Abstract
BACKGROUND
Stroke is the most common cause of disability in Western Countries. It can lead to loss of mobility, capability to walk and ultimately loss of independence in activities of daily living (ADL). Several rehabilitative approaches have been proposed in these years. Robot-assisted gait rehabilitation (RAGT) plays a crucial role to perform a repetitive, intensive, and task-oriented treatment in stroke survivors. However, there are still few data on its role in subacute stroke patients.
AIM
The aim of the present study was to assess the efficacy of RAGT for gait recovery in subacute stroke survivors.
DESIGN
Systematic review with meta-analysis.
SETTING
The setting of the study included Units of Rehabilitation.
POPULATION
The analyzed population was represented by subacute stroke patients.
METHODS
PubMed, Scopus, Web of Science, CENTRAL, and PEDro were systematically searched until January 18, 2021, to identify randomized controlled trials (RCTs) presenting: stroke survivors in subacute phase (≤6 months) as participants; exoskeleton robots devices as intervention; conventional rehabilitation as a comparator; gait assessment, through qualitative scales, quantitative gait scales or quantitative parameters, as outcome measures. We also performed a meta-analysis of the mean difference in the functional ambulation category (FAC) via the random effect method.
RESULTS
Out of 3188 records, 14 RCTs were analyzed in this systematic review. The 14 studies have been published in the last 14 years (from 2006 to 2021) and included 576 stroke survivors, of which 306 received RAGT, and 270 underwent conventional rehabilitation. Lokomat robotic system was the most investigated robotic exoskeleton by the RCTs included (N.=9), albeit the meta-analysis demonstrated a non-significant difference of -0.09 in FAC (95% CI: -0.22.0.03) between Lokomat and conventional therapy. According to the PEDro Scale, 11 (78.5%) were classified as good-quality studies, two as fair-quality studies (14.3%), and one as poor-quality study (7.1%).
CONCLUSIONS
Taken together, these findings showed that RAGT might have a potential role in gait recovery in subacute stroke survivors. However, further RCTs comparing the efficacy of RAGT with conventional physical therapy are still warranted in the neurorehabilitation field.
CLINICAL REHABILITATION IMPACT
This systematic review provides information on the efficacy of RAGT in allowing subacute stroke patients to perform high-intensity gait training with a lower physical burden on PRM professionals.
Key words: Robotics, Stroke, Gait, Stroke rehabilitation, Rehabilitation
Introduction
Stroke is the most common cause of disability in the Western Countries, taking into account that about 40% of stroke patients suffer from moderate disability, while 10 to 30% results in severe disability.1
Walking is considered the cornerstone of autonomous mobility, thus making necessary gait recovery an essential determinant in stroke survivors. Walking ability and the resulting independence in ADL are crucial in many aspects, such as improving psychological well-being,2 reducing the risk of further cognitive decline,3 and increasing overall physical activity.4 Restoring gait, as far as quantity and quality are concerned, is considered a target of primary importance.5
Thus, gait rehabilitation is an essential treatment for stroke survivors with moderate to severe disabilities.6 Several approaches have been proposed in recent years.7-10 The most classical approach consists of a bottom-up, patient-oriented approach. Traditional therapeutic exercises are performed to improve gait pattern, recover balance, strength and endurance.10, 11 Indeed, given the high variability of functional conditions and gait patterns in stroke patients, all the approaches need a high degree of individualization, making standardization of clinical studies particularly hard. Until now, no approach is superior to the others.10
Recent technological advancements provide physicians with tools to compare high technology approaches to traditional gait rehabilitation.12 Technology (e.g. robotic devices, virtual reality) could facilitate plasticity-related recovery by increasing sensory feedback and supporting the motor system.13-15 Virtual reality and interactive video gaming have been proposed as rehabilitation tools for both upper limb functionality16 and gait ability and balance,17 however, their effectiveness is still not certain.14 Motor imagery is not a recent concept, as it has been used for motor rehabilitation for almost 20 years,18 not only in stroke survivors but even in other musculoskeletal diseases.19 However, brain-computer interface,20 transcranial direct current stimulation,21 and functional magnetic resonance imaging22 demonstrated to improve the efficacy of motor imagery.
Miniaturized technologies and advances in robotics offered robotic-assisted training as a novel approach in gait rehabilitation, where the need for prolonged, repeated training is crucial.23 Moreover, it has demonstrated that augmented exercise therapy effectively improves ADL, particularly within the first six months after stroke.24, 25
Robotic devices are well suited to produce intensive, task-oriented motor training for moving the patient’s limbs, supervised by physical therapists, enhancing conventional rehabilitation.26
Robot-assisted gait rehabilitation (RAGT) could involve end-effector-type devices or exoskeleton-type devices.27 End-effector devices might work by applying mechanical forces to the distal segments of limbs, offering the advantage of easy setup albeit suffering from limited control of the proximal joints of the limb, leading to abnormal movement patterns. On the contrary, robotic exoskeletons, consisting of axes aligned with the anatomical axes of the wearer, provide a direct control of individual joints, thus minimizing abnormal posture or movement.28 Robotic exoskeletons for gait rehabilitation might guide the legs through a planned gait pattern, while the patient experiences near-normal proprioceptive input during limb loading.28 In fact, previous studies have shown the efficacy of RAGT in improving muscular performance and walking measure during walking assessed by surface electromyography29-31 and gait analysis.32 Furthermore, RAGT can induce peculiar neurophysiologic modulation.32 Moreover, the robotic exoskeleton for the lower limb could be divided into “static robots,” that allow patients to perform gait training in a fixed and confined area (e.g., treadmill), and “overground exoskeleton,” either unilateral or bilateral robots, that allow the patients to walk overground exploring the environment.27 In this context, RAGT allows the patients to perform high-intensity rehabilitation sessions with considerable safety and with a relatively low load for physiotherapists, both in terms of time and physical effort.27 The physical and rehabilitation medicine (PRM) physician has the ability to set all important kinematic parameters, in order to both personalize and allow consistent repeatability of rehabilitative sessions. New evidence has been published on the topic at a fast rate, because of the topic’s novelty, without clear recommendations, especially in chronic stroke survivors.25, 33, 34 A recent meta-analysis, performed by Mehrholz et al., affirmed that stroke patients who received electromechanical-assisted gait training combined with physiotherapy were more likely to achieve independent walking than those with gait training alone. More in detail, patients with subacute stroke are expected to benefit more from RAGT than patients with chronic stroke.23
Therefore, in this systematic review, we aimed to assess the efficacy of RAGT in patients with subacute stroke to provide PRM physicians with the state-of-the-art on this crucial topic.
Evidence acquistion
Search strategy
PubMed, Scopus, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and Physiotherapy Evidence Database (PEDro) databases were systematically searched for English-language articles, according to each specific thesaurus, adopting the strategy depicted by Table I. Data search was performed from the inception until January 18, 2021. This systematic review has been performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.35 The protocol has been submitted for registration in the International prospective register of systematic reviews (PROSPERO).
Table I. —Search strategy.
| PubMed |
| (“exoskeleton” OR “robotic”) AND (“gait” OR “ambulation”) AND (“rehabilitation” OR “exercise”) AND (“stroke” OR “hemiparesis”) |
| Scopus |
| Title-abs-key ([“exoskeleton” OR “robotic”] AND [“gait” OR “ambulation”] AND [“rehabilitation” OR “exercise”] AND [“stroke” OR “hemiparesis”]) |
| Web of Science |
| ([“exoskeleton” OR “robotic”] AND [“gait” OR “ambulation”] AND [“rehabilitation” OR “exercise”] AND [“stroke” OR “hemiparesis”]) |
| CENTRAL |
| *(“exoskeleton” OR “robotic”) AND (“gait” OR “ambulation”) AND (“rehabilitation” OR “exercise”) AND (“stroke” OR “hemiparesis”) |
| PEDro |
| (exoskeleton) AND (stroke) AND (gait) |
Selection criteria
Studies were considered eligible if responding to the questions defined according to the PICO model:
participants – stroke survivors in subacute phase (not more than six months from the event);
intervention – all robotic exoskeleton devices;
comparator – conventional rehabilitation (strengthening exercises for tibialis anterior, quadriceps, gluteus maximus and medius muscles, muscle stretching, Bobath approach, postural control training, parallel walking training, and manual walking training-assisted overground therapy);
outcome measure – gait assessment, through qualitative scales (Functional Ambulation Scale: FAC; Modified Emory Functional Ambulation Profile: mEFAP; Mobility Milestones, Walking Handicap Scale: WHS), quantitative gait scale (10-Meter Walk Test: 10MWT; 2-Minutes Walking Test:2MWT; 6-Minute Walking Test: 6MWT; Time Up and Go: TUG; quantitative parameters (walking speed, maximal walking speed, stride duration cadence, stance duration and single support time of both legs, and walking distance).
We included only papers with randomized controlled trial (RCT) as the study design. Exclusion criteria were: 1) robotic devices different from exoskeleton (e.g., end effectors); 2) time after stroke more than six months; 3) studies written in a language different from English; 4) full-text unavailability (i.e., posters and conference abstracts); and 5) studies involving animals.
Data extraction
Two reviewers independently extracted data from included studies using a customized data extraction table in Microsoft Excel. In case of disagreement, a consensus was achieved by the decision of another reviewer.
The following data were extracted: 1) first author; 2) publication year; 3) nationality; 4) type and name of RAGT; 6) comparator; 7) population and the number of patients included; 8) age of study participants; 9) days from the stroke; 10) side of stroke; 11) type of stroke; 12) gait outcome measures; and 13) main findings.
Data synthesis
The selected studies have been synthetized describing extracted data. Two reviewers independently assessed study the quality of the RCTs according to the PEDro scale.36 In case of disagreement, a consensus was achieved involving another reviewer in the decisional process. According to the PEDro scale,24 studies included were classified as excellent (9-10 points), good (6-8 points), fair (4-5 points) or poor (<4 points). Furthermore, the risk of bias assessment was performed using GRADE model.37
Statistical synthesis
Data analysis was performed by Review Manager version 5.3.5 (Cochrane Collaboration, Copenhagen, Denmark). A prespecified alpha of 0.05 was used for all statistical tests and confidence intervals; statistical heterogeneity was assessed by the I2 statistic. For statistical homogeneity, medians and Inter-quartile ranges (IQRs) were converted to means with Standard Deviations (SD) to maximize the number of studies eligible for meta-analysis.38 For such continuous data, we computed means and SDs for each study and undertook meta-analysis via mean difference and random effect model to produce pooled measures of association, corresponding 95% confidence intervals (95% CI), and respective forest plot.
Evidence synthesis
At the end of the search, 3188 studies were identified, 162 of which were considered suitable for the title and abstract screening, after removing duplicates. Out of these, 98 were excluded after the title and abstract screening, according to the PICO model. Thus, 64 articles were assessed for eligibility and 50 of them were excluded with reasons: study type different than RCT (N.=6), full text unavailability (N.=10), withdrawn or unfinished articles (N.=3), RCT study protocol design (N.=1), articles not including gait assessment as an outcome (N.=10), studies including patients with chronic stroke (N.=13), articles using end-effector robots as intervention (N.=7).
Therefore, fourteen RCTs39-52 were included in this systematic review, as illustrated by the PRISMA flowchart in Figure 1. The main characteristics of these studies are described in detail in Supplementary Digital Material 1 (Supplementary Table I).
Figure 1.

—PRISMA 2009 Flow Diagram.
The included studies39-52 have been published in the last 15 years (from 2006 to 2021). Seven (50.0%)39, 43, 44, 46-48, 52 were conducted in Europe (2 from Italy, 2 from Germany, 1 from Austria-Italy, 1 from the Netherlands, and 1 from Sweden),39, 43, 44, 46-48, 51 one42, in the United States of America (7.1%) and six40, 41, 45, 49, 50, 52 (42.8%) in Asia (3 in South Korea, 1 in Israel and 2 in Japan).40, 41, 45, 49, 50, 52
A total of 576 subjects were analyzed, of which 306 received RAGT rehabilitation and 270 were included in the control group (undergone conventional rehabilitation). Study cohorts of the RCTs included ranged from 22 to 75 patients,49, 51 with a mean age ranging from 50.0±9.6 years47 to 76.8±13.8 years.50 The average time from the onset of stroke ranged between 16.1±4.9 days40 and 139.9±60.942 days, including stroke survivors in subacute phase (<6 months from onset). At the baseline, FAC was <1 in six studies,39-41, 43, 45, 48 ranged from 1 to 3 in seven RCTs,46, 49-52 FAC was >3 in 1 study.42 Concerning the follow-up evaluations, one RCT performed a follow-up at 4 weeks from baseline,39 one at 8 weeks,52 one RCTs at 12 weeks,42 one at 17,46 one study at 24 weeks,48 one study at 8 and 12 weeks,50 and one RCT at 24 and 36 weeks.47 The duration of robotic rehabilitation was heterogeneous, varying from 241 to 844 weeks, with the duration of the interventions ranging from a total of 449, 50 to 3044 hours (Supplementary Table I). Nine RCTs39-47 investigated the effectiveness of Lokomat robotic system (Hocoma AG, Volketswil, Switzerland), three used the unilateral Hybrid Assistive Limb-HAL (Cyberdyne, Tsukuba, Japan),48-50 one the Ekso (Ekso GT™, Ekso Bionics Inc., Richmond, CA, USA),51 and one the Walkbot (P&S Mechanics, Seoul, Korea).52
According to the abovementioned the PEDro scale,36 we assessed the study quality reporting that eleven RCTs (78.5%)39-46, 48, 51, 52 were classified as good-quality studies, two RCTs (14.3%)49, 50 as fair-quality studies, and one RCT (7.4%)47 as poor-quality study (the quality scoring for each assessment criteria is showed in detail Supplementary Digital Material 2: Supplementary Table II). Concerning the risk of bias assessment by GRADE (Supplementary Digital Material 3: Supplementary Table III), in total ten studies had adequate random sequence generation,39, 40, 44, 47-51 our did not give enough information.45, 47, 52 Regarding “selection” bias, five studies had adequate allocation concealment,39, 43, 44, 46, 51 five did not give enough information41, 42, 45, 47, 52 and four described risky allocation concealment.40, 48-50 Overall, fourteen studies declared non-blinding participants and personnel.39-52 eight studies described blinding of outcome assessments,39-41, 43, 44, 46, 48, 52 two not give information,49, 50 four described no blinding of outcome assessment.42, 47, 49-51 Five reported complete outcome data,40, 45, 46, 48, 51 one did not give enough information44 and eight reported incomplete data.39, 41-43, 47, 49, 50 For most studies, reporting bias was not mentioned because the protocol was not described.39, 41-45, 47, 49, 50, 52 Of the fourteen studies, only four provided enough information to be considered at low risk.40, 46, 48, 51
A meta-analysis is performed only for six RCTs39-45, 47 comparing Lokomat vs. conventional rehabilitation in terms of the mean difference in FAC outcome. All the other papers were heterogeneous, adopting different systems for RAGT, and investigating different outcomes (e.g., 6MWT, 10MWT, FAC, etc.).
Lokomat vs. conventional rehabilitation
Nine RCTs39-47 assessed Lokomat as an intervention, two of which39, 42 compared the only Lokomat versus the only conventional rehabilitation in terms of efficacy on gait parameters in patients with subacute stroke. Bergman et al.39 showed a significant improvement in FAC only in the control group (P=0.023). Similar results were found by Hidler et al.42 that reported a significant lower increase in RAGT group than control group in walking speed at postintervention (0.12±0.03 m/s vs. 0.35±0.03 m/s; P=0.002) and at the 3-month follow-up (0.15±0.04 m/s vs. 0.30±0.03 m/s; P=0.006). Moreover, Lokomat group also showed significantly lower increase at 6MWT after intervention than conventional rehabilitation (164.6±32.5 feet vs. 274±35.4 feet P=0.03).
Furthermore, seven studies40, 41, 43-47 compared the effectiveness of Lokomat combined with conventional rehabilitation with the only conventional rehabilitation in terms of gait in patients with subacute stroke.
Schwartz et al.45 showed a greater percentage of patients who achieved independent walking (FAC≥3) in the RAGT group than the control group (54.1% vs. 28.6%; P=0.03) at the end of the treatment.
All the other RCTs40, 41, 43, 44, 46, 47 comparing Lokomat plus conventional therapy versus only conventional therapy reported no statistically significant differences between groups.
Considering the intra-group analysis, Han et al.41 found a statistically significant improvement in FAC in both groups (RAGT: 0.10±0.31 vs. 1.33±1.21; P<0.001; control: 0.27±0.46 vs. 1.95±1.75; P=0.001). Husemann et al.43 showed a significant improvement in both groups in the following gait outcome measures: stride duration (RAGT: 4.02±0.049 s vs. 3.15±0.3 s; P=0.028; control: 5.5±0.81 s vs. 3.58±0.4 s; P=0.011), stance duration of unaffected leg (RAGT: 3.67±0.58 s vs. 2.67±0.28 s; P=0.033; control: 4.75±0.7 s vs. 3.24±0.38 s; P=0.019), and independent walking defined by FAC (RAGT: 0±1 vs. 1±3; P=0.001; control: 0±0 vs. 1±3; P=0.001). Mayr et al.44 found a significant improvement of mEFAP in both groups (RAGT: -145; P=0.001; control: -151; P=0.001). Taveggia et al.46 showed a significant improvement of 10MWT in RAGT group after intervention (P=0.014) and at the follow-up (P<0.01), and a significant improvement of 6MWT in subjects undergoing conventional rehabilitation at the follow-up (P=0.017).
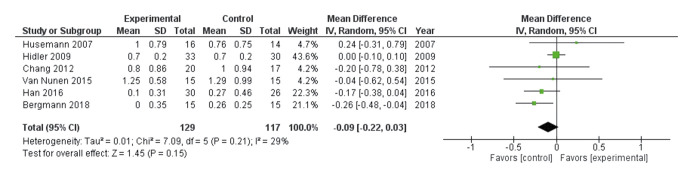
Lastly, we performed a meta-analysis of the mean difference in FAC outcome via the random effect method. Although the heterogeneity of the results of the six RCTs39-43, 47 was low (I2 = 29%), we reported the conversion of the medians and IQRs to means±SD in the studies by Husemann et al.43 and Bergmann et al.39 Thus, we found a non-significant difference of -0.09 in FAC [95% CI: -0.22.0.03] in favor of the control compared to the Lokomat group (see the forest plot depicted by Figure 2).39-43, 47
Figure 2.
—Forest plot of meta-analysis of the mean difference in functional ambulation category comparing Lokomat versus conventional therapy.39-43, 47
Unilateral hybrid assisted limb vs. conventional rehabilitation
Three studies48-50 have investigated the efficacy of unilateral HAL as a robotic exoskeleton in combination with bodyweight supported treadmill compared to conventional rehabilitation in terms of gait in subacute stroke survivors.
Watanabe et al.49 in 2014 reported a statistically significant difference between groups; thus, the RAGT group showed a significantly higher improvement in FAC than the control group (between-group difference = 0.45; P=0.04). The same study group in 201750 found that the unilateral HAL group had a significant improvement in FAC after 12 sessions (4 weeks) and at 8 and 12 weeks postintervention compared to the conventional group (P=0.026). On the other hand, Wall et al.48 did not report significant between-group differences in FAC and 2MWT in their RCT.
Focusing on the intra-group analysis, the first RCT performed by Watanabe et al.49 showed a significant improvement only in the HAL group in terms of maximal walking speed (0.61±0.43 m/s vs. 0.85±0.43 m/s; P<0.05), cadence (86.1±35.9 m vs. 108.4±33.3 m; P<0.05) and 6MWT (97.7±107.6 m vs. 156.7±137.9 m; P<0.05); however, TUG (RE: 27.8±14.3 s vs. 16.8±7.0 s; P<0.05; CT: 45.8±25.7 s vs. 29.9±18.4 s; P<0.01) and FAC (RE: 2.0±1.1 vs. 3.1±1.4; P<0.001; CT: 2.0±1.0 vs. 2.6±1.4; P<0.01) significantly improved in both groups.
Ekso vs. conventional rehabilitation
In 2021, Molteni et al.51 compared Ekso vs. conventional therapy, showing a statistically significant difference between-groups in terms of WHS at the end of the 3-week-treatment in favour of the RAGT group (P=0.001).
Considering the intra-group differences, the authors found a statistically significant improvement (P<0.001) after a 3-week-treatment in terms of 6MWT, 10MWT, FAC, and WHS in both groups (Ekso exoskeleton group and conventional therapy group).
Walkbot vs. conventional rehabilitation
In 2015, Kim et al.52 compared Walkbot plus conventional rehabilitation with only conventional rehabilitation. The authors found a significant interaction effect (time×group) for FAC (P=0.002) between groups in favor of the RAGT group.
Discussion
Rehabilitation with exoskeletons aimed to improve gait in patients with neurological diseases is a hot topic, with a quickly expanding literature.26, 27, 53, 54 Thus, by this systematic review, we aimed at summarizing the evidence on the efficacy of robotic exoskeletons in gait training for sub-acute stroke patients. This update was needed because of the relative novelty of the topic (the first selected RCT was published less than 15 years ago, in 2007)43 and because of the recent development of the technologies that provide physicians with more mature and refined robotic exoskeletons in the last few years. More than half of the studies (7 out of 14)39, 41, 44, 46, 48, 50, 51 was published in the last 4 years (from 2016 to January, 2021).
Studies were generally of sound quality, as they scored reasonably well in the PEDro scale, with eleven studies reaching the grade of good-quality study;39-46, 48, 51, 52 the most represented flaws were the lack of blinding of subjects (14/14), therapists (14/14), and assessors (5/14). In a technology such as robotics, it is almost inevitable to hide to patients and therapists the intervention because of its inherent intrusiveness and the impossibility of creating sham treatment. Unfortunately, another common flaw in the studies included was the lack of concealed allocation, revealed to be absent in 8 out of 14 studies. Authors of future studies on the RAGT should consider the importance of avoiding allocation bias through appropriate concealment.
A recent systematic review, performed by Moucheboeuf et al.54 showed that robotic gait training could be beneficial for stroke patients; however, differently from their work, our systematic review focused both on stroke patients during the subacute phase, with the duration of stroke shorter than 6 months, and on a specific robotic exoskeleton. Stroke patients in this phase are undergoing a process of spontaneous recovery, so it is harder to discern natural history from the effect of rehabilitation. For this reason, control groups with similar characteristics are of the utmost importance; all the studies included in this review reported a control group composed of patients with subacute stroke.
However, it might be expected in the first phase of adopting new technology since it is still not clear how different features affect different aspects of gait, and how technology and outcomes researchers should focus their studies. For example, two technologies such as Lokomat/Walkbot and HAL/Ekso are both robotic exoskeletons but have a crucial difference: the first one is a static robot and adopt a fixed treadmill. In contrast, the second one is an overground robot and is not linked to a fixed structure, allowing gait in a more ecological setting with the patients able to walk overground and to explore the environment.27 Indeed, static and overground robots are not entirely different, as both have all the characteristics of RAGT. Still, their peculiar and unique features could be more beneficial for some subclasses of patients and aspects of gait, which will be understood only after the completion of further studies. Only after the completion of this first exploratory phase researchers will focus more on specific patients and outcomes.
Moreover, in all RCTs, it is not adequately specified how the robot setting has been performed concerning the patient’s characteristics; we retain that promoting a “tailored” RAGT could improve the efficacy of robotic rehabilitation.27 In this context, instrumental tools (e.g., functional magnetic resonance, electroencephalography, transcranial magnetic stimulation paradigm and electromyography) might help the clinicians to highlight the usefulness of RAGT on gait re-learning.27 It would be very important to identify electromyographic parameters to define the optimal exoskeleton setting and optimize the interaction between the user and the robot.
Limitations of the study
The main limitation of the current systematic review is the lack of meta-analysis for all the RAGT interventions assessed. Indeed, except for studies investigated the Lokomat (with FAC as outcome), all the other RCTs were very heterogeneous, adopting different systems for RAGT and studied different outcomes, in some cases related to endurance (e.g., 6MWT), in other cases linked to gait speed (e.g., 10MWT) or gait independence (e.g., FAC). Furthermore, all studies showed a high heterogeneity of protocol session and duration of intervention.
Conclusions
In conclusion, early gait training is crucial to recover for stroke survivors, albeit, to date, the scientific literature lacks RCTs on the efficacy of RAGT. These present findings suggest that the use of RAGT associated with CT is effective on gait recovery in subacute stroke patients, albeit not superior at only CT. Further RCTs are warranted to confirm the efficacy of RAGT in stroke survivors.
References
- 1.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke 2005;36:e100–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16120836&dopt=Abstract 10.1161/01.STR.0000180861.54180.FF [DOI] [PubMed] [Google Scholar]
- 2.Trompetto C, Marinelli L, Mori L, Cossu E, Zilioli R, Simonini M, et al. Postactivation depression changes after robotic-assisted gait training in hemiplegic stroke patients. Gait Posture 2013;38:729–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23570893&dopt=Abstract 10.1016/j.gaitpost.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 3.Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr 2019;19:186. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31277579&dopt=Abstract 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer SF, Hung SH, Brodtmann A. The Impact of Physical Activity Before and After Stroke on Stroke Risk and Recovery: a Narrative Review. Curr Neurol Neurosci Rep 2019;19:28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31011851&dopt=Abstract 10.1007/s11910-019-0949-4 [DOI] [PubMed] [Google Scholar]
- 5.Negrini F, Gasperini G, Guanziroli E, Vitale JA, Banfi G, Molteni F. Using an Accelerometer-Based Step Counter in Post-Stroke Patients: Validation of a Low-Cost Tool. Int J Environ Res Public Health 2020;17:E3177. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32370210&dopt=Abstract 10.3390/ijerph17093177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693–702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21571152&dopt=Abstract 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 7.Mehrholz J, Thomas S, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev 2017;8:CD002840. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28815562&dopt=Abstract 10.1002/14651858.CD002840.pub4 [DOI] [PMC free article] [PubMed]
- 8.States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev 2009;(3):CD006075. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19588381&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva S, Borges LR, Santiago L, Lucena L, Lindquist AR, Ribeiro T. Motor imagery for gait rehabilitation after stroke. Cochrane Database Syst Rev 2020;9:CD013019. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32970328&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 10.Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014;(4):CD001920. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24756870&dopt=Abstract 10.1002/14651858.CD001920.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tollár J, Nagy F, Csutorás B, Prontvai N, Nagy Z, Török K, et al. High Frequency and Intensity Rehabilitation in 641 Subacute Ischemic Stroke Patients. Arch Phys Med Rehabil 2021;102:9–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32861668&dopt=Abstract 10.1016/j.apmr.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 12.Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2017;11:CD008349. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29156493&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 13.Morone G, Tramontano M, Iosa M, Shofany J, Iemma A, Musicco M, et al. The efficacy of balance training with video game-based therapy in subacute stroke patients: a randomized controlled trial. BioMed Res Int 2014;2014:580861. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24877116&dopt=Abstract 10.1155/2014/580861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010;362:1772–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20400552&dopt=Abstract 10.1056/NEJMoa0911341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klamroth-Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol 2014;13:159–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24382580&dopt=Abstract 10.1016/S1474-4422(13)70305-3 [DOI] [PubMed] [Google Scholar]
- 16.Johnson L, Bird ML, Muthalib M, Teo WP. An Innovative STRoke Interactive Virtual thErapy (STRIVE) Online Platform for Community-Dwelling Stroke Survivors: A Randomized Controlled Trial. Arch Phys Med Rehabil 2020;101:1131–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32283048&dopt=Abstract 10.1016/j.apmr.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 17.Lloréns R, Noé E, Colomer C, Alcañiz M. Effectiveness, usability, and cost-benefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: a randomized controlled trial. Arch Phys Med Rehabil 2015;96:418–425.e2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25448245&dopt=Abstract 10.1016/j.apmr.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 18.Stevens JA, Stoykov ME. Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil 2003;84:1090–2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12881842&dopt=Abstract 10.1016/S0003-9993(03)00042-X [DOI] [PubMed] [Google Scholar]
- 19.Zapparoli L, Sacheli LM, Seghezzi S, Preti M, Stucovitz E, Negrini F, et al. Motor imagery training speeds up gait recovery and decreases the risk of falls in patients submitted to total knee arthroplasty. Sci Rep 2020;10:8917. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32488010&dopt=Abstract 10.1038/s41598-020-65820-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao Y, Chen S, Zhang X, Jin J, Xu R, Daly I, et al. BCI-Based Rehabilitation on the Stroke in Sequela Stage. Neural Plast 2020;2020:8882764. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33414824&dopt=Abstract 10.1155/2020/8882764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew E, Teo WP, Tang N, Ang KK, Ng YS, Zhou JH, et al. Using Transcranial Direct Current Stimulation to Augment the Effect of Motor Imagery-Assisted Brain-Computer Interface Training in Chronic Stroke Patients-Cortical Reorganization Considerations. Front Neurol 2020;11:948. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32973672&dopt=Abstract 10.3389/fneur.2020.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehler DM, Williams AN, Whittaker JR, Krause F, Lührs M, Kunas S, et al. Graded fMRI Neurofeedback Training of Motor Imagery in Middle Cerebral Artery Stroke Patients: A Preregistered Proof-of-Concept Study. Front Hum Neurosci 2020;14:226. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32760259&dopt=Abstract 10.3389/fnhum.2020.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev 2020;10:CD006185. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33091160&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 24.Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 2004;35:2529–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15472114&dopt=Abstract 10.1161/01.STR.0000143153.76460.7d [DOI] [PubMed] [Google Scholar]
- 25.Nam YG, Lee JW, Park JW, Lee HJ, Nam KY, Park JH, et al. Effects of Electromechanical Exoskeleton-Assisted Gait Training on Walking Ability of Stroke Patients: A Randomized Controlled Trial. Arch Phys Med Rehabil 2019;100:26–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30055163&dopt=Abstract 10.1016/j.apmr.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 26.Morone G, Paolucci S, Cherubini A, De Angelis D, Venturiero V, Coiro P, et al. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat 2017;13:1303–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28553117&dopt=Abstract 10.2147/NDT.S114102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molteni F, Gasperini G, Cannaviello G, Guanziroli E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: narrative Review. PM R 2018;10:S174–88. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30269804&dopt=Abstract 10.1016/j.pmrj.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 28.Chang WH, Kim YH. Robot-assisted Therapy in Stroke Rehabilitation. J Stroke 2013;15:174–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24396811&dopt=Abstract 10.5853/jos.2013.15.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenzi T, Carrozza MC, Agrawal SK. Powered hip exoskeletons can reduce the user’s hip and ankle muscle activations during walking. IEEE Trans Neural Syst Rehabil Eng 2013;21:938–48. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23529105&dopt=Abstract 10.1109/TNSRE.2013.2248749 [DOI] [PubMed] [Google Scholar]
- 30.Escalona MJ, Bourbonnais D, Goyette M, Duclos C, Gagnon DH. Wearable exoskeleton control modes selected during overground walking affect muscle synergies in adults with a chronic incomplete spinal cord injury. Spinal Cord Ser Cases 2020;6:26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32332703&dopt=Abstract 10.1038/s41394-020-0269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contreras-Vidal JL, A, Bhagat N, Brantley J, Cruz-Garza JG, He Y, Manley Q, et al. Powered exoskeletons for bipedal locomotion after spinal cord injury. J Neural Eng 2016;13:031001. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27064508&dopt=Abstract 10.1088/1741-2560/13/3/031001 [DOI] [PubMed] [Google Scholar]
- 32.Calabrò RS, Naro A, Russo M, Bramanti P, Carioti L, Balletta T, et al. Shaping neuroplasticity by using powered exoskeletons in patients with stroke: a randomized clinical trial. J Neuroeng Rehabil 2018;15:35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29695280&dopt=Abstract 10.1186/s12984-018-0377-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayaraman A, O’Brien MK, Madhavan S, Mummidisetty CK, Roth HR, Hohl K, et al. Stride management assist exoskeleton vs functional gait training in stroke: A randomized trial. Neurology 2019;92:e263–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30568009&dopt=Abstract 10.1212/WNL.0000000000006782 [DOI] [PubMed] [Google Scholar]
- 34.Rojek A, Mika A, Oleksy Ł, Stolarczyk A, Kielnar R. Effects of Exoskeleton Gait Training on Balance, Load Distribution, and Functional Status in Stroke: A Randomized Controlled Trial. Front Neurol 2020;10:1344. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32010039&dopt=Abstract 10.3389/fneur.2019.01344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19631508&dopt=Abstract 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 36.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009;55:129–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19463084&dopt=Abstract 10.1016/S0004-9514(09)70043-1 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22008217&dopt=Abstract 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25524443&dopt=Abstract 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergmann J, Krewer C, Jahn K, Müller F. Robot-assisted gait training to reduce pusher behavior: A randomized controlled trial. Neurology 2018;91:e1319–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30171076&dopt=Abstract 10.1212/WNL.0000000000006276 [DOI] [PubMed] [Google Scholar]
- 40.Chang WH, Kim MS, Huh JP, Lee PK, Kim YH. Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: a randomized controlled study. Neurorehabil Neural Repair 2012;26:318–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22086903&dopt=Abstract 10.1177/1545968311408916 [DOI] [PubMed] [Google Scholar]
- 41.Han EY, Im SH, Kim BR, Seo MJ, Kim MO. Robot-assisted gait training improves brachial-ankle pulse wave velocity and peak aerobic capacity in subacute stroke patients with totally dependent ambulation: randomized controlled trial. Medicine (Baltimore) 2016;95:e5078. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27741123&dopt=Abstract 10.1097/MD.0000000000005078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair 2009;23:5–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19109447&dopt=Abstract 10.1177/1545968308326632 [DOI] [PubMed] [Google Scholar]
- 43.Husemann B, Müller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke 2007;38:349–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17204680&dopt=Abstract 10.1161/01.STR.0000254607.48765.cb [DOI] [PubMed] [Google Scholar]
- 44.Mayr A, Quirbach E, Picelli A, Kofler M, Smania N, Saltuari L. Early robot-assisted gait retraining in non-ambulatory patients with stroke: a single blind randomized controlled trial. Eur J Phys Rehabil Med 2018;54:819–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29600688&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 45.Schwartz I, Sajin A, Fisher I, Neeb M, Shochina M, Katz-Leurer M, et al. The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trial. PM R 2009;1:516–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19627940&dopt=Abstract 10.1016/j.pmrj.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 46.Taveggia G, Borboni A, Mulé C, Villafañe JH, Negrini S. Conflicting results of robot-assisted versus usual gait training during postacute rehabilitation of stroke patients: a randomized clinical trial. Int J Rehabil Res 2016;39:29–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26512928&dopt=Abstract 10.1097/MRR.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Nunen MP, Gerrits KH, Konijnenbelt M, Janssen TW, de Haan A. Recovery of walking ability using a robotic device in subacute stroke patients: a randomized controlled study. Disabil Rehabil Assist Technol 2015;10:141–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24611590&dopt=Abstract 10.3109/17483107.2013.873489 [DOI] [PubMed] [Google Scholar]
- 48.Wall A, Borg J, Vreede K, Palmcrantz S. A randomized controlled study incorporating an electromechanical gait machine, the Hybrid Assistive Limb, in gait training of patients with severe limitations in walking in the subacute phase after stroke. PLoS One 2020;15:e0229707. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32109255&dopt=Abstract 10.1371/journal.pone.0229707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe H, Tanaka N, Inuta T, Saitou H, Yanagi H. Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch Phys Med Rehabil 2014;95:2006–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25010538&dopt=Abstract 10.1016/j.apmr.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 50.Watanabe H, Goto R, Tanaka N, Matsumura A, Yanagi H. Effects of gait training using the Hybrid Assistive Limb® in recovery-phase stroke patients: A 2-month follow-up, randomized, controlled study. NeuroRehabilitation 2017;40:363–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28222558&dopt=Abstract 10.3233/NRE-161424 [DOI] [PubMed] [Google Scholar]
- 51.Molteni F, Guanziroli E, Goffredo M, Calabrò RS, Pournajaf S, Gaffuri M, et al. On Behalf Of Italian Eksogait Study Group . Gait Recovery with an Overground Powered Exoskeleton: A Randomized Controlled Trial on Subacute Stroke Subjects. Brain Sci 2021;11:104. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33466749&dopt=Abstract 10.3390/brainsci11010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SY, Yang L, Park IJ, Kim EJ, JoshuaPark MS, You SH, et al. Effects of Innovative WALKBOT Robotic-Assisted Locomotor Training on Balance and Gait Recovery in Hemiparetic Stroke: A Prospective, Randomized, Experimenter Blinded Case Control Study With a Four-Week Follow-Up. IEEE Trans Neural Syst Rehabil Eng 2015;23:636–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25850089&dopt=Abstract 10.1109/TNSRE.2015.2404936 [DOI] [PubMed]
- 53.Dijkers MP, Akers KG, Dieffenbach S, Galen SS. Systematic Reviews of Clinical Benefits of Exoskeleton Use for Gait and Mobility in Neurologic Disorders: A Tertiary Study. Arch Phys Med Rehabil 2021;102:300–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30849306&dopt=Abstract 10.1016/j.apmr.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 54.Moucheboeuf G, Griffier R, Gasq D, Glize B, Bouyer L, Dehail P, et al. Effects of robotic gait training after stroke: A meta-analysis. Ann Phys Rehabil Med 2020;63:518–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32229177&dopt=Abstract 10.1016/j.rehab.2020.02.008 [DOI] [PubMed] [Google Scholar]