Abstract
BACKGROUND
The assessment of patients with severe Acquired Brain Injury (sABI) is mandatory in every phase and setting of care, and requires a multidimensional and interdisciplinary approach, to develop the individual rehabilitation project, and monitor long-term functional outcomes. In 2001 the Italian Society of Physical and Rehabilitation Medicine (SIMFER) published the minimal assessment protocol for traumatic sABI, providing a comprehensive, standardized functional assessment based on the International Classification of Functioning, Disability and Health (ICF), 2001. In 2007, a new protocol was published, extended to all sABI patients (PMGCA). In 2019, the SIMFER appointed a working group to provide a revised, updated version: the PMGCA2020.
AIM
The purpose of this study was to describe the minimal assessment protocol to be applied at every stage and setting of the care process of patients with sABI.
METHODS
The working group, including one neurologist and 11 physiatrists experts in sABI rehabilitation, performed a review of the international recommendations for sABI assessment focusing on the following key words: “sABI assessment,” “sABI rehabilitation,” “sABI prognostic factors,” “sABI rehabilitation assessment,” “sABI outcome,” in MEDLINE. Revision and integration proposals by each member were written and motivated, discussed and voted.
RESULTS
The PMGCA2020 is addressed to sABI adult patients. It investigates the main clinical problems of sABI at any time of the rehabilitation pathway. It includes a demographic/anamnestic section, a clinical/functional assessment section and an outcome measures section following the ICF model of functioning and the model of the construction of the rehabilitation project.
CONCLUSIONS
The PMGCA2020 provides an updated tool for the multidimensional rehabilitation assessment of sABI patients, at any stage of the rehabilitation pathway. Further studies will allow the validation of this minimum set of variables paving the way to an assessment standardization of patients with sABI in the rehabilitation settings.
CLINICAL REHABILITATION IMPACT
This minimum set of variables, defining patient’s functioning and clinical status and outcomes, at every stage and setting of the care process to provide a framework for the standardization of the clinical evaluation of patients with sABI in rehabilitation settings.
Key words: Brain injuries, Rehabilitation, Patient care planning, Patient-reported outcome measures
Severe acquired brain injury (sABI) is a brain damage related to a pathological event of a non-congenital, perinatal or degenerative nature, such as to determine a coma condition, with Glasgow Coma Scale score - acute phase (GCS) ≤8 and lasting more than 24 hours. This damage can be of vascular, traumatic, anoxic, infectious, toxic-metabolic, neoplastic origin, and can cause multiple complex sensory-motor, cognitive and/or behavioral impairments leading to severe disability.
SABIs have a high worldwide prevalence and often result in severe chronic disability, with a significant impact on health systems, families and society.1, 2 In Europe, an incidence rate of 235 persons per 100,000 population per year is estimated for ABI of traumatic etiology, of which about 9% are severe, while for non-traumatic ABI an incidence of 114-350/100,000 population per year is estimated.3 In Italy, 3-5 people/100,000 inhabitants/year affected by head trauma require hospitalization in intensive rehabilitation, while the incidence and prevalence of sABI with very severe disability with a state of prolonged Disorder of Consciousness (DoC), including the Unresponsive Waking State (UWS)/Vegetative State (VS) and the Minimal Conscious State (MCS), are estimated to be 1.8-1.9/100,000 inhabitants and 2.0-2.1/100,000 inhabitants, respectively, although with regional differences.4
SABI survivors need a long-term management, and the rehabilitation of these patients and the support to their families are a demanding and extremely complex task.5 The comprehensive multidimensional assessment of the person with sABI is a necessary step for defining and developing the patient’s individual rehabilitation plan, and defining the integrated care pathway of sABI patients. The assessment requires a multidimensional and interprofessional approach, that addresses the multiple factors affecting functioning, including psychosocial and environmental factors, according to the International Classification of Functioning, Disability and Health-World Health Organization (ICF 2001, WHO).6 An accurate assessment must use of reliable tools, possibly validated into the adopted language.
In 1998 a working group (WG) of the Italian Society of Physical and Rehabilitative Medicine (SIMFER) had published an original protocol called “Minimum Protocol for the Rehabilitation of the Patient with Traumatic Brain Injury”,7 later revised in 2007 to develop a minimum rehabilitation assessment protocol of persons with sABI (PMGCA);8 these protocols were proposed to provide to rehabilitation professionals with a sort of “toolbox” for the evaluation of people with sABI in the different care settings along the Integrated rehabilitation pathway.
Since then, dedicated sABI Registers9-12 have been adopted in Italy and three National Consensus Conferences on sABI were promoted by SIMFER from 2000 to the 2010, for each of the phases of the treatment pathway.13-16 Many multicentric trials17-20 were also carried out, involving several centers of the national territory, confirming the need to adopt shared standardized assessment protocols, to perform quality assessment and benchmarking as well as to improve professional understanding of these patients’ needs and outcomes.
This second revision of the protocol by newly appointed SIMFER WG stems from the need to update the version developed in 20078 with the following aims:
to verify over time the relevance and real-practice use of the tools contained in the protocol, in light of the evolution in the rehabilitation protocols and pathways of persons with sABI;
to provide an update of the assessment tools in line with the changes and innovations in clinical practice and literature, including the SIMFER Consensus conferences and the International Guidelines.
Materials and methods
The WG was appointed in March 2019 by the elected Coordinator (SL) from the board of the SIMFER sABI section, including 10 experts and two external reviewers, PB and FC. Six of them were authors of the previous versions of the protocol. All members of WG (one neurologist and 11 physiatrists) were experts in rehabilitation of patients with sABI.
The group operated by call conferences, collegial meetings and email communications with the following steps:
critical revision of the PMGCA: most members had direct extensive clinical expertise in the clinical management of sABI patients;
collegial discussion on the PMGCA main critical issues;
collegial agreement on the objectives of the protocol revision;
update of systematic review of sABI clinical practice guidelines and consensus conferences’ recommendations involving assessment tools from 2007 to 2018;9-12
update 2007-2018 of systematic search of literature to verify the selection of relevant variables and appropriate tools to include in the PMGCA; to the purpose of this revision the search was focused on the following keywords “sABI assessment,” “sABI rehabilitation,” “sABI prognostic factors,” “sABI rehabilitation assessment,”, “sABI outcome,” in MED-LINE databank (any date, English Language, Human, Adults 18+);
advancement of the proposed changes: each proposed change was written and motivated and voted by all group members: when total agreement was not reached, the change was approved or refused by majority;
drafting, collegial revision, and final editing of the revised version,
The reference framework maintained the adherence to the ICF 2001, WHO6 conceptual model of functioning21 however, the protocol structure has been organized following the model of the construction of the rehabilitation project, by problem/intervention areas, including:
clinical stability area: referring to complications/problems in progress at the time of the evaluation;
basic vital functions area: including aspects relating to respiratory function, nutrition and sphincter function;
area of communicative, relational and cognitive behavioral functions: including the level of consciousness and environmental interactions, basic communication skills, orientation and general awareness of situation, initiative, basic social skills;
area of sensory-motor functions, mobility and transfers: referring to motor aspects (muscular strength, range of motion, balance, coordination, dexterity, etc.) and sensory functions, evaluation of motor patterns/altered sensitives and conserved components, displacements-transfers, walking and locomotion;
area of autonomy in everyday life activities;
social adaptation and reintegration area: including the assessment of participation, intended as social, family, school and work reintegration.
As in the previous versions, in selecting the variables, the following features were considered:
information value;
need to keep the assessment protocol minimal;
retrievability of data in most contexts;
validated tools, preferably in Italian version.
Results
The protocol consists of three parts: 1) anamnestic Information; 2) clinical assessment tools; 3) global tools for multidimensional outcome assessment. All evaluation tools references are provided in Supplementary Digital Material 1 (Supplementary Text File 1).
Part 1: anamnestic information
This part includes the minimum significant information to be collected at the time of first contact with the person with sABI. It includes demographic and anamnestic data, such as date of birth; gender; nationality; residence; mother tongue; education; occupation; premorbid living situation; housing conditions; premorbid conditions that required assistance; pre-existing socio-environmental problems; certificate of civil disability; support administrator; other forms of social protection. It also includes: event date; etiology (multiple answers possible); brain damage (neuroradiology: widespread; focal: hemispheric; bilateral; posterior cranial fossa; brainstem). SABI severity is assessed by the GCS. Clinical information includes also comorbidities prior to damage; any damage associated with the event; any surgery on primary brain damage and/or associated damage: neurosurgery, orthopedic surgery, other.
Part 2: clinical evaluation tools
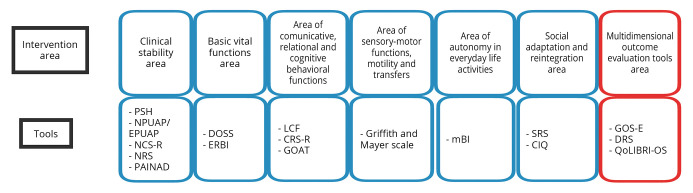
Clinical evaluation tools are applicable in the different phases and different rehabilitation settings of the treatment process. For each area of intervention,21 the main potential problems that may require an evaluation or therapeutic intervention and the related assessment tools are indicated. Evaluation tools are summarized in Figure 1.
Figure 1.
—Intervention areas and tools. PSH: paroxystic sympathetic hyperactivity; NPUAP/EPUAP: National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel; NCS-R: Nociception Coma Scale Revised; NRS: Nociception Rating Scale, PAINAD: Pain Assessment in Advanced Dementia; DOSS: Dysphagia Outcome and Severity Scale; ERBI: Early Rehabilitation Barthel Index; LCF: Levels of Cognitive Functioning, CRS-R: Coma Recovery Scale-Revised; GOAT: Galveston Orientation and Amnesia Test; mBI: modified Barthel Index; SRS: Supervision Rating Scale; CIQ: Community Integration Questionnaire; GOS-E: Glasgow Outcome Scale-Expanded; DRS: Disability Rating Scale; QoLIBRI-OS: Quality of LIfe after BRain Injury Overall Scale.
Part 3: multidimensional outcome evaluation tools
The third part of the protocol is aimed to global outcome assessment. It includes: the Glasgow Outcome Scale-Expanded (GOS-E) and the Disability Rating Scale (DRS)
These tools measure the overall outcome after a sABI, which can be used throughout the personal care process, and in the different settings. This part also includes an assessment the quality of life dimension, as an outcome indicator that is particularly relevant in the chronic phase of sABI, by the Quality Of LIfe after BRain Injury Overall Scale (QOLIBRI-OS)
Discussion
The new version of the minimal assessment protocol for patients with sABI (PMGCA 2020) sees many confirmations compared to the first version of the protocol (the 2007 version)8, but also some important innovations.
The protocol maintains firm reference to the ICF classification’ conceptual model of functioning, but its organization has been totally revised following the methodology of the construction of the Individual Rehabilitation Project (IRP) oriented to the outcome.21This choice was motivated by the ever wider diffusion of the construction model of the rehabilitation project in intensive rehabilitation units dedicated to sABI patients in Italy by problem areas/interventions, to develop the IRP and define outcomes of persons with sABI, as recommended by all international guidelines5 and by the results of the Italian Consensus Conferences.13-16 Structuring the protocol according to the areas of problem and intervention that can be found in the different settings and phases of the path,21 seemed an innovative and useful proposal to guide the professionals step by step in assessing and addressing the rehabilitation needs of the patient with sABI, to provide a common methodological and cultural ground to the construction and implementation of the rehabilitation project, and to improve communication between the different centers and territories.
The choice of assessment tools included in the final protocol is the result of a careful review of the literature and of a mutual comparison based also on the specific clinical experience of the members of the WG17-20, 22 and on experiences of Italian national and regional registries on sABI (GISCAR, GRACER...).9-12
In the 2001 and 2007 versions there were some contents within the protocols with reference to the evaluation of the etiology of brain damage, that required a structured list of possible ICD9CM codes. However, the consolidated use of the hospital discharge form, requiring the ICD codes for all hospital rehabilitation discharges, led the WG to remove those variables from the PMGCA2020.
Although preference was always given to tools that have been developed and validated into Italian for all sABI patients, when these were unavailable, the WG chose also some tools that were validated only for traumatic brain injury or for stroke patients.
The new assessment tools, exploring also care complexity, clinical complexity and vital functions, include: pain measure23 (Nociception Coma Scale-Revised-NCS-R, Nociception Rating Scale-NRS and Pain Assessment in Advanced Dementia -PAINAD), paroxysmal sympathetic hyperactivity evaluation-Assessment Measure-PSH-AM), clinical complexity evaluation (Early Rehabilitation Barthel Index-ERBI), dysphagia assessment (Dysphagia Outcome and Severity Scale-DOSS), recovery of disorders of consciousness evaluation (Coma Recovery Scale-Revised -CRS-R), Pressure Sores (PS) assessment (National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel -NPUAP/EPUAP), and quality of life assessment (QOLIBRI-OS). As to the ICF domain: structures and functions, in the Area of Clinical Stability several new assessment tools have been introduced.
The ratio for changing, introducing or maintaining some clinical tools in the new version of the PMGCA was based on the literature as discussed below.24
PSH-AM
Individuals with sABI may develop a state of excessive sympathetic nervous system activity characterized by sudden increases in heart rate, blood pressure, respiratory rate, temperature, dystonia, rigidity and spasticity. The term PSH was shared by the scientific community, and a scale able to standardize the clinical criteria of differential diagnosis and quantification of the syndrome was recently developed: The Diagnosis Likelihood Tool. PSH persistence can be the cause of fewer rehabilitative treatments, longer hospitalization period has negative prognostic value on sABI outcomes.25
NPUAP/EPUAP
sABI patients are at risk for development of PS due to immobility, malnutrition, metabolic changes, urinary and fecal incontinence; bedsores were found to be significantly associated with mortality at 21 days and recovery status at 3 months.26 The NPUAP has developed evidence-based recommendations for the prevention and treatment of PS, including the recommendation to provide a standardized assessment of the risk of developing a PS and of the severity of lesions existing.
NCS-R/NRS/PAINAD
Pain diagnosis and treatment are crucial during the rehabilitation pathway.23 In the present protocol, different tools were proposed for the assessment of pain: the pain NRS for communicant and cognitively intact patient who report their pain in the absence of stimulus; the PAINAD for patients unable to communicate verbally their pain. Since recent neuroimaging studies in patients with DoC support the presence of pain perception capacity highlighting that these patients need analgesic treatment and monitoring,27 the Nociception Coma Scale (NCS), developed specifically for patients with DoC due to sABIs was introduced. A revised version of the NCS (or NCS-R) was proposed, including the motor, verbal and facial subscores and excluding the visual subscore, highly sensitive to responses to noxious stimulation in sABI patients, tested on patients with DoC and tracheotomy28 and strongly related with the level of consciousness as measured by the CRS-R.
DOSS
In the basic vital function assessment of the new protocol of sABIs it is required to specify the presence or absence of dysphagia and its severity with the DOSS. Dysphagia complicates a variety of ABIs.29 Severity of dysphagia can be assessed clinically at bedside, with the Modified Evans blue dye test before decannulation, and/or by instrumentation, mainly Videofluoroscopy (VFS) or Flexible Endoscopic Evaluation of Swallowing. The DOSS is a simple, easy-to-use, 7-point scale developed to systematically rate the functional severity of dysphagia, providing recommendations for diet level, independence level and type of nutrition. DOSS is the only clinical severity assessment scale that is based on objective assessment with VFS. A recent study investigated inter-rater reliability (IRR) of the DOSS concluded that clinical experience and audio recording tend to improve IRR, but some doubt was expressed relative to the objectivity of the scale despite the use of instrumentation.
CRS-R
As to the Area of Relational Communicative and Cognitive Behavioral function, the CRS-R has been introduced in the PMGCA2020 to better stratify the level of consciousness of patients with DoC. The CRS-R is currently acknowledged as the gold standard clinical tool to disentangle SV/UWS from MCS and to define the exit from the DoC, in accordance with the Aspen Workgroup criteria.30 It consists of 23 hierarchically organized items, parcellated into six sub-scales, assessing different. For each sub-scale, there are threshold values corresponding to the various states of consciousness: UWS/VS, MCS, or emergent from the MCS. CRS-R scale is a reference tool worldwide indicated by many international guidelines; the Italian version was adopted.
Galveston Orientation and Amnesia Test (GOAT)
For Post Traumatic Amnesia (PTA) assessment,31 the GOAT, the Artiola Scale32 and its evolution in the Westmead Post-Traumatic Amnesia Scale, that have been developed for brain trauma, but later widely used for all sABI33 were o considered. The final choice confirmed the GOAT, already present in the first version of the protocol, since, despite being an apparently more “crude” test than the others, it assesses not only orientation and anterograde amnesia, but also retrograde amnesia, often being more sensitive to PTA recovery and less influenced by interfering drug therapies.34
List of disorders and alterations regarding sensorimotor functions
In the face of a great multiplicity and variability of possible disorders and in consideration of the different types of etiology, the WG decided to suggest a list of possible disorders and alterations, observable in clinical practice, leaving the choice of tools validated for specific assessment, such as spasticity, muscle strength, gait alterations, axial control etc. The classification proposed by Griffith and Mayer which identifies some patterns of motor impairment in patients with traumatic etiology that recur with greater frequency, can always be useful in clinical evaluation.35
Modified Barthel Index and ERBI
As a measure of the ICF domain of activity the BI (PMCGCA2002) was changed to the modified BI (mBI).
The BI is one of the most widely used measures of self-care performances, mainly for its high sensitivity, simplicity, communicability and ease of scoring.36 The BI is used in most of the Italian Regional Health Systems to determine discharge placement of patients, the burden of care, the efficiency and effectiveness of rehabilitation.
The mBI in the version provided by Shah et al. was chosen in this protocol as it achieved greater sensitivity and improved reliability than the original version, without causing additional difficulty or affecting the implementation time. This assessment allows more discriminant levels to quantify the need for help, and provides an accurate description of the abilities required to classify for each score in any single level. The mBI can be scored by observing the patient performance (recommended in the inpatient and home settings) and by recording the patient’s and/or caregiver’s report (allowed for outpatient assessment).
Routine clinical use of the BI/mBI in many settings is feasible and responds to clinically important change but floor and ceiling effects are a particular issue for stroke trials and limit the potential utility of the scale.37
Therefore, considering the functional status of patients with sABI, both in the acute phase and in inpatient rehabilitation, a supplementary tool was introduced: the ERBI.
The ERBI is composed of the BI and the Early Rehabilitation Index (ERI). The ERI was introduced by Schönle to address several clinically important aspects among early rehabilitation patients, such as the presence of mechanical ventilation, tracheotomy and dysphagia. For this reason, it can be particularly useful in the immediate post-acute phase of the course of care of people with sABI, allowing changes in the clinical complexity of care related to the sphere of basic vital functions to be assessed. The sum of the BI and the ERI results in the ERBI with a range from -325 to 100, with lower values indicating higher impairments. ERBI is associated with morbidity and length of stay.
In recent literature, ERBI was applied to assess the functional status in intensive care unit and early rehabilitation of sABI.38, 39 Boltzmann showed that ERBI value on admission in Neurological and neurosurgical early rehabilitation, had the strongest predictive value for gains in functional outcome.40
Supervision Rating Scale (SRS) and Community Integration Questionnaire (CIQ)
The assessment of the domain of participation is often difficult to be included in routine clinical practice; since by the previous experience with the PMGCA-2007 we found that the SRS was sparsely collected, we searched for a more feasible tool but we did not find any. So, we decided to maintain the SRS.41
This tool was chosen for inclusion in the protocol because it is the only tool we found that identifies the sABI patient’s needs at the time of discharge, when this information is crucial for the planning of car.42, 43
Also, the CIQ tool has been maintained in the domain of participation, that need to be administrated only at follow-ups. The tool is extensively known and used in clinical and research practice as an outcome tool after head trauma;44 it has been validated and widely used for the evaluation of interventions also in other populations.45, 46 The CIQ was chosen because it allows to explore the integration with respect to primary and secondary ADL, for its simplicity of administration as it may be administered by telephone, and can be completed by a cohabitant and the CIQ.
GOS and GOS-E
The Glasgow Outcome Scale (GOS) and the DRS were designed for the multidimensional assessment of sABI patients’ outcome. These tools can be used alongside the treatment path to monitor the outcome during the different phases of recovery as well as in the follow-up assessments.
The original GOS was the first of two scales developed as a practical index of social outcome following head injury designed to complement the GCS. The original GOS includes five categories of disability: dead (D=1), vegetative state (VS=2), severe disability (SD=3), moderate disability (MD=4), good recovery (GR=5). However, the GOS has been criticized for its lack of sensitivity to detect small but clinically relevant changes in outcome. The other scale, the GOS-E, presents an increased number of categories by subdividing SD, MD and GR through additional questions. The GOS-E scores have been reported to have higher validity and greater sensitivity to change and to produce a small consistent increase in efficiency compared to other outcome scales.
DRS
The DRS was also developed as an instrument to quantitatively assess the disability of severe head trauma patients, so that their rehabilitative progress could be followed from coma through different levels of awareness and functioning and until their return to the community. The DRS instrument was designed to be easily learned, quickly completed, valid, predictive of outcome and to have a high inter-rater reliability. The DRS is more sensitive than the GOS in detecting and measuring clinical changes in individuals who have sustained severe head trauma. The DRS identifies, with a score from 0 complete autonomy to 30 death.
QOLIBRI
QOLIBRI is a quality of life assessment tool specifically created for the head injury population. The scale is the outcome of an international work that led to the initial validation in English and then in Italian.47 For the minimum protocol, its reduced version was chosen, the QOLIBRI Overall Scale (QOLIBRI-OS) for its greater ease of use and speed of administration while maintaining good psychometric characteristics such as reliability and construct validity, similar to the original version.48 This last version has been subsequently validated also in the populations of patients suffering from subarachnoid hemorrhage and from stroke.49, 50
Conclusions
The PMGCA2020 includes a minimum set of variables defining patient’s functioning and clinical problems, needs and outcomes, at every stage of the care process and in every care setting, the protocol is organized according to the ICF model of functioning and to problem areas/interventions of the IRP. The PMGCA2020 provides an updated tool for the multidimensional rehabilitation assessment of sABI patients, at any stage of the rehabilitation pathway to share data and information among the health professional community.
Supplementary Digital Material 1
Supplementary Table I
Evaluation tool references.
References
- 1.Cuthbert JP, Corrigan JD, Harrison-Felix C, Coronado V, Dijkers MP, Heinemann AW, et al. Factors that predict acute hospitalization discharge disposition for adults with moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2011;92:721–730.e3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21530719&dopt=Abstract 10.1016/j.apmr.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) . Incidence rates of hospitalization related to traumatic brain injury—12 states, 2002. MMWR Morb Mortal Wkly Rep 2006;55:201–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16511440&dopt=Abstract [PubMed] [Google Scholar]
- 3.Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006;148:255–68, discussion 268. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16311842&dopt=Abstract 10.1007/s00701-005-0651-y [DOI] [PubMed] [Google Scholar]
- 4.Servadei F, Verlicchi A, Soldano F, Zanotti B, Piffer S. Descriptive epidemiology of head injury in Romagna and Trentino. Comparison between two geographically different Italian regions. Neuroepidemiology 2002;21:297–304. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12411733&dopt=Abstract 10.1159/000065523 [DOI] [PubMed] [Google Scholar]
- 5.The ERABI Group. A systematic review of the rehabilitation of moderate to severe acquired brain injuries, Module Introduction and Methodology, version 13.0; 2019 [Internet]. Available from: https://erabi.ca/wp-content/uploads/2019/09/Module-1_Introduction-and-Methodology-V13.pdf [cited 2022, May 31].
- 6.World Health Organization. ICF International Classification of Functioning, Disability and Health; 2001 [Internet]. Available from: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health/ [cited 2022, May 31].
- 7.Sezione SIMFER sulla Riabilitazione del Traumatizzato Cranio-encefalico. Raccomandazioni per la valutazione riabilitativa del paziente con esito di trauma cranio-encefalico. Protocollo di valutazione riabilitativa di minima per il paziente con esito di trauma cranio-encefalico. Giornale Italiano di Medicina Riabilitativa 1998;3:5–25. [Google Scholar]
- 8.Taricco M, Bargellesi S. Protocollo di valutazione riabilitativa di minima della persona con grave cerebrolesione acquisita (GCA): presentazione. Ital J Rehab Med- MR 2007;21. [Google Scholar]
- 9.Zampolini M. Lo studio GISCAR sulle gravi cerebrolesioni acquisite. Aspetti metodologici e dati preliminari. Giornale Italiano di Medicina Riabilitativa 2003;17:15–30. [Google Scholar]
- 10.Boldrini P, Maietti A, Basaglia N. Progettazione e realizzazione di un registro regionale delle gravi cerebrolesioni acquisite in Emilia-Romagna. Eura Medicophys 2004;40(Suppl 1):9–11.16030488 [Google Scholar]
- 11.Chiavaroli F, Derraik JG, Zani G, Lavezzi S, Chiavaroli V, Sherwin E, et al. Epidemiology and clinical outcomes in a multicentre regional cohort of patients with severe acquired brain injury. Disabil Rehabil 2016;38:2038–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26729309&dopt=Abstract 10.3109/09638288.2015.1111439 [DOI] [PubMed] [Google Scholar]
- 12.Avesani R, Roncari L, Khansefid M, Formisano R, Boldrini P, Zampolini M, et al. The Italian National Registry of severe acquired brain injury: epidemiological, clinical and functional data of 1469 patients. Eur J Phys Rehabil Med 2013;49:611–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23558700&dopt=Abstract [PubMed] [Google Scholar]
- 13.Giuria della Consensus Conference. Modalità di trattamento riabilitativo del traumatizzato cranio-encefalico in fase acuta, criteri di trasferibilità in strutture riabilitative e indicazioni a percorsi appropriati - Documento Conclusivo della Giuria e Raccomandazioni. Giornale Italiano di Medicina Riabilitativa 2001;15:29–42. [Google Scholar]
- 14.Taricco M, De Tanti A, Boldrini P, Gatta G. National Consensus Conference. The rehabilitation management of traumatic brain injury patients during the acute phase: criteria for referral and transfer from intensive care units to rehabilitative facilities (Modena June 20-21, 2000). Eura Medicophys 2006;42:73–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16565689&dopt=Abstract [PubMed] [Google Scholar]
- 15.Avesani R, Taricco M, Gambini M, De Tanti A, Fogar P. Documento preparatorio alla Conferenza Nazionale di Consenso - Bisogni riabilitativi ed assistenziali delle persone con disabilità da grave cerebro-lesione acquisita (GCA) e delle loro famiglie, nella fase post-ospedaliera; 2005 [Internet]. Available from: http://www.simferweb.net/varie_sito_simfer_allegati/varie/lineeGuida/GCLA/Consensus%20Conference%202%20GCADEFINITIVOp.pdf [cited 2022, May 31].
- 16.De Tanti A, Zampolini M, Pregno S; CC3 Group. Recommendations for clinical practice and research in severe brain injury in intensive rehabilitation: the Italian Consensus Conference. Eur J Phys Rehabil Med 2015;51:89–103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25184800&dopt=Abstract [PubMed] [Google Scholar]
- 17.Bargellesi S, Reverberi C, De Tanti A, Pregno S. La gestione della cannula tracheostomica nelle persone con grave cerebrolesione acquisita: consenso a un protocollo condiviso. Ital J Rehab Med- MR 2013;27:9–16. [Google Scholar]
- 18.De Tanti A, Scarponi F, Bertoni M, Gasperini G, Lanzillo B, Molteni F, et al. ITB Italian Group . Management of intrathecal baclofen therapy for severe acquired brain injury: consensus and recommendations for good clinical practice. Neurol Sci 2017;38:1429–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28478498&dopt=Abstract 10.1007/s10072-017-2972-z [DOI] [PubMed] [Google Scholar]
- 19.Bargellesi S, Cavasin L, Scarponi F, De Tanti A, Bonaiuti D, Bartolo M, et al. ; Heterotopic Ossification Cross Sectional Survey group (HOCSS) *. Occurrence and predictive factors of heterotopic ossification in severe acquired brain injured patients during rehabilitation stay: cross-sectional survey. Clin Rehabil 2018;32:255–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28805078&dopt=Abstract 10.1177/0269215517723161 [DOI] [PubMed] [Google Scholar]
- 20.Scarponi F, Zampolini M, Zucchella C, Bargellesi S, Fassio C, Pistoia F, et al. ; C.I.R.C.LE (Comorbidità in Ingresso in Riabilitazione nei pazienti con grave CerebroLEsione acquisita) study group. Identifying clinical complexity in patients affected by severe acquired brain injury in neurorehabilitation: a cross sectional survey. Eur J Phys Rehabil Med 2019;55:191–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30543265&dopt=Abstract 10.23736/S1973-9087.18.05342-X [DOI] [PubMed] [Google Scholar]
- 21.Basaglia N. Progettare la Riabilitazione – Il lavoro in team interprofessionale. Milan: Edi-Ermes; 2002. [Google Scholar]
- 22.Estraneo A, Masotta O, Bartolo M, Pistoia F, Perin C, Marino S, et al. Multi-center study on overall clinical complexity of patients with prolonged disorders of consciousness of different etiologies. Brain Inj 2021;35:1–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33331792&dopt=Abstract 10.1080/02699052.2020.1861652 [DOI] [PubMed] [Google Scholar]
- 23.Bartolo M, Chiò A, Ferrari S, Tassorelli C, Tamburin S, Avenali M, et al. Italian Consensus Conference on Pain in Neurorehabilitation (ICCPN) . Assessing and treating pain in movement disorders, amyotrophic lateral sclerosis, severe acquired brain injury, disorders of consciousness, dementia, oncology and neuroinfectivology. Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Eur J Phys Rehabil Med 2016;52:841–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27579582&dopt=Abstract [PubMed] [Google Scholar]
- 24.Lavezzi S, Bargellesi S, Cassio A, De Tanti A, Gatta G, Lombardi F, et al. Protocollo di Minima GCA 2020 [Internet]. Available from: https://springerhealthcare.it/mr/archivio/protocollo-di-minima-gca-2020/ [cited 2022, May 31].
- 25.Lucca LF, De Tanti A, Cava F, Romoli A, Formisano R, Scarponi F, et al. Predicting Outcome of Acquired Brain Injury by the Evolution of Paroxysmal Sympathetic Hyperactivity Signs. J Neurotrauma 2021;38:1988–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33371784&dopt=Abstract 10.1089/neu.2020.7302 [DOI] [PubMed] [Google Scholar]
- 26.Dhandapani M, Dhandapani S, Agarwal M, Mahapatra AK. Pressure ulcer in patients with severe traumatic brain injury: significant factors and association with neurological outcome. J Clin Nurs 2014;23:1114–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24112115&dopt=Abstract 10.1111/jocn.12396 [DOI] [PubMed] [Google Scholar]
- 27.Pistoia F, Sacco S, Stewart J, Sarà M, Carolei A. Disorders of Consciousness: Painless or Painful Conditions?-Evidence from Neuroimaging Studies. Brain Sci 2016;6:47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27740600&dopt=Abstract 10.3390/brainsci6040047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lejeune N, Thibaut A, Martens G, Martial C, Wannez S, Laureys S, et al. Can the Nociception Coma Scale-Revised Be Used in Patients With a Tracheostomy? Arch Phys Med Rehabil 2020;101:1064–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31765612&dopt=Abstract 10.1016/j.apmr.2019.09.020 [DOI] [PubMed] [Google Scholar]
- 29.Reverberi C, Lombardi F, Lusuardi M, Pratesi A, Di Bari M. Development of the Decannulation Prediction Tool in Patients With Dysphagia After Acquired Brain Injury. J Am Med Dir Assoc 2019;20:470–475.e1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30455047&dopt=Abstract 10.1016/j.jamda.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 30.Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018;91:450–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30089618&dopt=Abstract 10.1212/WNL.0000000000005926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forrester G, Encel J, Geffen G. Measuring post-traumatic amnesia (PTA): an historical review. Brain Inj 1994;8:175–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8193637&dopt=Abstract 10.3109/02699059409150969 [DOI] [PubMed] [Google Scholar]
- 32.Fortuny LA, Briggs M, Newcombe F, Ratcliff G, Thomas C. Measuring the duration of post traumatic amnesia. J Neurol Neurosurg Psychiatry 1980;43:377–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7420085&dopt=Abstract 10.1136/jnnp.43.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marosszeky NE, Ryan L, Shores EA. The PTA Protocol. Guidelines focusing the Westmead Post-Traumatic Amnesia (PTA) Scale. Sydney: Wild &Wooley; 1998 [Google Scholar]
- 34.Marshman LA, Hennessy M, Delle Baite L, Britton G. Utility of Retrograde Amnesia Assessment Alone, Compared with Anterograde Amnesia Assessment in Determining Recovery After Traumatic Brain Injury: Prospective Cohort Study. World Neurosurg 2018;110:e830–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29191531&dopt=Abstract 10.1016/j.wneu.2017.11.131 [DOI] [PubMed] [Google Scholar]
- 35.Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J Rehabil Res Dev 2007;44:975–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18075954&dopt=Abstract 10.1682/JRRD.2006.12.0158 [DOI] [PubMed] [Google Scholar]
- 36.Castiglia SF, Galeoto G, Lauta A, Palumbo A, Tirinelli F, Viselli F, et al. The culturally adapted Italian version of the Barthel Index (IcaBI): assessment of structural validity, inter-rater reliability and responsiveness to clinically relevant improvements in patients admitted to inpatient rehabilitation centers. Funct Neurol 2017;22:221–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29306359&dopt=Abstract 10.11138/FNeur/2017.32.4.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakao S, Takata S, Uemura H, Kashihara M, Osawa T, Komatsu K, et al. Relationship between Barthel Index scores during the acute phase of rehabilitation and subsequent ADL in stroke patients. J Med Invest 2010;57:81–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20299746&dopt=Abstract 10.2152/jmi.57.81 [DOI] [PubMed] [Google Scholar]
- 38.Bartolo M, Bargellesi S, Castioni CA, Bonaiuti D, Antenucci R, Benedetti A, et al. Intensive Care and Neurorehabilitation Italian Study Group . Early rehabilitation for severe acquired brain injury in intensive care unit: multicenter observational study. Eur J Phys Rehabil Med 2016;52:90–100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26530213&dopt=Abstract [PubMed] [Google Scholar]
- 39.Boltzmann M, Schmidt SB, Gutenbrunner C, Krauss JK, Stangel M, Höglinger GU, et al. The influence of the CRS-R score on functional outcome in patients with severe brain injury receiving early rehabilitation. BMC Neurol 2021;21:44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33514337&dopt=Abstract 10.1186/s12883-021-02063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boltzmann M, Schmidt SB, Rollnik JD. Impact of Thyroid Hormone Levels on Functional Outcome in Neurological and Neurosurgical Early Rehabilitation Patients. BioMed Res Int 2017;2017:4719279. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28900623&dopt=Abstract 10.1155/2017/4719279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogner JA, Whiteneck GG, MacDonald J, Juengst SB, Brown AW, Philippus AM, et al. Test-Retest Reliability of Traumatic Brain Injury Outcome Measures: A Traumatic Brain Injury Model Systems Study. J Head Trauma Rehabil 2017;32:E1–16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28195954&dopt=Abstract 10.1097/HTR.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 42.Benge JF, Caroselli JS, Reed K, Zgaljardic DJ. Changes in supervision needs following participation in a residential post-acute brain injury rehabilitation programme. Brain Inj 2010;24:844–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20377342&dopt=Abstract 10.3109/02699051003724960 [DOI] [PubMed] [Google Scholar]
- 43.Bailey EK, Nakase-Richardson R, Patel N, Dillahunt-Aspillaga C, Ropacki SA, Sander AM, et al. Supervision Needs Following Veteran and Service Member Moderate to Severe Traumatic Brain Injury: A VA TBI Model Systems Study. J Head Trauma Rehabil 2017;32:245–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28520667&dopt=Abstract 10.1097/HTR.0000000000000317 [DOI] [PubMed] [Google Scholar]
- 44.Ritchie L, Wright-St Clair VA, Keogh J, Gray M. Community integration after traumatic brain injury: a systematic review of the clinical implications of measurement and service provision for older adults. Arch Phys Med Rehabil 2014;95:163–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24016401&dopt=Abstract 10.1016/j.apmr.2013.08.237 [DOI] [PubMed] [Google Scholar]
- 45.Lee H, Lee Y, Choi H, Pyun SB. Community integration and quality of life in aphasia after stroke. Yonsei Med J 2015;56:1694–702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26446656&dopt=Abstract 10.3349/ymj.2015.56.6.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turcotte S, Beaudoin M, Vallée C, Vincent C, Routhier F. Psychometric properties of the Community Integration Questionnaire: a systematic review of five populations. Clin Rehabil 2019;33:1775–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31397182&dopt=Abstract 10.1177/0269215519867998 [DOI] [PubMed] [Google Scholar]
- 47.Giustini M, Longo E, Azicnuda E, Silvestro D, D’Ippolito M, Rigon J, et al. Health-related quality of life after traumatic brain injury: italian validation of the QOLIBRI. Funct Neurol 2014;29:167–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25473736&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 48.von Steinbuechel N, Wilson L, Gibbons H, Muehlan H, Schmidt H, Schmidt S, et al. QOLIBRI overall scale: a brief index of health-related quality of life after traumatic brain injury. J Neurol Neurosurg Psychiatry 2012;83:1041–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22851609&dopt=Abstract 10.1136/jnnp-2012-302361 [DOI] [PubMed] [Google Scholar]
- 49.Heiberg G, Pedersen SG, Friborg O, Nielsen JF, Holm HS, Steinbüchel von N, et al. Can the health related quality of life measure QOLIBRI- overall scale (OS) be of use after stroke? A validation study. BMC Neurol 2018;18:98. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30021558&dopt=Abstract 10.1186/s12883-018-1101-9 [DOI] [PMC free article] [PubMed]
- 50.Wong GK, Lam SW, Ngai K, Wong A, Mok V, Poon WS. Quality of Life after Brain Injury (QOLIBRI) Overall Scale for patients after aneurysmal subarachnoid hemorrhage. J Clin Neurosci 2014;21:954–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24373816&dopt=Abstract 10.1016/j.jocn.2013.09.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Evaluation tool references.