Abstract
BACKGROUND
Coronavirus disease (COVID-19) is characterized by different clinical pictures that may require prolonged hospitalization and produce disabilities challenging the recovery of previous independence.
AIM
The aim is to evaluate the impact of an early assisted rehabilitation program on the functional status of an acutely hospitalized population affected by COVID-19.
DESIGN
Single-institution retrospective longitudinal study.
SETTING
Inpatient intensive care units (ICU) and medical care units (MCU).
POPULATION
Acute COVID-19 patients.
METHODS
General information was collected; age-adjusted Charlson Comorbidity Index was used for comorbidities. Duration of hospital stay, the length of stay in ICU and/or MCU, the length of the rehabilitative treatment, and the destination at the discharge were collected. Evaluation was performed when patients were clinically stable (T0), and at hospital discharge (T1); for subjects enrolled in ICU functional status was assessed at the time of transfer to the MCU. Muscle strength of the four limbs was measured with the Medical Research Council (MRC) sum-score. Functional status was assessed using the 3-item Barthel Index (BI-3) and the General Physical Mobility Score (GPMS). Early assisted-tailored rehabilitation protocol was applied in ICU and in MCU: the aims were the maintenance (or recovery) of the range of motion and of the strength and the recovery of sitting/standing position and gait.
RESULTS
We evaluated 116 patients (mean age 65, SD 11) (65% male), 68 in ICU (mean age 60, SD 10), 48 in MCU (mean age 73, SD 9). At discharge, BI-3 and GPMS significantly improved in both ICU (P<0.001) and MCU (P<0.001) subgroups of patients. MRC sum-score significantly improved in ICU patients (P<0.001). Patients hospitalized in ICU had a significantly longer hospital stay. At discharge, patients admitted to the ICU reach a functional state that is close to that of patients admitted to the MCU.
CONCLUSIONS
The results suggest that an early assisted rehabilitation program may be helpful in improving the short-term functional status of an acutely hospitalized population affected by COVID-19, with discharge at home of 48%
CLINICAL REHABILITATION IMPACT
this study focuses on a functional assessment method to be used to identify the rehabilitation needs and verify the results of an early rehabilitation protocol applied to the acute COVID-19 patient admitted to ICU and MCU.
Key words: COVID-19, SARS-CoV-2, Rehabilitation
Coronavirus disease (COVID-19) is an infectious disease first detected in Wuhan, China. It first appeared in Italy in January 2020, the first case with secondary transmission being diagnosed in February 2020. In March 2020, W.H.O. declared a pandemic state.1 COVID-19 is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and is characterized by a broad spectrum of clinical features, ranging from a complete absence of symptoms to mild symptoms such as fever, cough, fatigue, headache, myalgia, gastrointestinal disorders and anosmia up to dyspnea in case of bilateral pneumonia of varying grade of severity.2-5 COVID-19, whose symptoms are marked by the expression of the viral infection and by the host’s anti-inflammatory response, can progress into three pathophysiological stages: the infection phase, the pulmonary phase and the inflammatory phase.3-8 In severe cases, interstitial pneumonia may be accompanied by multiorgan failure and by phenomena of thrombosis, microangiopathic skin lesions at the extremities and disseminated intravascular coagulation.7 The compromise of respiratory function may express itself with pictures of different severity requiring oxygen therapy or non-invasive ventilatory assistance; in case of acute respiratory distress syndrome (ARDS), invasive mechanical ventilation with prolonged hospitalization in intensive wards is required.2, 3 In addition to advanced age and male gender, comorbidities such as type 2 diabetes, hypertension, obesity and pre-existing impaired immune function are considered risk factors for the development of a severe SARS-CoV-2 infection.6
Patients with severe pictures of SARS-CoV-2 infection may therefore require prolonged hospitalization in intensive care unit (ICU) which, as already reported in the literature regarding patients with respiratory disease not related to COVID-19, is cause of myopathy with loss of body mass of about 20% after the first week and with rhabdomyolysis, hypotrophy and hyposthenia being due to mitochondrial dysfunction and metabolic alterations in the satellite cells that are necessary for muscle regeneration.9-12 Moreover, muscle atrophy is in turn a complication of hypercapnia due to lung damage.13 The pathophysiological mechanisms of ICU-acquired weakness are believed to be multifactorial and muscle damage is associated with neuronal damage and axonal degeneration from microcirculatory dysfunction.11 In ICU-acquired weakness neuromuscular recovery is delayed and 65% of patients have been estimated to have functional limitation at the discharge, the neuromuscular damage lasting for years.14, 15 Previous reports about severe form of Coronavirus 1 infection have shown long recovery times, even 12 years, in case of critical illness.6
In order to reduce the effects of prolonged immobility and to improve functional outcomes, rehabilitation is actually considered an integral part of the management of critically ill patients.16-18
With the spread of the covid 19 pandemic, greater knowledge on the clinical characteristics, treatment and sequelae of this pathology have led to a growing scientific literature regarding the rehabilitation approach of the SARS-CoV-2 patient, and a series of systematic rapid living reviews on rehabilitation needs has been published. The main fields of intervention concern the rehabilitation treatment of acute respiratory failure that characterizes the initial stages of the disease, the containment of the effects related to prolonged immobility with an early mobilization of the patient admitted to the ICU, the recovery of mobility prior to hospitalization through continuation of rehabilitation treatment both in the postacute phase and at home, where continuous monitoring of the motor, neurological, cognitive sequelae is necessary.19, 20
The aim of this study is to evaluate the impact of an early assisted rehabilitation program on the functional status of an acutely hospitalized population affected by COVID-19.
Materials and methods
Population and study design
This study was conducted as a single-institution retrospective longitudinal study and was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Guidelines. It was approved by the local ethical board (“Area Vasta Pavia” Bioethics Committee, protocol number: 20200069920 - date of approval: 05/08/2020) and was drawn up in accordance with the current version of the World Medical Association Declaration of Helsinki (2013). For informed consent to study participation we refer to the consent that all patients sign upon admission to the ICU or MCU ward, as approved by the bioethics committee. The trial was carried out in accordance with the standards of good clinical practice.
Data were collected, from March to June 2020, on subjects admitted to COVID Units of the IRCCS Policlinico San Matteo Foundation (Pavia, Lombardia, Italy) with a diagnosis of COVID-19 related pneumonia. COVID Units included Intensive Care Units (ICU) and Medical Care Units (MCU). As soon as the patients were considered clinically stable, they were evaluated for inclusion in an assisted rehabilitation program (see procedures for details) by a Physical and Rehabilitation Medicine (PRM) specialized physician both in ICU and in MCU. Inclusion criteria were: age 18 or older and a diagnosis of COVID-19 related pneumonia. ICU patients were included with a diagnosis of COVID-19 related pneumonia with ARDS (in phase of weaning from mechanical ventilation). Exclusion criteria were a previous diagnosis of neuromuscular diseases and general contraindications to mobilization (cardiorespiratory or neurological instability, need for Extra Corporeal Membrane Oxygenation assistance).
Procedures
As part of the recruitment procedure, general information was collected on basic demographics and the burden of previous clinical history was determined using the age-adjusted Charlson Comorbidity Index.21, 22 The PRM clinical evaluation included sensorium, general compliance, active and passive motility of all four limbs, sitting/standing position and gait (if possible). We specifically measured muscle strength in all four limbs, applying the Medical Research Council (MRC) sum-score, one of the most commonly used clinical grading tool to assess global muscle strength.23, 24 The MRC sum-score is the summation of the strength of six muscle groups (arm abduction, elbow flexion, wrist extension, hip flexion, knee extension and ankle dorsiflexion), bilaterally tested according to the MRC scale.25 The sum-score ranges from 0 to 60 points, the range 0-36 indicating severe muscular weakness, the range 36-48 indicating significant muscular weakness.23, 24 The functional evaluation (see outcome section for details) was performed at the first PRM evaluation (T0) and at the discharge from hospital (T1); for subjects enrolled in ICU and then transferred to MCU, the functional status and the MRC sum-score were also assessed at the discharge from ICU. We also registered the total duration of hospital stay, the length of stay in ICU and/or MCU, the length of the rehabilitative treatment (expressed as number of sessions of physiotherapy) and the destination at the discharge.
The early rehabilitation project had two aims: the maintenance (or recovery) of the range of motion and of the strength and the recovery of sitting/standing position and gait. The exercise program involved daily a 30-minute session assisted by a physiotherapist. In Table I, II we reported the stages of the rehabilitation program we carried out in ICU and MCU respectively.
Table I. —Rehabilitation protocol in Intensive Care Covid Units.
| Intensive Care Units rehabilitation program 1- Postural alignment in bed of the head, neck, trunk, shoulder and pelvic girdle; 2- Passive/active assisted range of motion exercises of the upper and lower limbs. Selective exercises on the shoulder girdle musculature without involving the pelvic girdle, and vice versa; 3- If the patient is intubated via tracheostomy, passive mobilization of the cervical spine; awareness of the oral cavity by mobilizing the tongue and the temporomandibular joint; 4- Active exercises to strengthen the muscles of the upper and lower limbs 5- Balance bed exercises of the trunk, shoulder and pelvic girdle in the sitting position in bed; gradual recovery of the sitting position at the edge of the bed; training in the upright standing position and gradual recovery of the bed-chair transition 6- Reached the standing position, walking training with load transfer and walking on the spot with direct assistance or supervision 7- Cognitive exercise for the tidal volume breathing pattern; sequential breathing exercise Active Cycle of Breathing Techniques |
| Transfer to Medical Care Units 1- Continuation of the motor rehabilitation program performed in ICU until ambulation is recovered 2- Sequential breathing exercise Active Cycle of Breathing Techniques, breathing exercises in a sitting position, incentive spirometers using volume - oriented device. |
Table II. —Rehabilitation protocol in Medical Care Covid Units.
| 1- Passive/active assisted range of motion exercises of the upper and lower limbs; exercise for cervical spine 2- Active exercises to strengthen the muscles of the upper and lower limbs 3- Balance bed exercises of the trunk, shoulder and pelvic girdle in the sitting position in bed; gradual recovery of the sitting position at the edge of the bed, training in the upright standing position and gradual recovery of the bed-chair transition 4- Reached the standing position, walking training with load transfer and walking on the spot with direct assistance or supervision until ambulation is recovered 5- Sequential breathing exercise Active Cycle of Breathing Techniques, breathing exercises in a sitting position, incentive spirometers using volume - oriented device. |
Outcome assessment
As primary outcome, the functional status was assessed using the 3-item Barthel index (BI-3) and the General Physical Mobility Score (GPMS). The Barthel Index is a worldwide known standard measure of functional ability.26 Information on functional status is essential for assessing rehabilitation practice, but during COVID-19 spreading, it was impractical to obtain a full assessment in the acute units, so we chose the BI-3, a simplified and validated alternative to the full Barthel score, based on three items only (transfers, walking, bladder control). The BI-3 is reported to predict total Barthel Index score in around 90% of cases.27 The GPMS was instead created as a new tool, made up of single item, in order to assess in more detail than the item “transfers” of the BI-3 the maximum grade of ability in autonomously performing transfers (Table III).
Table III. —General Physical Mobility Score (GPMS).
| 0= patient confined to bed 1= patient performs movements on the bed 2= patient reaches the sitting position on the edge of the bed 3= patient reaches the upright position 4= patient walks with assistance 5= patient walk independently |
As secondary outcomes, we assessed variations of MRC sum-score in the ICU subgroup of patients at the first PRM evaluation (T0) and at the transfer to MCU. Moreover, we assessed differences in terms of length of hospital stay and functional status at discharge between patients admitted in ICU and those admitted in MCU. Finally, we evaluated the impact of functional status on the destination at discharge.
Statistical analysis
The Shapiro-Wilk Test was used to test the normal distribution of quantitative variables. If normally distributed, the results were expressed as mean and standard deviation (SD), otherwise median and interquartile range (IQR: 25-75th percentiles); qualitative variables were summarized as counts and percentages.
Functional outcomes (BI-3 and GPMS) are compared between admission and discharge (separately in ICU and MCU patients) with paired t-test.
Multivariate linear regression models were fitted to evaluate the effect of the length of the rehabilitative treatment on improvement of functional outcome (BI-3 and GPMS); covariate were sex and age-adjusted Charlson Index and being admitted to ICU. Results are expressed as coefficient with their 95% confidence interval (CI) and presented with term specific P values; the coefficient represents the mean variation of outcomes for unit change of quantitative predictors or between levels of categorical or ordinal predictors.
For MRC sum score multi level linear regression with patients as random factors are fitted to evaluate the effect of the length of the rehabilitative treatment on improvement of this functional outcome covariate were sex and age-adjusted Charlson Index. Results are expressed as coefficient with their 95% CI and presented with term specific P-values; the coefficient represents the mean variation of outcomes for unit change of quantitative predictors or between levels of categorical or ordinal predictors.
For MRC sum score categories multi level ordinal logistic regression with patients as random factors are fitted to evaluate the effect of the length of the rehabilitative treatment on improvement of this functional outcome covariate were sex and age-adjusted Charlson Index. Results are expressed as as odds ratio (OR) with their 95% CI and presented with term specific P values.
Multivariate logistic regression models are fitted to evaluate effect of improvement of functional outcome on discharge at home (covariate sex age-adjusted Charlson Index). Results are expressed as OR with their 95% CI and presented with term specific P values.
P values <0.05 were considered to be statistically significant. Data analysis was performed with STATA statistical package (release 16.1, 2013, Stata Corporation, College Station, TX, USA).
Results
Descriptive data
General characteristics of the population at the first PRM evaluation are described in Table IV.
Table IV. —Demographic, anamnestic ad functional characteristics of the population.
| Sample (N.) | ICU subgroup (68) |
MCU subgroup (48) |
All patients (116) |
|---|---|---|---|
| Age, years (SD) | 60 (10) | 73 (9) | 65 (11) |
| Sex, male (%) | 51 (75) | 25 (52) | 76 (65) |
| Charlson Comorbidity Index (SD) | 1 (0-2) | 1.5 (1-2) | 1 (0-2) |
| BI-3 median (range) | 0 (0-2) | 0 (0-7) | 0 (0-7) |
| GPMS median (range) | 0 (0-2) | 1 (0-4) | 0 (0-4) |
| MRC sum-score (SD) | 23 (12) | 41 (8) | 31 (13) |
Value are expressed as means±SD and as median (IQR) for continous data and counts (percentage) for categorica data. ICU: Intensive Care Unit; MCU: Medical Care Unit; BI-3: 3-item Barthel Index; GPMS: General Physical Mobility Score; MRC sum-score: Medical Research Council sum score.
The rehabilitative program was generally well tolerated by the patients. In subjects diagnosed for thrombosis or pulmonary embolism after the beginning of the rehabilitation protocol, assisted physiotherapy was stopped and started again 48 hours after the beginning of a proper anticoagulant therapy. Two subjects presented hematomas, respectively located at the thigh and at the iliopsoas, but they regularly followed the protocol.
In five ICU patients, a critical illness polyneuropathy and miopathy was clinically diagnosed and confirmed by an EMG/ENG examination during the hospital stay. A persistent clinical picture of ICU acquired weakness at discharge from hospital was observed in further thirteen ICU cases. Finally, two subjects presented neurological complications, respectively a Guillain-Barré Syndrome and a facial nerve paralysis.
Primary outcome
The BI-3 total score and the GPMS showed a statistically significant improvement in both ICU (P<0.001) and MCU (P<0.001) subgroups of patients, as reported in Figure 1, 2 respectively.
Figure 1.
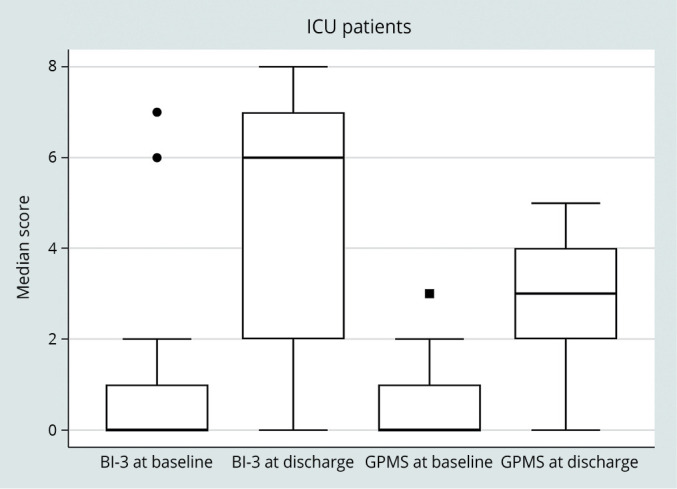
—BI-3 total score and GPMS score at baseline and at discarge in ICU patients.
Figure 2.
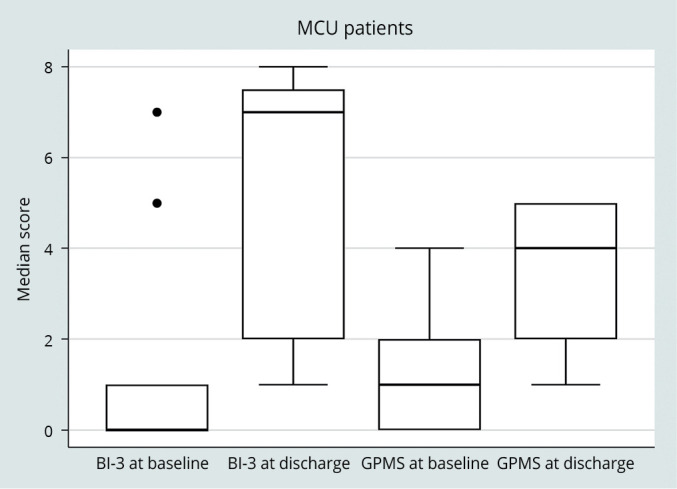
—BI-3 total score and GPMS score at baseline and at discarge in MCU patients.
Regarding the regression analysis, the length of the rehabilitative treatment (BI-3 beta 0.07 for 1 day increase 95%CI 0.01-0.12 P=0.014; GPMS beta 0.03 95%CI 0.01-0.06 P≥0.015) and the age-adjusted Charlson Index (P<0.001) are significantly positively and negatively correlated to the improvement of functional outcome.
Secondary outcome
Data about monitoring of MRC sum-score in ICU patients are summarized in Figure 3.
Figure 3.
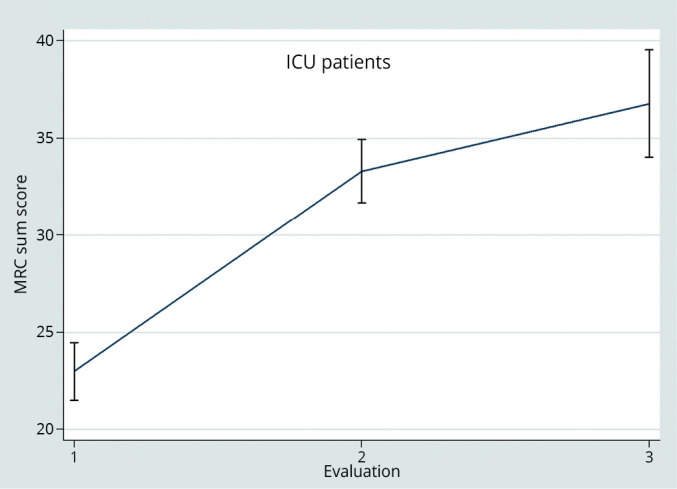
—Monitoring of MRC sum score in ICU patients.
MRC sum-score significantly improved in ICU (P<0.001). For what regards the regression analysis, the length of the rehabilitative treatment (beta 0.29 for 1 day increase; 95%CI 0.06-0.53 P=0.012) is also significantly correlated to the improvement of muscular strength. On the contrary, the MRC sum-score variation resulted negatively affected by the burden of comorbidity, the coefficient value decreasing by -3,7 points as the age-adjusted Charlson Index point-by-point increase. Analysing the MRC sum-score by categories (0-36, 36-48, 48-60), results about the influence of the cited variables did not change (Figure 4).
Figure 4.
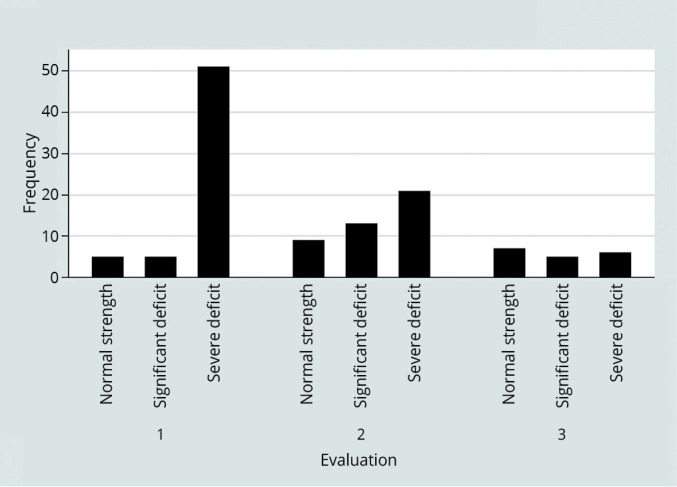
—Monitoring of MRC sum score categories in ICU patients.
Total duration of the hospital stay, the length of stay in ICU and/or MCU, the length of the rehabilitative treatment and the destination at the discharge are listed in Table V.
Table V. —Duration of the hospital stay, length of the rehabilitative treatment, destination at the discharge.
| Sample (N.) | ICU subgroup (68) |
MCU subgroup (48) |
All patients (116) |
|---|---|---|---|
| ICU hospital stay, days median (IQR) (available only for ICU subgroup) | 36 (28-50) | - | - |
| Total Hospital stay, days median (IQR) | 50 (38-67) | 29 (21-37) | 41 (26-57) |
| Rehabilitative treatment session median (IQR) | 12 (9-20) | 6 (4-9) | 10 (5-16) |
| Discharged to Subacute Units (%) | 42 (62) | 12 (27) | 54 (48) |
| Discharged to home (%) | 21 (31) | 33 (73) | 54 (48) |
| Dead (%) | 5 (7) | 0 | 5 (4) |
Value are expressed as median (IQR) for continous data and counts (percentage) for categorica data. ICU: Intensive Care Unit; MCU: Medical Care Unit.
Patients hospitalized in ICU had a significantly longer hospital stay. At discharge, patients admitted to the ICU reach a functional state that is close to that of patients admitted to the MCU. The improvement of functional outcome (BI-3) (OR 1.67 95%CI 1.33-2.10 P<0.001), the age-adjusted Charlson Index (P<0.001) and ICU stay (P<0.001) are significantly positively and negatively correlated to discharge to home.
Discussion
The results of this observational study highlight a major impairment of functional status in patients with severe SARS-CoV-2 infection that required hospitalization, however with a significant functional improvement at discharge the degree of the improvement being correlated to comorbidity and to the length of the rehabilitative treatment.
Our early, structured and assisted rehabilitation protocol was feasible, tailored to the patient and continued throughout the hospital stay.
As reported by Curci et al., postacute COVID-19 patients admitted to the Rehabilitation Unit from ICU presented a severe disability in terms of pulmonary function and motor impairment for which an early rehabilitation treatment adapted to the clinical conditions becomes necessary. The need of rehabilitation care already in the acute stages of COVID-19 disease is reported in the scientific literature that has grown with the spread of the COVID-19 pandemic.28 McWilliams et al. report that rehabilitation protocols implemented in intensive care on patients with stabilized clinical picture lead to increased levels of mobility before ICU discharge.29
Despite the severity of the COVID-19 and the geriatric average age (65 years) of the sample, 48% of the patients were discharged home. Despite patients hospitalized in ICU resulted to have a significantly longer hospital stay and a major exposure to prolonged immobility, we observed in this subgroup of patients a significant and progressive improvement of MRC sum-score, with a changeover from a severe muscular weakness (0-36) to a significant muscular weakness (36-48) during the rehabilitative treatment.
During the COVID-19 pandemic outbreak in March 2020, the urge to provide a rehabilitative support to an increasing number of patients and lack of scientific evidence about the rehabilitative treatment of subject affected by COVID-19 infection, led us to plan, in a short space of time, a rehabilitative program based on our skills and previous clinical experience in treating acutely hospitalized patients.16, 17, 30-34
Subjects hospitalized in ICU because of a SARS-CoV-2 infection usually show a respiratory impairment similar to patients affected by ARDS of different etiologies, the typical restrictive pulmonary picture requiring respiratory support and, in some cases, bringing to delayed weaning from mechanical ventilation and to prolonged hospitalization in intensive wards. As direct consequence, we observed a progressive and diffuse muscular hypotrophy, with concomitant weakness to the trunk and the four limbs, sometimes leading to the most severe picture of the critical illness polyneuromyopathy. Moreover, prolonged mechanical ventilation cause diaphragm muscle atrophy with concomitant hyposthenia and further lengthening of the weaning procedures.35 The infection related inflammatory state, the multiorgan involvement and the use of sedatives and neuromuscular blocking drugs worsen, in turn, muscular weakness into a complex motor impairment, requiring a rehabilitative intervention, aimed to recover muscle strength, sitting/standing position and gait. Considering that the loss of muscle strength is greatest in the first week of immobility,36 the rehabilitative intervention should begin as early as possible. Moreover patients with COVID-19 demonstrated improved mobility at hospital discharge and higher probability of discharging home with increased frequency and longer mean duration of physical therapy treatment.37
Patients hospitalized in MCU also showed a partial loss of muscle strength, caused by the concomitant action of inflammatory state, hypoxemia and stay in isolation wards. Besides, evidences from literature clearly reported a reduction of general mobility during hospital stay for any reason.38 On that basis, the beginning of the rehabilitative treatment should be early and adaptable to the rapid evolution of clinical conditions in MCU too. Moreover, we suggest to educate patients to repeat active exercises during day time and to preserve their functional skills (“transfers” and activities of daily living), depending on the need to stay in isolation wards. The learning process of a rehabilitative program during the hospital stay allows patients discharged home to carry on their exercises in order to complete their functional recovery.
Analyzing MRC sum-score data, patients hospitalized in ICU showed a severe muscular weakness (0-36); those hospitalized in MCU showed instead a significant muscular weakness (36-48). Despite patients hospitalized in ICU resulted to have a significantly longer hospital stay and a major exposure to immobility, we observed in this subgroup of patients a significant and progressive improvement of muscular strength during the rehabilitative treatment, as a proof that an early participation in a rehabilitative program is helpful in limiting the sequelae of prolonged immobility and in facilitating functional recovery, as showed by the improvement of BI-3 and GPMS total score. The GPMS resulted to be an easy-to-fill in tool, useful in assessing patients’ skills to perform “transfers” and residual functional needs.
The discharge from hospital to home is considered an optimal indicator of functional recovery: in the present study, despite the geriatric age of the sample, 48% of the patients were discharged to home. In the subgroup hospitalized in MCU, the percentage of patients discharged to home resulted to be 73%, in spite of a major number of comorbidities and average age (73 years).
Limitations of the study
The present study has several limitations. For ethical reasons we could not plan a placebo-controlled trial with a control group (not performing rehabilitation), since we enrolled subjects with COVID-19 related functional limitation during an emergency time. Consequently, we couldn’t demonstrate the full efficacy of the intervention on the observed functional evolution, in particular on muscle recovery and on discharge at home.
The lack of a longer follow-up prevented further consideration about long-lasting functional limitations in patients affected by COVID-19 related pneumonia. We also have to specify that we chose the 3-item Barthel Index and the GPMS to assess function for practical reasons only (in an emergency setting). Further studies, with a controlled design and with a more exhaustive functional evaluation are certainly justified to better understand the efficacy of rehabilitation in this context.
Conclusions
In patients affected by COVID-19 related pneumonia the respiratory impairment is often associated to a progressive muscular weakness with a concomitant loss of function. An early rehabilitation treatment, tailored to the clinical setting and adaptable to the rapid evolution of clinical conditions is desirable to be applied in acute patients with SARS-CoV-2 infection.
References
- 1.European Centre for Disease Prevention and Control (ECDC). Download todays data on the geographic distribution of Covid-19 cases worldwide (April 22nd, 2020); 2020 [Internet]. Available from: www. Ecdc. Europa. eu/en/publications-data/download-todays-data-geographic-distribution-covid-19-cases worldwide [cited 2020, Jul 20].
- 2.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32113704&dopt=Abstract 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:562–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28792873&dopt=Abstract 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant 2020;39:405–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32362390&dopt=Abstract 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020;383:1757–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32329974&dopt=Abstract 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 6.Ayres JS. A metabolic handbook for the COVID-19 pandemic. Nat Metab 2020;2:572–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32694793&dopt=Abstract 10.1038/s42255-020-0237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32437596&dopt=Abstract 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. Sars-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32142651&dopt=Abstract 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network . Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32250385&dopt=Abstract 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020;116:1666–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32352535&dopt=Abstract 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014;370:1626–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24758618&dopt=Abstract 10.1056/NEJMra1209390 [DOI] [PubMed] [Google Scholar]
- 12.Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun 2015;6:10145. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26666572&dopt=Abstract 10.1038/ncomms10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 2020;26:1618–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32197060&dopt=Abstract 10.3201/eid2607.200445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. Canadian Critical Care Trials Group . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12594312&dopt=Abstract 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- 15.Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, et al. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med 2003;31:1012–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12682465&dopt=Abstract 10.1097/01.CCM.0000053651.38421.D9 [DOI] [PubMed] [Google Scholar]
- 16.Gosselink R, Bott J, Johnson M, Dean E, Nava S, Norrenberg M, et al. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med 2008;34:1188–99. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18283429&dopt=Abstract 10.1007/s00134-008-1026-7 [DOI] [PubMed] [Google Scholar]
- 17.Green M, Marzano V, Leditschke IA, Mitchell I, Bissett B. Mobilization of intensive care patients: a multidisciplinary practical guide for clinicians. J Multidiscip Healthc 2016;9:247–56. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27307746&dopt=Abstract 10.2147/JMDH.S99811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care 2014;18:658. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25475522&dopt=Abstract 10.1186/s13054-014-0658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Sire A, Andrenelli E, Negrini F, Patrini M, Lazzarini SG, Ceravolo MG, International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER Action . Rehabilitation and COVID-19: a rapid living systematic review by Cochrane Rehabilitation Field updated as of December 31st, 2020 and synthesis of the scientific literature of 2020. Eur J Phys Rehabil Med 2021;57:181–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33599442&dopt=Abstract 10.23736/S1973-9087.21.06870-2 [DOI] [PubMed] [Google Scholar]
- 20.Ceravolo MG, Arienti C, de Sire A, Andrenelli E, Negrini F, Lazzarini SG, et al. ; International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER action. Rehabilitation and COVID-19: the Cochrane Rehabilitation 2020 rapid living systematic review. Eur J Phys Rehabil Med 2020;56:642–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32705860&dopt=Abstract 10.23736/S1973-9087.20.06501-6 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3558716&dopt=Abstract 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22.Frenkel WJ, Jongerius EJ, Mandjes-van Uitert MJ, van Munster BC, de Rooij SE. Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: a prospective cohort study. J Am Geriatr Soc 2014;62:342–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24521366&dopt=Abstract 10.1111/jgs.12635 [DOI] [PubMed] [Google Scholar]
- 23.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991;14:1103–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1745285&dopt=Abstract 10.1002/mus.880141111 [DOI] [PubMed] [Google Scholar]
- 24.Sommers J, Vredeveld T, Lindeboom R, Nollet F, Engelbert RH, van der Schaaf M. de Morton Mobility Index is feasible, reliable, and valid in patients with critical illness. Phys Ther 2016;96:1658–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27081202&dopt=Abstract 10.2522/ptj.20150339 [DOI] [PubMed] [Google Scholar]
- 25.Medical research Council. Aids to the examination of the peripheral nervous system, Memorandum no 45. London: Her Majesty’s Stationery Office; 1981.
- 26.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965;14:61–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14258950&dopt=Abstract [PubMed] [Google Scholar]
- 27.Ellul J, Watkins C, Barer D. Estimating total Barthel scores from just three items: the European Stroke Database ‘minimum dataset’ for assessing functional status at discharge from hospital. Age Ageing 1998;27:115–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16296670&dopt=Abstract 10.1093/ageing/27.2.115 [DOI] [PubMed] [Google Scholar]
- 28.Curci C, Pisano F, Bonacci E, Camozzi DM, Ceravolo C, Bergonzi R, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehabil Med 2020;56:633–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32667150&dopt=Abstract 10.23736/S1973-9087.20.06339-X [DOI] [PubMed] [Google Scholar]
- 29.McWilliams D, Weblin J, Hodson J, Veenith T, Whitehouse T, Snelson C, Queen Elizabeth Hospital Birmingham COVID-19 Research Team . Rehabilitation Levels in Patients with COVID-19 Admitted to Intensive Care Requiring Invasive Ventilation. An Observational Study. Ann Am Thorac Soc 2021;18:122–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32915072&dopt=Abstract 10.1513/AnnalsATS.202005-560OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrucci L, Monteleone S, Ricotti S, Giromini E, Gullace M, Ambrosini E, et al. Disability after major abdominal surgery: determinants of recovery of walking ability in elderly patients. Eur J Phys Rehabil Med 2018;54:683–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29898583&dopt=Abstract 10.23736/S1973-9087.18.04348-4 [DOI] [PubMed] [Google Scholar]
- 31.Sala V, Petrucci L, Monteleone S, Dall’Angelo A, Miracca S, Conte T, et al. Oxygen saturation and heart rate monitoring during a single session of early rehabilitation after cardiac surgery. Eur J Phys Rehabil Med 2016;52:12–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26220328&dopt=Abstract [PubMed] [Google Scholar]
- 32.Petrucci L, Carlisi E, Ricotti S, Zanellato S, Klersy C, D’Armini AM, et al. Functional assessment and quality of life before and after pulmonary endoarterectomy. G Ital Med Lav Ergon 2015;37:170–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26749979&dopt=Abstract [PubMed] [Google Scholar]
- 33.Petrucci L, Ramella FC, Ricotti S, Carlisi E, Di Natali G, Messina S, et al. Early rehabilitative treatment in aortocoronary bypass surgery. G Ital Med Lav Ergon 2013;35:125–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23914605&dopt=Abstract [PubMed] [Google Scholar]
- 34.Jang MH, Shin MJ, Shin YB. Pulmonary and Physical Rehabilitation in Critically Ill Patients. Acute Crit Care 2019;34:1–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31723900&dopt=Abstract 10.4266/acc.2019.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18367735&dopt=Abstract 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]
- 36.Rochester CL. Rehabilitation in the intensive care unit. Semin Respir Crit Care Med 2009;30:656–69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19941223&dopt=Abstract 10.1055/s-0029-1242635 [DOI] [PubMed] [Google Scholar]
- 37.Johnson JK. lapin B, Green K, Stilphen M. Frequency of physical therapist intervention is associated with mobility status and disposition at hospital discharge for patients with COVID-19. Phys Ther 2021;101:1–8. 10.1093/ptj/pzaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim SH, Ang SY, Ong HK, Lee TZ, Lee TX, Luo EZ, et al. Promotion of mobility among hospitalised older adults: an exploratory study on perceptions of patients, carers and nurses. Geriatr Nurs 2020;41:608–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32268947&dopt=Abstract 10.1016/j.gerinurse.2020.03.015 [DOI] [PubMed] [Google Scholar]


