Abstract
BACKGROUND
Ankle-foot orthoses are used to improve gait stability in patients with post-stroke gait; however, there is not enough evidence to support their beneficial impact on gait stability.
AIM
To investigate the effects of ankle-foot orthoses on post-stroke gait stability.
DESIGN
An experimental study with repeated measurements of gait parameters with and without orthosis.
SETTING
Inpatients and outpatients in the Fujita Health University Hospital, Toyoake, Japan.
POPULATION
Thirty-two patients (22 males; mean age 48.3±20.0 years) with post-stroke hemiparesis participated in the study.
METHODS
Three-dimensional treadmill gait analysis was performed with and without ankle-foot orthosis for each participant. Spatiotemporal parameters, their coefficient of variation, and margin of stability were evaluated. Toe clearance, another major target of orthosis, was also examined. The effect of orthosis in the patients with severe (not able to move within the full range of motion, defying gravity) and mild ankle impairment (able to move within the full range but have problem with speed and/or smoothness of the ankle movement) was compared.
RESULTS
In the total group comparison, the decrease in the coefficient of variation of step width (P=0.012), and margin of stability on the paretic side (P=0.023) were observed. In the severe ankle impairment groups, the decreased in the coefficient of variation of the non-paretic step length (P=0.007), stride length (P=0.037), and step width (P=0.033) and margin of stability on the paretic side (P=0.006) were observed. No significant effects were observed in the mild ankle impairment group; rather, the coefficient of variation of non-paretic step length increased with the use of orthosis in this group (P=0.043); however, toe clearance increased with the use of ankle-foot orthosis (P=0.041).
CONCLUSIONS
Ankle-foot orthoses improved gait stability indices; however, the effect was either not significant or showed possible worsening in the patients with mild ankle impairment, while the effect on toe clearance was significant. These results suggest that the effects of using orthoses in patients with mild impairment should be carefully evaluated.
CLINICAL REHABILITATION IMPACT
Understanding the effects of ankle-foot orthoses on the stability of post-stroke gait and their relationship with ankle impairment severity may support clinical decision-making while prescribing orthosis for post-stroke hemiparesis.
Key words: Hemiplegia, Stroke, Gait, Rehabilitation
Ankle-foot orthoses (AFOs) are widely used as clinical devices to support ankle function and improve gait ability in hemiparetic stroke patients. The benefits of AFOs on various parameters related to hemiparetic gait have been observed, such as gait velocity, gait symmetry, and walking efficiency,1-3 which may be due to the improved ankle joint stability and subsequent increase in postural stability and toe clearance. Previous studies have reported an increase in toe clearance and reduction in compensatory movements using AFOs.4, 5 Regarding stability, several reports have evaluated the effect of AFOs on static balance improvement.6, 7 However, dynamic balance improvement during walking is not well-understood. A previous study supported the reduction in variability of spatiotemporal parameters,8 revealing an improvement in the stability of gait patterns with the use of AFO; however, there is a lack of more direct evidence on the effect of the dynamic balance control during gait. The AFO’s ability to improve the static balance may support the improvement in gait balance control; however, in healthy individuals, restricting ankle motion with AFO has reportedly had a negative influence on dynamic balance.9 It is also possible that AFO’s effects on stability vary depending on the kinematic function of the ankle joint.
In this study, we examined stability improvement with AFOs in stroke patients and whether the effect of AFOs differed with varying degrees of ankle joint motor impairment. To evaluate the stability of gait patterns, we assessed the coefficient of variation (CV) in spatiotemporal gait parameters in stroke patients with and without AFOs and the margin of stability (MoS), based on the relationship between the center of mass (COM) and the base of support, using three-dimensional gait analysis systems. Furthermore, the relationship between the gait parameters, including the stability and toe clearance indices, and the severity of ankle paralysis was also examined.
Materials and methods
Participants
The patients with post-stroke hemiparesis who underwent rehabilitation at the Fujita Health University Hospital were recruited, using the convenience sampling method. The measurements were performed between April 2009 and May 2020. Inclusion criteria were: 1) unilateral hemiparesis caused by cerebrovascular disease; 2) more than 60 days after onset; 3) continuous use of an AFO for walking for more than a week; and 4) ability to walk independently on a treadmill without orthoses, handrails, or assistive devices. Exclusion criteria were: 1) a history of previous neuromuscular diseases and/or orthopedic conditions that could interfere with the walking ability; and 2) impaired cognition and/or communication affecting the ability to follow instructions. Neurological motor impairments were evaluated using the stroke impairment assessment set.10, 11 Lower limb motor function was evaluated by the hip-flexion, knee-extension, and foot-tap tests included in the stroke impairment assessment set. For each test, motor function was rated from 0 (severely impaired) to 5 (normal). The scores in the foot-tap test were used to divide participants into two groups according to ankle motor function: the participants who scored ≥3 points, indicating that they could perform the full range of dorsiflex motion, were classified as having a mild ankle impairment, while the participants who scored ≤2 points, indicating insufficient dorsiflexion or severer impairment, were classified as having a severe ankle impairment.
The sample size was calculated using the G*power software (G*power; Aichach, Germany) version 3.1.9.2,12, 13 and it was based on the effect of AFO on limitation of ankle movement described in a previous study with a similar setting.5 The effect size was 0.71, and accordingly, the minimum sample size was calculated to be 14 (alpha 0.05, 1-beta 0.80). Consequently, we recruited more than 14 patients for the severe and mild ankle impairment groups. All participants were prescribed either a thermoplastic AFO (tAFO) or an adjustable posterior strut AFO (APS-AFO) (Figure 1). The APS-AFO is an orthosis with a posterior strut made by carbon, which is generally a stiffer ankle orthosis than tAFO.
Figure 1.

—Adjustable posterior strut ankle-foot orthosis (APS-AFO).
Data collection
This study was conducted as an experimental study with repeated measurements of gait parameters with and without orthosis.
The KinemaTracer®, a three-dimensional motion analysis system (Kissei Comtec Co., Ltd.; Matsumoto, Japan), was used to record kinematic data.5, 14, 15 This system comprises a computer for data recording and analysis and four charge-coupled device cameras with 60 Hz frame rates installed around the treadmill. Twelve markers (30-mm diameter) were placed bilaterally on the acromia, iliac crests, hip joints (one-third distance from the greater trochanter to anterior superior iliac spine), knee joints (midpoint of the anteroposterior diameter of the lateral femoral epicondyle), ankle joints (lateral malleolus), and toes (fifth metatarsal head). The treadmill speed was set at each participant’s comfortable gait speed. The treadmill speed was initially set at the mean of two comfortable 10-m over ground gait speeds without AFO and then adjusted according to the participant’s comfort level. During the measurements, the patients used AFOs that had been previously prescribed for themselves and worn in daily life. All the patients used same type of flat-soled shoes. To prevent the sole height differences from affecting the results of the examination (with and without AFO), the thickness of the shoes was adjusted using insoles such that the sole heights for the patients were the same regardless of the use of AFO.
The participants practiced walking on the treadmill prior to the measurements. The order of examination for the two conditions, with and without AFO, was randomly determined using a computer-based random number generator. The treadmill was set at the same speed in both conditions. The use of the handrails of the treadmill or additional assistive devices was not allowed during the measurements. The data collection started once participants achieved a steady-state of walking speed, and the duration of the measurement was 20 s for each trial. To prevent falls, safety harnesses and handrails were prepared, and the participant was supervised by a physiotherapist.
Data analysis
Heel strikes and toe-offs were automatically identified by ankle and toe marker trajectories using the KinemaTracer® (Kissei Comtec Co., Ltd.). Temporal parameters, including the stride time, single limb stance time, and double stance time on the paretic and non-paretic side, as well as spatial factors, such as cadence, stride length, step length, and step widths of both sides, were calculated. Step width was defined as the mediolateral distance between ankle joints upon the heel strike of the paretic and non-paretic sides. The spatial parameters were normalized to the participants’ body height. The CVs of these spatiotemporal parameters, except for cadence, were calculated to evaluate walking variability as a percentage by dividing the standard deviation by the mean. Stability was evaluated using MoS16 as an index of dynamic stability, interpreted as the ‘safety margin’ of the base of support during gait (the distance between the boundaries of the base of support and the extrapolated COM [XCOM]). The XCOM is a state of the COM in which both the position and velocity are considered, and it is calculated using the following equation:
Where PCOM is the mediolateral position of the COM, VCOM is the mediolateral velocity of the COM, g is the gravitational constant (9.81 m/s2), and l is the pendulum length (leg length × 1.34).17 The boundary of the base of support was defined as the mediolateral position of the ankle marker of the leading foot.18 The MoS was calculated at every heel strike and averaged for each foot separately (Figure 2).
Figure 2.
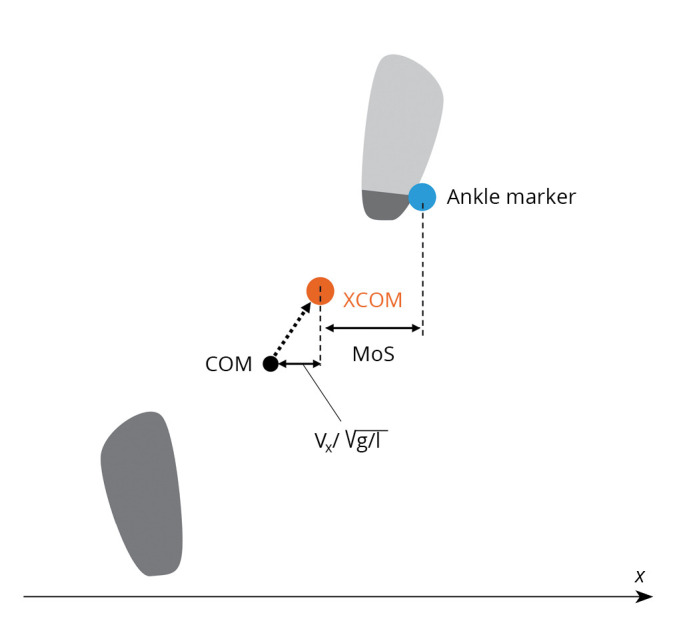
—Schematic illustration of the margin of stability (MoS) and the extrapolated center of mass (XCOM) at heel strike. MoS represents the mediolateral distance between the XCOM and the ankle marker, XCOM represents the range that COM does not exceed at any timing under the assumption of the inverted pendulum model, Vx is the mediolateral velocity of the COM, g is the gravitational constant (9.81 m/s2), l is the pendulum length (the height of the COM).
The COM was calculated by the equation described by Ehara and Yamamoto.19 The body was divided into seven segments (trunk, both thighs, both lower thighs and both feet) using the markers, and the centers of these segments were calculated. The mass ratios of each segment were estimated as 0.66, 0.10, 0.05 and 0.02 for the trunk, thigh, lower thigh and foot, respectively. The COM was calculated as a composite of the centres of the seven segments. The mediolateral COM movement, measured as the peak-to-peak displacement over a gait cycle, was also analyzed to assess lateral stability during walking.
A component analysis of toe clearance was also conducted. The parameters of toe clearance and its components in the paretic limb during the swing phase were calculated on the basis of previously reported procedures.5, 20 These studies have shown that the value of toe clearance is equivalent to the sum of the following vertical components: 1) limb-shortening; 2) non-paretic hip elevation; 3) hip elevation due to pelvic obliquity; and 4) foot elevation due to circumduction. Parameters 2, 3 and 4 are considered as movements compensating for limb-shortening due to hemiparesis.5, 20, 21 From the breakdown of toe clearance into its individual components, the dependence on the compensatory movements can be evaluated. Using this methodology, the value of toe clearance and the extent of the dependence on compensatory movements were examined.
Statistical analysis
The normality of each parameter was checked using the Shapiro-Wilk Test. For comparison between the severe and mild ankle impairment groups, Student’s t-test (unpaired t-test) and the Wilcoxon Rank-Sum and χ2 tests were performed for continuous and categorical variables, respectively. Within-group differences in gait parameters with and without AFO were assessed using the paired t-test or the Wilcoxon’s Signed-Rank test. As the time since stroke onset could be a confounding factor for the effects of AFO on hemiparetic gait, correlational analyses using Spearman’s rank correlation coefficients were performed between the time after stroke and the value differences with- and without-AFO for the parameters showing significant differences between conditions. Any P value less than 0.05 were considered statistically significant. Statistical analyses were conducted using JMP 13 (SAS Institute Inc.; Cary, NC, USA).
Ethical considerations
This study conformed to the declaration of Helsinki and was approved by the Medical Ethics Committee of Fujita Health University. All participants provided written informed consent prior to participation.
Data availability
The data collected and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Thirty-two patients (15 and 17 with severe and mild ankle impairment, respectively) participated in this study. The overall patient demographics are shown in Table I. The time after stroke was significantly longer and stroke impairment assessment set scores were significantly worse in the severe than in the mild ankle impairment group.
Table I. —Patient demographics.
| Variables | Total | Severe ankle impairment group (N.=15) |
Mild ankle impairment group (N.=17) |
P value |
|---|---|---|---|---|
| Age, years | 48.3±20.0 | 44.0±20.6 | 52.0±19.2 | 0.265 |
| Height, cm | 165.3±8.6 | 166.3±8.1 | 164.4±9.1 | 0.529 |
| Weight, kg | 60.8±14.4 | 60.5±15.2 | 61.0±14.1 | 0.934 |
| Sex, male/female | 22/10 | 11/4 | 11/6 | 0.599 |
| Diagnosis, intracerebral hemorrhage/cerebral infarction | 11/21 | 6/9 | 5/12 | 0.529 |
| Time after stroke, days | 140 (65-2439) | 322 (70-2439) | 82 (65-1768) | 0.025* |
| Affected side, right/left | 17/15 | 6/9 | 11/6 | 0.162 |
| Score of lower extremity motor function on the SIAS | 9.9±1.8 | 8.5±1.3 | 11.1±1.2 | <0.0001** |
| Hip-flexion test | 3.7±0.5 | 3.5±0.5 | 3.9±0.3 | 0.033* |
| Knee-extension test | 3.5±0.6 | 3.3±0.6 | 3.6±0.6 | 0.058 |
| Foot-tap test | 2.7±1.1 | 1.7±0.6 | 3.5±0.6 | <0.0001** |
| Treadmill speed, km/h | 2.7±0.8 | 2.4±0.7 | 2.9±0.7 | 0.101 |
| Functional ambulation categories | 4.6±0.5 | 4.7±0.5 | 4.5±0.5 | 0.451 |
| Type of AFO, tAFOs/APS-AFOs | 15/17 | 7/8 | 8/9 | 0.982 |
*P<0.05; **P<0.01. AFO: ankle-foot orthosis; APS-AFO: adjustable posterior strut-AFO; SIAS: stroke impairment assessment set; tAFO: thermoplastic AFO.
Spatiotemporal parameters for the total study population are presented in Table II. The use of the AFO significantly increased the stride time, duration of non-paretic limb double stance, stride length, and step length on the non-paretic side; it also significantly decreased the cadence. No significant differences were observed in other parameters between patients with and without AFO.
Table II. — Spatiotemporal gait parameters in total study population.
| Variables | Without AFO | With AFO | P value |
|---|---|---|---|
| Stride time, s | 1.16±0.17 | 1.19±0.19 | 0.032* |
| Cadence, steps/min | 105.1±14.4 | 103.0±14.7 | 0.023* |
| Single stance time, s | |||
| Paretic side | 0.31±0.04 | 0.32±0.05 | 0.076 |
| Non-paretic side | 0.43±0.09 | 0.43±0.09 | 0.613 |
| Double stance time, s | |||
| Paretic side | 0.23±0.06 | 0.23±0.06 | 0.849 |
| Non-paretic side | 0.19±0.05 | 0.20±0.05 | 0.026* |
| Stride length, % body height | 51.0±11.8 | 51.8±11.8 | 0.042* |
| Step length, % body height | |||
| Paretic side | 26.2±7.4 | 26.2±7.1 | 0.846 |
| Non-paretic side | 24.8±5.6 | 25.6±5.4 | 0.029* |
| Step width, % body height | 14.4±3.3 | 13.8±2.9 | 0.059 |
*P<0.05; **P<0.01. AFO: ankle-foot orthosis.
Furthermore, the spatiotemporal parameters were compared between the mild and severe ankle impairment groups (Table III). The stride time, single stance time of the paretic side, and step length on non-paretic side increased, and the cadence decreased significantly with the use of AFO in the severe ankle impairment group. There were no significant changes in the spatiotemporal parameters with or without the use of AFO in the mild ankle impairment group. We also assessed the CVs of these parameters. In the total study population, the step width variability decreased significantly with the use of AFO (Table IV).
Table III. —Comparisons of spatiotemporal gait parameters in severe vs. mild impairment groups.
| Variables | Severe impairment group (N.=15) |
Mild impairment group (N.=17) |
||||
|---|---|---|---|---|---|---|
| Without AFO | With AFO | P value | Without AFO | With AFO | P value | |
| Stride time, s | 1.22±0.15 | 1.26±0.15 | 0.024* | 1.11±0.18 | 1.12±0.20 | 0.463 |
| Cadence, steps/min | 99.7±11.5 | 96.0±10.3 | 0.028* | 109.9±15.3 | 109.2±15.5 | 0.738 |
| Single stance time, s | ||||||
| Paretic side | 0.30±0.04 | 0.32±0.04 | 0.008** | 0.32±0.04 | 0.33±0.04 | 0.904 |
| Non-paretic side | 0.45±0.08 | 0.46±0.06 | 0.410 | 0.41±0.09 | 0.41±0.10 | 0.953 |
| Double stance time, s | ||||||
| Paretic side | 0.26±0.06 | 0.26±0.06 | 0.935 | 0.20±0.05 | 0.20±0.04 | 0.702 |
| Non-paretic side | 0.20±0.06 | 0.22±0.07 | 0.089 | 0.18±0.03 | 0.19±0.03 | 0.158 |
| Stride length, % body height | 48.9±13.1 | 50.0±12.3 | 0.094 | 52.8±10.6 | 53.3±11.5 | 0.272 |
| Step length, % body height | ||||||
| Paretic side | 25.9±9.1 | 25.5±7.6 | 0.476 | 26.5±5.7 | 26.8±6.8 | 0.580 |
| Non-paretic side | 23.0±5.2 | 24.5±5.3 | 0.042* | 26.3±5.5 | 26.6±5.5 | 0.432 |
| Step width, % body height | 15.5±2.5 | 14.4±2.0 | 0.058 | 13.4±3.6 | 13.3±3.5 | 0.702 |
Values are presented as mean±standard deviation. *P<0.05; **P<0.01. AFO: ankle-foot orthosis.
Table IV. —Coefficients of variation for spatiotemporal gait parameters in total study population.
| Variables | Without AFO | With AFO | P value |
|---|---|---|---|
| Stride time, % | 3.7±2.6 | 3.4±1.1 | 0.681 |
| Single stance time, % | |||
| Paretic side | 9.2±4.0 | 8.2±2.5 | 0.120 |
| Non-paretic side | 7.5±4.0 | 7.5±2.7 | 0.805 |
| Double stance time, % | |||
| Paretic side | 10.9±8.2 | 9.2±3.1 | 0.215 |
| Non-paretic side | 9.0±2.7 | 10.3±5.0 | 0.403 |
| Stride length, % | 5.3±3.1 | 4.6±1.9 | 0.339 |
| Step length, % | |||
| Paretic side | 7.9±7.8 | 7.5±4.5 | 0.358 |
| Non-paretic side | 8.1±4.6 | 7.1±2.6 | 0.498 |
| Step width, % | 8.2±4.3 | 7.3±3.4 | 0.012* |
*P<0.05, **P<0.01. AFO: ankle-foot orthosis.
In addition, AFO decreased stride, step length on the non-paretic side, and step width variabilities in the severe ankle impairment group, but increased step length variability on the non-paretic side in the mild ankle impairment group (Table V).
Table V. —Coefficients of variation for spatiotemporal gait parameters in severe vs. mild impairment groups.
| Variables | Severe ankle impairment group (N.=15) | Mild ankle impairment group (N.=17) | ||||
|---|---|---|---|---|---|---|
| Without AFO | With AFO | P value | Without AFO | With AFO | P value | |
| Stride time, % | 4.3±3.4 | 3.3±1.0 | 0.177 | 3.2±1.5 | 3.4±1.3 | 0.619 |
| Single stance time, % | ||||||
| Paretic side | 10.4±4.5 | 9.2±2.5 | 0.264 | 8.2±3.3 | 7.3±2.2 | 0.229 |
| Non-paretic side | 8.5±4.9 | 7.6±3.2 | 0.600 | 6.7±2.8 | 7.3±2.4 | 0.249 |
| Double stance time, % | ||||||
| Paretic side | 12.4±11.4 | 8.7±3.6 | 0.091 | 9.6±3.6 | 9.6±2.7 | 0.982 |
| Non-paretic side | 7.9±2.6 | 9.3±4.0 | 0.212 | 9.9±2.5 | 11.2±5.8 | 0.910 |
| Stride length, % | 6.5±3.8 | 4.8±2.0 | 0.037* | 4.2±1.6 | 4.5±1.9 | 0.330 |
| Step length, % | ||||||
| Paretic side | 9.9±10.9 | 7.7±4.3 | 0.858 | 6.2±2.6 | 7.2±4.8 | 0.226 |
| Non-paretic side | 10.2±5.5 | 6.9±2.4 | 0.007** | 6.2±2.5 | 7.3±2.8 | 0.043* |
| Step width, % | 8.7±2.8 | 7.4±2.4 | 0.033* | 7.8±5.3 | 7.3±4.1 | 0.231 |
Values are presented as mean±standard deviation. *P<0.05; **P<0.01. AFO: ankle-foot orthosis.
The MoS on the paretic side decreased with AFO in the total study population (Figure 3A), as well as in patients with severe ankle impairment (Figure 3B) (11.6±2.3 vs. 9.7±1.8 cm, P=0.006), but there was no difference with or without AFO in the mild ankle impairment group (Figure 3B) (8.7±2.7 vs. 8.7±3.4 cm, P=0.900). No significant differences were observed for MoS on the non-paretic side between the patients with and without AFO in each group (Figure 3C) (severe ankle impairment group: 9.2±2.1 vs. 9.2±2.2 cm, P=0.979; mild ankle impairment group: 7.7±2.4 vs. 7.8±1.9 cm, P=0.705).
Figure 3.
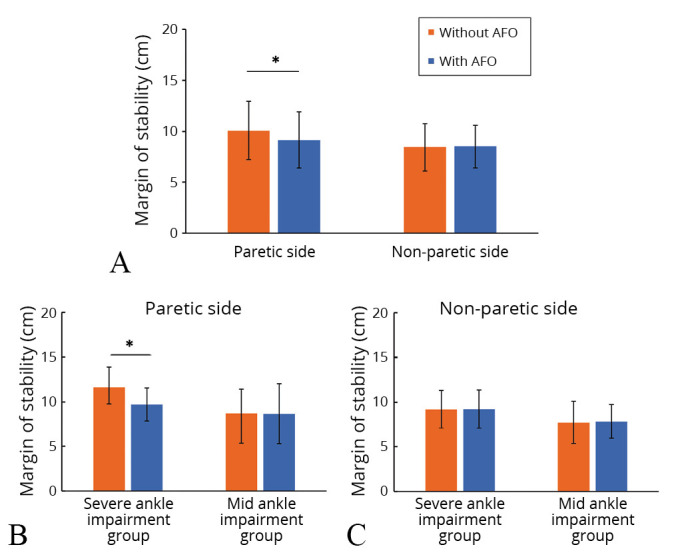
—A) Margin of stability (MoS) with or without using an ankle-foot orthosis (AFO) in paretic and non-paretic sides in total study population; B) and subgroup comparisons in the severe ankle impairment group and mild ankle impairment group in paretic side; and C) and non-paretic side. The bars indicate the average values of MoS at paretic/non-paretic heel strikes with and without an AFO in each group. The MoS on the paretic side was significantly smaller than that without an AFO in the overall comparison (A) and in the severe impairment group (B). No significant difference was observed in the mild impairment group (C). Error bar indicates the standard deviation. *P< 0.01.
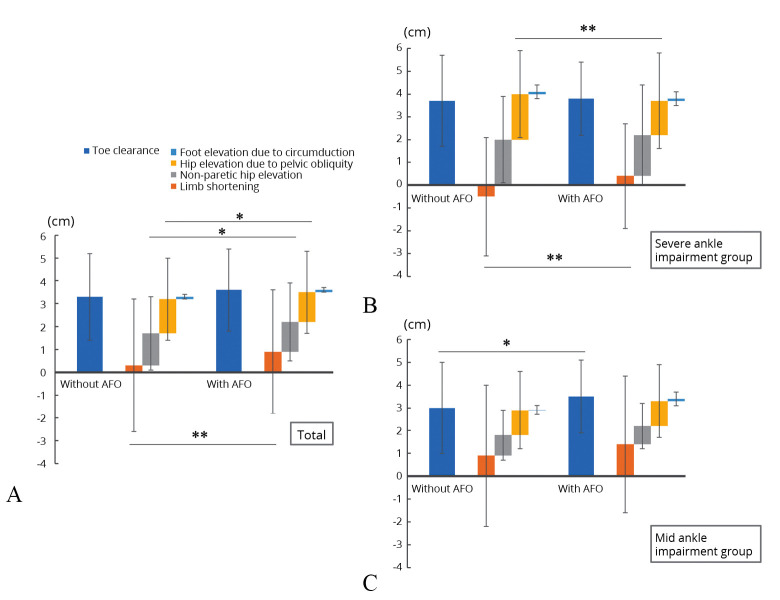
The analysis results of toe clearance and its break down into components are shown in Figure 4. In the comparison in the total study population (Figure 4A), a significant increase in the component of limb-shortening (without AFO vs. with AFO: 0.3±2.9 vs. 0.9±2.7 cm, P=0.001) and a significant decrease in the component of hip elevation due to pelvic obliquity (1.5±1.8 vs. 1.3±1.8 cm, P=0.024) and the component of non-paretic hip elevation (1.4±1.6 vs. 1.3±1.7 cm, P=0.048) were observed. In addition, there was also an increasing tendency in the toe clearance (3.3±1.9 vs. 3.6±1.8 cm, P=0.065). No significant effects on the component of circumduction with the use of AFO were observed. In the severe ankle impairment group, there was no difference in toe clearance between patients with and without AFO (Figure 4B) (without AFO vs. with AFO: 3.7±2.0 vs. 3.8±1.6 cm, P=0.806). However, the component of limb-shortening increased (-0.5±2.6 vs. 0.4±2.3 cm, P=0.002) and the component of hip elevation due to pelvic obliquity (2.0±1.9 vs. 1.5±2.1 cm, P=0.005) decreased when wearing an AFO. No significant differences were observed in other components of compensatory movements (non-paretic hip elevation: 2.0±1.9 vs. 1.8±2.2 cm, P=0.165; foot elevation due to circumduction: 0.1±0.3 vs. 0.1±0.3 cm, P=0.394). Toe clearance significantly increased when wearing an AFO in the mild ankle impairment group (Figure 4C) (3.0±1.8 vs. 3.5±2.0 cm, P=0.041), and there was also an increasing tendency in the limb-shortening (0.9±3.1 vs. 1.4±3.0 cm, P=0.081), but there were no differences in the component of compensatory movements with or without an AFO (non-paretic hip elevation: 0.9±1.1 vs. 0.8±1.0 cm, P=0.194; hip elevation due to pelvic obliquity: 1.1±1.7 vs. 1.1±1.6 cm, P=0.649; foot elevation due to circumduction: 0.0±0.2 vs. 0.1±0.3 cm, P=0.973).
Figure 4.
—A) Toe clearance and its components with and without ankle-foot orthosis (AFO) in the total study population; B) the severe ankle impairment group; and C) the mild ankle impairment group. The comparison in the total study population shows a significant increase in the component of limb-shortening and significant decrease in the component of hip elevation due to pelvic obliquity and the component of non-paretic hip elevation (A). The comparison in the severe ankle impairment group shows a significant increase in the component of limb-shortening and significant decrease in the component of hip elevation due to pelvic obliquity (B). The comparison in the mild ankle impairment groups shows a significant increase in toe clearance (C). Error bar indicates standard deviation. *P<0.05; **P< 0.01.
In correlational analyses, the changes by AFO in the component of limb-shortening and the vertical component of hip elevation due to pelvic obliquity significantly correlated with the time after stroke (Table VI: limb-shortening: r=0.46, P=0.009; hip elevation due to pelvic obliquity: r=-0.39, P=0.028). All other variables did not significantly correlate with time after stroke.
Table VI. —Spearman’s ranked correlation coefficient between the time after stroke and the value differences with and without AFO.
| Variables | ρ | P value |
|---|---|---|
| Stride time | 0.28 | 0.127 |
| Cadence | -0.12 | 0.499 |
| Single stance time | ||
| Paretic side | 0.15 | 0.418 |
| Non-paretic side | 0.14 | 0.461 |
| Double stance time | ||
| Paretic side | 0.05 | 0.792 |
| Non-paretic side | 0.18 | 0.319 |
| Stride length | 0.06 | 0.756 |
| Step length | ||
| Paretic side | -0.03 | 0.867 |
| Non-paretic side | 0.20 | 0.274 |
| Step width | -0.34 | 0.060 |
| Stride time CV | -0.03 | 0.866 |
| Single stance time CV | ||
| Paretic side | -0.09 | 0.640 |
| Non-paretic side | 0.15 | 0.409 |
| Double stance time CV | ||
| Paretic side | -0.02 | 0.932 |
| Non-paretic side | 0.07 | 0.698 |
| Stride length CV | 0.01 | 0.956 |
| Step length CV | ||
| Paretic side | 0.03 | 0.884 |
| Non-paretic side | -0.14 | 0.458 |
| Step width CV | -0.09 | 0.611 |
| Margin of stability | ||
| Paretic side | -0.33 | 0.067 |
| Non-paretic side | -0.07 | 0.723 |
| Toe clearance | 0.33 | 0.065 |
| Limb shortening | 0.46 | 0.009** |
| Non-paretic hip elevation | 0.03 | 0.872 |
| Hip elevation due to pelvic obliquity | -0.39 | 0.028* |
| Foot elevation due to circumduction | -0.21 | 0.258 |
*P<0.05; **P<0.01. CV: Coefficient of variation.
Discussion
This study investigated the effects of AFO on stability in the post-stroke gait and the differences in these effects related to the severity of ankle impairment. The results showed that the variabilities in spatiotemporal parameters and MoS, which were higher in the paretic side compared to the non-paretic side, decreased with AFO, indicating the improvement in the dynamic balance control during gait. These effects on gait differed with varying ankle impairment severity and were more evident in the severe ankle impairment group. Conversely, these effects were not significant in the mild ankle impairment group, instead, the variability seemed to slightly worsen with the use of AFO. The effects on the toe clearance were more universal, being present both in severe and mild impairment groups.
In post-stroke patients, impairments such as muscle weakness, abnormal movement synergies and spasticity cause instability in the paretic limb.22, 23 Such impairments lead to a decline in gait performance. In this study, the patients with post-stroke hemiparesis had a slower walking speed, shorter step length, shorter paretic single stance and longer double stance time compared to those of the healthy subjects shown previously;24, 25 these findings are consistent with previous reports.26 The decline in gait performance caused by paretic limb impairments may improve with AFO, which adjusts the alignment and supports paretic ankle stability. Previous studies have demonstrated the effect of AFO on spatiotemporal parameters, including an increase in step length and paretic single stance time, which can be attributed to improved ankle stability.3, 27, 28 These effects were also observed in the present study: there was a significant increase in non-paretic step length in the total population and an increase in the paretic single stance time in the severe impairment group. However, we also observed a decrease in cadence and increase in double stance time in the total population, which contradicts the findings of a previous study.3 This can be attributed to using the identical walking speeds to compare the gait in patients with and without the AFO. With the same walking speed, the increase in step length with AFO may have resulted in the decreased cadence, which caused an increase in the stride time, and a subsequent increase in double stance time, as it is a component of the stride time.
Further, we observed several changes in the gait variability parameters that support the effects of AFOs on paretic stance stability. In the total study population, the step width CV decreased in patients using AFOs. The difference in the variability was even more evident in the severe impairment group, exhibiting a significant decrease in stride length, non-paretic step length, and step width CVs, which is in line with the findings of a previous study.8 As the non-paretic step is an action that occurs during the paretic single stance, the instability of the paretic single stance due to paresis may have been reflected on the non-paretic step length CV.
The variabilities in these spatiotemporal parameters reflect the fall risk. For example, the increase in the variability in stride length, step length, step width, and stride time is related to the fall risk among the elderly and patients with central nervous system disorders.29-32 Accordingly, the decreased stride CV and step length CV on the non-paretic side in this study indicate the beneficial effect of AFO in improving paretic limb stability in patients with severe ankle impairment. In addition, the use of AFO also increased the paretic single stance time, which is a period of less stability in stroke patients.33 Contrary to the findings observed in the severe ankle impairment group, the paretic single stance time did not significantly change, and the step length CV increased with the use of an AFO in the mild ankle impairment group. This may be attributable to the impaired dynamic balance control by AFO due to the restricted plantar flexor muscle force generation, which is seen when healthy individuals wear the AFO.9 Future studies with kinetic measurements would help clarify the mechanism of this impairment severity-related differences in the effect of AFO on the dynamic stability during walking.
Another parameter employed to evaluate the dynamic balance during gait was the MoS. The MoS reflects the ‘safety margin’ of the base of support, i.e., dealing with the movement of the COM, and is used to evaluate dynamic balance during walking.18, 34-37 A higher MoS in neurological patients indicates a compensatory strategy to increase the ‘safety margin’ of the base of support, thus reflecting poor dynamic balance.38 In the present study, the AFO decreased the MoS on the paretic side, suggesting that it may have contributed to dynamic balance improvement, and thus reduced the compensation to maintain the balance during gait. The subgroup analysis showed that this effect was robust in the severe impairment group, while there was no significant effect in the mild impairment group. In combination with the changes observed in the spatiotemporal parameters, these results support the benefit of AFOs on dynamic balance control during gait in patients, especially those with severe ankle impairment.
In contrast to the robust merits in the severe impairment group, the present results on dynamic balance failed to show the merits of using AFOs in the mild ankle impairment group. However, the AFO also plays an important role in supporting toe clearance in patients post stroke,4, 5 which was also investigated in the present study. Post-stroke gait is characterized by decreased knee flexion and ankle dorsiflexion during the swing phase on the paretic side.39-41 Impaired leg movement decreases toe clearance and increases the risk of falling; therefore, individuals post-stroke adopt compensatory movement strategies (e.g., pelvic hiking and circumduction).22, 39, 42 In this study, the results regarding the total study population were consistent with earlier findings,5 revealing a significant increase in the vertical component of limb-shortening and decrease in the vertical component of compensatory movements with the use of AFO, with an increasing tendency (P=0.065) in toe clearance. In the mild impairment groups, a significant increase in toe clearance and an increased tendency (P=0.080) in the limb-shortening component with the use of AFO were observed, showing the merits of using AFO in improving gait performance even in this group. However, the effect of AFO was relatively small compared to the effects on the severe ankle impairment group; this may be because the extent of the paresis was mild involving fewer problems in limb-shortening. In addition, the AFO used by the patients with mild paresis may be less solid and thus, had a smaller effect in limiting ankle plantar flexion, though the type of the orthosis (tAFOs and APS-AFOs) was not significantly different between the groups (Table I). In any case, the weaker effect of AFO both in stability and toe clearance may suggest that the use of AFO should be carefully evaluated in the mild impairment groups. Further investigation on the effects of AFO in combination with its detailed setting would facilitate our understanding of AFO in the post-stroke gait.
Clinical implications
The study findings support the benefits of AFO in improving both stance stability and toe clearance in patients. The AFO presents significant merit in toe clearance but may impede stance stability in patients with mild ankle impairment; therefore, the effects of AFOs should be carefully evaluated in these patients. Thus, the effects on stability, which are occasionally difficult to detect visually, need particular attention. Technologies, such as three-dimensional motion analysis, to quantify changes in performance may help improve the clinicians’ assessments on the effects of AFOs and facilitate appropriate individualized prescribing.
Limitations of the study
There are several limitations to this study. First, the participants had relatively preserved gait ability, as we only included those who were able to independently walk on the treadmill without gait aids or orthoses. Thus, our findings may not be generalizable to patients with more severe hemiplegia. Second, the fixed walking speed used while comparing the gait performance in the patients with and without the AFOs may have influenced the results of this study. The walking speed was identical for the gait performance examinations because dynamic stability indices, which were main outcome indices of this study, can be influenced by the walking speed.43 However, this may have masked the positive effects of the AFO on the spatiotemporal indices. Third, AFO types were inconsistent across participants. In fact, the effect of orthosis may differ according to their types, and the weak effect of orthosis in the mild paresis group may be due to the use of a simpler orthosis with less stiffness. Though the ratio of tAFOs and APS-AFOs was not significantly different between the groups (Table I), the setting of the AFOs (thickness, trimming, etc.) might have yielded some variation in stiffness. Therefore, it should be noted that the effect of the AFO on severe and mild impairment cannot be directly comparable. Even so, the fact that the patients with mild ankle impairment presented with only slight benefits with the use of AFO, and even had some negative effects related to stability, indicates the importance of gait evaluation before prescribing AFO for patients with mild ankle impairment. Fourth, the time after stroke in this study varied widely, including that for cases of both subacute and chronic stroke. In addition, the time after stroke was significantly longer in the severe than in the mild ankle impairment group. The effects on limb shortening and hip elevation due to pelvic obliquity were correlated with the time after stroke; thus, the longer time after stroke in the severe ankle impairment group may have some influence in the differences in limb shortening and hip elevation due to pelvic obliquity between the severe and mild impairment groups shown in this study. However, none of the gait stability parameters presented a significant correlation with the time after onset within each group. Thus, the differences in the effects of AFO between severe and mild impairment groups on the stability during gait were not likely affected by the difference in the time after stroke. Comparisons under more controlled conditions are needed to further clarify the complex relationship between the severity of ankle impairment and the effects of AFO.
The small number of recruited subjects may also be a limitation of this study. The sample size of this study was calculated based on the robust effect of AFO on ankle movement limitation shown previously;5 however, it may have been insufficient to demonstrate the differences in the with and without AFO conditions for some parameters with a small effect size, especially in the subgroup analysis of the severe and mild impairment groups. Further analysis, such as one examining the differences in the effects of AFO between patients with different levels of paresis or between sub-acute and chronic cases should be performed using a larger sample size. Finally, the ground reaction force was not investigated in this study; therefore, it was not possible to clarify the influence of kinetic changes due to ankle movement restriction on the gait parameters. Further studies are required to quantify and analyze these kinetic changes to help better understand the effect of AFO on dynamic stability during the stance phase.
Conclusions
In this study, we investigated the effects of AFO on stability during gait and the differences in the effects between patients with severe and mild ankle impairment. AFO improved MoS and step width CV, which suggests an improvement in stability. Subgroup analysis showed that this effect was robust in the severe ankle impairment group. On the contrary, the parameters of gait stability were not evident and were even slightly worse in patients with mild ankle impairment, though the effect of AFO on toe clearance was also significant in the mild impairment group. These results suggest that the effects of using AFOs in patients with mild impairment should be carefully evaluated. Further detailed studies on the effect of AFO in relation to the severity of impairment and the settings of AFO (i.e., stiffness or joint types of AFO) are warranted to further understand the effects of AFOs.
References
- 1.Esquenazi A, Ofluoglu D, Hirai B, Kim S. The effect of an ankle-foot orthosis on temporal spatial parameters and asymmetry of gait in hemiparetic patients. PM R 2009;1:1014–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19942187&dopt=Abstract 10.1016/j.pmrj.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 2.Leung J, Moseley A. Impact of ankle-foot orthoses on gait and leg muscle activity in adults with hemiplegia: systematic literature review. Physiotherapy 2003;89:39–55. 10.1016/S0031-9406(05)60668-2 [DOI] [Google Scholar]
- 3.Franceschini M, Massucci M, Ferrari L, Agosti M, Paroli C. Effects of an ankle-foot orthosis on spatiotemporal parameters and energy cost of hemiparetic gait. Clin Rehabil 2003;17:368–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12785244&dopt=Abstract 10.1191/0269215503cr622oa [DOI] [PubMed] [Google Scholar]
- 4.Cruz TH, Dhaher YY. Impact of ankle-foot-orthosis on frontal plane behaviors post-stroke. Gait Posture 2009;30:312–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19570678&dopt=Abstract 10.1016/j.gaitpost.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pongpipatpaiboon K, Mukaino M, Matsuda F, Ohtsuka K, Tanikawa H, Yamada J, et al. The impact of ankle-foot orthoses on toe clearance strategy in hemiparetic gait: a cross-sectional study. J Neuroeng Rehabil 2018;15:41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29792211&dopt=Abstract 10.1186/s12984-018-0382-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cakar E, Durmus O, Tekin L, Dincer U, Kiralp MZ. The ankle-foot orthosis improves balance and reduces fall risk of chronic spastic hemiparetic patients. Eur J Phys Rehabil Med 2010;46:363–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20927002&dopt=Abstract [PubMed] [Google Scholar]
- 7.Chen CK, Hong WH, Chu NK, Lau YC, Lew HL, Tang SF. Effects of an anterior ankle-foot orthosis on postural stability in stroke patients with hemiplegia. Am J Phys Med Rehabil 2008;87:815–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18617863&dopt=Abstract 10.1097/PHM.0b013e31817c150e [DOI] [PubMed] [Google Scholar]
- 8.Abe H, Michimata A, Sugawara K, Sugaya N, Izumi S. Improving gait stability in stroke hemiplegic patients with a plastic ankle-foot orthosis. Tohoku J Exp Med 2009;218:193–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19561389&dopt=Abstract 10.1620/tjem.218.193 [DOI] [PubMed] [Google Scholar]
- 9.Vistamehr A, Kautz SA, Neptune RR. The influence of solid ankle-foot-orthoses on forward propulsion and dynamic balance in healthy adults during walking. Clin Biomech (Bristol, Avon) 2014;29:583–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24698166&dopt=Abstract 10.1016/j.clinbiomech.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chino N, Sonoda S, Domen K, Saitoh E, Kimura A. Stroke Impairment Assessment Set (SIAS) - A new evaluation instrument for stroke patients -. Jpn J Rehabil Med. 1994;31:119–25. 10.2490/jjrm1963.31.119 [DOI] [Google Scholar]
- 11.Hachisuka K, Umezu Y, Ogata H. Disuse muscle atrophy of lower limbs in hemiplegic patients. Arch Phys Med Rehabil 1997;78:13–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9014951&dopt=Abstract 10.1016/S0003-9993(97)90003-4 [DOI] [PubMed] [Google Scholar]
- 12.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19897823&dopt=Abstract 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 13.Faul F, Erdfelder E, Lang AG, Buchner A. G.*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17695343&dopt=Abstract 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 14.Mukaino M, Ohtsuka K, Tanikawa H, Matsuda F, Yamada J, Itoh N, et al. Clinical-oriented Three-dimensional Gait Analysis Method for Evaluating Gait Disorder. J Vis Exp 2018;133:57063. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29553535&dopt=Abstract 10.3791/57063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtsuka K, Saitoh E, Kagaya H, Itoh N, Tanabe S, Matsuda F, et al. Application of Lissajous overview picture in treadmill gait analysis. Jpn J Compr Rehabil Sci. 2015;6:33–42. [Google Scholar]
- 16.Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech 2005;38:1–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15519333&dopt=Abstract 10.1016/j.jbiomech.2004.03.025 [DOI] [PubMed] [Google Scholar]
- 17.Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture 2007;25:250–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16740390&dopt=Abstract 10.1016/j.gaitpost.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 18.McAndrew Young PM, Dingwell JB. Voluntary changes in step width and step length during human walking affect dynamic margins of stability. Gait Posture 2012;36:219–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22472707&dopt=Abstract 10.1016/j.gaitpost.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehara Y, Yamamoto S. Introduction to body dynamics-analysis of standing up movement. First Edition. Tokyo: Ishiyaku Publishers; 2001. p.65–8. [Google Scholar]
- 20.Matsuda F, Mukaino M, Ohtsuka K, Tanikawa H, Tsuchiyama K, Teranishi T, et al. Analysis of strategies used by hemiplegic stroke patients to achieve toe clearance. Jpn J Compr Rehabil Sci. 2016;7:111–8. [Google Scholar]
- 21.Matsuda F, Mukaino M, Ohtsuka K, Tanikawa H, Tsuchiyama K, Teranishi T, et al. Biomechanical factors behind toe clearance during the swing phase in hemiparetic patients. Top Stroke Rehabil 2017;24:177–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27671158&dopt=Abstract 10.1080/10749357.2016.1234192 [DOI] [PubMed] [Google Scholar]
- 22.Balaban B, Tok F. Gait disturbances in patients with stroke. PM R 2014;6:635–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24451335&dopt=Abstract 10.1016/j.pmrj.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 23.Rahimzadeh Khiabani R, Mochizuki G, Ismail F, Boulias C, Phadke CP, Gage WH. Impact of Spasticity on Balance Control during Quiet Standing in Persons after Stroke. Stroke Res Treat 2017;2017:6153714. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29098109&dopt=Abstract 10.1155/2017/6153714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10-79 years of age. J Rehabil Res Dev 1993;30:210–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8035350&dopt=Abstract [PubMed] [Google Scholar]
- 25.Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011;34:111–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21531139&dopt=Abstract 10.1016/j.gaitpost.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheffler LR, Chae J. Hemiparetic Gait. Phys Med Rehabil Clin N Am 2015;26:611–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26522901&dopt=Abstract 10.1016/j.pmr.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 27.Gök H, Küçükdeveci A, Altinkaynak H, Yavuzer G, Ergin S. Effects of ankle-foot orthoses on hemiparetic gait. Clin Rehabil 2003;17:137–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12625653&dopt=Abstract 10.1191/0269215503cr605oa [DOI] [PubMed] [Google Scholar]
- 28.Nikamp CD, Hobbelink MS, van der Palen J, Hermens HJ, Rietman JS, Buurke JH. A randomized controlled trial on providing ankle-foot orthoses in patients with (sub-)acute stroke: short-term kinematic and spatiotemporal effects and effects of timing. Gait Posture 2017;55:15–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28407505&dopt=Abstract 10.1016/j.gaitpost.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 29.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci 2009;64:896–901. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19349593&dopt=Abstract 10.1093/gerona/glp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc 1997;45:313–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9063277&dopt=Abstract 10.1111/j.1532-5415.1997.tb00946.x [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology 1996;42:108–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9138973&dopt=Abstract 10.1159/000213780 [DOI] [PubMed] [Google Scholar]
- 32.Johansson J, Nordström A, Nordström P. Greater Fall Risk in Elderly Women Than in Men Is Associated With Increased Gait Variability During Multitasking. J Am Med Dir Assoc 2016;17:535–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27006336&dopt=Abstract 10.1016/j.jamda.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Nott CR, Neptune RR, Kautz SA. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture 2014;39:129–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23820449&dopt=Abstract 10.1016/j.gaitpost.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devetak GF, Bohrer RC, Rodacki AL, Manffra EF. Center of mass in analysis of dynamic stability during gait following stroke: A systematic review. Gait Posture 2019;72:154–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31202025&dopt=Abstract 10.1016/j.gaitpost.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 35.Kao PC, Dingwell JB, Higginson JS, Binder-Macleod S. Dynamic instability during post-stroke hemiparetic walking. Gait Posture 2014;40:457–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24931112&dopt=Abstract 10.1016/j.gaitpost.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peebles AT, Reinholdt A, Bruetsch AP, Lynch SG, Huisinga JM. Dynamic margin of stability during gait is altered in persons with multiple sclerosis. J Biomech 2016;49:3949–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27889188&dopt=Abstract 10.1016/j.jbiomech.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stimpson KH, Heitkamp LN, Embry AE, Dean JC. Post-stroke deficits in the step-by-step control of paretic step width. Gait Posture 2019;70:136–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30856525&dopt=Abstract 10.1016/j.gaitpost.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vistamehr A, Kautz SA, Bowden MG, Neptune RR. Correlations between measures of dynamic balance in individuals with post-stroke hemiparesis. J Biomech 2016;49:396–400. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26795124&dopt=Abstract 10.1016/j.jbiomech.2015.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005;22:51–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15996592&dopt=Abstract 10.1016/j.gaitpost.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto S, Ibayashi S, Fuchi M, Yasui T. Immediate-term effects of use of an ankle-foot orthosis with an oil damper on the gait of stroke patients when walking without the device. Prosthet Orthot Int 2015;39:140–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24469429&dopt=Abstract 10.1177/0309364613518340 [DOI] [PubMed] [Google Scholar]
- 41.Lamontagne A, Malouin F, Richards CL, Dumas F. Mechanisms of disturbed motor control in ankle weakness during gait after stroke. Gait Posture 2002;15:244–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11983499&dopt=Abstract 10.1016/S0966-6362(01)00190-4 [DOI] [PubMed] [Google Scholar]
- 42.Stanhope VA, Knarr BA, Reisman DS, Higginson JS. Frontal plane compensatory strategies associated with self-selected walking speed in individuals post-stroke. Clin Biomech (Bristol, Avon) 2014;29:518–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24768223&dopt=Abstract 10.1016/j.clinbiomech.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Mukaino M, Ohtsuka K, Otaka Y, Tanikawa H, Matsuda F, et al. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int J Rehabil Res 2020;43:69–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31855899&dopt=Abstract 10.1097/MRR.0000000000000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data collected and analyzed during the current study are available from the corresponding author on reasonable request.