Abstract
BACKGROUND
Chronic obstructive pulmonary disease (COPD), a progressive lung disease, might improve with neuromuscular electrical stimulation. No trials on inspiratory plus expiratory neuromuscular electrical stimulation have been conducted yet.
AIM
The aim of this study was to evaluate the safety and effectiveness of inspiratory plus expiratory neuromuscular electrical stimulation in subjects with severe COPD.
DESIGN
This was a multicenter, prospective, randomized controlled trial.
SETTING
The subjects were outpatients enrolled from Beijing Chao-Yang Hospital affiliated with Capital Medical University, Tianjin Chest Hospital, and the First Hospital of Hebei Medical University.
POPULATION
Subjects had stable COPD with severe respiratory impairment.
METHODS
Using a computer statistical software, 120 stable subjects were randomly allocated (1:1) to receive inspiratory plus expiratory neuromuscular electrical stimulation (study group) and diaphragm pacing (control group). Demographic and clinical data were collected before, and after 2, and 4 weeks of the trial. The intention-to-treat analysis was conducted. The primary outcome was to analyze the changes in functional exercise capacity, estimated as six-minute walk distance (6MWD), following electrical stimulation for 4 weeks. The secondary outcomes were changes in modified Medical Research Council score, forced expiratory volume in 1 second (FEV1), FEV1% predicted, and FEV1 ratio forced vital capacity (FEV1/FVC) following electrical stimulation for 4 weeks.
RESULTS
The change in 6MWD was greater in the study group (65.53±39.45 m) than in the control group (26.66±32.65 m). The mean between-group difference at the fourth week was 29.07 m (95% confidence interval, 16.098-42.035; P<0.001). There were no significant between-group differences in the secondary outcomes after 4 weeks of electrical stimulation. For GOLD-4 COPD subjects, FEV1 and FEV1/FVC improved in the study group (P<0.05). No electrical stimulation-related serious adverse events were observed in either group.
CONCLUSIONS
6MWD were increased significantly, without adverse events, after four weeks of treatment of inspiratory plus expiratory neuromuscular electrical stimulation in stable patients with severe COPD, suggesting that this protocol benefits COPD rehabilitation.
CLINICAL REHABILITATION IMPACT
The results of this study suggest that the simultaneous use of inspiratory plus expiratory neuromuscular electrical stimulation as an adjunct therapy may improve the functional exercise capacity of severe stable COPD subjects.
Key words: Pulmonary disease, chronic obstructive; Electric stimulation therapy; Respiratory Function Tests
Chronic obstructive pulmonary disease (COPD) is a significant contributor to global morbidity and mortality currently, and is responsible for a considerable economic and social burden.1, 2 Respiratory muscle dysfunction is a common consequence of COPD.3 Pulmonary rehabilitation plays an important role in COPD patients. Reports have shown that expiratory muscle strength4 and endurance5 are impaired in patients with COPD. The contraction of the expiratory muscles increases the intrathoracic pressure, diminishes the lung volume, and facilitates expiratory flow in the absence of flow limitation. In addition, the expiratory muscles should be effective for cough. Previous studies have shown that inspiratory plus expiratory muscle training can enhance respiratory muscle strength and improve respiratory exercise capacity in subjects with severe to very severe COPD.6 However, in COPD patients with prominent ventilation dysfunction, engagement in active muscle training is restricted. As a result, respiratory neuromuscular electrical stimulation therapy, is being studied as an alternative method for respiratory muscle training.
An increase in respiratory minute volume has been observed in patients with COPD, with the use of transcutaneous electrical diaphragmatic stimulation, a form of inspiratory neuromuscular electrical stimulation.7 Studies have confirmed that abdominal electrical stimulation can improve the ventilation parameters, such as peak expiratory flow rate, in COPD patients.8 Therefore, inspiratory plus expiratory neuromuscular electrical stimulation in these patients was a potentially rational protocol to reduce the severity of breathlessness and improve exercise tolerance in COPD patients. However, until now, there have been no studies on the evaluation of inspiratory plus expiratory neuromuscular (diaphragmatic and abdominal muscles) electrical stimulation in COPD patients.
This trial aimed to determine the effectiveness and safety of inspiratory plus expiratory neuromuscular electrical stimulation on functional exercise capacity in severe COPD patients. The trial engaged a novel strategy of stimulating the diaphragm and abdominal muscles (rectus abdominis muscle and obliquus externus abdominis muscle) during inspiration and expiration, respectively. We hypothesized that patients who received inspiratory plus expiratory neuromuscular electrical stimulation for four weeks would show a more significant increase in functional exercise capacity when compared with the patients who received stimulation of the phrenic nerve, called diaphragm pacing.
Materials and methods
Study design and participants
This was a multicentre, prospective, and randomized controlled trial investigating the effects of inspiratory plus expiratory neuromuscular electrical stimulation over diaphragm pacing in subjects with severe COPD.
The subjects were enrolled from the Beijing Chaoyang Hospital affiliated with the Capital Medical University, Tianjin Chest Hospital, and the First Hospital of Hebei Medical University between May 2018 and September 2019. Subjects diagnosed with severe COPD9 who met the following criteria were included: 1) capacity to sign an informed consent; 2) aged 40-75 years; 3) diagnosed with COPD, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommended spirometric criterion, postbronchodilator ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) below 0.70; 4) severe respiratory impairment, postbronchodilator FEV1% predicted value of less than 50%; 5) a modified Medical Research Council (mMRC) Score of 1-3. The subjects who met the following criteria were excluded: 1) unable to complete the experiment; 2) contraindication of respiratory neuromuscular stimulator or diaphragm pacemaker; 3) an acute exacerbation four weeks prior to the screening; 4) presence of other lung diseases, such as pneumothorax, tuberculosis, lung cancer; 5) breathing ambient air at rest [partial pressure of oxygen (PaO2) <60 mmHg and partial pressure of carbon dioxide (PaCO2) >50 mmHg at sea level]; 6) use of invasive or non-invasive mechanical ventilation; 7) heart function level III-IV, according to the New York Heart Association functional classification; 8) episode of unstable angina or myocardial infarction within 30 days before the screening; 9) renal function at the time of screening, creatinine clearance rate of ≤30 mL/min according to the Chronic Kidney Disease Epidemiology Collaboration formula; 10) liver function at the time of screening, aspartate aminotransferase, alanine aminotransferase, or total bilirubin ≥1.5-fold the upper limit of the normal value; 11) participation in other clinical trials within 30 days before the screening; 12) unable to cooperate due to conditions such as mental disorders and cognitive disorders.
The trial protocol was approved by the ethics committee of all three participating hospitals and was registered as ChiCTR2000032681. All participants provided written informed consent.
Data collection
The demographic characteristics, including age, gender, body mass index, and general information, were recorded. In addition, the clinical details, including patient medical history, vital signs, laboratory test data, 6-minute walk distance (6MWD),10 mMRC dyspnea score (a 5-point [0–4] scale, based on the severity, utilized to assess the sensation of dyspnoea during activities in daily living),11 spirometry (Masterscreen PFT System; Jaeger; Wurzburg, Germany),12 arterial blood gas, and diaphragmatic motions during quiet breathing and deep breathing, measured by ultrasound (APLIO 500 TUS-A500, Toshiba, Japan), were recorded.13 These measurements were recorded before and after (2-week and 4-week) electrical stimulation. Further, the subjects were sub-grouped according to the classification of airflow limitation severity, baseline FEV1% predicted; GOLD-3, 30% ≤FEV1% predicted <50%; and GOLD-4, FEV1% predicted <30%.
Study intervention
In the study intervention group, the subjects were treated with inspiratory plus expiratory neuromuscular electrical stimulation (by Yaguo Technology Co., Ltd, Beijing, China). The device contains one diaphragmatic stimulation channel and two abdominal muscle stimulation channels. The procedure was conducted by a doctor, according to the following instructions. The phrenic nerve electrodes consisted of one pair of stimulating electrodes and one pair of reference electrodes. The stimulating electrodes were placed bilaterally on the lower 1/3rd of the lateral sternocleidomastoid muscle of the neck, and the reference electrodes were placed bilaterally on the pectoralis major muscle in the middle of the second rib. The abdominal muscle electrodes consisted of two pairs of stimulating electrodes and two pairs of reference electrodes. One pair of stimulating electrodes were placed bilaterally and symmetrically on the top of the rectus abdominis muscle, and the reference electrodes were placed bilaterally and symmetrically on the bottom of the rectus abdominal muscle. In contrast, the stimulating electrodes of the other pair were placed bilaterally and symmetrically on the outer edge of the rectus abdominis muscle to stimulate the obliquus externus abdominis muscle, and the pair of reference electrodes were parallelly placed on the outside of the stimulating electrodes (Supplementary Digital Material 1: Supplementary Text File 1). The positions of the electrodes and the treatment administered in the study intervention group are shown in Supplementary Digital Material 2 (Supplementary Figure 1).
The phrenic nerves were stimulated when the device gave the acousto-optic prompt to inhale, while the diaphragm contracted to assist inspiration. The rectus abdominis and the oblique abdominis were stimulated when the device gave the acousto-optic prompt to exhale, and the abdominal muscles contracted to assist expiration. The subjects inhaled and exhaled synchronously at the acousto-optic prompt.
In the control intervention group, the subjects were treated with diaphragm pacing (by USCON, Jilin, China). The position of the phrenic nerve electrodes was the same as that in the study intervention group.
We used “all or none” stimulation. In both intervention groups, electrical stimulation was set at the frequency of 40 Hz,14 stimulation duration of 1 second, and pulse duration of 300 µs. The intensity of the stimulation was increased gradually, according to the subject’s tolerance level, from a minimum intensity of 10-20 mA. Each stimulation intensity was recorded by a nurse (Supplementary Digital Material 3; Supplementary Text File 2). The procedure was conducted by a doctor according to the instructions. Each patient was stimulated for 30 minutes a day, continuously for 4 weeks.15, 16
Outcome analysis
The primary outcome was the increment in 6MWD from the baseline to four weeks after the stimulation. The secondary outcomes were: 1) improvement in pulmonary function; 2) improvement in symptoms of dyspnoea; 3) changes in diaphragmatic motion during quiet breathing and deep breathing, at four weeks after the electrical stimulation, in the two groups. The safety endpoints were the adverse events observed in either group during the study period.
Sample size
In a previous study, an increment of 35 meters was observed in the 6MWD of the subjects who underwent inspiratory muscle training.17 We expected an increment of 20 meters more in the 6MWD as the primary outcome, in the study intervention group, in comparison to the control intervention group. The power was at 80% for a 0.05 level of significance. The estimated sample size, for each group, was 50; on considering attrition of less than 20%, the number required, in each group, was 60.
Randomization procedure
All enrolled subjects were randomly allocated (1:1) to either inspiratory plus expiratory neuromuscular electrical stimulation (study intervention group) or diaphragm pacing (control intervention group) using stratified block randomization. Intervention assignments were generated using the statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and communicated using sealed opaque envelopes by a statistical agency; the doctors were responsible for the enrolment of participants.
Statistical analysis
SAS 9.4 software was used for the statistical analysis. A P value of <0.05 was considered statistically significant. The primary outcome analysis was done, on an intention-to-treat basis, in all randomly assigned subjects. The results were expressed as the mean±SD for the normally distributed variables. For the quantitative data, intra-group comparisons were performed using the paired Student’s t-test for normally distributed variables, and the Wilkinson Rank Test for non-normally distributed variables. Inter-group comparisons were performed using unpaired Student’s t-tests for the normally distributed data, and the Mann-Whitney U Test for the non-normally distributed data. Correlation analyses between the pulmonary function parameters and 6MWD were performed by the Spearman correlation. The categorical variables were presented as percentages and compared using the χ2 tests or Fisher’s Exact Test. For subgroup analysis, the subjects with GOLD-3 and GOLD-4 COPD were compared.9
Results
Recruitment and baseline characteristics
The flowchart of the patient enrolment is shown in Figure 1. Among the 162 people screened for eligibility, 37 did not meet the inclusion criteria, one met one of the exclusion criteria, four declined participations. Thus, a total of 120 subjects were randomly assigned either to the study intervention group (N.=60) or the control intervention group (N.=60). Five discontinued the intervention: four withdrew consent, and one had influenza during the study period. The missing data was divided and processed according to the data set: the last observation carried forward (LOCF) was used to translate the missing data for data efficacy. Missing values are not translated for baseline characteristics and safety data.
Figure 1.
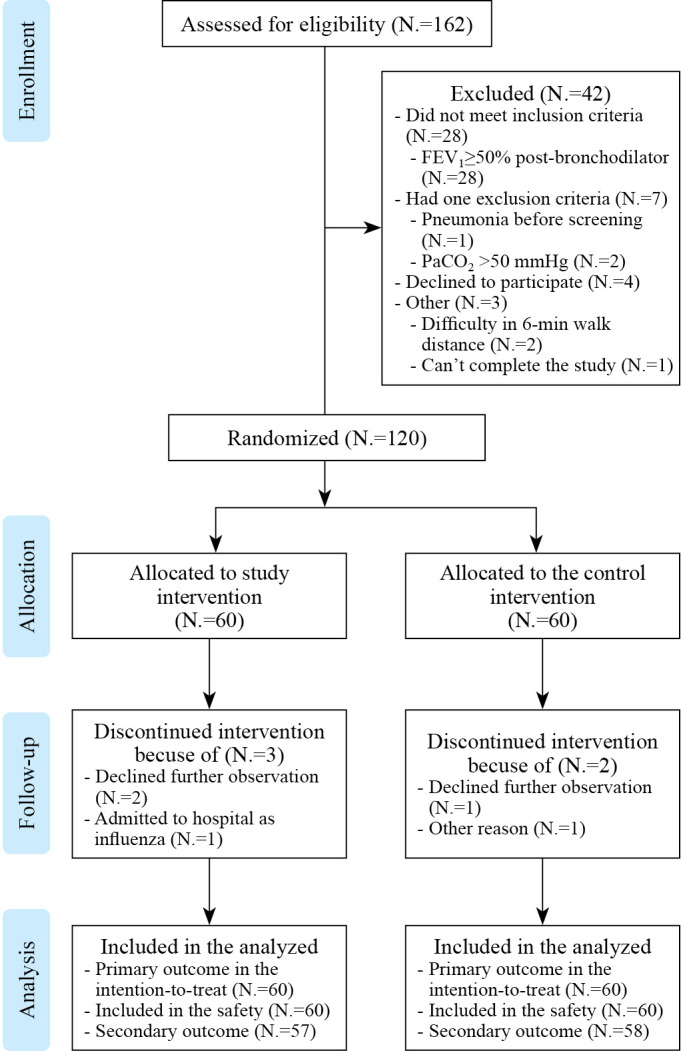
—CONSORT diagram for study flow. Flow diagram illustrating the number of participants in each group.
The participants had a mean age of 64.59±5.69 years, and 110 (91.6%) were males. The mean 6MWD was 431.28±57.06 m. The mean value of FEV1% predicted for all participants was 40.82% (32.00-47.00%). At enrolment, 78.5% of the participants were receiving inhaled anticholinergic agents or long-acting β2 adrenergic agonists. The baseline characteristics of the subjects were similar across the two intervention groups (Table I).
Table I. —Baseline characteristics for both groups according to allocation.
| Variables | Study intervention (N.=60) |
Control intervention (N.=60) |
Ρ |
|---|---|---|---|
| Anthropometric data | |||
| Age (years) * | 65.7 (61.66, 68.33) | 64.7 (60.93, 69.26) | 0.910 |
| Gender: male N (%) † | 53 (88.33%) | 57 (95.00%) | 0.186 |
| Height (cm) * | 170.00 (166.00, 173.00) | 170.00 (165.00, 172.50) | 0.499 |
| Weight (kg) ‡ | 67.74±10.95 | 66.17±9.45 | 0.401 |
| Comorbidity, N. (%) | |||
| Hypertension † | 7 (11.67%) | 3 (5.00%) | 0.186 |
| Ischemic heart disease † | 4 (6.66%) | 7 (11.67%) | 0.194 |
| Heart failure † | 4 (6.66%) | 7 (11.67%) | 0.194 |
| Smoking status: never/current N (%) † | 11 (18.33%)/49 (81.67%) | 9 (15.00%)/51 (85.00%) | 0.624 |
| Medical treatment, N. (%) | |||
| Inhaled long-acting bronchodilators (%) † | 49 (83.05%) | 45 (75.00%) | 1.000 |
| 6-minute walking distance (m) ‡ | 423.27±59.34 | 439.30±53.98 | 0.124 |
| Spirometry | |||
| FEV1 (L) ‡ | 1.15±0.27 | 1.10±0.26 | 0.316 |
| FEV1%predicted (%) * | 40.92 (32.10, 47.40) | 40.40 (31.97, 45.75) | 0.495 |
| FVC (L) ‡ | 2.50±0.52 | 2.45±0.55 | 0.570 |
| FEV1 / FVC (%) * | 43.80 (39.83, 53.66) | 45.24 (39.75, 52.31) | 0.998 |
| Maxima ventilation volume (L/min) ‡ | 44.97±13.74 | 42.68±13.10 | 0.384 |
| Peak expiratory flow (L/s) ‡ | 3.47±1.09 | 3.39±1.05 | 0.686 |
| mMRC grade1 N. (%)† | 31 (51.67%) | 27 (45.00%) | 0.383 |
| mMRC grade2 N. (%) † | 20 (33.33%) | 27 (45.00%) | |
| mMRC grade3 N. (%) † | 9 (15.00%) | 6 (10.00%) | |
| PaCO2 (mmHg) * | 40.80 (38.50, 43.20) | 39.55 (36.85, 43.05) | 0.233 |
| PaO2 (mmHg) * | 77.20 (72.00, 84.95) | 77.60 (73.00, 84.15) | 0.787 |
| Diaphragm motion in quiet breathing (cm) ‡ | 2.26±0.69 | 2.29±0.60 | 0.789 |
| Diaphragm motion in deep breathing (cm) ‡ | 4.67±1.22 | 4.52±0.94 | 0.450 |
Data are reported as the mean±standard deviation, or median (25th centile, 75th centile) unless stated otherwise. FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; mMRC: modified Medical Research Council; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; inhaled long-acting bronchodilators: long-acting β2 adrenergic agonists or anticholinergic agents. *Wilkinson Rank Test for non-normally distributed data; ‡Independent t-test; †χ2 test was used to compare groups.
Outcomes
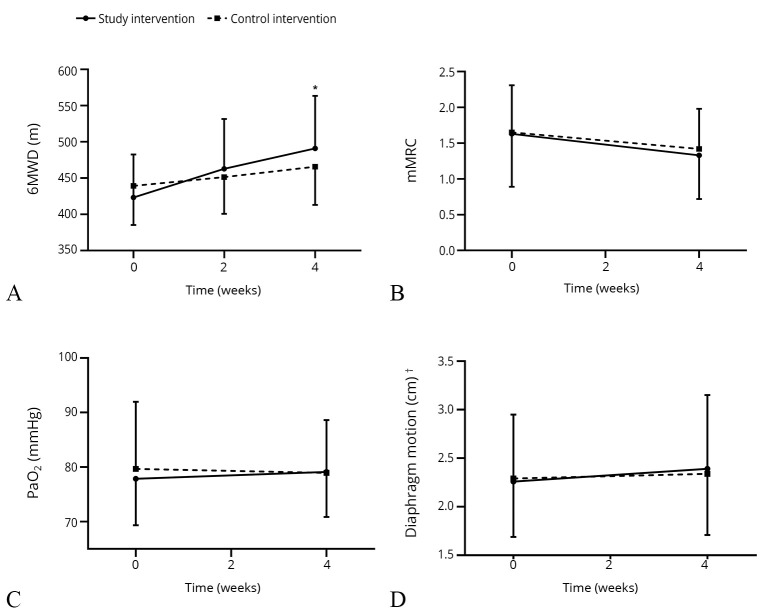
A statistically significant increase in 6MWD was observed in the study intervention group (65.53±39.45 m, P<0.001), which was twice that observed in the control intervention group (26.66±32.65 m, P<0.05). The between-group comparisons of the changes in the 6MWD showed a significant difference (29.07 m; 95% CI: 16.098-42.035), P<0.001). The trend of differences in the measurement between the two groups is shown in Figure 2.
Figure 2.
—Functional exercise capacity changes and secondary outcomes after 2- and 4-week of electrical stimulation between-group. A) Functional exercise capacity; B) differences in mMRC; C) changes in PaO2; D) changes in diaphragmatic motion after 2- and 4-week electrical stimulation. mMRC: modified Medical Research Council; PaO2: partial pressure of oxygen. *Note a significant between-group difference of P<0.05 at the end of 4 weeks; †changes in diaphragmatic motion in quiet breathing between-group.
A moderate positive significant correlation, between changes in 6MWD and changes in pulmonary function test parameters after 4-weeks, was observed in FEV1(r=0.32435, Ρ=0.0138), FEV1% predicted (r=0.30526, Ρ=0.0209), and maximal voluntary ventilation (MVV; r=0.30928, Ρ=0.0306).
Table II summarizes the results of the major secondary outcomes. For the secondary outcomes, after 4 weeks of electrical stimulation, the subjects in the study intervention group had a greater improvement in mMRC grade than the control intervention group, but the difference was not statistically significant (-0.3±0.57 vs. -0.22±0.53, Ρ=0.526).
Table II. —Variables of subjects in major secondary outcome measures at four weeks after intervention according to group allocation.
| Variables | Study intervention (N.=57) | Control intervention (N.=58) | Comparisons of changes | ||||
|---|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Post-pre | Preintervention | Postintervention | Post-pre | P value § | |
| Spirometry | |||||||
| FEV1 (L) * | 1.15±0.27 | 1.21±0.32 | 0.06±0.17 | 1.10±0.26 | 1.19±0.32 | 0.08±0.17 | 0.553 |
| FEV1%predicted (%)† | 40.83 (32.80, 47.20) | 39.80 (34.60, 49.51) | 1.50 (-2.10, 6.30) | 40.40 (32.20, 45.20) | 42.05 (34.40, 50.30) | 1.75 (-1.20, 5.20) | 0.673 |
| FVC (L) * | 2.50±0.52 | 2.63±0.47 | 0.12±0.32 | 2.46±0.55 | 2.60±0.50 | 0.14±0.35 | 0.725 |
| FEV1/FVC (%) † | 43.71 (40.50, 53.63) | 43.63(38.33, 53.52) | -0.12 (-3.15, 3.26) | 45.24 (39.83, 53.03) | 44.91 (38.79, 52.89) | 0.20 (-4.46, 3.71) | 0.717 |
| Maxima ventilation volume (L/min) * | 44.97±13.74 | 48.03±13.42 | 3.37±10.75 | 42.74±13.23 | 45.08±14.45 | 2.97±9.07 | 0.503 |
| Peak expiratory flow (L/s) * | 3.47±1.11 | 3.70±1.25 | 0.23±0.77 | 3.40±1.06 | 3.61±1.08 | 0.21±0.68 | 0.982 |
| mMRC* | 1.63±0.74 | 1.33±0.61 | -0.30±0.57 | 1.65±0.66 | 1.42±0.56 | -0.22±0.53 | 0.526 |
| mMRC grade 0 N. (%) ‡ | 0 (0.00%) | 1 (1.75%) | 0 (0.00%) | 0 (0.00%) | |||
| mMRC grade 1 N. (%) ‡ | 29 (50.88%) | 39 (68.42%) | 26 (44.83%) | 35(60.34%) | |||
| mMRC grade 2 N. (%) ‡ | 20 (35.09%) | 14 (24.56%) | 26(44.83%) | 21(36.21%) | |||
| mMRC grade 3 N. (%) ‡ | 8 (14.04%) | 3 (5.26%) | 6 (10.34%) | 2 (3.45%) | |||
| PaCO2 (mmHg) † | 40.80 (38.60, 43.40) | 41.60(39.30, 43.90) | 1.20 (-1.10, 2.50) | 39.15 (36.70, 42.80) | 39.90 (36.50, 44.10) | 0.60 (-2.30, 2.60) | 0.524 |
| PaO2 (mmHg) † | 77.20 (72.50, 85.10) | 78.30 (74.10, 84.80) | 1.10 (-3.40, 5.30) | 77.70 (73.00, 84.20) | 79.20 (72.50, 84.50) | 1.00 (-5.60, 6.40) | 0.566 |
| Diaphragm motion in quiet breathing (cm) * | 2.26±0.69 | 2.39±0.76 | 0.13±0.98 | 2.28±0.60 | 2.34±0.63 | 0.06±0.77 | 0.672 |
| Diaphragm motion in deep breathing (cm) * | 4.70±1.24 | 5.01±0.93 | 0.31±1.27 | 4.53±0.96 | 4.85±0.98 | 0.32±1.03 | 0.938 |
Data are reported as the mean±standard deviation, or median (25th centile, 75th centile) unless stated otherwise; P>0.05 in inter-group analysis and intra-group analysis after four weeks of intervention. mMRC: modified Medical Research Council; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen. *Independent t-Test; †Wilkinson Rank Test for non-normally distributed data; ‡χ2 test was used to compare groups; §comparisons of changes in post minus preintervention between groups.
We observed no significant differences in the intra-group FEV1% predicted, FVC, FEV1/FVC, and MVV values following four weeks of intervention. Although there were no statistical differences in the diaphragmatic motion, in quiet breathing, there was a two-fold improvement in the study intervention group when compared to the control intervention group (0.13±0.98 cm and 0.06±0.77 cm, respectively, P=0.672).
Subgroup analysis
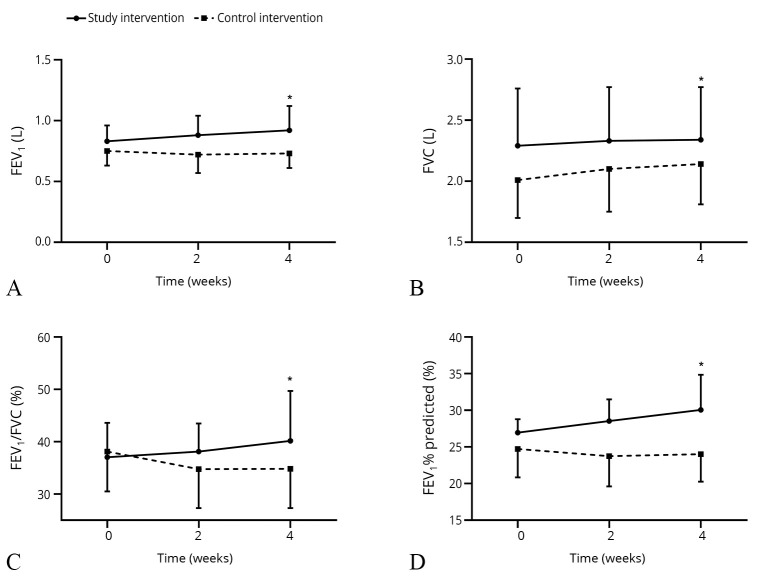
Forty-eight subjects in the study intervention group and 49 subjects in the control intervention group had GOLD-3 COPD. Twelve and 11 subjects had GOLD-4 COPD in the study intervention and the control intervention group, respectively. There were no significant differences in the baseline variables of the subjects within the subgroups. Details of baseline characteristics are outlined in Supplementary Digital Material 4 (Supplementary Table I). For GOLD-3 COPD subjects, 6MWD following four-week electrical stimulation was significantly increased in the study intervention group than in the control intervention group (495.88±74.61 m vs. 469.04±52.59 m, P=0.049), but there were no significant differences in the secondary outcomes between two groups; the details are outlined in Supplementary Digital Material 5 (Supplementary Table II). In GOLD-4 COPD subjects, no significant differences were found in the baseline variables; the details are outlined in Supplementary Digital Material 6 (Supplementary Table III). However, FEV1, FEV1% predicted, and FEV1/FVC levels were improved in the study intervention group P<0.05; Table III).
Table III. —Estimates of the effect of pulmonary function of GOLD-4 COPD group at four weeks.
| Variables | Study intervention (N.=12) |
Control intervention (N.=9) |
Ρ |
|---|---|---|---|
| 6-minute walking distance (m) * | 472.37±63.71 | 447.44±52.68 | 0.353 |
| mMRC | |||
| mMRC grade 0 N. (%) † | 1 (8.33%) | 0 (0.00%) | 0.227 |
| mMRC grade 1 N. (%) † | 5 (41.67%) | 2 (22.22%) | |
| mMRC grade 2 N. (%) † | 5 (41.67%) | 6 (66.67%) | |
| mMRC grade 3 N. (%) † | 1 (8.33%) | 1 (11.11%) | |
| Spirometry | |||
| FEV1 (L) * | 0.92±0.20 | 0.73±0.12 | 0.018 |
| FEV1%predicted (%) ‡ | 29.30 (25.89, 34.40) | 25.03 (20.70, 26.40) | 0.005 |
| FVC (L) * | 2.34±0.43 | 2.14±0.33 | 0.259 |
| FEV1 / FVC (%) ‡ | 38.90 (34.71, 42.56) | 38.02 (28.39, 39.54) | 0.180 |
| Maxima ventilation volume (L / min) * | 37.05±6.84 | 31.06±5.93 | 0.050 |
| Peak expiratory flow (L / s) * | 2.88±0.81 | 2.81±0.61 | 0.849 |
| PaCO2 (mmHg) ‡ | 43.50 (41.00, 45.15) | 39.80 (37.30, 45.20) | 0.276 |
| PaO2 (mmHg) ‡ | 73.05 (70.45, 79.40) | 79.20 (73.70, 85.80) | 0.431 |
| Diaphragm motion in quiet breathing (cm) * | 2.16±0.62 | 2.37±0.78 | 0.493 |
| Diaphragm motion in deep breathing (cm) * | 4.84±1.17 | 5.23±0.51 | 0.317 |
Data are reported as the mean±standard deviation, or median (25th centile, 75th centile) unless stated otherwise. mMRC: modified Medical Research Council; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen. *Independent t-Test; †Wilkinson Rank Test for non-normally distributed data; ‡χ2 test was used to compare groups. *Independent t-Test; †Wilkinson Rank Test for non-normally distributed data; ‡χ2 test was used to compare groups.
An increasing trend in the pulmonary function was observed in the GOLD-4 COPD group, following 2- and 4-week of electrical stimulation (Figure 3).
Figure 3.
—Pulmonary function changes in GOLD-4 COPD group following 2- and 4-week electrical stimulation. A-D) FEV1, FVC, FEV1/FVC, and FEV1% predicted measured retrospectively. COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease. *Note a significant between-group difference of P<0.05 at the end of 4 weeks.
Adverse events
The occurrence of adverse events related to the intervention was minimal. The risk of adverse events between the two intervention groups was similar; nine (15%) in the study intervention group and nine (15%) in the control intervention group (P=1.0) had the risk of adverse events. One participant in the study intervention group suffered from influenza during the treatment and therefore quit the trial due to hospital admission. None of these subjects had any acute exacerbations, requiring antibiotics, during the study period. Supplementary Digital Material 7 (Supplementary Table IV) shows the data on adverse events in more detail.
Discussion
This study aimed to evaluate whether the electrical stimulation of the diaphragm and the abdominal muscles had an advantage over the electrical stimulation of the diaphragm alone. The subjects with severe COPD showed a more significant improvement in their functional exercise capacity after 4 weeks of inspiratory plus expiratory neuromuscular electrical stimulation than after the diaphragmatic stimulation; this was primarily observed in the GOLD-3 subgroup.
At present, respiratory muscle electrical stimulation, which can improve the patient’s respiratory muscle endurance and strength, to some extent, has replaced muscle training, for pulmonary rehabilitation, in selective COPD patients.8 A systematic retrospective study on respiratory muscle training recommends that each training should last 20-30 minutes.18 The frequency in both techniques used in this trial was set at 40 Hz in the beginning, and the treatment duration was set at 30 minutes per day; this increased respiratory muscle strength and avoided respiratory muscle fatigue.14 Previous clinical trials have shown that neuromuscular electrical stimulation can improve respiratory muscle strength after 4 weeks of the intervention.16 Therefore, we also observed the outcomes after 4 weeks of intervention.
The 6MWD was used as a primary observational variable to evaluate the therapeutic effects of electrical stimulation in subjects with severe COPD.
The improvement in 6MWD was more significant in the study intervention group (65.53±39.45 m) when compared with the control intervention group (26.66±32.65 m). According to previous research, the increment in 6MWD, with the use of inspiratory neuromuscular electrostimulation, ranged from 25-33 m.19, 20 The reason for a greater improvement in 6MWD, within the study intervention group of this trial, could be due to the combined electrical stimulation of the diaphragmatic and abdominal muscles. These findings are consistent with the results of previous studies.21 According to some meta-analysis, the combination of inspiratory and expiratory muscle training was more effective in enhancing exercise performance than inspiratory muscle training alone.6, 22
It is known that the expiratory muscles are activated mostly at the end of expiration in subjects with COPD.23 Here, the study intervention group showed a trend towards a greater improvement in the mMRC Score, in comparison to the control intervention group. The lack of significant statistical differences may be related to the short trial period and the small sample size. Electrostimulation might prevent muscle function deterioration,24 improve muscle strength and dyspnea in individuals with low Body Mass Index.25
Although the therapeutic effects of COPD are often judged by the changes in FEV1, there was no significant improvement in the pulmonary functions after 4 weeks of intervention. Weiner et al. demonstrated that, when inspiratory muscle training was used alone, there was no improvement in the maximum expiratory pressure.26 However, the authors observed that the combined inspiratory and expiratory muscle training provided higher gains in the maximum inspiratory and expiratory pressure of the intervention group when compared to the control group.6 These studies emphasized the importance of improving the efficiency of both inspiratory and expiratory muscles in subjects with COPD. Meanwhile, as reported previously, short-term pulmonary rehabilitation may not have a significant impact on FEV1.27 Longer-term clinical trials are warranted in the future to validate this hypothesis.
The main expected benefit of the intervention in subjects with severe COPD is likely to be related to muscle activity and reinforcement. In our study, although the increase of diaphragm motion did not amount to a statistically significant difference, we observed a greater increase in diaphragmatic motion during quiet breathing and deep breathing after 4 weeks of the study intervention. This could be due to several reasons. First, Prieur et al. evaluated the effects of electrical stimulation, on skeletal muscle oxygenation in patients with COPD.28 They determined that deoxygenation and increased oxygen uptake occurred in the muscle and tissue during electrical stimulation. The deoxygenation might reflect a lower level of voluntary muscle activity during the electrical stimulation, suggesting that the metabolic load of the muscle was increased by electrical stimulation. In addition, electrical stimulation can improve muscle strength in peripheral muscles.25
By subgrouping the participants into GOLD-3 versus GOLD-4, we could explore whether the effects differed between the subjects with different severities. The results showed a trend towards favorable outcomes in GOLD-4 COPD subjects, indicating that individuals with lower pulmonary function may achieve significant gains in pulmonary function, which could be explained by the trend towards an increment in the range of diaphragmatic motion. This indicates that subjects with a high level of impairment might respond favorably.
No severe adverse events related to the intervention were observed in both groups. There was only one adverse event related to the intervention in the control group; the participant reported persistent erythema, which was possibly related to the use of adhesive electrodes. Overall, the risk of electrical stimulation appeared to be minimal, and related-adverse events were a few, suggesting that this technique was well tolerated by the subjects, regardless of their disease severity.
The improvement in respiratory muscle strength is important for patients with COPD. Pulmonary rehabilitation has been demonstrated to reduce dyspnoea, increase exercise capacity, and improve the quality of life in subjects with COPD.6, 10 Respiratory muscle training is an effective way for COPD patients to recover. However, many training exercises require patients to perform active work, resulting in poor patient compliance and limited treatment effects. Furthermore, many patients do not participate in these training sessions due to personal reasons. Neuromuscular electrical stimulation is a passive training method that allows selective muscles to contract without the need for active work. It has been found that electrical stimulation could partly mimic the muscle training procedure and had a similar, although partial, effect on muscle function.21
The results suggest that inspiratory plus expiratory neuromuscular electrical stimulation may represent a novel form of neuromuscular electrical stimulation and could be a promising method of pulmonary rehabilitation. Electrical stimulation of the respiratory muscles has also been utilized in the treatment of other diseases: a study found that functional electrical stimulation of the abdominal muscles could shorten ventilation duration and intensive care unit length of staying in mechanically ventilated patients;29 in addition, a new review showed that neuromuscular or functional electrical stimulation could slightly reduce the duration of invasive mechanical ventilation . Further studies, to evaluate the effects of electrical stimulation in critically ill patients should be undertaken.
Limitations of the study
There are some limitations in the present study. First, this study was not blinded as there were significant differences in the function and appearance of the devices used in the study intervention and the control intervention group. Second, the subjects in this trial demonstrated a higher 6MWD when compared to the European subjects. One possible reason for this discrepancy might be due to all participants being recruited from a stable outpatient group. In the multicentre study conducted by Waschki et al., the subjects achieved a mean 6MWD of 364 m with a similar FEV1 level; the results may have been biased due to the inclusion of relatively severe subjects.30 Future studies need to recruit subjects with lower functional exercise capacity. Third, our study involved intervention for only 4 weeks and did not include a longer observation and follow-up period. Based on this research, future work should consider longer programs of intervention. Fourth, we did not take into account the improvement in the muscles, functional performance, symptoms other than dyspnoea, and other health-related changes affecting the patient’s quality of life. Future studies should preferably be designed to permit more effects to be perceived.
Conclusions
The preliminary results demonstrate an advantage in improving the functional exercise capacity of severe, stable COPD subjects, after 4 weeks of treatment with inspiratory plus expiratory neuromuscular electrical stimulation. Our study further consolidates the important role of neuromuscular electrical stimulation in pulmonary rehabilitation. More extensive, long-term studies are necessary to confirm the clinical and functional benefits of this technique and define the precise therapeutic indications.
Supplementary Digital Material 1
Supplementary Text File 1
Instructions of inspiratory plus expiratory neuromusclar electrical stimulation
Supplementary Digital Material 2
Supplementary Figure 1
The positions of the electrodes and the treatment status of the study intervention.
Supplementary Digital Material 3
Supplementary Text File 2
Instructions of inspiratory neuromuscular electrical stimulation.
Supplementary Digital Material 4
Supplementary Table I
Baseline characteristics of GOLD-3 COPD subjects in both groups.
Supplementary Digital Material 5
Supplementary Table II
Characteristics of GOLD-3 COPD subjects after 4-week intervention between-group.
Supplementary Digital Material 6
Supplementary Table III
Baseline characteristics of GOLD-4 COPD subjects in both groups.
Supplementary Digital Material 7
Supplementary Table IV
Adverse Events of participants in both groups.
References
- 1.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. U.S. Burden of Disease Collaborators . The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–608. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23842577&dopt=Abstract 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. China Pulmonary Health Study Group . Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29650248&dopt=Abstract 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 3.Weiner P, McConnell A. Respiratory muscle training in chronic obstructive pulmonary disease: inspiratory, expiratory, or both? Curr Opin Pulm Med 2005;11:140–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15699786&dopt=Abstract 10.1097/01.mcp.0000152999.18959.8a [DOI] [PubMed] [Google Scholar]
- 4.Ferrari K, Goti P, Misuri G, Amendola M, Rosi E, Grazzini M, et al. Chronic exertional dyspnea and respiratory muscle function in patients with chronic obstructive pulmonary disease. Lung 1997;175:311–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9270988&dopt=Abstract 10.1007/PL00007577 [DOI] [PubMed] [Google Scholar]
- 5.Ramírez-Sarmiento A, Orozco-Levi M, Barreiro E, Méndez R, Ferrer A, Broquetas J, et al. Expiratory muscle endurance in chronic obstructive pulmonary disease. Thorax 2002;57:132–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11828042&dopt=Abstract 10.1136/thorax.57.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neves LF, Reis MH, Plentz RD, Matte DL, Coronel CC, Sbruzzi G. Expiratory and expiratory plus inspiratory muscle training improves respiratory muscle strength in subjects with COPD: systematic review. Respir Care 2014;59:1381–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24782553&dopt=Abstract https://doi.org/ 10.4187/respcare.02793 [DOI] [PubMed] [Google Scholar]
- 7.Cancelliero-Gaiad KM, Ike D, Pantoni CB, Mendes RG, Borghi-Silva A, Costa D. Acute effects of transcutaneous electrical diaphragmatic stimulation on respiratory pattern in COPD patients: cross-sectional and comparative clinical trial. Braz J Phys Ther 2013;17:547–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24271095&dopt=Abstract 10.1590/S1413-35552012005000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sewa Y, Tomita K, Okuno Y, Ose H, Imura S. Respiratory flow and vital signs associated with the intensity of functional electrical stimulation delivered to human abdominal muscles during quiet breathing. J Phys Ther Sci 2016;28:3337–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28174447&dopt=Abstract 10.1589/jpts.28.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020 [Internet]. Available from: www.goldcopd.org [cited 2022, Jan 21].
- 10.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25359355&dopt=Abstract 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 11.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30846476&dopt=Abstract 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 12.Lange NE, Mulholland M, Kreider ME. Spirometry: don’t blow it! Chest 2009;136:608–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19666760&dopt=Abstract 10.1378/chest.08-2315 [DOI] [PubMed] [Google Scholar]
- 13.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest 2009;135:391–400. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19017880&dopt=Abstract 10.1378/chest.08-1541 [DOI] [PubMed] [Google Scholar]
- 14.Rochester DF. The diaphragm: contractile properties and fatigue. J Clin Invest 1985;75:1397–402. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3889054&dopt=Abstract 10.1172/JCI111841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BB, Boswell-Ruys C, Butler JE, Gandevia SC. Surface functional electrical stimulation of the abdominal muscles to enhance cough and assist tracheostomy decannulation after high-level spinal cord injury. J Spinal Cord Med 2008;31:78–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18533416&dopt=Abstract https://doi.org/ 10.1080/10790268.2008.11753985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng PT, Chen CL, Wang CM, Chung CY. Effect of neuromuscular electrical stimulation on cough capacity and pulmonary function in patients with acute cervical cord injury. J Rehabil Med 2006;38:32–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16548084&dopt=Abstract https://doi.org/ 10.1080/16501970510043387 [DOI] [PubMed] [Google Scholar]
- 17.Izumizaki M, Satake M, Takahashi H, Sugawara K, Shioya T, Homma I. Effects of inspiratory muscle thixotropy on the 6-min walk distance in COPD. Respir Med 2008;102:970–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18396028&dopt=Abstract 10.1016/j.rmed.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 18.Menezes KK, Nascimento LR, Ada L, Polese JC, Avelino PR, Teixeira-Salmela LF. Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: a systematic review. J Physiother 2016;62:138–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27320833&dopt=Abstract 10.1016/j.jphys.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Maddocks M, Nolan CM, Man WD, Polkey MI, Hart N, Gao W, et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial. Lancet Respir Med 2016;4:27–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26701362&dopt=Abstract 10.1016/S2213-2600(15)00503-2 [DOI] [PubMed] [Google Scholar]
- 20.Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1447–78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25359356&dopt=Abstract 10.1183/09031936.00150414 [DOI] [PubMed] [Google Scholar]
- 21.Xu W, Li R, Guan L, Wang K, Hu Y, Xu L, et al. Combination of inspiratory and expiratory muscle training in same respiratory cycle versus different cycles in COPD patients: a randomized trial. Respir Res 2018;19:225. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30458805&dopt=Abstract 10.1186/s12931-018-0917-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Illi SK, Held U, Frank I, Spengler CM. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med 2012;42:707–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22765281&dopt=Abstract 10.1007/BF03262290 [DOI] [PubMed] [Google Scholar]
- 23.Ninane V, Rypens F, Yernault JC, De Troyer A. Abdominal muscle use during breathing in patients with chronic airflow obstruction. Am Rev Respir Dis 1992;146:16–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1385684&dopt=Abstract 10.1164/ajrccm/146.1.16 [DOI] [PubMed] [Google Scholar]
- 24.Giavedoni S, Deans A, McCaughey P, Drost E, MacNee W, Rabinovich RA. Neuromuscular electrical stimulation prevents muscle function deterioration in exacerbated COPD: a pilot study. Respir Med 2012;106:1429–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22726566&dopt=Abstract 10.1016/j.rmed.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 25.Vivodtzev I, Pépin JL, Vottero G, Mayer V, Porsin B, Lévy P, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006;129:1540–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16778272&dopt=Abstract 10.1378/chest.129.6.1540 [DOI] [PubMed] [Google Scholar]
- 26.Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest 2003;124:1357–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14555566&dopt=Abstract 10.1378/chest.124.4.1357 [DOI] [PubMed] [Google Scholar]
- 27.Leelarungrayub J, Pinkaew D, Puntumetakul R, Klaphajone J. Effects of a simple prototype respiratory muscle trainer on respiratory muscle strength, quality of life and dyspnea, and oxidative stress in COPD patients: a preliminary study. Int J Chron Obstruct Pulmon Dis 2017;12:1415–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28553094&dopt=Abstract 10.2147/COPD.S131062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieur G, Combret Y, Bonnevie T, Gravier FE, Robledo Quesada A, Quieffin J, et al. Functional Electrical Stimulation Changes Muscle Oxygenation in Patients with Chronic Obstructive Pulmonary Disease During Moderate-Intensity Exercise: A Secondary Analysis. COPD 2019;16:30–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30821515&dopt=Abstract 10.1080/15412555.2018.1560402 [DOI] [PubMed] [Google Scholar]
- 29.McCaughey EJ, Jonkman AH, Boswell-Ruys CL, McBain RA, Bye EA, Hudson AL, et al. Abdominal functional electrical stimulation to assist ventilator weaning in critical illness: a double-blinded, randomised, sham-controlled pilot study. Crit Care 2019;23:261. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31340846&dopt=Abstract 10.1186/s13054-019-2544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waschki B, Spruit MA, Watz H, Albert PS, Shrikrishna D, Groenen M, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med 2012;106:522–30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22118987&dopt=Abstract 10.1016/j.rmed.2011.10.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text File 1
Instructions of inspiratory plus expiratory neuromusclar electrical stimulation
Supplementary Figure 1
The positions of the electrodes and the treatment status of the study intervention.
Supplementary Text File 2
Instructions of inspiratory neuromuscular electrical stimulation.
Supplementary Table I
Baseline characteristics of GOLD-3 COPD subjects in both groups.
Supplementary Table II
Characteristics of GOLD-3 COPD subjects after 4-week intervention between-group.
Supplementary Table III
Baseline characteristics of GOLD-4 COPD subjects in both groups.
Supplementary Table IV
Adverse Events of participants in both groups.