62 for high intensity interval training had more positive results. Despite inconsistencies, the summary effect size for the 16 studies indicated a standardized mean difference of 0.272 (95% CI: 0.199-0.346), P=0.001, favoring the interventions overall. Consistent with the range of results and interventions, between-study heterogeneity was also high in this analysis (Q=25.67, P=0.059, I2=37.67%).
Thirteen of these studies focused on breast cancer survivors and when analyzed separately the standard difference in means was 0.260 (95% CI: 0.183-0.337), P=0.001, and between study heterogeneity of Q=23.029, P=0.06, I2=39.206%.
Supportive therapies
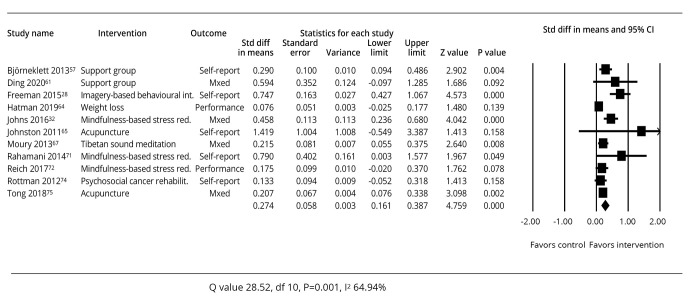
A summary of the study outcomes related to studies about supportive therapies is provided in Figure 5.28, 32, 66-74
Figure 5.
—Meta-analysis of effect sizes for studies of supportive therapies.28, 32, 66-74
Eleven studies were included using a wide range of supportive therapies with some inconsistencies in effect size results. Those demonstrating a P value of >0.05 indicating an insignificant difference in the intervention and control groups included Ding67 for a support group, Hartman et al.68 for weight loss, Johnston et al.69 for acupuncture, Reich et al.72 for mindfulness-based stress reduction, and Rottman et al.73 for psychosocial rehabilitation.
However, other interventions had more positive effect sizes such as Bjorneklett66 for a support group, Freeman28 for an imagery based behavioral intervention, Johns et al.32 and Rahmani et al.71 for mindfulness-based stress reduction, Milbury70 for Tibetan sound meditation, and Tong et al.74 for acupuncture, had more positive results.
Despite inconsistencies, the overall summary effect size for the 11 studies indicated a standardized mean difference of 0.274 (95% CI: 0.161-0.387), P=0.001, favoring the interventions overall. Consistent with the range of results and interventions, between-study heterogeneity was also high in this analysis (Q=28.52, P=0.001, I2=64.94%).
Seven of these studies were focused on breast cancer survivors and when analysed separately, the standard difference in means was 0.284 (95% CI: 0.120-0.447), P=0.001, and between study heterogeneity of Q=20.493, P=0.002, I2=70.721%.
Sensitivity analysis
All 29 (65%) studies had quality scores under 70% and 16 (35%) had quality scores over 70%. The lower quality studies were analyzed together and had a standard mean difference of 0.313 (95% CI: 0.237-0.389), P=0.001 and between study heterogeneity of Q=156.923, P=0.001, I2=80.882. Eight studies in this group had p values of >0.05 indicating an insignificant difference in the intervention and control groups. The high-quality studies had an overall standard mean difference of 0.330 (95% CI: 0.245-0.416, P=0.001 and between study heterogeneity of Q=33.25, P=0.004, I2=54.907%. Five studies in this group had p values of >0.05 indicating an insignificant difference in the intervention and control groups.
Discussion
This systematic review set out to identify and evaluate published RCTs examining interventions to manage CRCI in adult populations, to analyze their effectiveness via the outcome measures used, the impact of these interventions, and to investigate the quality of the studies. Whilst summary effect sizes indicated overall effectiveness in each category of interventions, the results were not unequivocal for individual studies. A total of 32 studies demonstrated a significant difference in cognitive outcomes (depending on the outcome measure used) such as changes in language, learning and memory, complex attention (e.g. sustained attention, divided attention, selective attention, processing speed), and executive function, between intervention and control groups, with 14 studies individually failing to demonstrate effectiveness.
Identified CRCI interventions
The key categories of cognitive interventions that were identified in this review were cognitive training, cognitive behavioral therapy, physical activity and supportive interventions. Cognitive training appeared to be the most consistently effective category of intervention. CBT is a widely used therapy that has origins in psychology and is commonly used in mental health settings. However, the results for CBT interventions in this review were mixed in relation to cognitive functioning for CRCI. Various methods of delivery were used for interventions included in this review including group and individual training and rehabilitation, in-person training, and online interventions. They also included the use of compensatory strategies that aim to maintain functionality in daily life, despite subtle cognitive impairment.75 Findings of the meta-analysis in this study suggest that directly addressing cognitive deficits through cognitive training is an effective method of improving cognition in cancer survivors.
The physical activity and supportive therapies categories encompassed a range of interventions. Key inclusions within this category are mind-body interventions (e.g. yoga, Tai Chi, Qigong) and meditation-based interventions. Although the usefulness and legitimacy of complementary therapies is often questioned, findings from this review demonstrated that a number of these interventions significantly improved cognition. One concern relating to studies in these categories is difficulties with adherence to, or standardization of the intervention. As these interventions are often self-managed by participants and completed at home, monitoring adherence to the intervention protocol can be difficult. Physical activity interventions included a range of types contributing to improved cognition. Some physical activity interventions aimed to address cancer-related fatigue, with cognitive functioning as a secondary outcome.38 Due to the link between fatigue and cognition it is important to consider interventions where cognition may not be the primary outcome. A high proportion of physical activity related studies were published in 2018-2020, suggesting a recent interest in linking exercise and physical activity with CRCI. Obesity and diabetes are related to cognitive decline in the general population and have also been linked to cognitive challenges experienced by breast cancer survivors by Hartman et al.68 As physical activity can help control these issues, this may have an effect on cognition for cancer survivors. Another hypothesis for the effectiveness of physical activity on improving cognition suggests that exercise leads to the production of brain derived neurotrophic factor, increased oxygenation via blood flow to the brain or decreases in inflammatory cytokines72 that can lead to improved cognitive function. Issues relating to home-based independently applied interventions and supervised individual and group interventions may affect adherence to an exercise program.
The studies included in this review were dominated by those targeted on breast cancer (BC) (31 studies) with BC also included in studies targeting any cancer types. This means that results cannot be generalized to all cancer survivors. When analyzed separately, the studies involving only BC survivors did not perform differently to the analysis of the studies together, indicating that the dominance of BC in the studies did not influence the results of which interventions were the most effective. There are many reasons why there are more studies involving BC survivors. There are large numbers of breast cancer diagnoses annually amounting to 14% of all cancers diagnosed, and survivorship rates are high.65 Cognitive impairments are frequently reported by breast cancer survivors, and these can be linked to chemotherapy treatments and the resulting induced menopause. Estrogen-reduction strategies postactive treatment such as ongoing endocrine treatments may also be associated with cognitive impairments.73 Anxiety and distress are also common symptoms reported by breast cancer survivors that may contribute to cognitive impairments.74 However, this does not negate the need to address CRCI in other types of cancer.
The review did not identify a relationship between study effectiveness and the duration of the intervention. Therefore, it is difficult to determine the minimum dose of an intervention that is required to be effective. Recommendations on intervention dosage have not been made in other reviews of CRCI management2, 15 and the gold standard of treatment and timing is yet to be determined.76
Comparison with other reviews
The current review intended to explore a comprehensive range of interventions for CRCI using cognitive outcomes and located 46 RCTs for review. Treanor et al.16 differed from this review as their selection criteria required cancer survivors to have completed their treatment and reviewed five RCTs. All the studies involved BC survivors and supported cognitive training as beneficial as well as compensatory strategy training. Zeng et al.17 conducted a review using a selection criterion of self-reported cognitive function only as a primary outcome measure and reviewed 29 RCTs. They also reported that the most effective interventions were meditation, cognitive training, cognitive rehabilitation, and exercise interventions. The current review included both self-reported and performance-based outcome measures of cognitive functioning and included studies at any point of the cancer treatment continuum. The selection criteria for the current review, resulted in a broader range of 14 countries represented within the review, including studies from Asian and Middle Eastern countries compared to other reviews. All the RCTs included in the Treanor et al.16 review and 27 of the 29 RCTs in the Zeng et al.17 review met the selection criteria for this review.
Outcome measurement
Studies included in this review used 16 self-reported cognitive outcome measures and 35 performance-based cognitive outcome measures (Supplementary Table II). Some of the performance-based measures were originally designed for a different treatment group which may limit their application to the CRCI group where impairments may be much more subtle and difficult to assess. The capacity of performance-based test results to translate into functional activity is questionable.
There may be a lack of agreement between scores on self-reported and performance-based cognitive outcome measures. This may be attributed to people needing to work harder to perform a neuropsychological performance test following chemotherapy, as evidenced by functional MRI data.77 This may then mask the level of cognitive impairment present as scores will remain high. However, there appears to be no consistent difference in effect sizes for studies using only self-reported outcome measures, only performance-based outcome measures or a mix of both types in this review. As so many outcome measures were used by studies included in this review, a consensus is needed to select the most appropriate measures so that real comparisons can be made. The International Cognition and Cancer Task Force has published their recommendations for outcome measures used for CRCI.78 They recommended the use of the Hopkins Verbal Learning Test-Revised (HVLT-R), Trail Making Test (TMT), and the Controlled Oral Word Association (COWA) test, to measure learning and memory, processing speed, and executive function. These tests were used in only two studies in this review.
Quality of research and risk of bias
The quality of studies included in systematic reviews is important to prevent an exaggeration of the overall treatment effect. Quality analysis of the studies identified poor concealment of allocation and blinding of participants and researchers, leading to potential selection bias. However, there may be methodological barriers to blinding participants participating in groups and undertaking certain activities related to improving cognitive functioning. Quality ratings for the studies that were common to this review and the Zeng et al.17 reviews were consistent. Of the studies included in this review, most received scores of over 50% for total quality. The comparison of studies deemed as high quality (score of over 70%) and low quality (score of under 70%) indicated that the studies did not demonstrate differences in effect sizes. The interventions in this review were compared with both usual care or waitlist populations as well as alternative interventions or sham interventions. Therefore, interpretation of effect size results for studies should take this into account.
Implications for clinicians and cancer survivors
This study provides insight into current options for the management of CRCI, which may improve the lives of those who experience it. For health professionals developing a rehabilitation program for people with CRCI, the choice of interventions and outcome measures to use is complex. The findings of this review can help inform clinical practice and assist practitioners in recommending and developing interventions and deciding how to evaluate them. A multidisciplinary approach to managing CRCI is important.76 Awareness of limitations in the scope of the research, and the financial and time burdens associated with interventions will allow practitioners to make informed decisions around implementing any interventions. There is need for further, high-quality research in this area to determine the best duration and intensity of an intervention to achieve the best gains in cognitive improvement, and to identify clinically significant effect sizes.
Limitations of the study
One limitation of this review is the restriction to studies written in English, as there was no opportunity for translation of studies in other languages, and there may have been other studies conducted in other languages. Whilst the exclusion of head and neck cancers was important for excluding cognitive dysfunction caused by the location of the cancer, this may have excluded other relevant studies of effective cognitive interventions. The review conclusions may also be affected by the variety of cognitive outcomes used across the studies, and whether they were used as primary or secondary cognitive outcomes. Furthermore, the time points when cognition was measured were varied. There were methodological limitations in the included studies as there was no consistency in reporting. The sample sizes of individual studies varied considerably which would contribute to the effect size reported. The applicability of interventions to different individuals at different stages of their cancer recovery may also have affected results as interventions that are effective at one stage may not be relevant for another stage.79-83 Included studies also provided interventions at different stages of the survivorship phase making comparison difficult.
Conclusions
The findings of this review are important in clinical decision making for practitioners involved in cancer care and survivorship for people with cognitive concerns. Health professionals are faced with a large choice of interventions for CRCI, some with inconsistent evidence about their effectiveness. The results suggest that, of the current interventions, best practice for improving cancer-related cognitive functioning includes cognitive training or some supportive therapies. As each of the meta-analyses indicated overall efficacy for different categories of interventions, there is insufficient evidence to reject any of the types of cognitive interventions. Clinicians need to consider the dominance of breast cancer populations in this review. To date, there are no guidelines about which cognitive interventions should be offered, and in this review several studies demonstrated high potential for selection bias. Clinicians also need to consider the time and financial burden of the interventions recommended to patients. The review has not identified the optimal dosage required for the interventions to be most effective. In some cases, this may mean longer (and more costly) interventions than those included in the review. CRCI can disrupt everyday activities for cancer survivors and interventions to address impairments are needed as part of a rehabilitation program. This comprehensive review has outlined potential interventions for CRCI, some of which require a multidisciplinary approach. Outcome measures commonly used for CRCI interventions are identified that can assist health professionals to address CRCI. The findings of this review provide preliminary evidence supporting the need for services beyond medical interventions for individuals experiencing CRCI. However, there is also a lack of consensus about which outcome measures reliably measure CRCI. The findings also suggest the need for further high-quality RCTs including a broader spectrum of cancer survivor populations.
Supplementary Digital Material 1
Supplementary Table I
Characteristics of included studies (N.=45).
Supplementary Digital Material 2
Supplementary Table II
Cognitive outcomes measures used in the studies to determine efficacy of interventions.
Supplementary Digital Material 3
Supplementary Table III
Quality of studies included in the review.
References
- 1.Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum 2012;39:E31–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22201666&dopt=Abstract 10.1188/12.ONF.E31-E40 [DOI] [PubMed] [Google Scholar]
- 2.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 2014;26:102–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24716504&dopt=Abstract 10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanaskie ML. Chemotherapy-related cognitive change: a principle-based concept analysis. Oncol Nurs Forum 2012;39:E241–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22543395&dopt=Abstract 10.1188/12.ONF.E241-E248 [DOI] [PubMed] [Google Scholar]
- 4.Pergolotti M, Williams GR, Campbell C, Munoz LA, Muss HB. Occupational therapy for adults with cancer: why it matters. Oncologist 2016;21:314–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26865588&dopt=Abstract 10.1634/theoncologist.2015-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nott MT, Barden HL, Chapparo C, Ranka JL. Evidence based practice and knowledge translation: A survey of Australian occupational therapy practice with clients experiencing neurocognitive impairments. Aust Occup Ther J 2020;67:74–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31758581&dopt=Abstract 10.1111/1440-1630.12625 [DOI] [PubMed] [Google Scholar]
- 6.Wallis A, Meredith P, Stanley M. Cancer care and occupational therapy: A scoping review. Aust Occup Ther J 2020;67:172–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31957031&dopt=Abstract 10.1111/1440-1630.12633 [DOI] [PubMed] [Google Scholar]
- 7.Faithfull S, Samuel C, Lemanska A, Warnock C, Greenfield D. Self-reported competence in long term care provision for adult cancer survivors: A cross sectional survey of nursing and allied health care professionals. Int J Nurs Stud 2016;53:85–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26412775&dopt=Abstract 10.1016/j.ijnurstu.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Mulcahy S, Prendergast J, Foley G, O, Hare A, Murphy E, Guinan EM, et al. Exercise rehabilitation services provided by physiotherapy departments in cancer care in Ireland. Ir Med J 2018;111:818. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30556666&dopt=Abstract [PubMed] [Google Scholar]
- 9.Ranka J, Chapparo C. Definition of terms. In J. Ranka, C Chapparo. Occupational Performance Model (Australia): Monograph 1. Sydney: Occupational Performance Network; 1997. p.58–60. [Google Scholar]
- 10.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 2009;3:223–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19760150&dopt=Abstract 10.1007/s11764-009-0098-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvio L, Peugeot M, Bruns GL, Todd BL, Feuerstein M. Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med 2010;52:219–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20134340&dopt=Abstract 10.1097/JOM.0b013e3181d0bef7 [DOI] [PubMed] [Google Scholar]
- 12.Selamat MH, Loh SY, Mackenzie L, Vardy J. Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PLoS One 2014;9:e108002. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25259847&dopt=Abstract 10.1371/journal.pone.0108002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, et al. Do “brain-training” programs work? Psychol Sci Public Interest 2016;17:103–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27697851&dopt=Abstract 10.1177/1529100616661983 [DOI] [PubMed] [Google Scholar]
- 14.Sala G, Gobet F. Cognitive training does not enhance general cognition. Trends Cogn Sci 2019;23:9–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30471868&dopt=Abstract 10.1016/j.tics.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol 2019;30:1925–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31617564&dopt=Abstract 10.1093/annonc/mdz410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treanor CJ, McMenamin UC, O’Neill RF, Cardwell CR, Clarke MJ, Cantwell M, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev 2016;(8):CD011325. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27529826&dopt=Abstract 10.1002/14651858.CD011325.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Y, Dong J, Huang M, Zhang JE, Zhang X, Xie M, et al. Nonpharmacological interventions for cancer-related cognitive impairment in adult cancer patients: A network meta-analysis. Int J Nurs Stud 2020;104:103514. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32004776&dopt=Abstract 10.1016/j.ijnurstu.2019.103514 [DOI] [PubMed] [Google Scholar]
- 18.Ehlers DK, Aguiñaga S, Cosman J, Severson J, Kramer AF, McAuley E. The effects of physical activity and fatigue on cognitive performance in breast cancer survivors. Breast Cancer Res Treat 2017;165:699–707. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28677009&dopt=Abstract 10.1007/s10549-017-4363-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers JS. Review complementary and integrative interventions for cancer-related cognitive changes. Asia Pac J Oncol Nurs 2015;2:215–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26719850&dopt=Abstract 10.4103/2347-5625.162825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton DL, Burger K, Novotny PJ, Fitch TR, Kohli S, Soori G, et al. The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support Care Cancer 2013;21:1185–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23150188&dopt=Abstract 10.1007/s00520-012-1647-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Ah D, Jansen CE, Allen DH. Evidence-based interventions for cancer- and treatment-related cognitive impairment. Clin J Oncol Nurs 2014;18(Suppl):17–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25427606&dopt=Abstract 10.1188/14.CJON.S3.17-25 [DOI] [PubMed] [Google Scholar]
- 22.Nathan PC, Patel SK, Dilley K, Goldsby R, Harvey J, Jacobsen C, et al. Children’s Oncology Group Long-term Follow-up Guidelines Task Force on Neurocognitive/Behavioral Complications After Childhood Cancer . Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med 2007;161:798–806. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17679663&dopt=Abstract 10.1001/archpedi.161.8.798 [DOI] [PubMed] [Google Scholar]
- 23.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol 2004;3:159–68. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14980531&dopt=Abstract 10.1016/S1474-4422(04)00680-5 [DOI] [PubMed] [Google Scholar]
- 24.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 25.Checklist for randomized controlled trials. Joanna Briggs Institute; 2017 [Internet]. Available from: https://joannabriggs.org/sites/default/files/2019-05/JBI_RCTs_Appraisal_tool2017_0.pdf [cited 2021, Sep 09].
- 26.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to meta-analysis. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 27.Dos Santos M, Hardy-Léger I, Rigal O, Licaj I, Dauchy S, Levy C, et al. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: A 3-arm randomized trial. Cancer 2020;126:5328–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32996583&dopt=Abstract 10.1002/cncr.33186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman LW, White R, Ratcliff CG, Sutton S, Stewart M, Palmer JL, et al. A randomized trial comparing live and telemedicine deliveries of an imagery-based behavioral intervention for breast cancer survivors: reducing symptoms and barriers to care. Psychooncology 2015;24:910–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25146413&dopt=Abstract 10.1002/pon.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 2012;135:799–809. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22918524&dopt=Abstract 10.1007/s10549-012-2210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson BM, Johnson C, Case KR, Shackelford DY, Brown JM, Lalonde TL, et al. Feasibility of a combined aerobic and cognitive training intervention on cognitive function in cancer survivors: a pilot investigation. Pilot Feasibility Stud 2018;4:50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29468085&dopt=Abstract 10.1186/s40814-018-0242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson RJ, Sigmon ST, Pritchard AJ, LaBrie SL, Goetze RE, Fink CM, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer 2016;122:1782–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27135464&dopt=Abstract 10.1002/cncr.29891 [DOI] [PubMed] [Google Scholar]
- 32.Johns SA, Von Ah D, Brown LF, Beck-Coon K, Talib TL, Alyea JM, et al. Randomized controlled pilot trial of mindfulness-based stress reduction for breast and colorectal cancer survivors: effects on cancer-related cognitive impairment. J Cancer Surviv 2016;10:437–48. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26586494&dopt=Abstract 10.1007/s11764-015-0494-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larkey LK, Roe DJ, Smith L, Millstine D. Exploratory outcome assessment of Qigong/Tai Chi Easy on breast cancer survivors. Complement Ther Med 2016;29:196–203. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27912947&dopt=Abstract 10.1016/j.ctim.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May AM, Korstjens I, van Weert E, van den Borne B, Hoekstra-Weebers JE, van der Schans CP, et al. Long-term effects on cancer survivors’ quality of life of physical training versus physical training combined with cognitive-behavioral therapy: results from a randomized trial. Support Care Cancer 2009;17:653–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18953578&dopt=Abstract 10.1007/s00520-008-0519-9 [DOI] [PubMed] [Google Scholar]
- 35.McDougall GJ, Becker H, Acee TW, Vaughan PW, Delville CL. Symptom management of affective and cognitive disturbance with a group of cancer survivors. Arch Psychiatr Nurs 2011;25:24–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21251599&dopt=Abstract 10.1016/j.apnu.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers JS, Mitchell M, Krigel S, Steinhoff A, Boyce-White A, Van Goethem K, et al. Qigong intervention for breast cancer survivors with complaints of decreased cognitive function. Support Care Cancer 2019;27:1395–403. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30128855&dopt=Abstract 10.1007/s00520-018-4430-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer 2015;137:471–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25484317&dopt=Abstract 10.1002/ijc.29383 [DOI] [PubMed] [Google Scholar]
- 38.Steindorf K, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Habermann N, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25096607&dopt=Abstract 10.1093/annonc/mdu374 [DOI] [PubMed] [Google Scholar]
- 39.Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med 2009;17:274–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19942107&dopt=Abstract 10.1016/j.ctim.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 40.Functional assessment of cancer therapy-cognition FACT-Cog. FACIT; 2016 [Internet]. Available from: https://www.facit.org/measures/FACT-Cog [cited 2021, Sep 09].
- 41.European Organization for Research and Treatment of Cancer Quality of life questionnaire EORTC-QLQ-C30. EORTC; 1995 [Internet]. Available from: https://www.eortc.org/research_field/quality-of-life/ [cited 2021, Sep 09].
- 42.WISC-IV Clinical Use and Interpretation. Wechsler Adult Intelligence Scale; 2008 [Internet]. Available from: https://www.sciencedirect.com/topics/neuroscience/wechsler-adult-intelligence-scale [cited 2021, Sep 09].
- 43.Controlled Oral Word Association Test. Encyclopedia of Clinical Neuropsychology; 2011 [Internet]. Available from: https://link.springer.com/referenceworkentry/10.1007%2F978-0-387-79948-3_876 [cited 2021, Sep 09].
- 44.Bray VJ, Dhillon HM, Bell ML, Kabourakis M, Fiero MH, Yip D, et al. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol 2017;35:217–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28056205&dopt=Abstract 10.1200/JCO.2016.67.8201 [DOI] [PubMed] [Google Scholar]
- 45.Cherrier MM, Anderson K, David D, Higano CS, Gray H, Church A, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci 2013;93:617–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24012579&dopt=Abstract 10.1016/j.lfs.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damholdt MF, Mehlsen M, O’Toole MS, Andreasen RK, Pedersen AD, Zachariae R. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psychooncology 2016;25:1293–300. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26763774&dopt=Abstract 10.1002/pon.4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ercoli LM, Petersen L, Hunter AM, Castellon SA, Kwan L, Kahn-Mills BA, et al. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology 2015;24:1360–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25759235&dopt=Abstract 10.1002/pon.3769 [DOI] [PubMed] [Google Scholar]
- 48.Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer 2013;13:299–306. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23647804&dopt=Abstract 10.1016/j.clbc.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King S, Green HJ. Psychological intervention for improving cognitive function in cancer survivors: a literature review and randomized controlled trial. Front Oncol 2015;5:72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25859431&dopt=Abstract 10.3389/fonc.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meneses K, Benz R, Bail JR, Vo JB, Triebel K, Fazeli P, et al. Speed of processing training in middle-aged and older breast cancer survivors (SOAR): results of a randomized controlled pilot. Breast Cancer Res Treat 2018;168:259–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29128897&dopt=Abstract 10.1007/s10549-017-4564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mihuta ME, Green HJ, Shum DH. Web-based cognitive rehabilitation for survivors of adult cancer: A randomised controlled trial. Psychooncology 2018;27:1172–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29266524&dopt=Abstract 10.1002/pon.4615 [DOI] [PubMed] [Google Scholar]
- 52.Park JH, Jung YS, Kim KS, Bae SH. Effects of compensatory cognitive training intervention for breast cancer patients undergoing chemotherapy: a pilot study. Support Care Cancer 2017;25:1887–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28132089&dopt=Abstract 10.1007/s00520-017-3589-8 [DOI] [PubMed] [Google Scholar]
- 53.Wu LM, Amidi A, Tanenbaum ML, Winkel G, Gordon WA, Hall SJ, et al. Computerized cognitive training in prostate cancer patients on androgen deprivation therapy: a pilot study. Support Care Cancer 2018;26:1917–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29282534&dopt=Abstract 10.1007/s00520-017-4026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology 2012;21:176–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22271538&dopt=Abstract 10.1002/pon.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goedendorp MM, Knoop H, Gielissen MF, Verhagen CA, Bleijenberg G. The effects of cognitive behavioral therapy for postcancer fatigue on perceived cognitive disabilities and neuropsychological test performance. J Pain Symptom Manage 2014;47:35–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23707383&dopt=Abstract 10.1016/j.jpainsymman.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 56.Campbell KL, Kam JW, Neil-Sztramko SE, Liu Ambrose T, Handy TC, Lim HJ, et al. Effect of aerobic exercise on cancer-associated cognitive impairment: A proof-of-concept RCT. Psychooncology 2018;27:53–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28075038&dopt=Abstract 10.1002/pon.4370 [DOI] [PubMed] [Google Scholar]
- 57.Carayol M, Ninot G, Senesse P, Bleuse JP, Gourgou S, Sancho-Garnier H, et al. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the “APAD1” randomized controlled trial. BMC Cancer 2019;19:737. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31345179&dopt=Abstract 10.1186/s12885-019-5896-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derry HM, Jaremka LM, Bennett JM, Peng J, Andridge R, Shapiro C, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology 2015;24:958–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25336068&dopt=Abstract 10.1002/pon.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gokal K, Munir F, Ahmed S, Kancherla K, Wallis D. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS One 2018;13:e0206874. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30485297&dopt=Abstract 10.1371/journal.pone.0206874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartman SJ, Nelson SH, Myers E, Natarajan L, Sears DD, Palmer BW, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer 2018;124:192–202. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28926676&dopt=Abstract 10.1002/cncr.30987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miki E, Kataoka T, Okamura H. Feasibility and efficacy of speed-feedback therapy with a bicycle ergometer on cognitive function in elderly cancer patients in Japan. Psychooncology 2014;23:906–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24532471&dopt=Abstract 10.1002/pon.3501 [DOI] [PubMed] [Google Scholar]
- 62.Northey JM, Pumpa KL, Quinlan C, Ikin A, Toohey K, Smee DJ, et al. Cognition in breast cancer survivors: A pilot study of interval and continuous exercise. J Sci Med Sport 2019;22:580–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30554923&dopt=Abstract 10.1016/j.jsams.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 63.Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, et al. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer 2012;20:1235–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21688163&dopt=Abstract 10.1007/s00520-011-1209-6 [DOI] [PubMed] [Google Scholar]
- 64.Pasyar N, Barshan Tashnizi N, Mansouri P, Tahmasebi S. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: A pilot study. Eur J Oncol Nurs 2019;42:103–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31479846&dopt=Abstract 10.1016/j.ejon.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 65.Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc 2009;41:935–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19276838&dopt=Abstract 10.1249/MSS.0b013e31818e0e1b [DOI] [PubMed] [Google Scholar]
- 66.Björneklett HG, Rosenblad A, Lindemalm C, Ojutkangas ML, Letocha H, Strang P, et al. Long-term follow-up of a randomized study of support group intervention in women with primary breast cancer. J Psychosom Res 2013;74:346–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23497838&dopt=Abstract 10.1016/j.jpsychores.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 67.Ding K, Zhang X, Zhao J, Zuo H, Bi Z, Cheng H. Managing Cancer and Living Meaningfully (CALM) intervention on chemotherapy-related cognitive impairment in breast cancer survivors. Integr Cancer Ther 2020;19:1534735420938450. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32683997&dopt=Abstract 10.1177/1534735420938450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartman SJ, Nelson SH, Marinac CR, Natarajan L, Parker BA, Patterson RE. The effects of weight loss and metformin on cognition among breast cancer survivors: Evidence from the Reach for Health study. Psychooncology 2019;28:1640–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31140202&dopt=Abstract 10.1002/pon.5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnston MF, Hays RD, Subramanian SK, Elashoff RM, Axe EK, Li JJ, et al. Patient education integrated with acupuncture for relief of cancer-related fatigue randomized controlled feasibility study. BMC Complement Altern Med 2011;11:49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21703001&dopt=Abstract 10.1186/1472-6882-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milbury K, Chaoul A, Biegler K, Wangyal T, Spelman A, Meyers CA, et al. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psychooncology 2013;22:2354–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23657969&dopt=Abstract 10.1002/pon.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahmani S, Talepasand S, Ghanbary-Motlagh A. Comparison of effectiveness of the metacognition treatment and the mindfulness-based stress reduction treatment on global and specific life quality of women with breast cancer. Iran J Cancer Prev 2014;7:184–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25628839&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 72.Reich RR, Lengacher CA, Alinat CB, Kip KE, Paterson C, Ramesar S, et al. Mindfulness-based stress reduction in post-treatment breast cancer patients: immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage 2017;53:85–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27720794&dopt=Abstract 10.1016/j.jpainsymman.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rottmann N, Dalton SO, Bidstrup PE, Würtzen H, Høybye MT, Ross L, et al. No improvement in distress and quality of life following psychosocial cancer rehabilitation. A randomised trial. Psychooncology 2012;21:505–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21308859&dopt=Abstract 10.1002/pon.1924 [DOI] [PubMed] [Google Scholar]
- 74.Tong T, Pei C, Chen J, Lv Q, Zhang F, Cheng Z. Efficacy of acupuncture therapy for chemotherapy-related cognitive impairment in breast cancer patients. Med Sci Monit 2018;24:2919–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29735975&dopt=Abstract 10.12659/MSM.909712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellens A, Roelant E, Sabbe B, Peeters M, van Dam PA. A video-game based cognitive training for breast cancer survivors with cognitive impairment: A prospective randomized pilot trial. Breast 2020;53:23–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32554133&dopt=Abstract 10.1016/j.breast.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmer P, Baumann FT, Oberste M, Wright P, Garthe A, Schenk A, et al. Effects of Exercise Interventions and Physical Activity Behavior on Cancer Related Cognitive Impairments: A Systematic Review. BioMed Res Int 2016;2016:1820954. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27144158&dopt=Abstract 10.1155/2016/1820954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Breast cancer in Australia statistics. Cancer Australia; 2020 [Internet]. Available from: https://www.canceraustralia.gov.au/affected-cancer/cancer-types/breast-cancer/statistics [cited 2021, Sep 09].
- 78.Ganz PA. Understanding the impact of breast cancer adjuvant endocrine therapy on cognitive function: a work in progress. Br J Cancer 2016;114:953–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27115566&dopt=Abstract 10.1038/bjc.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y, Hendrix CC. Cancer-related cognitive impairment in breast cancer patients: influences of psychological variables. Asia Pac J Oncol Nurs 2018;5:296–306. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29963592&dopt=Abstract 10.4103/apjon.apjon_16_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padgett L, Van Dyk K, Kelly N, Newman R, Hite S, Asher A. Addressing cancer-related cognitive impairment in cancer survivorship. Oncol Issues 2020;35:52–7. 10.1080/10463356.2020.1692601 [DOI]
- 81.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol 2007;25:3866–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17761972&dopt=Abstract 10.1200/JCO.2007.10.8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 2011;12:703–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21354373&dopt=Abstract 10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- 83.Duncan M, Moschopoulou E, Herrington E, Deane J, Roylance R, Jones L, et al. ; SURECAN Investigators. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 2017;7:e015860. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29187408&dopt=Abstract 10.1136/bmjopen-2017-015860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Characteristics of included studies (N.=45).
Supplementary Table II
Cognitive outcomes measures used in the studies to determine efficacy of interventions.
Supplementary Table III
Quality of studies included in the review.