Abstract
Lymphedema is a disorder characterized by the accumulation of protein-rich lymphatic fluid in the cutaneous and subcutaneous tissue. Based on the underlying causes, it is classified into primary and secondary forms. The use of ultrasound has recently become widespread in the field of lymphedema – especially for its diagnosis and treatment planning. In this study, we briefly reviewed the anatomy and histology of the skin and subcutaneous tissue – to propose a standardized ultrasound assessment of the superficial tissues in patients with upper-/lower-limb lymphedema. We believe that identification of the sono-histological patterns of the dermo-epidermal complex and subcutaneous tissue has place to serve as a simple and reproducible strategy to evaluate their edema diseases that are often subject to an inaccurate diagnosis in daily clinical practice.
Key words: Extremities, Edema, Ultrasonography, Dermis, Subcutaneous tissue
Due to several reasons/diseases, lymphedema (LE) represents a chronic and disabling pathological condition with increasing frequency in the population. With its primary and secondary forms, LE has therefore a significant impact on the health and social assistance systems.1 In this sense, prompt diagnosing/staging is paramount to accurately plan for conservative/surgical treatment. Recently, the commonly used clinical tools to evaluate these patients in daily practice (e.g., limb circumference/volume, skin indentation, soft tissue stiffness, Stemmer sign) are coupled with specific US features to optimize the management of LE.2 Actually, several authors have already reported the potentiality of US assessment to accurately evaluate the presence and spatial distribution of fluids, cellular infiltration, and fibrotic tissue in the lymphedematous limbs.2-8
To this end, comprehensive knowledge of the anatomy of the peripheral lymphatic system and the histological architecture of the superficial tissues would be paramount to better understand the sonographic patterns of LE. In this regard, similar to the former protocols of the European Musculoskeletal Ultrasound Study Group in Physical and Rehabilitation Medicine/Ultrasound Study Group of the International Society of Physical and Rehabilitation Medicine (EURO-MUSCULUS/USPRM),9-15 we have briefly reviewed the anatomy and histology of the skin and the subcutaneous tissue to allow a standardized US examination as well as correct interpretation of the sonographic findings.
Anatomy
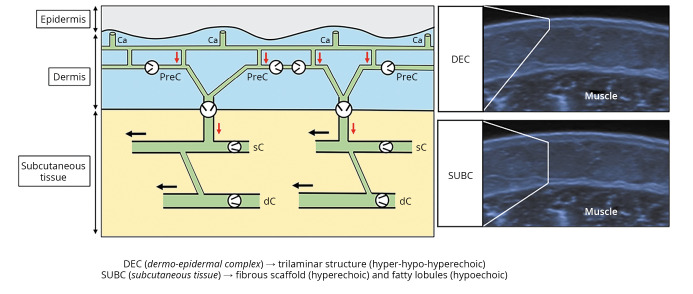
Lymphatic capillaries - located immediately below the epidermis - appear as thin tubular structures without an endo-luminal valve system and are characterized by gaps between the endothelial cells that ensure high permeability to the interstitial fluid. Additionally, they have an open-ended superficial edge defining the peripheral lymphatic system as an open system.16, 17 Fluids drained from the interstitial space are then directed – by the network of lymphatic capillaries – towards a second level canalicular system that consists of precollectors. The latter are located in the deep dermis and equipped with unidirectional valves that guarantee a “vertical” lymphatic flow towards the lymphatic collectors in the subcutaneous tissue (Figure 1).16, 17 Lymphatic collectors, housed in the fibrous scaffold interposed between the adipose lobules of the subcutaneous tissue, are organized in superficial and deep networks interconnected by vertical/oblique communications. Through endo-luminal valves and smooth muscle cells (with peristaltic activity), they ensure a “horizontal” lymphatic flow conveying the fluids to the relative lymph node stations (Figure 1).16-18
Figure 1.
—Schematic drawing shows the lymphatic capillaries in communication with the precollectors in the dermis, and the superficial and deep lymphatic collectors in the subcutaneous tissue. Corresponding sonograms show the trilaminar structure of the normal dermo-epidermal complex and the echotexture of the subcutaneous tissue. Light grey arrow (red in the online version): vertical lymphatic flow; black arrow: horizontal lymphatic flow; black circle: unidirectional valve. Ca: capillaries; PreC: precollectors; sC: superficial; dC: deep; DEC: dermo-epidermal complex; SUBC: subcutaneous tissue.
Histology
Normal skin presents a multilayered composition consisting of epidermis, dermis, and subcutis.19, 20 The dermis consists of two layers: the papillary dermis and the reticular one.19, 20 The papillary dermis represents a thin layer – beneath the epidermis and between the rete ridges – that comprises a unique morpho-functional unit with the epidermis. Conversely, the reticular dermis is thicker and lies between the papillary dermis and the subcutaneous tissue (Figure 2).19-21 The papillary dermis presents a mixture of finely woven meshwork - composed of type III collagen fibers and thin elastic fibers - arranged in a complex network wrapping the blood and lymphatic vessels running beneath the dermal-epidermal junction.19-21
Figure 2.
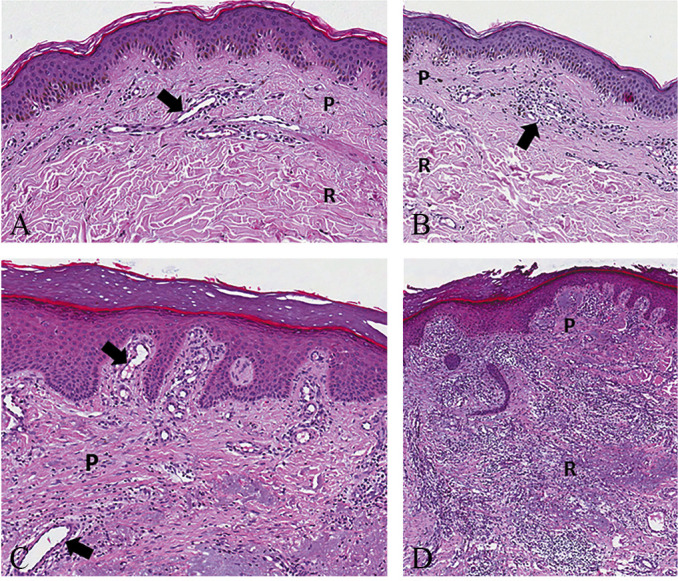
—Normal papillary and reticular dermis with the superficial lymphovascular plexus (black arrow) made of blood and lymphatic vessels (A) (H&E, Original magnification ×100). Mild inflammatory infiltrate (black arrow) of the papillary dermis with no involvement of the reticular dermis (B) (H&E, Original magnification ×80). Edema and moderate inflammatory infiltrate of the papillary dermis with telangiectasia (black arrow) of the superficial lymphovascular plexus (C) (H&E, Original magnification ×100). Severe inflammatory infiltrate of the papillary and reticular dermis with disorganization of the physiological architecture of the dermo-epidermal complex (D) (H&E, Original magnification ×80). H&E: hematoxylin and eosin; P: papillary; R: reticular.
The reticular dermis, on the other hand, is characterized by thicker elastic fibers and type I collagen bundles extending horizontally in various directions.19-21 Of note, the two layers of the dermis greatly differ from each other, not only for the spatial architecture but also for the distribution of lymphovascular components.22, 23 The papillary dermis contains the superficial lymphovascular plexus consisting of an intricated network of arterioles, venules, capillaries, and lymphatic vessels – highly anastomosed to each other – in close proximity to the epidermis and extending into each dermal papilla.22, 23 Instead, the reticular dermis presents a lower number of (small) lymphovascular structures required for its metabolic sustenance. Herein, it is mainly characterized by vertically-oriented blood and lymphatic vessels that directly connect the superficial plexus to the deep one located in the subcutis.22, 23 The vertical vessels are of larger caliber, have different and more complex multilayer composition and, are in continuity with lymphovascular branches extending within fibrous septae (the fibrous scaffold) located between the adipose lobules of underlying subcutaneous tissue.19, 23 The aforementioned differences in the lymphovascular networks and composition of the extracellular matrix of the stroma make the reticular dermis more compact and less sensitive to edematous and inflammatory processes compared to the papillary dermis (Figure 2).19, 23
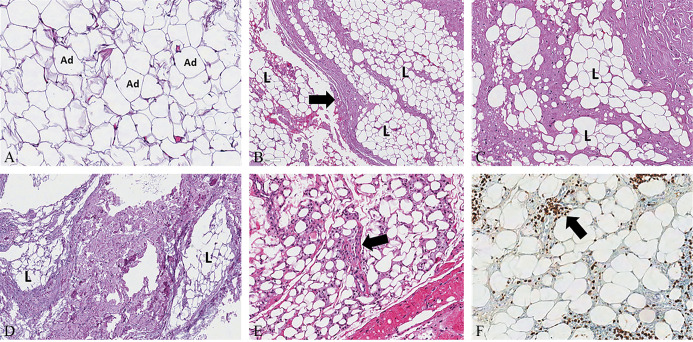
Subcutaneous tissue consists of lobules of mature adipocytes separated from each other by thin fibrous septae (the intercellular matrix) that form a complex net through which small lymphovascular structures course (Figure 3).19, 23 This complex and thin structural network provides mechanical stability by compartmentalizing subcutaneous fat and connecting it to the reticular dermis. Moreover, the intercellular matrix is directly connected to thicker fibrous septae that separate the fatty lobules (the fibrous scaffold) within which blood and lymphatic vessels of larger caliber course - draining the subcutaneous fat and communicating with the lymphovascular components of the reticular dermis (Figure 4).19, 23
Figure 3.
—Mature adipocites – separated by a thin intercellular matrix – are arranged in fatty lobules sustained by a thicker fibrous scaffold (A, B) (black arrow): A) H&E, Original magnification ×200; B) H&E, Original magnification ×50. Moderate (C) and severe (D) thickening of the fibrous scaffold with rarefaction of the adipocytic lobules: C) H&E, Original magnification ×100; D) H&E, Original magnification ×50. Edema, telangiectasia of the lymphatic and blood vessels, and inflammatory infiltrate (black arrow) (E, F) of the intercellular matrix of adipose lobules: E) H&E, Original magnification ×80; and F) CD3, Original magnification ×100). H&E: hematoxylin and eosin; CD3: cluster of differentiation 3, immunohistochemical stain for T-cell lymphocytes; Ad: adipocites; L: lobule.
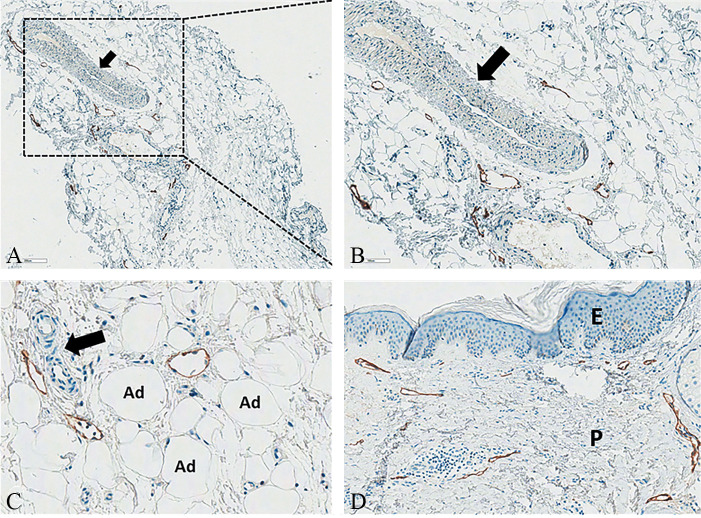
Figure 4.
—Not-stained large venous vessel (black arrow) and stained small lymphatic vessels in the normal fibrous scaffold: A) D2-40, Original magnification ×50; B) D2-40, Original magnification ×100). Not-stained small capillaries (black arrow) and stained small lymphatic vessels in the intercellular matrix of the fatty lobule (C) (D2-40, Original magnification ×200). Normal papillary dermis with stained small lymphatic vessels forming the superficial lymphovascular plexus just beneath the epidermis (D) (D2-40, Original magnification ×100). D2-40: sialoglycoprotein, immunohistochemical podoplanin stain for the lymphatic endothelium, Ad: adipocyte; P: papillary; E: epidermis.
Ultrasound imaging
First and foremost, the use of a large amount of gel (i.e., gel standoff technique) is a prerequisite to avoid compression of the cutis/subcutis during imaging. This is important for correct assessment of the real thickness of different layers and the spatial arrangement of fluids. Moreover, comparative scanning (pathological vs. normal side) and identification of the specific anatomical areas of the limbs to image (i.e. target zones related to the distribution of the lymphosomes) will guarantee reproducibility of the US features as well as their precision during follow-up.2-5 In this regard, the authors suggest performing a standardized sonographic approach both as regards the anatomic areas (Table I) and the different tissue layers in each and every area (starting from the DEC and progressively evaluating the deeper histological components of the subcutaneous layers) using various probe maneuvers (e.g. lateral shift, tilting).
Table I. —Ultrasound imaging zones and features in lymphedema.
| Anatomical areas | ||
|---|---|---|
| Upper Limb | Arm | Proximal half of the medial quadrant |
| Distal half of the medial quadrant | ||
| Proximal half of the lateral quadrant | ||
| Distal half of the lateral quadrant | ||
| Forearm | Proximal half of the volar quadrant | |
| Distal half of the volar quadrant | ||
| Proximal half of the dorsal quadrant | ||
| Distal half of the dorsal quadrant | ||
| Hand | Dorsum of the hand/fingers | |
| Lower Limb | Thigh | Proximal half of the medial quadrant |
| Distal half of the medial quadrant | ||
| Proximal half of the lateral quadrant | ||
| Distal half of the lateral quadrant | ||
| Leg | Proximal half of the medial quadrant | |
| Distal half of the medial quadrant | ||
| Proximal half of the lateral quadrant | ||
| Distal half of the lateral quadrant | ||
| Foot | Dorsum of the foot/fingers | |
| Sonographic features | ||
| Dermo-epidermal complex | Thickness* | |
| Sonographic pattern | ||
| Subcutaneous tissue | Thickness* | |
| Sonographic pattern | ||
| Extra findings§ | ||
*In patients with fibrotic involution, the dermo-hypodermal junction may not be easily detectable. In that case, the total thickness of (the superficial) soft tissues – from the muscular fascia to the interface between the gel and the stratum corneum of the epidermis – can be measured; §lymphatic lakes, fascial delamination, superficial venous thrombosis, positive color/power Doppler findings compatible with infectious/inflammatory phenomena.
Of note, the more superficial canalicular system - lymphatic capillaries and precollectors located in the dermis (i.e., subepidermal lymphatic plexus) - is not directly visible with the linear US probe (6-18 MHz) commonly used in musculoskeletal practice. Herewith, indirect signs of pathology (e.g., changes in thickness and/or echogenicity of the tissues) can precisely be evaluated - knowing the trilaminar structure of the normal dermo-epidermal complex (DEC) i.e., hyperechoic - hypoechoic - hyperechoic layers (Figure 1).2, 3 The superficial hyperechoic band is generated by the reflective interface between the gel and the stratum corneum of the epidermis. The intermediate band – representing the papillary dermis – is hypoechoic due to the high-water content, and the deep hyperechoic layer is generated by the peculiar content/orientation of the collagen fibers of the reticular dermis.6 Immediately deep to the trilaminar structure of the DEC, the dermo-hypodermal junction is easily recognizable as a continuous line - due to the marked difference of the echotexture of the subcutaneous tissue.
On the other hand, the deeper canalicular component - harboring lymphatic collectors - is clearly visible in case of pathologies due to progressive dilatation of the lymphatic channels with disorganization of the hyperechoic fibrous scaffold of the subcutaneous tissue.2, 3
Dermo-epidermal complex
During the sonographic assessment, the following (main) patterns need to be sought (Figure 5, 6):
Figure 5.
—Pathological findings and sonographic features to be examined during the basic scanning protocol of the dermo-epidermal complex and the subcutaneous tissue. DEC: dermo-epidermal complex; SUBC: subcutaneous tissue.
Figure 6.
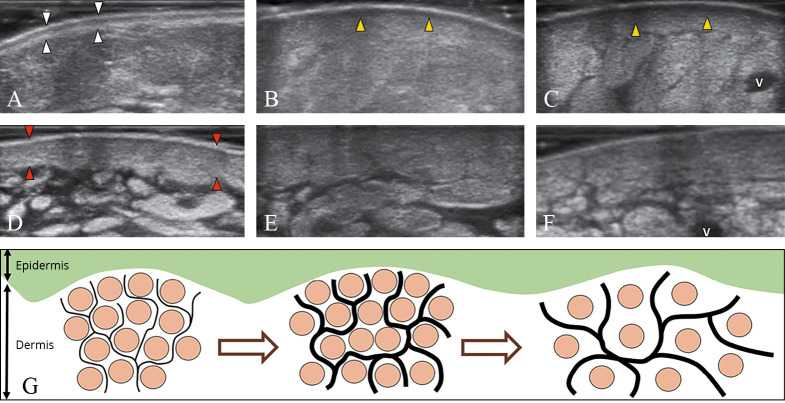
—The normal trilaminar structure of the dermo-epidermal complex (white arrowheads) (A). This pattern gradually disappears in dermal edema (arrowheads; yellow in the online version), usually starting from the papillary dermis (B) and progressively/also involving the reticular dermis (C). Dermal sclerosis (arrowheads; red in the online version) which is initially characterized by cellular infiltration and thickening (D), can lead to loss of the dermo-hypodermal interface (E) until the end-stage of dermal fibrosis (F). Schematic drawing shows the different phases of dermal backflow with distension of the subepidermal lymphatic plexus (black lines) and rarefaction of the collagen bundles (light grey dots; brown in the online version) of the interstitial space (G). V: vein.
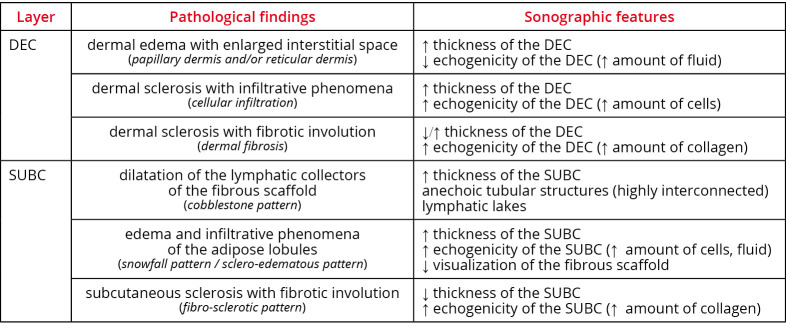
dermal edema with enlarged interstitial space – dermal edema is a complex phenomenon starting with dilatation of the subepidermal lymphatic plexus (i.e., lymphatic capillaries and precollectors). It progressively leads to extravasation of fluid in the interstitial space of dermis with the development of vessel-like structures among the endothelial cell-free collagen bundles (i.e., dermal backflow).24, 25 These histological changes may involve the papillary and/or the reticular dermis leading to an increase in the DEC thickness and a reduction of its echogenicity (i.e., hypoechoic dermis). Of note, dermal edema usually involves the papillary dermis earlier than the reticular dermis due to the different distributions of lymphatic and blood vessels and the different quality and spatial arrangement of the collagen fibers.19-23 Some authors have also/even suggested a link between the dermal edema and paresthesias, due to stretching and irritation of the dermal sensory terminations;6
dermal sclerosis with infiltrative phenomena – cellular infiltration of the dermis leading to a disorganization of the classical trilaminar structure of the DEC (i.e. hyper-hypo-hyperechoic layers) is seen along with a marked increase of the DEC thickness and echogenicity (i.e. hyperechoic dermis);
dermal sclerosis with fibrotic involution – in the chronic phase, progressive deposition of collagen fibers causes changes in the DEC thickness and increase of its echogenicity. This is true until the disappearance of the dermo-hypodermal interface (i.e., undifferentiated dermis), making the exact measurement of the different layers difficult or even impossible. Using the sono-histological classification of the DEC, it is possible to promptly differentiate a “fluid pattern” (typical for the acute phase of the disease and reversible with a targeted treatment) from a “sclerotic pattern”. The latter actually ensues in a more advanced phase of the disease and may progressively evolve to an irreversible condition, i.e., the dermal fibrosis.24
Subcutaneous tissue
During the sonographic assessment, some patterns need to be sought (Figure 5, 6, 7, 8).
Figure 7.
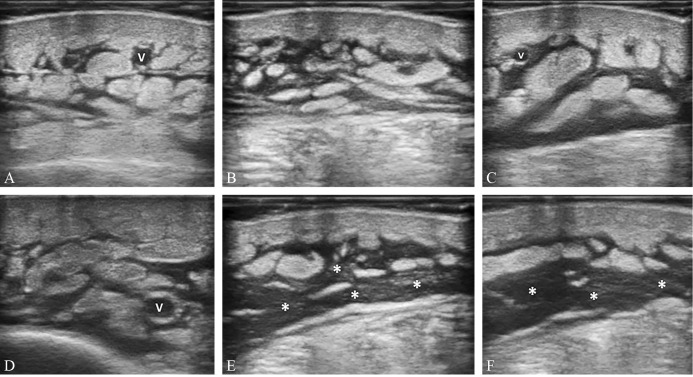
—Mild (A), moderate (B), and severe (C, D) dilatation of the lymphatic collectors - located inside the fibrous scaffold of the subcutaneous tissue - until the complete disorganization of the subcutis architecture and the development of the lymphatic lakes (white asterisks) (E, F). V: vein.
Figure 8.
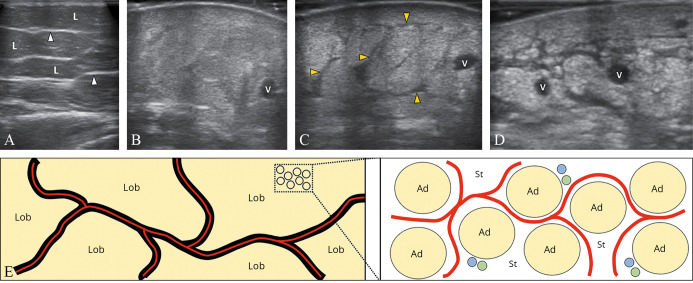
—The normal subcutaneous tissue shows hypoechoic fatty lobules and hyperechoic fibrous scaffold (white arrowheads) (A). Fluids and cells can infiltrate the intercellular matrix of the adipose lobules generating a “snowfall” pattern with poor visualization of the scaffold (B). Simultaneous dilatation of the lymphatic collectors (black arrowheads; yellow in the online version) can be detected in some patients (C). Fibrotic involution is the end-stage of the disease with a lamellar pattern of the subcutaneous tissue (D). Schematic drawing shows the spatial distribution of the fluids (light grey lines; red in the online version) inside the canalicular system of the fibrous scaffold (black lines) or as widely diffuse in the stromal matrix of the fatty lobules (E). V: vein, Ad: adipocyte, blue dots: blood channels, green dots: lymphatic channels; St: stromal matrix; L/Lob: lobules.
In the following paragraphs are indicated the main patterns.
Dilatation of the lymphatic collectors of the fibrous scaffold
Fluids progressively dilate the lymphatic collectors leading to disappearance of the (hyperechoic) fibrous scaffold of the subcutaneous tissue and increase in its thickness with multiple anechoic tubular structures. These are highly interconnected with each other and dissociate the adipose lobules (i.e., “cobblestone” pattern) (Figure 7). It is noteworthy that massive distension of the lymphatic collectors can cause mechanical disruption of the collagen fibers of the subcutaneous tissue (fibrous scaffold and intercellular stroma of the adipose lobules) with the formation of lymphatic lakes. Different from the dilatated lymphatic collectors that collapse during sono-palpation (and slowly refilled upon lifting the probe), fluids contained within the lymphatic lakes are not easily compressible with the probe.26
Edema and infiltrative phenomena of the adipose lobules
Fluid and cellular infiltration of the stromal component of the adipose lobules lead to an increase of the thickness and echogenicity of the subcutaneous tissue with poor visualization of the surrounding fibrous scaffold. Hypoechoic fatty lobules are progressively replaced by a diffuse and coarse hyperechoic background, also known as the “snowfall” (i.e., sclero-edematous) pattern (Figure 8). In this pathological condition, the fluid pressure induces a mechanical deformation of the stromal matrix of the adipose lobules until the development of a network of intercellular micro-cracks. Inside this novel tissue cavitation, fluids can accumulate; but – different from the lymphatic collectors which are equipped with smooth muscle cells and peristaltic activity – the “neo-channels” do not have their own propelling force.25
Subcutaneous sclerosis with fibrotic involution
In the chronic phase, adipose lobules are progressively replaced by collagen fibers and fibrotic tissue – leading to a reduction of the subcutaneous thickness and an increase of its echogenicity (i.e., fibro-sclerotic pattern). Herein, as previously described for dermal fibrosis, subcutis fibrosis can also be associated with the disappearance of the dermo-hypodermal interface – a sonographic sign of local atrophy (Figure 8). For sure, in clinical practice, two or more patterns of the subcutaneous tissue can coexist in the same patient, e.g., dilatation of the lymphatic collectors of the fibrous scaffold and sclero-edema of the adipose lobules. Accordingly, a detailed description of the mixed pattern is paramount to exactly define the pathological condition of that case. Of note, in patients with a predominant pattern of lymphatic collectors’ dilatation, fluids are mainly confined in a canalicular system (i.e., a collection system housed inside the fibrous scaffold). On the other hand, in patients with a predominant pattern of sclero-edema of the adipose lobules, fluids are widely diffuse in the stromal matrix of the fatty lobules (i.e., in the intercellular network). Herein, the different spatial distribution of the edema should be carefully considered during the management of these patients (Figure 8).
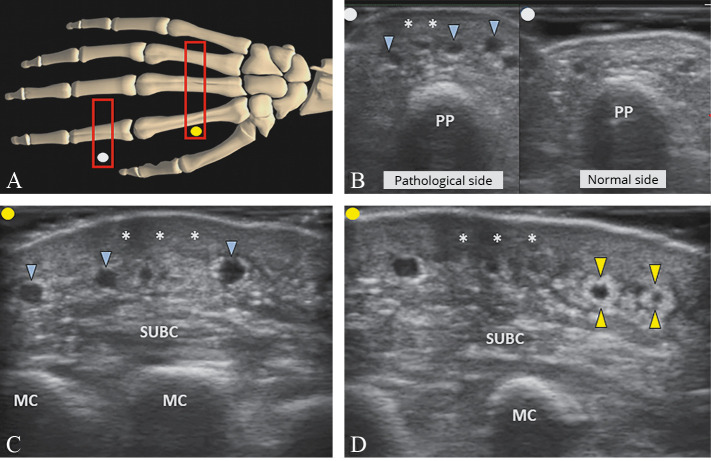
Lastly, in some anatomical areas (e.g., hand), special sonographic patterns may be detected due to the atypical arrangement of the superficial tissues. For instance, due to the presence of several dorsal fasciae of the hand (i.e., dorsal superficial, dorsal intermediate, dorsal deep and the subvenous fascia), pathological changes of the DEC are often associated with dilatation of the superficial dorsal venous plexus (i.e., “black bubbles” interface) and irregular edema of the subcutaneous tissue (i.e. “striped” pattern) (Figure 9).27 Likewise, in the advanced stage of the disease, fibrotic tissue can progressively envelop the wall of superficial veins (i.e., perivascular fibrosis) generating a peculiar sonographic finding also known as the “white ring sign.”
Figure 9.
—Positioning the probe (light grey rectangle; red in the online version) over the dorsal surface of the hand/fingers (A). Dermal sclerosis (white asterisks) associated with dilatation of the superficial dorsal veins (light grey arrowheads; blue in the online version) and striped edema of the subcutaneous tissue (SUBC) is clearly visualized (B, C). In the chronic phase, fibrotic tissue (light grey arrowheads; yellow in the online version) wrapping the superficial veins (i.e., perivascular fibrosis) can be identified as well (D). PP: proximal phalanx, MC: metacarpal bone.
Clinical rehabilitation impact
Physical examination and anthropometric measurements of the edematous limbs are still the cornerstones in the management of patients with LE. Herewith, owing to its convenience and the ever-developing technology providing high-frequency probes and high-resolution imaging, US evaluation is inevitable and already/rapidly taking its invaluable place in the management of LE.2-5
While the optimal timing and number of US evaluations are not well established,28-33 the authors believe that repeat US imaging (with close monitorization) could help better plan the rehabilitation strategies i.e. for selecting necessary therapeutic methods/orthoses in particular patients/scenarios at different disease stages.34 In one study, sonographic skin thickness measurements have shown that a combination of complex decongestive therapy (CDT) and progressive resistance exercises was found to be superior to CDT alone.28 Likewise, in another study, sonographic monitoring at two-week follow-ups after CDT clearly demonstrated significant reduction of the soft tissue thickness in lymphedematous limbs.3 Additionally, US measurements were also reported to be highly correlated with the commonly used anthropometric tools.4, 29
US examination always follows the clinical assessment in daily clinical practice. Indeed, sonographic mapping of the superficial tissues represents an easy tool to: 1) promptly define the histological layers involved in the pathological process; 2) quantify those tissues before and after the treatment; and 3) understand (especially) the sono-histological patterns of involvement.2, 6, 7, 24 To this end, using the US protocol, the authors believe that a specific diagnosis can be established which, in turn, would help tailor the treatment as well. Yet, several authors have clearly demonstrated that peculiar patterns of fluid distribution in the (sub)cutis could be refractory to some treatments.5, 30-33
Suehiro et al.5 have shown that the reduction of limb volume induced by CDT is limited if there is no sonographic evidence of lymphatic collector dilatation of the fibrous scaffold in the subcutaneous tissue, i.e. subcutaneous echo-free space (SEFS). Moreover, as the limb volume did not seem to increase without the use of compression sleeves, the authors concluded that regular compression therapy may not be necessary in LE in the absence of deep canalicular system distension.5 Of note, the SEFS is strongly correlated with the global status of extracellular fluids in the entire upper/lower limb evaluated via bioelectrical impedance analysis.31 In this sense, the presence/intensity of dilatation of the lymphatic collectors in the subcutaneous tissue may be considered as a quick and easy-available tool in order to promptly perform a panoramic quantification of the edematous status of a limb in the outpatient settings. Similarly, in another study, sonographic evidence of cobblestone pattern was found to be a predictor of good response to intensive DCT whereby early fluid evacuation also counteracted the fibrotic involution of the cutis/subcutis.32 Further, high echogenicity of the subcutaneous tissue (fibro-sclerotic and/or sclero-edematous pattern) was mentioned to be responsible for worse clinical and functional results after DCT.32 Based on the aforementioned studies; the “fluid” patterns seem to identify the early stage of the disease which is likely to be more responsive to CDT, compared to the more advanced phases (fibrotic involution and tissue hardening). Unfortunately, poor data are available as regards other sono-histological patterns of cutis/subcutis.
Using lymphoscintigraphy and immunohistopathology, Zaleska et al.25 have shown that in LE patients with fluids out of the lymphatic channels, long-term utilization of intermittent pneumatic compression might generate novel cavitation within the soft tissues – acting as novel pathways for evacuating edema, expelling local cytokines and slowing the fibrosis process and collagen fiber deposition.25 Therefore, we speculate that in case of sclero-edematous pattern of the adipose lobules with distribution of fluids within the intercellular matrix (i.e., snowfall pattern), technical adjustments of the intermittent pneumatic compression may be required in order to achieve better clinical/functional results.
Conclusions
Standardized US examination of the limbs in patients with LE represents a powerful tool in daily practice, better defining the diagnosis, planning for the treatment and diligent monitoring during the follow-up. As such, deep knowledge of the anatomy/histology of the superficial tissues and the peripheral lymphatic system as well as the sono-histological patterns of involvement is paramount in clinical practice. Finally, we believe that a standardized US approach (like described above) can also facilitate a common language between different medical specialties and/or health professionals. Accordingly, the inappropriate use of a huge number of “historical labels” (e.g., adiposalgia, adiposis edematosa, fatty edema, lipidosis, column leg) could be avoided as well.35, 36
References
- 1.Lopez M, Roberson ML, Strassle PD, Ogunleye A. Epidemiology of Lymphedema-related admissions in the United States: 2012-2017. Surg Oncol 2020;35:249–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32932222&dopt=Abstract 10.1016/j.suronc.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Mander A, Venosi S, Menegatti E, Byung-Boong L, Neuhardt D, Maietti E, et al. Upper limb secondary lymphedema ultrasound mapping and characterization. Int Angiol 2019;38:334–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31203598&dopt=Abstract 10.23736/S0392-9590.19.04176-2 [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Shin BW, Jeong HJ, Kim GC, Kim DK, Sim YJ. Ultrasonographic evaluation of therapeutic effects of complex decongestive therapy in breast cancer-related lymphedema. Ann Rehabil Med 2013;37:683–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24236256&dopt=Abstract 10.5535/arm.2013.37.5.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sezgin Ozcan D, Oken O, Dalyan Aras M, Koseoglu BF. Is ultrasonography a useful method to evaluate the effectiveness of complex decongestive therapy in breast cancer-related lymphedema? Lymphology 2017;50:84–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30234245&dopt=Abstract [PubMed] [Google Scholar]
- 5.Suehiro K, Morikage N, Harada T, Samura M, Nagase T, Mizoguchi T, et al. Regular compression therapy may not be necessary for lymphedema in arms without a subcutaneous echo-free space. Ann Vasc Surg 2020;62:258–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31494264&dopt=Abstract 10.1016/j.avsg.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 6.Caggiati A. Ultrasonography of skin changes in legs with chronic venous disease. Eur J Vasc Endovasc Surg 2016;52:534–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27117248&dopt=Abstract 10.1016/j.ejvs.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 7.Giray E, Yağcı İ. Interrater and intrarater reliability of subcutaneous echogenicity grade and subcutaneous echo-free space grade in breast cancer-related lymphedema. Lymphat Res Biol 2019;17:518–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30570358&dopt=Abstract 10.1089/lrb.2018.0053 [DOI] [PubMed] [Google Scholar]
- 8.Bianchi A, Visconti G, Hayashi A, Santoro A, Longo V, Salgarello M. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: A step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg 2020;73:1622–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32591265&dopt=Abstract 10.1016/j.bjps.2020.05.053 [DOI] [PubMed] [Google Scholar]
- 9.Özçakar L, Kara M, Chang KV, Tekin L, Hung CY, Ulaülı AM, et al. EURO-MUSCULUS/USPRM basic scanning protocols for shoulder. Eur J Phys Rehabil Med 2015;51:491–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26158915&dopt=Abstract [PubMed] [Google Scholar]
- 10.Özçakar L, Kara M, Chang KV, Hung CY, Tekın L, Ulaşlı AM, et al. EURO-MUSCULUS/USPRM basic scanning protocols for elbow. Eur J Phys Rehabil Med 2015;51:485–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26158916&dopt=Abstract [PubMed] [Google Scholar]
- 11.Özçakar L, Kara M, Chang KV, Ulaşlı AM, Hung CY, Tekin L, et al. EURO-MUSCULUS/USPRM basic scanning protocols for wrist and hand. Eur J Phys Rehabil Med 2015;51:479–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26158917&dopt=Abstract [PubMed] [Google Scholar]
- 12.Özçakar L, Kara M, Chang KV, Akkaya N, Hung CY, Tok F, et al. EURO-MUSCULUS/USPRM. Basic scanning protocols for hip. Eur J Phys Rehabil Med 2015;51:635–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26351107&dopt=Abstract [PubMed] [Google Scholar]
- 13.Özçakar L, Kara M, Chang KV, Tok F, Hung CY, Akkaya N, et al. EURO-MUSCULUS/USPRM. Basic scanning protocols for knee. Eur J Phys Rehabil Med 2015;51:641–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26351105&dopt=Abstract [PubMed] [Google Scholar]
- 14.Özçakar L, Kara M, Chang KV, Bayram Çarli A, Hung CY, Tok F, et al. EURO-MUSCULUS/USPRM. Basic Scanning Protocols for Ankle and foot. Eur J Phys Rehabil Med 2015;51:647–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26351106&dopt=Abstract [PubMed] [Google Scholar]
- 15.Chang KV, Şahin Onat Ş, Lee CW, Kara M, Hung CY, Özçakar L. EURO-MUSCULUS/USPRM basic scanning protocols revisited in children. Eur J Phys Rehabil Med 2016;52:887–901. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27098301&dopt=Abstract [PubMed] [Google Scholar]
- 16.Suami H. Anatomical theories of the pathophysiology of cancer-related lymphoedema. Cancers (Basel) 2020;12:1338. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32456209&dopt=Abstract 10.3390/cancers12051338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suami H, Scaglioni MF. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Semin Plast Surg 2018;32:5–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29636647&dopt=Abstract 10.1055/s-0038-1635118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von der Weid PY. Lymphatic vessel pumping. Adv Exp Med Biol 2019;1124:357–77. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31183835&dopt=Abstract 10.1007/978-981-13-5895-1_15 [DOI] [PubMed] [Google Scholar]
- 19.Meigel WN, Gay S, Weber L. Dermal architecture and collagen type distribution. Arch Dermatol Res 1977;259:1–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=71020&dopt=Abstract 10.1007/BF00562732 [DOI] [PubMed] [Google Scholar]
- 20.Stenn K. Collagen heterogeneity of skin. Am J Dermatopathol 1979;1:87–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=549484&dopt=Abstract 10.1097/00000372-197901010-00016 [DOI] [PubMed] [Google Scholar]
- 21.Deutsch TA, Esterly NB. Elastic fibers in fetal dermis. J Invest Dermatol 1975;65:320–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1159318&dopt=Abstract 10.1111/1523-1747.ep12598383 [DOI] [PubMed] [Google Scholar]
- 22.Yen A, Braverman IM. Ultrastructure of the human dermal microcirculation: the horizontal plexus of the papillary dermis. J Invest Dermatol 1976;66:131–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1249441&dopt=Abstract 10.1111/1523-1747.ep12481678 [DOI] [PubMed] [Google Scholar]
- 23.Hurley HJ, Jr, Mescon H, Moretti G. The anatomy and histochemistry of the arteriovenous anastomosis in human digital skin. J Invest Dermatol 1956;27:133–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13367521&dopt=Abstract 10.1038/jid.1956.85 [DOI] [PubMed] [Google Scholar]
- 24.Suehiro K, Morikage N, Yamashita O, Harada T, Samura M, Takeuchi Y, et al. Skin and subcutaneous tissue ultrasonography features in breast cancer-related lymphedema. Ann Vasc Dis 2016;9:312–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28018504&dopt=Abstract 10.3400/avd.oa.16-00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaleska M, Olszewski WL, Cakala M, Cwikla J, Budlewski T. Intermittent pneumatic compression enhances formation of edema tissue fluid channels in lymphedema of lower limbs. Lymphat Res Biol 2015;13:146–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25748341&dopt=Abstract 10.1089/lrb.2014.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi A, Giacalone G, Yamamoto T, Belva F, Visconti G, Hayashi N, et al. Ultra high-frequency ultrasonographic imaging with 70 MHz scanner for visualization of the lymphatic vessels. Plast Reconstr Surg Glob Open 2019;7:e2086. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30859043&dopt=Abstract 10.1097/GOX.0000000000002086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JA, Lee SH, Hwang SJ, Koh KS, Song WC. Anatomic, histologic, and ultrasound analyses of the dorsum of the hand for volumetric rejuvenation. J Plast Reconstr Aesthet Surg 2020;S1748-6815(20)30633-1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33303411&dopt=Abstract [DOI] [PubMed]
- 28.Bok SK, Jeon Y, Hwang PS. Ultrasonographic evaluation of the effects of progressive resistive exercise in breast cancer-related lymphedema. Lymphat Res Biol 2016;14:18–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26824517&dopt=Abstract 10.1089/lrb.2015.0021 [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Lee CH, Heo SJ, Moon MH. The clinical usefulness of lymphedema measurement technique using ultrasound. Lymphat Res Biol 2021. [Epub ahead of print] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33404351&dopt=Abstract 10.1089/lrb.2019.0070 [DOI] [PubMed]
- 30.Suehiro K, Morikage N, Ueda K, Samura M, Takeuchi Y, Nagase T, et al. Aggressive decongestion in limbs with lymphedema without subcutaneous echo-free space. Ann Vasc Surg 2018;53:205–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30012444&dopt=Abstract 10.1016/j.avsg.2018.04.033 [DOI] [PubMed] [Google Scholar]
- 31.Suehiro K, Morikage N, Ueda K, Samura M, Takeuchi Y, Nagase T, et al. Local echo-free space in a limb with lymphedema represents extracellular fluid in the entire limb. Lymphat Res Biol 2018;16:187–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29087773&dopt=Abstract 10.1089/lrb.2017.0053 [DOI] [PubMed] [Google Scholar]
- 32.Niimi K, Hirai M, Iwata H, Miyazaki K. Ultrasonographic findings and the clinical results of treatment for lymphedema. Ann Vasc Dis 2014;7:369–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25593621&dopt=Abstract 10.3400/avd.oa.14-00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tassenoy A, De Mey J, Stadnik T, De Ridder F, Peeters E, Van Schuerbeek P, et al. Histological findings compared with magnetic resonance and ultrasonographic imaging in irreversible postmastectomy lymphedema: a case study. Lymphat Res Biol 2009;7:145–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19778202&dopt=Abstract 10.1089/lrb.2008.1025 [DOI] [PubMed] [Google Scholar]
- 34.Paolucci T, Bernetti A, Bai AV, Capobianco SV, Bonifacino A, Maggi G, et al. The recovery of reaching movement in breast cancer survivors: two different rehabilitative protocols in comparison. Eur J Phys Rehabil Med 2021;57:137–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32406224&dopt=Abstract 10.23736/S1973-9087.20.06138-9 [DOI] [PubMed] [Google Scholar]
- 35.Byung-Boong Lee. Stanley G. Rockson, John Bergan. Lymphedema. A concise compendium of theory and practice. Amsterdam: Springer International Publishing AG, 2018. [Google Scholar]
- 36.Iker E, Mayfield CK, Gould DJ, Patel KM. Characterizing lower extremity lymphedema and lipedema with cutaneous ultrasonography and an objective computer-assisted measurement of dermal echogenicity. Lymphat Res Biol 2019;17:525–30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30615553&dopt=Abstract 10.1089/lrb.2017.0090 [DOI] [PubMed] [Google Scholar]