ABSTRACT
Many patients with hematological malignancies, such as acute myeloid leukemia, receive an allogeneic hematopoietic cell transplantation (HCT) to cure their underlying condition. Allogeneic HCT recipients are exposed to various elements during the pre-, peri- and post-transplant period that can disrupt intestinal microbiota, including chemo- and radiotherapy, antibiotics, and dietary changes. The dysbiotic post-HCT microbiome is characterized by low fecal microbial diversity, loss of anaerobic commensals, and intestinal domination, particularly by Enterococcus species, and is associated with poor transplant outcomes. Graft-versus-host disease (GvHD) is a frequent complication of allogeneic HCT caused by immunologic disparity between donor and host cells and results in tissue damage and inflammation. Microbiota injury is particularly pronounced in allogeneic HCT recipients who go on to develop GvHD. At present, manipulation of the microbiome for example, via dietary interventions, antibiotic stewardship, prebiotics, probiotics, or fecal microbiota transplantation, is widely being explored to prevent or treat gastrointestinal GvHD. This review discusses current insights into the role of the microbiome in GvHD pathogenesis and summarizes interventions to prevent and treat microbiota injury.
KEYWORDS: Allogeneic HCT, GvHD, gut microbiome, gut microbiota, FMT, mucosal immune system
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is used as consolidation therapy for many hematologic malignancies with the goal to induce a graft-versus-leukemia or graft-versus-lymphoma (GvL) response via alloreactive donor lymphocytes that eliminate residual tumor cells of the patient. These alloreactive lymphocytes, however, often also target the recipients’ tissues, inducing an inflammatory syndrome known as graft-versus-host disease (GvHD).1 GvHD is one of the major contributors to the high costs, the high morbidity, and the 10–30% transplantation-related mortality of allogeneic HCT, in addition to relapse of primary disease, organ failure, and infections.2–5 Treatment for GvHD consists of corticosteroids (prednisolone) and other immunosuppressants, such as the macrolide sirolimus, calcineurin inhibitors, and Janus-kinase-2 inhibitors such as ruxolitinib. Patients with steroid-refractory acute GvHD have a dismal prognosis.6 In search of new therapeutic strategies to improve the outcome for allogeneic HCT recipients, the intestinal microbiome has rapidly gained attention over the past decade. This review focuses specifically on the role of the intestinal microbiome in the development of GvHD and speculates on pre-transplant strategies to preserve a healthy microbiome or post-transplant therapies that target the gut flora to prevent or treat GvHD.
The pathogenesis of graft-versus-host disease
GvHD is classified into acute GvHD that develops early (<100 days) after allogeneic HCT, and late-onset acute or chronic GvHD that are considered late (>100 days) transplant-related complications. Acute GvHD primarily involves the skin, liver, and gastrointestinal tract, where symptoms typically include skin rash, jaundice, nausea, cramping, and diarrhea.7 Chronic GvHD is predominantly a sclerosing disease that can virtually affect any organ. Allogeneic HCT recipients routinely receive prophylactic immunosuppression, comprising (a combination of) anti-thymocyte globulin (ATG), post-transplantation cyclophosphamide, calcineurin inhibitors, mycophenolic acid, or methotrexate to prevent GvHD but despite these efforts, acute GvHD develops in 30–50% of the allogeneic HCT recipients.
In the classical view of acute GvHD pathophysiology, alloreactivity, and tissue damage are considered key driving factors (Figure 1).7–9 Chemo(radio)therapy preceding allogeneic HCT (referred to as ‘conditioning therapy’ as it conditions the recipient to receive the allograft) induces tissue damage and inflammation of the intestinal mucosa (mucositis), thereby weakening the intestinal mucosal barrier and increasing the risk of bacterial translocation. Together, this leads to the release of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) which activate hematopoietic and non-hematopoietic antigen-presenting cells (APCs). These APCs produce inflammatory cytokines and promote the priming and differentiation of donor immune cells. Activated neutrophils and alloreactive lymphocytes, including effector cells that are recruited into the tissues, also release inflammatory cytokines that enhance tissue damage and inflammation. It is assumed that chronic GvHD and the curative GvL response develop along the same pathways.10
Figure 1.
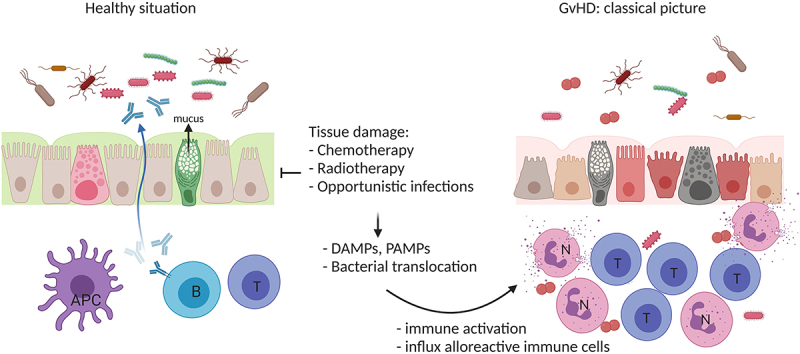
The classical picture of GvHD pathophysiology. Chemotherapy and/or radiotherapy (host conditioning) results in tissue damage, translocation of bacteria, and the release of damage-associated molecular patterns (DAMP) and pathogen-associated molecular patterns (PAMP), which activate host antigen-presenting cells (APC). APCs stimulate alloreactive donor lymphocytes (e.g. T cells (t)), which in turn produce inflammatory cytokines and recruit additional effector cells (e.g. neutrophils (n)) resulting in enhanced tissue damage and inflammation. Created with BioRender.com.
Evidence for a role for microbiota in GvHD pathophysiology and other transplantation-related complications
In steady-state conditions, a diverse collection of anaerobic commensal bacteria prevents the outgrowth of potential pathogens, produces essential nutrients, and orchestrates intestinal homeostasis through reciprocal interactions with the mucosal immune system.11 Gut microbiota contributes to a tolerant intestinal mucosal immune system for example, via the degradation of dietary polysaccharides into short-chain fatty acids (SCFA), such as butyrate, propionate, and acetate, which have an immunomodulatory function.12 SCFA stimulates the production of anti-inflammatory interleukin (IL)-10 by regulatory T cells (Treg, via inhibition of histone deacetylation) and IL-22 by both CD4+ T cells and innate lymphoid cells (ILC), which enhance epithelial integrity.13–15 SCFA also promote immunoglobulin A (IgA) secretion by B cells that provides a first line of defense against infiltrating pathogens.16 Furthermore, SCFA and other bacterial metabolites such as polyamines enhance gut barrier function by inducing Goblet cells to produce mucus and by stimulating the production of endothelial cell tight junction proteins.17,18
Advances in sequencing technology have allowed rapid and large-scale exploration of the compositional and to some extent functional configuration of the microbial communities in the human gut. This has initiated an impressive number of studies focusing on the relationship between microbiota and human health and led to the awareness that damage to the enteric bacterial population is associated with a variety of immune-mediated diseases, including GvHD.19 In 2012, it was reported that microbial diversity is reduced early after allogeneic HCT,20 and that this loss of diversity preceded the development of acute GvHD.21 A larger, prospective study later demonstrated that patients who maintained microbiota diversity had a significantly reduced probability of death due to GvHD or opportunistic infections, which translated into a better overall survival than patients who experienced loss of microbiome diversity.22 This association between low microbial diversity and survival disadvantage, more specifically higher transplantation-related and GvHD-related mortality, was recently confirmed in an international, multicenter study that included over 8000 samples from 1362 unique patients.23 Interestingly, this association was less apparent in patients receiving a lymphocyte-depleted allograft. The presence of alloreactive (donor-derived) adaptive immune cells thus appears to be a major contributor to the mortality associated with microbiota disruption. Indeed, in a murine allogeneic HCT model, it was demonstrated that radiotherapy-induced bacterial translocation leads to the recruitment of neutrophils and then, via the activation of alloreactive T cells, to acute GvHD.24
Loss of microbiome diversity may play a role in other aspects complicating transplantation outcomes that were noted in the past but less well understood. For example, obesity is associated with adverse outcome after allogeneic HCT. With the help of a murine HCT model, it was demonstrated that obesity, with its associated lack of microbiome diversity, leads to a pro-inflammatory intestinal milieu and an increased risk for GvHD.25 Moreover, allogeneic HCT recipients who survive the first 1–2 years after transplantation maintain a life-long increased risk for cardiovascular events and secondary malignancies. Loss of microbiome diversity is associated with a risk of cardiovascular events, malignancies, and poor health in general.26 In a cross-sectional study, it was demonstrated that the microbiome of allogeneic HCT survivors who developed secondary malignancies was more perturbed than long-term survivors without secondary malignancies.27 It is safe to assume that long-term complications in allogeneic HCT survivors are related to microbiome damage encountered early after transplantation, but evidence is lacking thus far.
Factors involved in microbiome disruption
Intestinal microbial diversity starts to decrease prior to transplantation, during the phase of remission-induced chemotherapy, and continues to decline in the first days after transplantation (Figure 2).20,28,29 The post-HCT intestinal microbiome commonly features low diversity, decreased abundance of commensals, and a high frequency of Enterococcus, Streptococcus, or Proteobacteria colonization compared to the microbiome of healthy individuals.23 A number of factors have been linked to early microbiota injury during allogeneic HCT. Allogeneic HCT recipients typically receive multiple rounds of chemotherapy, sometimes combined with total body irradiation (TBI), to reduce tumor burden and eliminate the recipients’ adaptive immune system to prevent graft rejection. The toxicity of both therapies has been implicated in the loss of microbial diversity and modulation of microbiome composition.30–36 This toxicity may act directly on gut microbes, and indirectly for example, through the death of Paneth cells. Paneth cells are secretory cells located at the intestinal crypt base where they produce antimicrobial factors to preserve host-microbial homeostasis.37 Patients with acute GvHD have reduced numbers of intestinal Paneth cells, while rescue of Paneth cells via treatment with glucagon-like-peptide-2 restored microbiome damage in a GvHD mouse model.38
Figure 2.
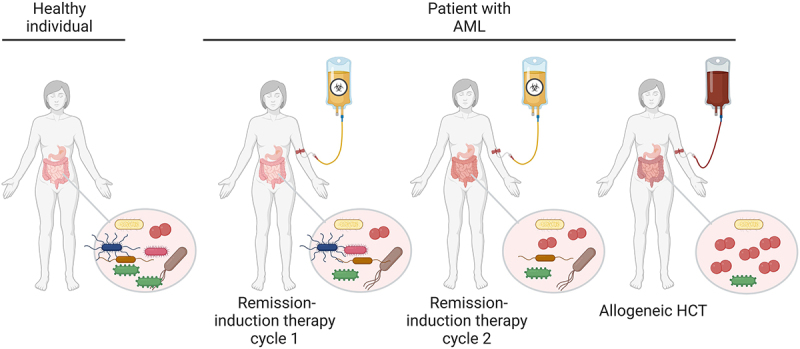
Peri-transplant microbiome injury. A typical schedule for the treatment of acute myeloid leukemia (AML) is depicted, with two AML remission-induction chemotherapy cycles followed by allogeneic HCT. Repeated cycles of remission-induction chemotherapy and subsequent allogeneic HCT are associated with alterations in diet and necessitate the use of antibiotics, anti-emetics, and other drugs that lead to loss of microbiome diversity. In addition, chemotherapy and radiotherapy directly damage the microbiome. Created with BioRender.com.
Most studied to date, however, is the microbiome-damaging effect of antibiotics, generally considered the culprit (but certainly not the only cause) of microbiome damage in allogeneic HCT recipients. Exposure to antibiotics is high and prolonged in allogeneic HCT recipients. Chemotherapy-induced mucositis of the oral cavity and gastrointestinal tract increases the risk of bacterial translocation,39 while remission-induction and conditioning chemotherapy regimens frequently lead to neutropenia. Prophylactic antibiotics are therefore routinely prescribed to prevent systemic infections, and patients receive empiric broad-spectrum antibiotics during episodes of neutropenic fever. In a retrospective study, the early administration of broad-spectrum antibiotics (between day −7 and day 0 before allogeneic HCT) led to a reduction in the abundance of Clostridiales and was associated with higher transplant-related mortality.40 Furthermore, the use of certain antibiotics was associated with a higher incidence of intestinal colonization and subsequent bloodstream infections by the respective dominating species.20,41 Antibiotics are, however, not the only drugs that can negatively impact microbiome diversity. Almost any drug can disturb the microbial community, as was demonstrated in two large observational studies in the healthy population of The Netherlands and Belgium.42,43 Commonly used drugs like anti-emetics, proton pump inhibitors, antidepressants, and opiates were demonstrated to affect the composition of the microbiome at a population level, and are therefore likely to impact the microbiome in allogeneic HCT recipients.
Another important factor that disturbs intestinal homeostasis may be the prolonged immunodeficiency that is characteristic of allogeneic HCT. The microbiome is shaped by microbes encountered from birth onwards and the interaction of these microbes with the developing immune system of the infant.44,45 After allogeneic HCT, this reciprocal interaction between the immune system and the microbiome comes under significant pressure as reconstitution of the donors’ adaptive immune system after transplantation typically takes months to years. This may significantly delay the recovery of the microbiome. In solid organ transplant recipients, the use of immunosuppressive drugs was the single most important determinant of microbiome damage, that lasted for years after transplantation.46 In allogeneic HCT, the microbiome was demonstrated to affect the recovery of innate and adaptive immunity in general and T cell recovery specifically, reinforcing the reciprocal nature of the interaction between the immune system and the microbiome.47,48
Finally, diet has a major influence on the constituents of the intestinal microbiome.49,50 The oral intake of allogeneic HCT recipients is frequently diminished due to the loss of appetite, pain, and nausea that is induced by mucositis, and this often requires parenteral feeding to ensure sufficient caloric intake.51 In two small cohorts, parenteral feeding was inferior to enteral nutrition in preserving microbiota diversity and composition.52,53 Moreover, two retrospective studies showed that parenteral nutrition was associated with a higher incidence of gastro-intestinal GvHD and lower survival rates compared to patients receiving adequate enteral nutrition.54,55
Collectively, these factors can drastically alter the gut microbiota community and disrupt intestinal homeostasis. The insights obtained from the different studies mentioned above led to a revised model of GvHD pathophysiology, where the importance of the gut microbiota as a mediator of immunologic tolerance and keeper of gut homeostasis was incorporated (Figure 3).56
Figure 3.
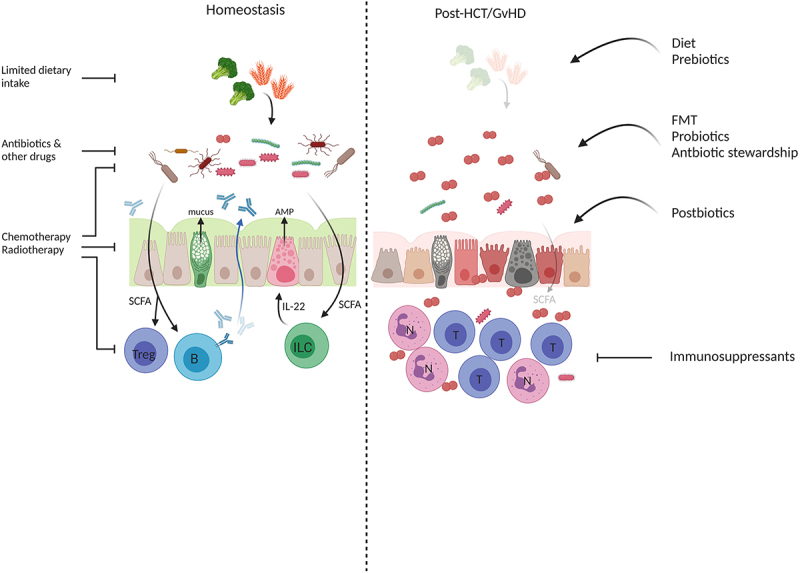
Disruption and restoration of intestinal homeostasis in GvHD. In the healthy situation (left panel), commensals metabolize dietary fibers to short-chain fatty acids (SCFA), which are important for immunologic tolerance via induction of regulatory T cells (Treg) and the production of secretory IgA by B cells (b). SFCA enhance IL-22 production via innate lymphoid cells (ILC) thereby supporting epithelial integrity and promoting anti-microbial peptide (AMP) production that are important in shaping the microbial community and the prevention of pathogen outgrowth. Goblet cells produce mucus to hamper bacterial translocation. All of these processes are impacted by factors that are inherent in cancer treatment, such as the use of antibiotics, changes in diet, etcetera. This results in dysbiosis, low levels of SCFA, hampered mucus production, damage to epithelial cells, activation and the influx of (alloreactive) T cells (t) and neutrophils (n), and inflammation (right panel). Where classic GvHD treatment predominantly focuses on tempering immune activation via immunosuppressants, novel approaches include therapies that target the microbiota to prevent or treat dysbiosis. Created with BioRender.com.
The ‘good’ and ‘bad’ guys associated with post-transplant complications
With the recognition of the intestinal microbiome as an important factor in GvHD pathogenesis, the question was raised whether gut homeostasis is regulated by the community as a whole, or by certain specific species. A number of studies demonstrated that certain species are in particular associated with worse outcome. The facultative anaerobe Enterococcus genus, which in the healthy microbiome represents only a very small minority (<0,1%),57 might be the best-studied pathobiont in relation to acute GvHD. Enterococci in particular, E. faecium and E. faecalis, are a prevalent cause of infection58 and, even more importantly in the context of GvHD, they have emerged as potent stimulators of intestinal inflammation.59 For example, E. faecalis compromises gut barrier integrity,60 induces the production of inflammatory cytokines,61 and causes colitis in IL-10 deficient mice.62 E. faecalis also induced donor T cell activation in a mouse GvHD model, suggesting a direct causal relationship.63 The frequency of intestinal Enterococcus domination is high in allogeneic HCT recipients and peaks at 1 week after HCT.23 In particular, there is a high abundance of E. faecium observed, which correlates with more severe acute GvHD and reduced survival.63 Other commonly dominating genera in allogeneic HCT recipients are Lactobacillus28 and Streptococcus. Streptococcus abundance is associated with induction of pro-inflammatory cytokines64 and has been shown to impair the growth of commensal bacteria in the oral microbiota.65 Streptococcus infections are more abundant in allogeneic HCT recipients with GvHD and are associated with higher mortality.66–68
Other species have been attributed to protective and/or anti-inflammatory capacities. For example, Barnesiella69 and Lactobacillus Johnsonii21 suppressed the outgrowth of Enterococcus species and thereby protect microbial homeostasis. A direct association with protection against lethal GvHD in allogeneic HCT recipients has been observed with the presence of Blautia, a genus from the Clostridia class.70 Preservation of Blautia was associated with reconstitution of gut epithelium protective mucosal-associated invariant T (MAIT) cells, which correlated with less acute GvHD and improved survival.71,72 An earlier study demonstrated that a mixture of 17 rationally selected Clostridia strains induced accumulation of Tregs73 and mitigated GvHD in mice,74 suggesting that this class of bacteria are important mediators in preserving intestinal homeostasis. One of the underlying mechanisms could be SCFA production; several Clostridia species, including Blautia spp., are well-known butyrate producers. A recent study reported that the fecal SCFA concentration is reduced in acute GvHD patients and correlates to the severity of disease.75 The concentration of circulating SCFAs also decreased, specifically in patients that go on to develop chronic GvHD.76 Loss of SCFA-producing bacteria and subsequent reduction of their beneficial metabolites thus appears to be important to limit conditioning or GvHD-mediated damage.74
Targeting the microbiome to prevent or treat acute GvHD
Together, these data suggest that maintenance of microbiome diversity in general, and specifically preservation or return of obligate anaerobe commensals, such as Blautia, could be of importance to prevent or treat acute GvHD. Given the many factors that affect the microbiome, there are a number of ways to prevent or treat microbiome damage during allogeneic HCT (Figure 3). Most of the studies referenced here are prospective trials or mechanistic (e.g. mouse model) studies, to avoid confounding factors that so often hamper proper interpretation of allogeneic HCT studies.
The most obvious first step is to limit damage to the intestinal microbiota. Antibiotic stewardship can play an important role in sparing the obligate anaerobe commensal community. In a retrospective study that included 161 allogeneic HCT recipients, it was demonstrated that the use of rifaximin preserved microbiota diversity, even when followed by systemic antibiotics.77 This was in contrast to other frequently used regimens, such as piperacillin/tazobactam or ciproxin/metronidazole, which were associated with significant microbiota disruption and damage to the intestinal epithelium.78 Patients who received rifaximin had a lower incidence of acute and chronic GvHD and better overall survival.79 Other studies investigating strategies to optimize antibiotic regimens to preserve intestinal microbiota composition are ongoing (for example, NCT03727113). Alternative strategies to prevent dysbiosis include compounds that neutralize antibiotic residues once they enter the colon80,81 and bacteria-specific antibody-antibiotic conjugates.82 These compounds have, however, not been tested in the context of allogeneic HCT. Novel approaches, such as CRISPR-Cas9 phagemids targeting pathogens by producing sequence-specific antimicrobials, are being developed.83
In healthy individuals, the effect of dietary intake on the microbiome is high and likely depends on the individual microbiome.50,84 Data obtained from mice and men demonstrate that Enterococcus domination is fed by dietary lactose, suggesting that lactose intake should be limited early after allogeneic HCT to prevent Enterococcus domination and acute GvHD.63 Thus far, reports on specific dietary interventions in allogeneic HCT are lacking but several studies are underway (for example, NCT0559009).
When microbiome damage cannot be prevented, ‘health-promoting’ species can be selectively or unselectively reintroduced. Probiotics are viable microorganisms that can be directly introduced in an attempt to restore bacterial diversity.85 Commercially available probiotics typically contain Lactobacillus and Bifidobacterium strains. In allogeneic HCT mouse models, administration of Lactobacillus rhamnosus GG resulted in less GvHD and increased survival.86 However, these findings were not reproducible in a study including allogeneic HCT recipients.87 In addition, the use of probiotics does raise concern in allogeneic HCT because of the risk of infectious complications.88,89,90,91,92 More importantly perhaps is the observation that probiotic therapy delayed rather than enhanced recovery of microbiome damage in healthy human volunteers.93 This raises serious questions regarding the efficacy of such therapy in humans.93,94 A rationally selected consortium of bacterial strains for example, containing Clostridia species, could offer an alternative for the commercial probiotics that are currently available. While further research might be needed to determine the optimal composition, the potential of bacterial consortia has been reported in a number of pre-clinical studies.73,74,95,96
Unselective introduction of bacterial species can be achieved by fecal microbiota transplantation (FMT). Donor FMT was first used in 1958 to treat colitis97 and is now applied to treat recurrent Clostridioides difficile infection.98 FMT resulted in restoration of microbiota diversity in patients who had received an allogeneic HCT.99,100 A few case reports suggested efficacy of FMT in steroid-refractory GvHD.101–103 We performed a prospective, single-arm study including 15 steroid-dependent or steroid-refractory GvHD demonstrating complete remission of GvHD in 10 patients.104 Interestingly, remission was sustained even after cessation of immunosuppressive therapy in 6 out of 10 responders. In this study, the use of antibiotics seemed associated with failure of FMT. Other, larger trials are underway and the results of these trials are expected soon (NCT03359980). Importantly, transfer of pathogens, including multidrug-resistant bacterial strains105 and infectious viruses,106 has been described, emphasizing the importance of careful selection and screening of FMT donors.107
Finally, not the microbial populations themselves but the metabolites they produce could be replenished. Prebiotics are defined as ‘substrates that are selectively utilized by host microorganisms conferring a health benefit’,108 and postbiotics are the bacterial components and metabolites that exert beneficial effects on other microbes, the gut epithelium, and the immune system.109 Given the reciprocal effects of pre- and postbiotics on homeostasis and composition of the microbiome, and on health-promoting factors produced by the microbiome, this distinction is somewhat arbitrary however.84A few groups have studied the effects of supplementation of microbiome products in allogeneic HCT recipients.110 Adequate intake of glutamine, fiber, oligosaccharides and resistant starch mixtures decreased diarrhea, mucositis, and bacteremia, and improved survival.111–113 Whether pre- and postbiotic therapy can restore healthy microbial populations, or whether additional support, for example in the form of FMT, is needed remains unanswered.
Conclusion
Efforts of the last couple of years have revealed important associations between the status of the gut microbiota and the prognosis of allogeneic HCT recipients. Outlines of the dysbiotic fecal microbiome in these patients have been drawn and the first careful steps have been taken to apply this knowledge in a clinical, interventional setting. Moving forward, there are several questions that will need to be answered.
First, a better understanding of the resilience of the microbiome is needed. Recent reports suggest that microbiome damage incurred upon transplantation is not reversible and persists over time.27,48 This is in contrast to observations in otherwise healthy individuals, in whom a single insult to the microbiome (e.g. course of antibiotics) induces microbiome damage that is reversible, and warrants further investigation into the causes of the irreversible nature of transplantation-related microbiome damage.93 Possible explanations could be persisting adaptive immunodeficiency or the permanent deletion of specific bacterial, archaeal, or viral species or populations that have a detrimental impact on microbiome homeostasis.45 With this knowledge, randomized controlled trials can be designed to prevent permanent damage to the microbiome. Additionally, we have to gain more insight into the mechanisms by which intestinal microbiota influence transplant-related complications to design more specific and tailor-made interventions.
Taken together, better care for the microbiome before and after allogeneic HCT, in addition to early interventions to repair microbiome damage after transplantation may significantly reduce transplantation-related morbidity and mortality, and improve allogeneic HCT outcomes in the short and long run.
Funding Statement
This work was supported by an anonymous donation via the AMC Foundation (YFvL), an intramural grant from the Academic Medical Center (YFvL), a VIDI grant (NWO ZonMW #91715362) (MDH), and a LSBR Fellowship (1438F) (MDH; Landsteiner Foundation for Blood Transfusion Research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Pasquini M, Wang Z, Horowitz MM, Gale RP.. Report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2013;2013:187–13. [PubMed] [Google Scholar]
- 2.D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, Devine S, Eapen M, Hamadani M, Hari P, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020. Aug;26(8):e177–e182. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penack O, Peczynski C, Mohty M, Yakoub-Agha I, Styczynski J, Montoto S, Duarte RF, Kröger N, Schoemans H, Koenecke C, et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020. Dec 22;4(24):6283–6290. doi: 10.1182/bloodadvances.2020003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gifford G, Gilroy N, Dyer G, Shouval R, Waters NR, Gomes ALC, Zuanelli Brambilla C, Fei T, Devlin SM, Nguyen CL, et al. The experience of survival following allogeneic haematopoietic stem cell transplantation in New South Wales, Australia. Bone Marrow Transplantation. 2016. Oct;51(10):1361–1368. doi: 10.1038/bmt.2016.135. [DOI] [PubMed] [Google Scholar]
- 5.Wolff D, Bardak J, Edinger M, Klinger-Schindler U, Holler E, Lawitschka A, Schoemans H, Herr W, Kröger N, Ayuk Ayuketang F. Evaluation of the Cost of survivorship care after allogeneic hematopoeitic stem cell transplantation-an analysis of 2 German transplantation centers. Front Public Health. 2020;8:572470. doi: 10.3389/fpubh.2020.572470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, Khouri IF, Shpall EJ, Anderlini P, Rondon G, et al. Steroid-Refractory Acute GVHD: predictors and Outcomes. Adv Hematol. 2011;2011:601953. doi: 10.1155/2011/601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeiser R, Blazar BR. Acute graft-versus-host disease - Biologic process, prevention, and therapy. N Engl J Med. 2017. Nov 30;377(22):2167–2179. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenq RR, van den Brink MRM. van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010. Mar;10(3):213–221. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 9.Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017. Dec 28;377(26):2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
- 10.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006. Mar;57(3):225–244. doi: 10.1016/j.critrevonc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016. May 27;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996. May;62(5):1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science. 2013. Aug 2;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018. Sep 3;9(1):3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020. Sep 8;11(1):4457. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016. Aug 10;20(2):202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudier E, Jarry A, Blottiere HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004. Dec;287(6):G1168–74. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 18.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003. Oct;52(10):1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruff WE, Greiling TM, Kriegel MA. Host-microbiota interactions in immune-mediated diseases. Nat Rev Microbiol. 2020. Sep;18(9):521–538. doi: 10.1038/s41579-020-0367-2. [DOI] [PubMed] [Google Scholar]
- 20.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012. Oct;55(7):905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012. May 7;209(5):902–910. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taur Y, Jenq RR, Perales M-A, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014. Aug 14;124(7):1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, Weber D, Hashimoto D, Slingerland AE, Slingerland JB, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020. Feb 27;382(9):822–834. doi: 10.1056/NEJMoa1900623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulsdunker J, Ottmuller KJ, Neeff HP, Koyama M, Gao Z, Thomas OS, Follo M, Al-Ahmad A, Prinz G, Duquesne S, et al. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset. Blood. 2018. Apr 19;131(16):1858–1869. doi: 10.1182/blood-2017-10-812891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khuat LT, Le CT, Pai CS, Shields-Cutler RR, Holtan SG, Rashidi A, Parker SL, Knights D, Luna JI, Dunai C, et al. Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation. Sci Transl Med. 2020. Nov 25;12(571). doi: 10.1126/scitranslmed.aay7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016. Oct 6;22(10):1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 27.Hino A, Fukushima K, Kusakabe S, Ueda T, Sudo T, Fujita J, Motooka D, Takeda AK, Shinozaki NO, Watanabe S, et al. Prolonged gut microbial alterations in post-transplant survivors of allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2022. Dec 5; doi: 10.1111/bjh.18574. [DOI] [PubMed] [Google Scholar]
- 28.Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Halaweish HF, Kaiser T, Holtan SG, Khoruts A, Weisdorf DJ, Staley C, et al. Lasting shift in the gut microbiota in patients with acute myeloid leukemia. Blood Adv. 2022. Jun 14;6(11):3451–3457. doi: 10.1182/bloodadvances.2021006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashidi A, Kaiser T, Graiziger C, Holtan SG, Rehman TU, Weisdorf DJ, Dunny GM, Khoruts A, Staley C. Gut dysbiosis during antileukemia chemotherapy versus allogeneic hematopoietic cell transplantation. Cancer. 2020. Apr 1;126(7):1434–1447. doi: 10.1002/cncr.32641. [DOI] [PubMed] [Google Scholar]
- 30.Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley Des Varannes S, Massart S, Moreau P, Potel G, de La Cochetière MF, Batard E. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015. Sep;42(5):515–528. doi: 10.1111/apt.13302. [DOI] [PubMed] [Google Scholar]
- 31.Galloway-Pena JR, Shi Y, Peterson CB, Sahasrabhojane P, Gopalakrishnan V, Brumlow CE, Daver NG, Alfayez M, Boddu PC, Khan MAW, et al. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute Myeloid Leukemia. Clin Infect Dis. 2020. Jun 24;71(1):63–71. doi: 10.1093/cid/ciz777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Vliet MJ, Tissing WJ, Dun CA, Meessen N, Kamps W, de Bont E, Harmsen H. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009. Jul 15;49(2):262–270. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 33.Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, Kitzweger E, Ruckser R, Haslberger AG. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One. 2011;6(12):e28654. doi: 10.1371/journal.pone.0028654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biagi E, Zama D, Nastasi C, Consolandi C, Fiori J, Rampelli S, Turroni S, Centanni M, Severgnini M, Peano C, et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplantation. 2015. Jul;50(7):992–998. doi: 10.1038/bmt.2015.16. [DOI] [PubMed] [Google Scholar]
- 35.Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O, Dheer R, et al. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut. 2018. Jan;67(1):97–107. doi: 10.1136/gutjnl-2017-313789. [DOI] [PubMed] [Google Scholar]
- 36.Shouval R, Waters NR, Gomes ALC, Zuanelli Brambilla C, Fei T, Devlin SM, Nguyen CL, Markey KA, Dai A, Slingerland JB, et al. Conditioning regimens are associated with distinct patterns of microbiota injury in allogeneic hematopoietic cell transplantation. Clin Cancer Res. 2023. Jan 4;29(1):165–173. doi: 10.1158/1078-0432.CCR-22-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cray P, Sheahan BJ, Dekaney CM. Secretory sorcery: paneth cell control of intestinal repair and homeostasis. Cell Mol Gastroenterol Hepatol. 2021;12(4):1239–1250. doi: 10.1016/j.jcmgh.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norona J, Apostolova P, Schmidt D, Ihlemann R, Reischmann N, Taylor G, Köhler N, de Heer J, Heeg S, Andrieux G, et al. Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans. Blood. 2020. Sep 17;136(12):1442–1455. doi: 10.1182/blood.2020005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamburini FB, Andermann TM, Tkachenko E, Senchyna F, Banaei N, Bhatt AS. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med. 2018. Dec;24(12):1809–1814. doi: 10.1038/s41591-018-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber D, Jenq RR, Peled JU, Taur Y, Hiergeist A, Koestler J, Dettmer K, Weber M, Wolff D, Hahn J, et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Tr. 2017. May;23(5):845–852. doi: 10.1016/j.bbmt.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MRM, Kamboj M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010. Dec;120(12):4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, et al. Population-level analysis of gut microbiome variation. Science. 2016. Apr 29;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 43.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016. Apr 29;352(6285):565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010. Jun 29;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006. Feb 24;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Swarte JC, Li Y, Hu S, Björk JR, Gacesa R, Vich Vila A, Douwes RM, Collij V, Kurilshikov A, Post A, et al. Gut microbiome dysbiosis is associated with increased mortality after solid organ transplantation. Sci Transl Med. 2022. Aug 31;14(660):eabn7566. doi: 10.1126/scitranslmed.abn7566. [DOI] [PubMed] [Google Scholar]
- 47.Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana E, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020. Dec;588(7837):303–307. doi: 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miltiadous O, Waters NR, Andrlova H, Dai A, Nguyen CL, Burgos da Silva M, Lindner S, Slingerland J, Giardina P, Clurman A, et al. Early intestinal microbial features are associated with CD4 T-cell recovery after allogeneic hematopoietic transplant. Blood. 2022. May 5;139(18):2758–2769. doi: 10.1182/blood.2021014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolodziejczyk AA, Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019. Dec;17(12):742–753. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- 50.Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Walter J, et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe. 2019. Jun 12;25(6):789–802 e5. doi: 10.1016/j.chom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Kyle UG, Chalandon Y, Miralbell R, Karsegard VL, Hans D, Trombetti A, Rizzoli R, Helg C, Pichard C. Longitudinal follow-up of body composition in hematopoietic stem cell transplant patients. Bone Marrow Transplant. 2005. Jun;35(12):1171–1177. doi: 10.1038/sj.bmt.1704996. [DOI] [PubMed] [Google Scholar]
- 52.D’Amico F, Biagi E, Rampelli S, Fiori J, Zama D, Soverini M, Barone M, Leardini D, Muratore E, Prete A, et al. Enteral nutrition in pediatric patients undergoing hematopoietic SCT promotes the recovery of gut microbiome homeostasis. Nutrients. 2019. Dec 4;11(12):doi: 10.3390/nu11122958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen S, Staudacher H, Weber N, Kennedy G, Varelias A, Banks M, Bauer J. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br J Haematol. 2020. Feb;188(4):570–581. doi: 10.1111/bjh.16218. [DOI] [PubMed] [Google Scholar]
- 54.Beckerson J, Szydlo RM, Hickson M, Mactier CE, Innes AJ, Gabriel IH, Palanicawandar R, Kanfer EJ, Macdonald DH, Milojkovic D, et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin Nutr. 2019. Apr;38(2):738–744. doi: 10.1016/j.clnu.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Gonzales F, Bruno B, Alarcon Fuentes M, De Berranger E, Guimber D, Behal H, Gandemar V, Spiegel A, Sirvent A, Yakoub-Agha I, et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin Nutr. 2018. Dec;37(6Pt A):2113–2121. doi: 10.1016/j.clnu.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Shono Y. Van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018. May;18(5):283–295. doi: 10.1038/nrc.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schloig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, et al. Genomic variation landscape of the human gut microbiome. Nature. 2013. Jan 3;493(7430):45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Tyne D, Gilmore MS. Friend turned foe: evolution of enterococcal virulence and antibiotic resistance. Annu Rev Microbiol. 2014;68(1):337–356. doi: 10.1146/annurev-micro-091213-113003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017. Feb 23;168(5):928–943 e11. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, Mair K, Krueger D, Pruteanu M, Shanahan F, et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 2011. Sep;141(3):959–971. doi: 10.1053/j.gastro.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 61.Molina MA, Diaz AM, Hesse C, Ginter W, Gentilini MV, Nuñez GG, Canellada AM, Sparwasser T, Berod L, Castro MS, et al. Immunostimulatory effects triggered by Enterococcus faecalis CECT7121 probiotic strain involve activation of dendritic cells and interferon-gamma production. PLoS One. 2015;10(5):e0127262. doi: 10.1371/journal.pone.0127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huyke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005. Apr;128(4):891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Stein-Thoeringer CK, Nichols KB, Lazrak A, Docampo MD, Slingerland AE, Slingerland JB, Clurman AG, Armijo G, Gomes ALC, Shono Y, et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science. 2019. Nov 29;366(6469):1143–1149. doi: 10.1126/science.aax3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Bogert B, Meijerink M, Zoetendal EG, Wells JM, Kleerebezem M. Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS One. 2014;9(12):e114277. doi: 10.1371/journal.pone.0114277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang X, Kudo Y, Baker JL, LaBonte S, Jordan PA, McKinnie SMK, Guo J, Huan T, Moore BS, Edlund A, et al. Cariogenic streptococcus mutans produces tetramic acid strain-specific antibiotics that impair commensal colonization. ACS Infect Dis. 2020. Apr 10;6(4):563–571. doi: 10.1021/acsinfecdis.9b00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kulkarni S, Powles R, Treleaven J, Riley U, Singhal S, Horton C, Sirohi B, Bhagwati N, Meller S, Saso R, et al. Chronic graft versus host disease is associated with long-term risk for pneumococcal infections in recipients of bone marrow transplants. Blood. 2000. Jun 15;95(12):3683–3686. doi: 10.1182/blood.V95.12.3683. [DOI] [PubMed] [Google Scholar]
- 67.Engelhard D, Cordonnier C, Shaw PJ, Parkalli T, Guenther C, Martino R, Dekker AW, Prentice HG, Gustavsson A, Nurnberger W, et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol. 2002. May;117(2):444–450. doi: 10.1046/j.1365-2141.2002.03457.x. [DOI] [PubMed] [Google Scholar]
- 68.Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, Rovira M, Martino R, Oriol A, Borrell N, Ruiz I, Martín-Dávila P, de la Cámara R, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant. 2011. Nov-Dec;25(6):E629–38. doi: 10.1111/j.1399-0012.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 69.Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Gobourne A, No D, Taur Y, et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun. 2013. Mar;81(3):965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Tr. 2015. Aug;21(8):1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhattacharyya A, Hanafi LA, Sheih A, Golob JL, Srinivasan S, Boeckh MJ, Pergam S, Mahmood S, Baker KK, Gooley TA, et al. Graft-derived reconstitution of mucosal-associated invariant T Cells after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018. Feb;24(2):242–251. doi: 10.1016/j.bbmt.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrlova H, Miltiadous O, Kousa AI, Dai A, DeWolf S, Violante S, Park H-Y, Janaki-Raman S, Gardner R, El Daker S, et al. MAIT and Vdelta2 unconventional T cells are supported by a diverse intestinal microbiome and correlate with favorable patient outcome after allogeneic HCT. Sci Transl Med. 2022. May 25;14(646):eabj2829. doi: 10.1126/scitranslmed.abj2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013. Aug 8;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 74.Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, Oravecz-Wilson K, Wu S-R, Sun Y, Rossi C, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016. May;17(5):505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Payen M, Nicolis I, Robin M, Michonneau D, Delannoye J, Mayeur C, Kapel N, Berçot B, Butel M-J, Le Goff J, et al. Functional and phylogenetic alterations in gut microbiome are linked to graft-versus-host disease severity. Blood Adv. 2020. May 12;4(9):1824–1832. doi: 10.1182/bloodadvances.2020001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Markey KA, Schluter J, Gomes ALC, Littmann, ER, Pickard, AJ, Taylor, BP , Giardana PA, Weber D, Dai A, Docampo MD, et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood. 2020. Jul 2;136(1):130–136. doi: 10.1182/blood.2019003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber D, Hiergeist A, Weber M, Dettmer K, Wolff D, Hahn J, Herr W, Gessner A, Holler E. Detrimental effect of broad-spectrum antibiotics on intestinal microbiome diversity in patients after allogeneic stem cell transplantation: lack of commensal sparing antibiotics. Clin Infect Dis. 2019. Apr 8;68(8):1303–1310. doi: 10.1093/cid/ciy711. [DOI] [PubMed] [Google Scholar]
- 78.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016. May 18;8(339):339ra71. doi: 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weber D, Oefner PJ, Dettmer K, Hiergeist A, Koestler J, Gessner A, Weber M, Stämmler F, Hahn J, Wolff D, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016. Aug;51(8):1087–1092. doi: 10.1038/bmt.2016.66. [DOI] [PubMed] [Google Scholar]
- 80.de Gunzburg J, Ghozlane A, Ducher A, Le Chatelier E, Duval X, Ruppé E, Armand-Lefevre L, Sablier-Gallis F, Burdet C, Alavoine L, et al. Protection of the human gut microbiome from antibiotics. J Infect Dis. 2018. Jan 30;217(4):628–636. doi: 10.1093/infdis/jix604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaleko M, Bristol JA, Hubert S, Parsley T, Widmer G, Tzipori S, Subramanian P, Hasan N, Koski P, Kokai-Kun J, et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe. 2016. Oct;41:58–67. doi: 10.1016/j.anaerobe.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 82.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Hiroshi Morisaki J, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015. Nov 19;527(7578):323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 83.Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, Fischetti VA, Marraffini LA. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014. Nov;32(11):1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018. Jun 13;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 85.De Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 86.Gerbitz A, Schultz M, Wilke A, Linde H-J, Scholmerich J, Andreesen R, Holler E. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004. Jun 1;103(11):4365–4367. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 87.Gorshein E, Wei C, Ambrosy S, Budney S, Vivas J, Shenkerman A, Manago J, McGrath MK, Tyno A, Lin Y. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2017;31(5):May. doi: 10.1111/ctr.12947. [DOI] [PubMed] [Google Scholar]
- 88.Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Jarvinen A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis. 2004. Jan 1;38(1):62–69. doi: 10.1086/380455. [DOI] [PubMed] [Google Scholar]
- 89.Robin F, Paillard C, Marchandin H, Demeocq F, Bonnet R, Hennequin C. Lactobacillus rhamnosus meningitis following recurrent episodes of bacteremia in a child undergoing allogeneic hematopoietic stem cell transplantation. J Clin Microbiol. 2010. Nov;48(11):4317–4319. doi: 10.1128/JCM.00250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vahabnezhad E, Mochon AB, Wozniak LJ, Ziring DA. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J Clin Gastroenterol. 2013. May-Jun;47(5):437–439. doi: 10.1097/MCG.0b013e318279abf0. [DOI] [PubMed] [Google Scholar]
- 91.Mehta A, Rangarajan S, Borate U. A cautionary tale for probiotic use in hematopoietic SCT patients-Lactobacillus acidophilus sepsis in a patient with mantle cell lymphoma undergoing hematopoietic SCT. Bone Marrow Transplant. 2013. Mar;48(3):461–462. doi: 10.1038/bmt.2012.153. [DOI] [PubMed] [Google Scholar]
- 92.Ambesh P, Stroud S, Franzova E, Gotesman J, Sharma K, Wolf L, Kamholz S. Recurrent lactobacillus bacteremia in a patient with leukemia. J Investig Med High Impact Case Rep. 2017. Oct-Dec;5(4):2324709617744233. doi: 10.1177/2324709617744233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018. Sep 6;174(6):1406–1423 e16. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 94.Cooper TE, Scholes-Robertson N, Craig JC, Hawley CM, Howell M, Johnson DW, Teixeira-Pinto A, Jaure A, Wong G. Synbiotics, prebiotics and probiotics for solid organ transplant recipients. Cochrane Database Syst Rev. 2022. Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simms-Waldrip TR, Sunkersett G, Coughlin LA, Savani MR, Arana C, Kim J, Kim M, Zhan X, Greenberg DE, Xie Y. Antibiotic-induced depletion of anti-inflammatory clostridia is associated with the development of graft-versus-host disease in pediatric stem cell transplantation patients. Biol Blood Marrow Transplant. 2017. May;23(5):820–829. doi: 10.1016/j.bbmt.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Kim SG, Becattini S, Moody TU, Shliaha PV, Littmann ER, Seok R, Gjonbalaj M, Eaton V, Fontana E, Amoretti L, et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature. 2019. Aug;572(7771):665–669. doi: 10.1038/s41586-019-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958. Nov;44(5):854–859. [PubMed] [Google Scholar]
- 98.Van Nood E, Dijkgraaf MG, Keller JJ, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med. 2013. May 30;368(22):2145. doi: 10.1056/NEJMc1303919. [DOI] [PubMed] [Google Scholar]
- 99.DeFilipp Z, Peled JU, Li S, Mahabamunuge J, Dagher Z, Slingerland AE, Del Rio C, Valles B, Kempner ME, Smith M, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018. Apr 10;2(7):745–753. doi: 10.1182/bloodadvances.2018017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taur Y, Coyte K, Schluter J, Robilotti E, Figueroa C, Gjonbalaj M, Littmann ER, Ling L, Miller L, Gyaltshen Y, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018. Sep 26;10(460). doi: 10.1126/scitranslmed.aap9489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spindelboeck W, Schulz E, Uhl B, Kashofer K, Aigelsreiter A, Zinke-Cerwenka W, Mulabecirovic A, Kump PK, Halwachs B, Gorkiewicz G, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft- versus -host-disease. Haematologica. 2017. May;102(5):e210–e213. doi: 10.3324/haematol.2016.154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaito S, Toya T, Yoshifuji K, Kurosawa S, Inamoto K, Takeshita K, Suda W, Kakihana K, Honda K, Hattori M, et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018. Nov 27;2(22):3097–3101. doi: 10.1182/bloodadvances.2018024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qi X, Li X, Zhao Y, Wu X, Chen F, Ma X, Zhang F, Wu D. Treating steroid refractory intestinal acute graft-vs.-host disease with fecal microbiota transplantation: a pilot study. Front Immunol. 2018;9:2195. doi: 10.3389/fimmu.2018.02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Lier YF, Davids M, Haverkate NJE, de Groot PF, Donker ML, Meijer E, Heubel-Moenen FCJI, Nur E, Zeerleder SS, Nieuwdorp M, et al. 2020. Aug 12. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med.12(556). doi: 10.1126/scitranslmed.aaz8926 [DOI] [PubMed] [Google Scholar]
- 105.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019. Nov 21;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 106.Bilinski J, Lis K, Tomaszewska A, Pechcinska A, Grzesiowski P, Dzieciatkowski T, Walesiak A, Gierej B, Ziarkiewicz-Wroblewska B, Tyszka M, et al. Eosinophilic gastroenteritis and graft-versus-host disease induced by transmission of Norovirus with fecal microbiota transplant. Transpl Infect Dis. 2020. Jun 23;e13386. doi: 10.1111/tid.13386. [DOI] [PubMed] [Google Scholar]
- 107.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019. Dec;68(12):2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017. Aug;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 109.Tsilingiri K, Rescigno M. Postbiotics: what else? Benef Microbes. 2013. Mar 1;4(1):101–107. doi: 10.3920/BM2012.0046. [DOI] [PubMed] [Google Scholar]
- 110.Riwes M, Reddy P. Short chain fatty acids: postbiotics/metabolites and graft versus host disease colitis. Semin Hematol. 2020. Jan;57(1):1–6. doi: 10.1053/j.seminhematol.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iyama S, Sato T, Tatsumi H, Hashimoto A, Tatekoshi A, Kamihara Y, Horiguchi H, Ibata S, Ono K, Murase K, et al. Efficacy of enteral supplementation enriched with glutamine, fiber, and oligosaccharide on mucosal injury following hematopoietic stem cell transplantation. Case Rep Oncol. 2014. Sep;7(3):692–699. doi: 10.1159/000368714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshifuji K, Inamoto K, Kiridoshi Y, Takeshita K, Sasajima S, Shiraishi Y, Yamashita Y, Nisaka Y, Ogura Y, Takeuchi R, et al. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv. 2020. Oct 13;4(19):4607–4617. doi: 10.1182/bloodadvances.2020002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andermann TM, Fouladi F, Tamburini FB, Sahaf B, Tkachenko E, Greene C, Buckley MT, Brooks EF, Hedlin H, Arai S, et al. A fructo-oligosaccharide prebiotic is well tolerated in adults undergoing allogeneic hematopoietic stem cell transplantation: a phase I dose-escalation trial. Transplant Cell Ther. 2021. Nov;27(11):932e1–932 e11. doi: 10.1016/j.jtct.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


