Abstract
BACKGROUND
The benefits of cardiac rehabilitation (CR) are already well established; however, such intervention has been underused, mainly in low- and middle-income countries.
AIM
To compare adherence, effectiveness, and cost of a home CR with the traditional CR (TCR) in a middle-income country (MIC).
DESIGN
Single-blind randomized control trial.
SETTING
A university hospital.
POPULATION
Individuals with coronary disease that were eligible were invited to participate. A randomized sample of 51 individuals was selected, where two participants were not included by not meeting inclusion criteria.
METHODS
The home-CR group participated in health education activities, carried out two supervised exercise sessions, and was instructed to carry out 58 sessions at home. Weekly telephone calls were made. The TCR group held 24 supervised exercise sessions and were instructed to carry out 36 sessions at home.
RESULTS
49 individuals (42 male, 56.37±10.35years) participated in the study, 23 in the home-CR group and 26 in the TCR group. After the intervention, adherence in the home-CR and TCR groups was 94.18% and 79.08%, respectively, with no significant difference (P=0.191). Both protocols were effective for the other variables, with no differences. The cost per patient for the service was lower in the home-CR (US$ 59.31) than in the TCR group (US$ 135.05).
CONCLUSIONS
CR performed at home in an MIC demonstrated similar adherence and effectiveness compared to the TCR program, but with a lower cost for the service. The results corroborate the possibility of using home CR programs, even in MICs, after exercise risk stratification and under remote supervision.
CLINICAL REHABILITATION IMPACT
Home-CR can contribute to overcome participants’ barriers with compatible cost. Home-CR is effective in improving functional capacity and risk factors control. Perform risk stratification and remote supervision are essential to offer Home-CR.
Key words: Cardiac rehabilitation, Patient compliance, Direct service costs, Home care services
The burden of cardiovascular diseases (CVD) is growing, particularly in low- and middle-income countries (LMICs). Cardiac rehabilitation (CR) is a multidisciplinary program designed to prevent and control CVD. The core components of CR have been established by national1 and international associations.2
Although the benefits of CR are well stablished,1, 2 the availability of CR programs worldwide are insufficient to meet the requirements of all patients,3 specially in LMICs.4 It is estimated that CR programs are available in half of countries around the world, concentrated in high-income countries (HICs).3 CR programs are available only in 40% of LMICs, but most of them offer only exercise-based programs, not comprehensive.4 Barriers in health systems, CR programs as well as related to health providers and patients, have been identified specifically in LMICs.5 Frequently identified barriers are related to difficulties in personal participation. These include the distance of the CR sites, transportation difficulties, and work schedule. Recently, another important barrier to participation in CR programs has occurred with the social isolation imposed by the COVID-19 pandemic, which reinforces the need for alternative models of CR.6, 7
The inclusion of mobile health technologies through cardiac telerehabilitation programs and the possibility of carrying out exercises at home can help to increase participation in CR programs. In HICs, home CR has been considered safe8, 9 and contributed to overcoming participation barriers with similar positive effects, as observed in CR delivered in the outpatient settings, in mortality,10 functional capacity,11-14 quality of life,10, 15 control of cardiac risk factors,13, 15 and depressive symptoms.15 Some studies have also shown a lower cost of CR delivered at home.16
Alternative models of offering CR in LMICs, including home-based programs, have been proposed to reduce the cost and improve the participation rate. CR offered without the requirement of a facility would be the most feasible and improve participation in LMICs. Thus, the objective of this study was to evaluate the adherence, effectiveness, and cost of a home CR (home-CR) program in comparison with the traditional CR (TCR) program in a middle-income country (MIC). It was hypothesized that the home-CR program would have the same adherence and effectiveness as the TCR, but with lower cost.
Materials and methods
This study was conducted according to the Declaration of Helsinki and was approved by the University Ethics Committee (#CAAE 51528615.3.0000.5149). The protocol was registered at clinicaltrials.gov (NCT03605992) and published.17
All the participants were asked to carefully read and sign an informed consent.
Design
A single-blind and single-site randomized control trial was conducted according to the Consolidated Standards Of Reporting Trials (CONSORT).18 All volunteers signed the informed consent form and were randomly allocated to two parallel groups: Home-CR and TCR. Adherence and cost were evaluated after 12 weeks of the CR program. Effectiveness related to control of risk factors and health behaviors were evaluated before randomization (pre-CR), after 12 weeks of CR programs (post-CR), and 12 weeks after completion of the CR programs (follow-up).
Setting
The trial was conducted in Brazil, one of the MICs with a high incidence of CVD and high CR demand.3, 19 Because of the low availability of CR programs, it is not included in the public CVD line of care,20 a system that is used by 80% of the Brazilian population.21 The study was undertaken in a publicly funded CR department of one academic center (Clinical Hospital of UFMG in Belo Horizonte, Minas Gerais, Brazil).
Participants
Volunteers ≥18 years, with coronary artery disease, post-myocardial-infarction, or those who had undergone percutaneous coronary intervention or coronary artery bypass graft surgery and had been advised CR or were eligible to enroll were invited to participate. Volunteers presented clinical stability and were stratified by cardiologist as low or moderate risk to physical exercise.22 The exclusion criteria were cardiac events<1 month previously; any comorbid physical or serious mental condition, such as heart failure, or any visual or cognitive condition; that could preclude the participant from participating in the CR programs proposed.
The sample size was determined to the primary outcome (adherence) for an alpha of 5% and a power of 80% in the Fisher exact t test and based on the results of previous study.14 The sample size should be at least 36 participants per group. After a pilot study with 20 volunteers, it was adjusted to 33 participants. Additional details and the calculation for the secondary outcome are described in the published protocol.17
Intervention groups
The Home-CR and TCR groups were evaluated with maximal treadmill exercise test, submaximal exercise test (Incremental Shuttle Walk Test) before and after the program, and offer risk factor management (e. g., lipid control, blood pressure (BP) control). Both programs had a duration of 12-week exercise regimen and systematized education programs.23, 24 There is no charge to the volunteers.
The parameters for monitoring the exercise prescription compliance were the same for the Home-CR and TCR groups. The sessions consisted of 5 to 10 min of warm-up, 40 min of aerobic activity with heart rate varying between 60% and 80% heart rate reserve (60%, 70% and 80% heart rate reserve in the first, second and third month, respectively) and 5-10 min cool-down.25
The difference in the exercise plan between groups was the number of supervised sessions delivered and the method of monitoring the exercise intensity. Both groups were instructed to reach the frequency of five times a week, totaling 60 sessions. TCR group (control) attended 24 (40%) supervised sessions at the CR center (3 per week in the first month, 2 per week in the second month and 1 per week in the third month) with exercise intensity monitored by HR monitor (G Pulse®) and based on the Borg Scale, and 36 non-supervised sessions at home (intensity based on the Borg Scale). Home-CR group did two supervised sessions at the CR center monitored by HR monitor and 58 (96.7%) non-supervised sessions at home also accompanied by a HR monitor. The HR monitor used in both groups monitored the HR through a strap attached to the participant’s chest and the HR was transmitted to a watch. The home-CR group also received weekly phone calls to check the correct use of the monitor as well as to encourage and check the correct execution of exercises and use of the pedometer (HJA-310-Omron®) as an incentive. Individual information about exercise intensity and the number of sessions during the week were registered manually in a personal diary.
Education program
Both groups received six 14-minute education sessions in person delivered at the CR center and a version of the Cardiac College Manual (available at https://www.healtheuniversity.ca/pt/CardiacCollege/Pages/default.aspx). Doubts related to physical exercise or symptoms were also clarified during these sessions.
After graduating from the 12-week CR program, participants were instructed to continuously practice physical exercise, controlling the risk factors, and to schedule an evaluation at the CR center, if necessary.
Procedure
Consecutive volunteers were approached between January 2017 and October 2018 by a doctorate student. After informed consent signed and physician evaluation, volunteers were scheduled for an initial assessment consisting of a functional test and a questionnaire about demographic and clinical characteristics. Additional information related to actual clinical conditions was obtained from the medical file.
After the initial assessment, participants were randomized to one of the research groups. The randomization sequence was generated by a professor not involved in the study using the website www.randomization.com in random blocks of four: for every 4 volunteers, 2 could be randomly allocated to the home-CR group and 2 to the TCR group. To ensure allocation concealment, the principal investigator had the allocation sequence in a password-protected file, and only provided randomization information to the PhD student once it was confirmed that the participant was eligible. Due to the nature of the intervention, participants could not be blinded to treatment allocation.
A physiotherapist blinded to the random allocation was responsible for pre and post-test assessment and data entry. Another physiotherapist was responsible for CR sessions at the center, according to the usual CR program. The home-CR program was controlled by physiotherapy students previously trained by the PhD student and based on a systematized protocol. To avoid missing the scheduled CR sessions at the center, volunteers received reminder calls.
Measures
Primary outcome
Adherence is one of the factors responsible for promoting positive behaviour changes.13, 26 It was analysed by the percentage of sessions realized by each group at the end of the intervention. The number of sessions was identified in the TCR and home-CR groups through the physical therapist’s record in the face-to-face sessions and the personal diary manually filled in by the participant himself in the home sessions.
Secondary outcomes
Functional capacity was evaluated using the incremental shuttle walk test (ISWT) as the distance walked.27 Morbidity was determined by the number of hospitalizations, complications or the presence of adverse clinical events.
The risk factors evaluated were BP, waist circumference, glucose, and lipids. BP was assessed using a validated 7670–06 mobile stand (Welch Allync). Mean systolic and diastolic BP values were recorded, and hypertension was considered where values exceeded 140/90 mmHg and/or participants were taking a BP-lowering medication. A weight scale and measuring tape were used to assess anthropometrics. Waist circumference was assessed at the superior border of the iliac crest.28 Glycaemia and lipid values were extracted from the clinical charts. Dysglycemia was considered present where fasting blood glucose exceeded 126 mg/dL and/or participants were taking a glucose-lowering medication. Dyslipidemia was considered present where total cholesterol values exceeded 240 mg/dL and/or participants were on a lipid-lowering agent.28
Health behavior: quality of life was evaluated using the Short Form-3629, 30 with scores calculated for the physical and mental domains30 on a scale of 0 (worst) to 100 (better). Depressive symptoms were identified by the Patient Health Questionnaire-9,31 which has a total score varying from 0 to 2732 and indicates major depression if the total score is ≥10. The Duke Activity Status Index Score (DASI)33 was used to evaluate the self-reported functional capacity. The final score is between 0 (worst) and 58.2 (better).34 Participants’ knowledge about cardiac disease was evaluated using the Coronary Artery Disease Education Questionnaire-Short Version,35 a questionnaire with 20 items related to the clinical condition, risk factors, exercise, nutrition, and psychosocial risk; each correct answer was equal to 1 point, with a maximum total of 20 points.
The cost for the service was defined as the expenditure to participate in the CR program. The expenditure for availing the 12 weeks of intervention were considered while analyzing the cost of the CR programs. This analysis considered the sum of the expenses of the procedures delivered for each group of the study based on the median price of health materials during the period and by the payment values considered by the hospital for each health procedure and service. The procedures delivered at the same cost to the two groups were not considered, as the intention was to analyze the difference between the groups.
Statistical analysis
A researcher who did not participate in the other stages performed the statistical analysis. Continuous data are presented as measures of central tendency and dispersion (95% confidence interval) and categorical data as frequency. To analyze the normal distribution of the data, the Shapiro–Wilk test was performed. To compare sociodemographic and clinical characteristics between groups, independent t-test or χ2 Test were performed.
Adherence (in percentage) was compared by independent t-test. For comparisons of the other variables between moments (within analysis - pre-CR, post-CR, and follow-up three months after the end of intervention) and between groups (between analysis - home-CR and TCR), a mixed factorial ANOVA was performed, and an α of 5% was considered significant. The power was calculated for within and between analysis to demonstrate the measure of confidence in detecting the effect, if it exists. Outcome analyses were performed on the basis of intention-to-treat using the last observation carried forward to mitigate bias and as per protocol.
Data availability
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Results
Participants characteristics
The flow diagram is shown in Figure 1. Of the 51 eligible volunteers, 49 were selected. As shown in Figure 1, among those randomized to one of the CR groups after baseline evaluation, 47 (96%) initiated the program, 23 with TCR, and 26 with home-CR. Five (22%) participants in the TCR and two (8%) in the home-CR had valid clinical reasons for missing sessions.
Figure 1.
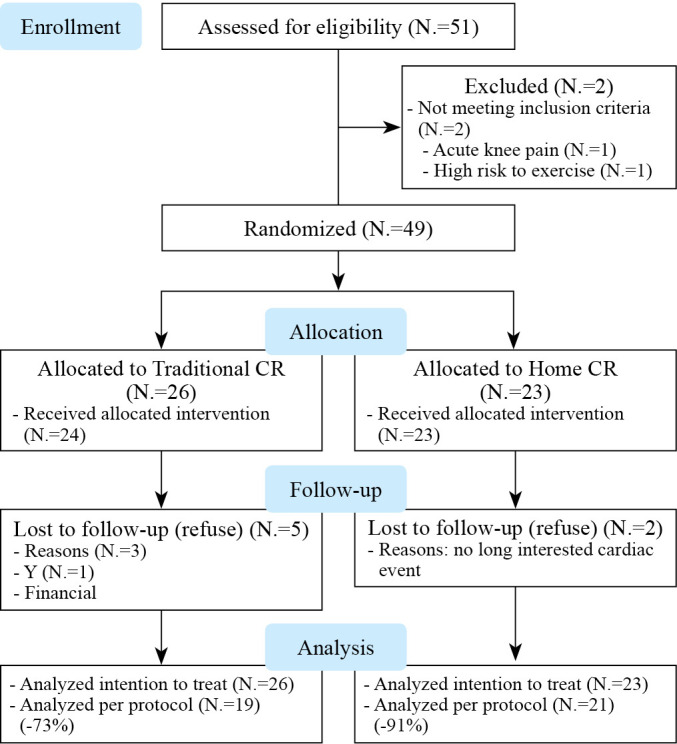
—Consolidated standards of reporting trials flow chart for cardiac rehabilitation.
Supplementary Digital Material 1, Supplementary Table I presents the sociodemographic and clinical characteristics of the 49 participants who attended the first day assessment and were randomly assigned to one of the CR programs. Randomization was effective in ensuring equivalence (P>0.05) across the groups in most characteristics.
Adherence
The retention rate at the end of the CR program (after 12 weeks) was 91% in the home-CR group and 73% in TCR. Participants in the home-CR group had adhered to 94.18% (95% CI 79.75-108.62) of the 60 sessions initially predicted versus 79.08% (95% CI 61.04-97.12) in TCR; the difference of 15.1% was not significant (P=0.191). In these sessions, 82.83% volunteers in the home-CR group achieved the exercise intensity prescribed (95% CI 66,57-99.08) versus 65.76% (95% CI 46.47-85.05) in TCR. The adherence to education activities was similar between groups (P=0.057): 81.27±30.50% in the TCR group versus 94.91±17.04% for home-CR. The home-CR participants had 100% adherence to phone calls.
Morbidity
No differences were observed between groups in the number of clinical events (11 in home-CR, including four by cardiac causes; compared to seven in TCR, one cardiac) as well as of hospitalizations (four in home-CR and one in TCR). None of these events was considered to be due to the CR intervention.
Effectiveness: functional capacity, clinical control of risk factors and health behaviors
Supplementary Digital Material 2, Supplementary Table II shows the functional capacity and risk factors pre-CR, post-CR (12 weeks) and follow-up (12 weeks after the end of the program). The functional capacity improved post-CR in both groups and was maintained at follow-up. Risk factors were controlled in the first assessment and no significant changes were observed post-CR or at follow-up in both groups, with no difference between them.
Supplementary Digital Material 3, Supplementary Table III shows an improvement in the quality of life with respect to physical and mental health from pre - to post-CR in both groups, with no significant differences between them. However, the reported physical health decreased at follow-up in both groups (P>0.05). The volunteers’ perception of functional capacity improved and the patient’s knowledge about the disease had the same pattern: improvement post-CR and maintenance at follow-up (Supplementary Table III).
Major depression was not identified on the initial assessment. Both groups showed reduced symptoms of depression post-CR and the results were maintained at follow-up (P=0.715 for group comparison).
Cost
As shown in Supplementary Digital Material 4, Supplementary Table IV, the total cost (12 weeks) for the service was approximately 44% lower for offering home-CR compared to TCR.
Discussion
In this randomized control trial, for the first time in Brazil and Latin America, we identified similar adherence and effectiveness, with lower cost, for the delivery of exercise and education through home-CR programs in comparison with traditional CR programs.
Besides the low availability of CR programs in LMICs, patient participation and adherence are also important challenges. Although home-CR volunteers participated in 15.1% more sessions than TCR, it was not statically significant. As we did not achieve the sample size calculated for this outcome, a type II error may have occurred, not finding a significant difference where it exists due to insufficient sample size. However, proving the hypothesis of equality between the home-CR and TCR groups already demonstrates clinical relevance. Other studies conducted in HICs showed higher adherence to home-CR compared to outpatient CR.9, 13, 14 The adherence per group in our study was similar to that in a previous study,14 wherein it was 94% in home-CR and 68% in traditional-CR. It could be related to the capacity to improve volunteers’ participation as it is possible to have flexibility to adapt the time dedicated to CR according and reduce the challenges related to transport.36 Moreover, barriers to participating in CR have been reported worldwide, including other LMICs and in Brazil, and frequently worries related to work, financial situation, and family issues were found.37, 38 Home-CR could contribute to overcoming these barriers. The high adherence of both groups to education activities also reinforces the viability of delivering home-CR when it is more appropriate because of different conditions, such as living in rural areas, transport issues, family demands, and recent situations like the COVID-19 pandemic.
Considering the clinical events identified during the study period, only one patient in the home-CR had to be hospitalized because of restenosis. This complication, independent of the CR model, can be expected after angioplasty, as it is more common between three to six months after the procedure.39 Furthermore, in a systematic review with meta-analysis, it was observed that the chances of having events during exercise at home were similar to those during exercise in a CR center when the risk stratification, adequate evaluation as well as individual exercise intensity are attended.40 Therefore, it is essential to perform risk stratification to determine if an individual can receive the CR program at home or if it is necessary to have direct supervision.
It is also important to note that in the risk stratification,22 one of the aspects evaluated is the capability of the patient to monitor and respect the exercise intensity prescribed as well as observe symptoms and promptly report them to the health staff. In the present study, we chose to give heart-rate monitors to monitor volunteers at home. A heart-rate monitor is a low-cost and easy-to-use device. Although HR monitors with electrodes are more accurate for monitoring during moderate to intensive training,41 the monitor with a strap attached to the participant’s chest allows usability in clinical practice. A strength of our study was the use of the pedometer, which ensured validity. Moreover, telephone calls allowed volunteers to improve safety and promote auto-efficacy during exercise.13 The home-CR model does not imply that volunteers will be unsupervised; rather it implies that the patient will be supervised remotely.22
As observed in previous studies, both CR programs are effective in improving the functional capacity14, 42 and contribute to maintaining control of risk factors15, 42 and health behavior.10-12, 14, 15 Recently because of the COVID-19 pandemic, the demand for home-CR services and programs has increased, showing that it is necessary to further explore such programs.6, 7
The low cost of delivering home-CR reinforces that it is viable to offer such programs in LMICs as a way to overcome barriers to patients’ participation in these programs, as well as to improve the availability of slots. Previous studies have also identified low cost of home-CR;11, 16 however, some studies show similar43 or even higher costs.44 This discrepancy could be related to the different models of intervention, number, type, and price of health professionals’ hours and equipment, considering that the characteristics of CR worldwide have variations.
To ensure the benefits of CR, it is necessary to continue exercising and changing habits to maintain the long-term effects. Other components besides aerobic exercise are usually included as the short component of CR. The protocol of study does not carry out strength training due to the difficulty of implementing and guaranteeing the performance of this type of exercise by the home-CR group. Therefore, we only performed aerobic exercise prescription to ensure the internal validity of the study. The performance of strength training without guaranteeing equality between the groups could be a bias for the analysis of the results and threaten the internal validity of the study.45
Limitations of the study
Limitations must be considered when interpreting the results of this study. Only volunteers with low and moderate risk for exercise were considered. Participants were selected from only one CR center of one LMIC. A type II error should be considered as the sample size for the primary outcome was not achieved. Manual recording of information on exercise intensity and number of sessions performed by home-CR participants in a diary. Finally, it is not possible to identify which technique was more effective in home-CR, as phone calls, pedometers, and heart-rate monitors were used simultaneously.
Conclusions
The home-CR program, based on physical exercise and patient education, showed similar adherence and effectiveness as the TCR program, but with low cost for the service in one MIC. The model of home-CR used in this study could be viable to be offered in other countries, since eligible volunteers were identified based on previous assessment for risk stratification as well as monitored remotely
Supplementary Digital Material 1
Supplementary Table I
Participant’s baseline sociodemographic and clinical characteristics by group.
Supplementary Digital Material 2
Supplementary Table II
Functional capacity and risk factors pre-, post-CR and follow-up. Per-protocol and intention-to-treat.
Supplementary Digital Material 3
Supplementary Table III
Quality of Life and health behaviors pre-, post-CR and at follow-up. Per-protocol and Intention-to-treat.
Supplementary Digital Material 4
Supplementary Table IV
Description of the service cost per participant to deliver the two models of CR.
References
- 1.Graham HL, Lac A, Lee H, Benton MJ. Predicting Long-Term Mortality, Morbidity, and Survival Outcomes Following a Cardiac Event: A Cardiac Rehabilitation Study. Rehabil Process Outcome 2019;8:1179572719827610. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34497458&dopt=Abstract 10.1177/1179572719827610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol 2016;67:1–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26764059&dopt=Abstract 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 3.Pesah E, Turk-Adawi K, Supervia M, Lopez-Jimenez F, Britto R, Ding R, et al. Cardiac rehabilitation delivery in low/middle-income countries. Heart 2019;105:1806–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31253695&dopt=Abstract 10.1136/heartjnl-2018-314486 [DOI] [PubMed] [Google Scholar]
- 4.Pesah E, Supervia M, Turk-Adawi K, Grace SL. A Review of Cardiac Rehabilitation Delivery Around the World. Prog Cardiovasc Dis 2017;60:267–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28844588&dopt=Abstract 10.1016/j.pcad.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 5.Sérvio TC, Britto RR, de Melo Ghisi GL, da Silva LP, Silva LD, Lima MM, et al. Barriers to cardiac rehabilitation delivery in a low-resource setting from the perspective of healthcare administrators, rehabilitation providers, and cardiac patients. BMC Health Serv Res 2019;19:615. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31477103&dopt=Abstract https://doi.org/ 10.1186/s12913-019-4463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanakis M, Batalik L, Papathanasiou J, Dipla L, Antoniou V, Pepera G. Exercise-based cardiac rehabilitation programs in the era of COVID-19: a critical review. Rev Cardiovasc Med 2021;22:1143–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34957758&dopt=Abstract 10.31083/j.rcm2204123 [DOI] [PubMed] [Google Scholar]
- 7.Scherrenberg M, Wilhelm M, Hansen D, Völler H, Cornelissen V, Frederix I, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;2047487320939671. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32615796&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 8.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol 2019;74:133–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31097258&dopt=Abstract 10.1016/j.jacc.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batalik L, Filakova K, Batalikova K, Dosbaba F. Remotely monitored telerehabilitation for cardiac patients: A review of the current situation. World J Clin Cases 2020;8:1818–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32518772&dopt=Abstract 10.12998/wjcc.v8.i10.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017;6:CD007130. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28665511&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 11.Maddison R, Rawstorn JC, Stewart RA, Benatar J, Whittaker R, Rolleston A, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30150328&dopt=Abstract https://doi.org/ 10.1136/heartjnl-2018-313189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moholdt T, Bekken Vold M, Grimsmo J, Slørdahl SA, Wisløff U. Home-based aerobic interval training improves peak oxygen uptake equal to residential cardiac rehabilitation: a randomized, controlled trial. PLoS One 2012;7:e41199. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22815970&dopt=Abstract 10.1371/journal.pone.0041199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart 2016;102:1183–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26936337&dopt=Abstract 10.1136/heartjnl-2015-308966 [DOI] [PubMed] [Google Scholar]
- 14.Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100:1770–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24973083&dopt=Abstract 10.1136/heartjnl-2014-305783 [DOI] [PubMed] [Google Scholar]
- 15.Gabelhouse J, Eves N, Grace SL, Reid RC, Caperchione CM. Traditional versus hybrid outpatient cardiac rehabilitation: A comparison of patient outcomes. J Cardiopulm Rehabil Prev 2018;38:231–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29135717&dopt=Abstract 10.1097/HCR.0000000000000253 [DOI] [PubMed] [Google Scholar]
- 16.Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, et al. Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: A cost-effectiveness analysis. Eur J Prev Cardiol 2016;23:674–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26289723&dopt=Abstract 10.1177/2047487315602257 [DOI] [PubMed] [Google Scholar]
- 17.Lima AP, Nascimento IO, Oliveira AC, Martins TH, Pereira DA, Britto RR. Home-based cardiac rehabilitation in Brazil’s public health care: protocol for a randomized controlled trial. JMIR Res Protoc 2019;8:e13901. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31697246&dopt=Abstract 10.2196/13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, CONSORT NPT Group . CONSORT Statement for randomized Trials of nonpharmacologic treatments: A 2017 update and a CONSORT extension for nonpharmacologic Trial Abstracts. Ann Intern Med 2017;167:40–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28630973&dopt=Abstract 10.7326/M17-0046 [DOI] [PubMed] [Google Scholar]
- 19.Britto RR, Supervia M, Turk-Adawi K, Chaves GS, Pesah E, Lopez-Jimenez F, et al. Cardiac rehabilitation availability and delivery in Brazil: a comparison to other upper middle-income countries. Braz J Phys Ther 2020;24:167–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30862431&dopt=Abstract 10.1016/j.bjpt.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro AL, Duncan BB, Brant LC, Lotufo PA, Mill JG, Barreto SM. Cardiovascular Health in Brazil. Circulation 2016;133:422–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26811272&dopt=Abstract 10.1161/CIRCULATIONAHA.114.008727 [DOI] [PubMed] [Google Scholar]
- 21.Viacava F, Oliveira RA, Carvalho CC, Laguardia J, Bellido JG. SUS: supply, access to and use of health services over the last 30 years. Cien Saude Colet 2018;23:1751–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29972484&dopt=Abstract 10.1590/1413-81232018236.06022018 [DOI] [PubMed] [Google Scholar]
- 22.American Association of Cardiovascular & Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- 23.Ghisi GL, Grace SL, Thomas S, Oh P. Behavior determinants among cardiac rehabilitation patients receiving educational interventions: an application of the health action process approach. Patient Educ Couns 2015;98:612–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25638305&dopt=Abstract 10.1016/j.pec.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Chaves GS, Ghisi GL, Grace SL, Oh P, Ribeiro AL, Britto RR. Effects of comprehensive cardiac rehabilitation on functional capacity in a middle-income country: a randomised controlled trial. Heart 2019;105:406–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30282639&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 25.Mezzani A, Hamm LF, Jones AM, McBride PE, Moholdt T, Stone JA, et al. European Association for Cardiovascular Prevention and Rehabilitation ; American Association of Cardiovascular and Pulmonary Rehabilitation; Canadian Association of Cardiac Rehabilitation. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol 2013;20:442–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23104970&dopt=Abstract 10.1177/2047487312460484 [DOI] [PubMed] [Google Scholar]
- 26.Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, et al. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol 2018;71:1814–37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29606402&dopt=Abstract 10.1016/j.jacc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1494764&dopt=Abstract 10.1136/thx.47.12.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herdy AH, López-Jiménez F, Terzic CP, Milani M, Stein R, Carvalho T, et al. South American guidelines for cardiovascular disease prevention and rehabilitation. Arq Bras Cardiol 2014;103(Suppl 1):1–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25387466&dopt=Abstract 10.5935/abc.2014S003 [DOI] [PubMed] [Google Scholar]
- 29.Ciconelli RM, Ferraz MB, Santos W, et al. Brazilian-Portuguese version of the SF-36 questionnaire: A reliable and valid quality of life outcome measure. Arthritis Rheum 1999;39:143–50. [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1593914&dopt=Abstract 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 31.Santos IS, Tavares BF, Munhoz TN, Almeida LS, Silva NT, Tams BD, et al. Sensibilidade e especificidade do Patient Health Questionnaire-9 (PHQ-9) entre adultos da população geral. Cad Saude Publica 2013;29:1533–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24005919&dopt=Abstract 10.1590/S0102-311X2013001200006 [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11556941&dopt=Abstract 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coutinho-Myrrha MA, Dias RC, Fernandes AA, et al. Duke activity status index em doenças cardiovasculares: validação de tradução em Português. Arq Bras Cardiol 2014;102:383–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24652056&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2782256&dopt=Abstract 10.1016/0002-9149(89)90496-7 [DOI] [PubMed] [Google Scholar]
- 35.Ghisi GL, Chaves GS, Loures JB, Bonfim GM, Britto R. Validation of the Brazilian-Portuguese version of a short questionnaire to assess knowledge in cardiovascular disease patients (CADE-Q SV). Arq Bras Cardiol 2018;111:841–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30281691&dopt=Abstract 10.5935/abc.20180169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knudsen MV, Laustsen S, Petersen AK, et al. Experience of cardiac tele-rehabilitation: analysis of patient narratives. Disabil Rehabil 2020. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31298957&dopt=Abstract [DOI] [PubMed]
- 37.Ghisi GL, dos Santos RZ, Aranha EE, Nunes AD, Oh P, Benetti M, et al. Perceptions of barriers to cardiac rehabilitation use in Brazil. Vasc Health Risk Manag 2013;9:485–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24039433&dopt=Abstract 10.2147/VHRM.S48213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragupathi L, Stribling J, Yakunina Y, Fuster V, McLaughlin MA, Vedanthan R. Availability, Use, and Barriers to Cardiac Rehabilitation in LMIC. Glob Heart 2017;12:323–334.e10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28302548&dopt=Abstract 10.1016/j.gheart.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 39.Nobuyoshi M, Kimura T, Nosaka H, Mioka S, Ueno K, Yokoi H, et al. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol 1988;12:616–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2969925&dopt=Abstract https://doi.org/ 10.1016/S0735-1097(88)80046-9 [DOI] [PubMed] [Google Scholar]
- 40.Dalal HM, Zawada A, Jolly K, Moxham T, Taylor RS. Home based versus centre based cardiac rehabilitation: cochrane systematic review and meta-analysis. BMJ 2010;340:b5631. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20085991&dopt=Abstract 10.1136/bmj.b5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etiwy M, Akhrass Z, Gillinov L, Alashi A, Wang R, Blackburn G, et al. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc Diagn Ther 2019;9:262–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31275816&dopt=Abstract 10.21037/cdt.2019.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jolly K, Lip GY, Taylor RS, Raftery J, Mant J, Lane D, et al. The Birmingham Rehabilitation Uptake Maximisation study (BRUM): a randomised controlled trial comparing home-based with centre-based cardiac rehabilitation. Heart 2009;95:36–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18332063&dopt=Abstract 10.1136/hrt.2007.127209 [DOI] [PubMed] [Google Scholar]
- 43.Wong WP, Feng J, Pwee KH, et al. A systematic review of economic evaluations of cardiac rehabilitation. BMC Health Serv Res 2021. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22873828&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 44.Kidholm K, Rasmussen MK, Andreasen JJ, Hansen J, Nielsen G, Spindler H, et al. Cost-Utility Analysis of a Cardiac Telerehabilitation Program: The Teledialog Project. Telemed J E Health 2016;22:553–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26713491&dopt=Abstract 10.1089/tmj.2015.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batalik L, Dosbaba F, Hartman M, Konecny V, Batalikova K, Spinar J. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur J Phys Rehabil Med 2021;57:807–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33619944&dopt=Abstract 10.23736/S1973-9087.21.06653-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Participant’s baseline sociodemographic and clinical characteristics by group.
Supplementary Table II
Functional capacity and risk factors pre-, post-CR and follow-up. Per-protocol and intention-to-treat.
Supplementary Table III
Quality of Life and health behaviors pre-, post-CR and at follow-up. Per-protocol and Intention-to-treat.
Supplementary Table IV
Description of the service cost per participant to deliver the two models of CR.
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.


