Abstract
Ovarian carcinoma is a common malignancy with a grim prognosis and a high mortality rate. Here, we report a rare case of an Iranian woman with four episodes of recurrent metastatic ovarian carcinoma. She was initially diagnosed with stage IVa high-grade serous ovarian adenocarcinoma (HGSOC), treated with paclitaxel-carboplatin and capecitabine, followed by total abdominal hysterectomy and bilateral salpingo-oophorectomy. Two years later, she developed cerebellar metastasis and received whole-brain radiotherapy and paclitaxel-carboplatin. Eighteen months later, she had peritoneal metastasis and had sequential gemcitabine-carboplatin-paclitaxel. One year later, she had splenic metastasis, treated with splenectomy and adjuvant carboplatin and nano-albumin bond paclitaxel. The patient remains in remission until now, 11 months after completing the most recent regimen. This report emphasizes the potential to successfully use chemoradiotherapy with sequential courses of platinum-based agents in patients with recurrent metastatic HGSOC.
Keywords: Adjuvant chemotherapy, carbotaxol, high-grade serous ovarian adenocarcinoma, ovarian cancer, ovarian cancer brain metastasis, whole-brain radiotherapy
Ovarian carcinoma is the second most common malignancy of the female reproductive system in the United States, with epithelial ovarian carcinoma (EOC) accounting for >90% of all ovarian carcinoma.1 Despite rapid advances in cancer treatments, EOC continues to have a grim prognosis.2–4 Here, we report a rare case of an Iranian woman with poorly differentiated EOC with four relapses successfully treated with paclitaxel, carboplatin, capecitabine, gemcitabine, and nano-albumin bond paclitaxel.
CASE DESCRIPTION
A 62-year-old Iranian woman presented with progressively enlarging supraclavicular lymphadenopathy. She had no known medical history and was not taking any medications. Ultrasound revealed the involvement of cervical lymph nodes levels III, IV, and VI. Her serum tumor markers revealed a normal α-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen (CA) 19-9 but elevated CA 15-3, CA 125, and human epididymis protein-4. An excisional biopsy of the right cervical lymph node revealed neoplastic cells with variable-sized vesicular nuclei and micropapillary features. Immunohistochemistry was positive for CK7, CK20, CA125, and WT1 and negative for GATA3, TTF1, and CDX2, consistent with poorly differentiated high-grade serous ovarian adenocarcinoma (HGSOC). Vaginal ultrasound revealed right ovarian enlargement, while computed tomography (CT) revealed diffuse abdominal lymphadenopathy. The patient was diagnosed with stage IVa HGSOC. She received six cycles of paclitaxel-carboplatin, followed by 6 months of capecitabine. She subsequently had a total abdominal hysterectomy and bilateral salpingo-oophorectomy and achieved complete clinical response.
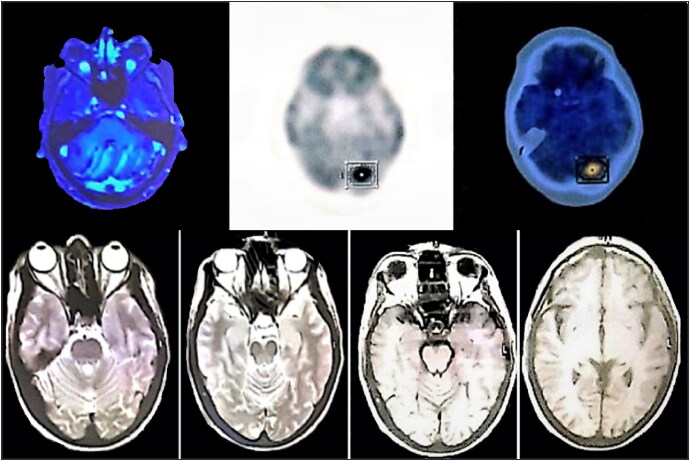
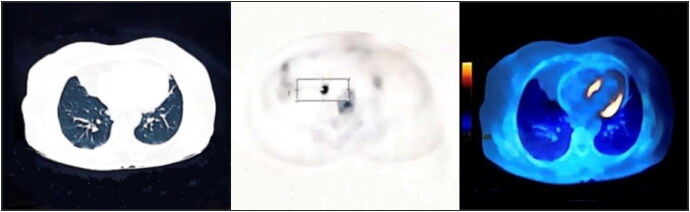
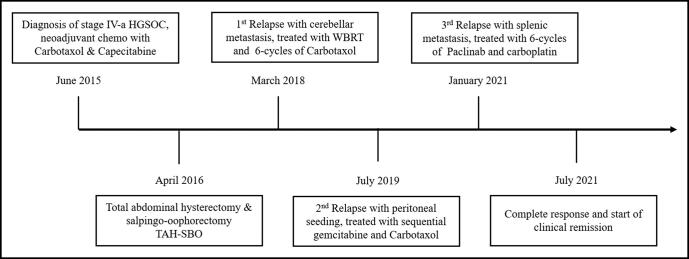
Approximately 18 months later, the patient presented with an ataxic gait. Brain magnetic resonance imaging (MRI) revealed multiple metastatic lesions in the pons and the cerebellum (Figure 1a). The patient was diagnosed with recurrent HGSOC with brain metastasis and had whole-brain radiotherapy and six cycles of paclitaxel-carboplatin. She achieved a complete response with no residual lesions on her brain MRI (Figure 1b). Approximately 18 months later, she presented again with abdominal lymphadenopathy and elevated tumor markers. Her positron emission tomography–CT scan revealed multifocal peritoneal seeding and hypermetabolic lymph nodes (Figure 2). The lymph node excisional biopsy revealed recurrent HGSOC. The patient had chemotherapy with six cycles of sequential gemcitabine-carboplatin-paclitaxel and had a complete response. One year later, the patient again presented with elevated tumor markers. Her abdominopelvic CT revealed a splenic lesion, suspicious for recurrent HGSOC. She had a splenectomy, followed by six cycles of nano-albumin bond paclitaxel and carboplatin. The patient achieved a complete response and has remained in remission for the past year. The clinical timeline of the patient’s diagnosis until her most recent treatment is shown in Figure 3.
Figure 1.
Top: Brain MRI showing several metastatic lesions in the pons and the cerebellum. Bottom: Posttreatment brain MRI showing no evidence of residual tumor.
Figure 2.
The patient’s positron emission tomography/CT scan showing multifocal hypermetabolic intraperitoneal metastatic seeding with hypermetabolic lymph nodes, with small sized bilateral axillary adenopathy with mild metabolic activity, most likely reactive in nature (maximum standardized uptake up to 2.3).
Figure 3.
Clinical timeline from the patient’s initial diagnosis of metastatic ovarian carcinoma until her most recent treatment and remission. HGSOC indicates high-grade serous ovarian adenocarcinoma; WBRT, whole-brain radiotherapy.
DISCUSSION
Ovarian carcinoma is the second most commonly diagnosed reproductive tract cancer, with a lifetime risk of 1.1%; it accounts for 2.1% of all cancer-related deaths, as >75% of patients present at an advanced disease stage.1,2 Despite initial response to treatment, nearly 70% of the patients with advanced ovarian carcinoma develop recurrent disease within 18 to 24 months.3 Risk factors include nulliparity, hormone replacement therapy, pelvic inflammatory disease, and mutations in the BRCA1, BRCA2, or mismatch repair genes MSH2, MLH1, and MSH6.4–7
EOC is often diagnosed in the advanced stages of the disease due to the ovaries’ location and the biological behavior of epithelial tumors.8 The recommended workup for patients includes abdominopelvic ultrasound followed by CT or MRI.9,10 The serum tumor markers for EOC diagnosis include CA 19-9, CA 125, carcinoembryonic antigen, α-fetoprotein, β-human chorionic gonadotropin, and lactate dehydrogenase, and occasionally CA 15-3 and human epididymis protein-4.11–13 The most common sites of EOC dissemination are the peritoneal cavity, abdominal or distant lymph nodes, colon, mediastinum, and hepatobiliary system.14 EOC metastasis to the central nervous system (CNS) is rare, with an incidence rate as low as 0.3%.15–17 Treatment for CNS metastasis often includes palliative chemotherapy with platinum agents.18 However, CNS metastasis portends a poor prognosis, with the median overall survival ranging from 3 to 15 months.17 In contrast, our patient has been in remission for 3 years after CNS metastasis. This highlights the potential for whole-brain radiotherapy and adjuvant chemotherapy using high-dose paclitaxel-carboplatin to successfully treat recurrent EOC with brain metastasis.
A multimodal therapeutic approach is used to treat metastatic EOC, combining chemoradiotherapy and cytoreductive surgical resection.4,19 Systemic chemotherapy regimens include a combination of fluoropyrimidines, platinum agents, taxanes, irinotecan, gemcitabine, and doxorubicin.20–22 One of the preferred chemotherapy regimens is a combination of fluoropyrimidines or taxanes and platinum agents.9,19–21 Patients with recurrent disease after 6 months of platinum therapy are considered to be platinum sensitive and may be treated again with paclitaxel and carboplatin, while patients with disease relapse <6 months after remission are considered platinum resistant and treated with other regimens (i.e., cyclophosphamide, irinotecan, or doxorubicin).23,24 Despite aggressive therapy, the 5-year survival in advanced EOC is <30% due to disease relapse and chemoresistance.3,4 This report emphasizes the potential to successfully use multiple courses of platinum-based agents in patients with recurrent metastatic HGSOC.
Disclosure statement/Funding
The authors report no funding or conflicts of interest. Informed consent was obtained from the patient for publication of this case.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A.. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Jelovac D, Armstrong DK.. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61(3):183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lheureux S, Gourley C, Vergote I, Oza AM.. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 5.Gaitskell V, Hermon K, Beral C, et al; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385(9980):1835–1842. doi: 10.1016/S0140-6736(14)61687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol. 2016;34(24):2888–2898. doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jervis S, Song H, Lee A, et al. Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. J Med Genet. 2014;51(2):108–113. doi: 10.1136/jmedgenet-2013-102015. [DOI] [PubMed] [Google Scholar]
- 8.Schorge JO, Modesitt SC, Coleman RL, et al. SGO white paper on ovarian cancer: etiology, screening and surveillance. Gynecol Oncol. 2010;119(1):7–17. doi: 10.1016/j.ygyno.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 10.Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Management of a suspicious adnexal mass: a clinical practice guideline. Curr Oncol. 2012;19(4):e244–e257. doi: 10.3747/co.19.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadowski EA, Rockall AG, Maturen KE, Robbins JB, Thomassin-Naggara I.. Adnexal lesions: imaging strategies for ultrasound and MR imaging. Diagn Interv Imaging. 2019;100(10):635–646. doi: 10.1016/j.diii.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Anthoulakis C, Nikoloudis N.. Pelvic MRI as the “gold standard” in the subsequent evaluation of ultrasound-indeterminate adnexal lesions: a systematic review. Gynecol Oncol. 2014;132(3):661–668. doi: 10.1016/j.ygyno.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Schummer M, Drescher C, Forrest R, et al. Evaluation of ovarian cancer remission markers HE4, MMP7 and Mesothelin by comparison to the established marker CA125. Gynecol Oncol. 2012;125(1):65–69. doi: 10.1016/j.ygyno.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao H, You D, Lan Z, Ye H, Hou M, Xi M.. Prognostic value of serum and tissue HE4 expression in ovarian cancer: a systematic review with meta-analysis of 90 studies. Expert Rev Mol Diagn. 2018;18(4):371–383. doi: 10.1080/14737159.2018.1457436. [DOI] [PubMed] [Google Scholar]
- 15.Teh BH, Yong SL, Sim WW, Lau KB, Suharjono HN.. Evaluation in the predictive value of serum human epididymal protein 4 (HE4), cancer antigen 125 (CA 125) and a combination of both in detecting ovarian malignancy. Horm Mol Biol Clin Investig. 2018;35(1):0029. doi: 10.1515/hmbci-2018-0029. [DOI] [PubMed] [Google Scholar]
- 16.Hogdall EV, Christensen L, Kjaer SK, et al. Protein expression levels of carcinoembryonic antigen (CEA) in Danish ovarian cancer patients: from the Danish ‘MALOVA’ ovarian cancer study. Pathology. 2008;40(5):487–492. doi: 10.1080/00313020802197889. [DOI] [PubMed] [Google Scholar]
- 17.Deng K, Yang C, Tan Q, et al. Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol. 2018;150(3):460–465. doi: 10.1016/j.ygyno.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Pectasides D, Aravantinos G, Fountzilas G, et al. Brain metastases from epithelial ovarian cancer. The Hellenic Cooperative Oncology Group (HeCOG) experience and review of the literature. Anticancer Res. 2005;25(5):3553–3558. [PubMed] [Google Scholar]
- 19.Wohl A, Kimchi G, Korach J, et al. Brain metastases from ovarian carcinoma: an evaluation of prognostic factors and treatment. Neurol India. 2019;67(6):1431–1436. doi: 10.4103/0028-3886.273627. [DOI] [PubMed] [Google Scholar]
- 20.Kim TJ, Song S, Kim CK, et al. Prognostic factors associated with brain metastases from epithelial ovarian carcinoma. Int J Gynecol Cancer. 2007;17(6):1252–1257. doi: 10.1111/j.1525-1438.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 21.Sehouli J, Pietzner K, Harter P, et al. Prognostic role of platinum sensitivity in patients with brain metastases from ovarian cancer: Results of a German multicenter study. Ann Oncol. 2010;21(11):2201–2205. doi: 10.1093/annonc/mdq229. [DOI] [PubMed] [Google Scholar]
- 22.Stopa BM, Cuoco JA, Adhikari S, Grider DJ, Rogers CM, Marvin EA.. Iatrogenic leptomeningeal carcinomatosis following craniotomy for resection of metastatic serous ovarian carcinoma: a systematic literature review and case report. Front Surg. 2022;9:850050. doi: 10.3389/fsurg.2022.850050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J, Sun X, Zhou J.. Insights into the role of mesothelin as a diagnostic and therapeutic target in ovarian carcinoma. Front Oncol. 2020;10:1263. doi: 10.3389/fonc.2020.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber EL, Zsiros E, Lurain JR, Rademaker A, Schink JC, Neubauer NL.. The combination of intravenous bevacizumab and metronomic oral cyclophosphamide is an effective regimen for platinum-resistant recurrent ovarian cancer. J Gynecol Oncol. 2013;24(3):258–264. doi: 10.3802/jgo.2013.24.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]