ABSTRACT
Ferroptosis has gained interest due to it immunogenicity and the higher sensitivity of cancer cells to it. However, it was recently shown that ferroptosis in tumor-associated neutrophils leads to immunosuppression and negatively impacts therapy. Here, we discuss the potential implications of the two sides (friend versus foe) of ferroptosis in cancer immunotherapy.
KEYWORDS: Immunogenic cell death, immunogenic ferroptosis, cancer immunosuppression
Cell death is an indispensable part of life since it is involved in various aspects of development, homeostasis and pathophysiological processes. For a long time, cell death had been classified dichotomously, with only two ways for a cell to die: by regulated apoptosis or by uncontrolled necrosis. However, during the last decades, several cell death modalities have been identified and classified, according to the Nomenclature Committee on Cell Death1, as accidental cell death (necrosis) or as regulated cell death (RCD), which includes ferroptosis.
Ferroptosis, first identified in 2012 by Brent Stockwell,2 is a non-apoptotic form of RCD driven by the iron-dependent accumulation of lipid reactive oxygen species and lipid peroxidation-mediated membrane damage. It results from a redox imbalance between the production of oxidants and antioxidants due to the abnormal expression and activity of multiple redox-active enzymes that produce or remove free radicals and lipid oxidation products.3,4
Since cancer cells have a high demand for iron uptake, which renders them vulnerable to ferroptosis induction, ferroptosis has recently gained widespread interest due to its therapeutically favorable role in cancer treatment. In this regard, it has been shown that early ferroptosis of cancer cells can be accompanied by signs of immunogenic cell death (ICD), such as emission of damage-associated molecular patterns (DAMPs), including ATP and HMGB1, which mediate the activation and maturation of dendritic cells and induce a strong anti-tumor immunity.5,6 The immunogenicity of ferroptotic cancer cells was further confirmed and other DAMPs required for ferroptotic immunogenicity (i.e. decorin) have been identified.7 However, it was recently demonstrated that ferroptosis in tumor-associated neutrophils is linked to cancer immunosuppression.8
The balance between immunogenic ferroptosis and immunosuppressive ferroptosis remains an open question. It was shown that ferroptosis induced by RSL3 in pathologically activated neutrophils (PMNs), named myeloid-derived suppressor cells (PMN-MDSCs), might have the opposite effect and induce cancer immunosuppression8 (Figure 1).
Figure 1.
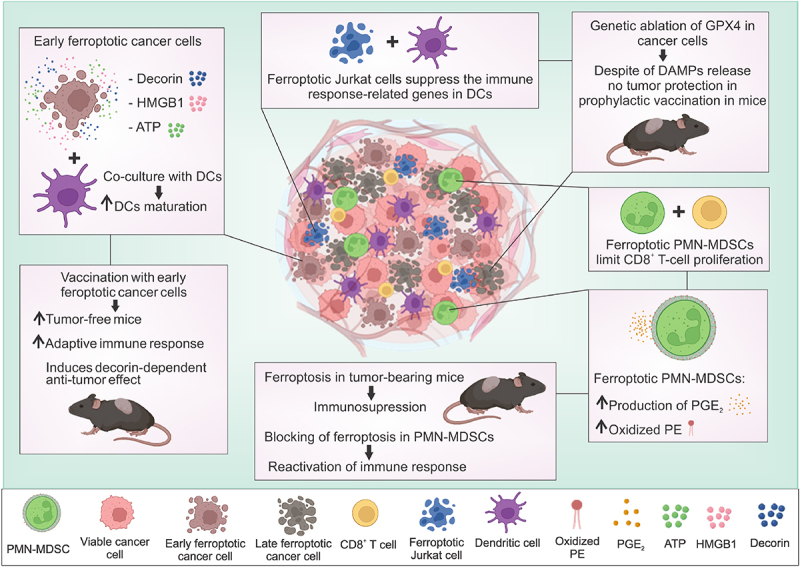
The multiple facets of ferroptosis in the modulation of immune responses in cancer. During ferroptotic cell death, and specifically in the early stages, certain DAMPs (i.e., stimulary DAMPs) are released, such as ATP, HMGB1 and decorin. When ferroptotic cancer cells are co-cultured with dendritic cells (DCs), they induce their activation and maturation (e.g., higher expression of MHCII, CD80 and CD86 markers). Moreover, prophylactic vaccination of mice with early ferroptotic cancer cells results in increased tumor protection and activation of an adaptive immune response. However, it has been shown that ferroptotic cancer cells can also be immunosuppressive under certain experimental conditions. The balance between inhibitory and stimulatory DAMPs during ferroptosis might determine the final immunological effect of ferroptotic cancer cells. Ferroptosis-derived PGE2 might function as an inhibitory DAMP (iDAMP) counteracting the immunostimulatory DAMPs in the context of ferroptosis. Importantly, the oxidative state of DAMPs and antigens during ferroptosis may also be changed, setting up a vicious cycle leading to immunosup-pressive ferroptosis. Tumor PMN-MDSCs have higher levels of oxidized PE in the cell membrane. Induction of ferroptosis in PMN-MDSCs causes the production and release of PGE2, and when ferroptotic PMN-MDSCs are co-cultured with CD8+ T cells, they decrease their proliferation. Moreover, in co-culture, ferroptotic Jurkat cells have been shown to limit the immune response-related genes of DCs. The knockdown of GPX4 in cancer cells induces lipid ROS production and DAMPs (e.g., ATP, HMGB1, CRT) release, however, ferroptotic cancer cells fail to protect mice against tumors in the prophylactic vaccination model. It was reported that blocking ferroptosis with liproxstatin-1 (together with anti-PD-1 therapy) in tumour-bearing mice reactivates the immune response and results in tumor regression.
In particular, tumor-infiltrating neutrophils are more susceptible to ferroptosis compared to peripheral blood neutrophils. Using RNA sequencing, a specific ferroptosis signature was detected in tumor neutrophils isolated from cancer patients but not in peripheral neutrophils, with eight ferroptosis-related genes being significantly upregulated. In addition, only tumor PMN-MDSCs contained higher levels of oxidized phosphatidylethanolamine, which is associated with ferroptosis.4 Compared to peripheral neutrophils, tumor PMN-MDSCs were also more sensitive to RSL3, an inhibitor of glutathione peroxidase 4 and thus an inducer of ferroptosis. These data clearly indicate that tumor-associated PMN-MDSCs undergo ferroptosis.
When PMN-MDSCs were treated with RSL3 for two hours, which is consistent with the condition of early ferroptosis,5 PMN-MDSCs did not gain immunogenic properties. Furthermore, CD8+ T-cell proliferation was reduced if the cells are brought into contact with the RSL3-treated PMN-MDSCs, indicating an immunosuppressive effect (Figure 1). To further confirm the role of ferroptosis in the immunosuppressive nature of PMN-MDSCs, the cells were treated with the ferroptosis inhibitor liproxstatin-1, which did block their immunosuppression. Importantly, this effect was specific to tumor PMN-MDSCs but absent in spleen PMN-MDSCs. It is conceivable that the immunogenic properties of ferroptosis are cell-type dependent and, as such, the tumor microenvironment might play an important role in the modulation of ferroptosis immunogenicity (Figure 1).
Ferroptosis-mediated immunosuppression can be caused by different factors, such as the release of proinflammatory factors or hypoxia. For instance, it was observed that genes involved in the synthesis of prostaglandin E2 (PGE2) were expressed at higher levels, and that inhibition of PGE2 production by blockade of cyclo-oxygenase-1 and -2 reduced the immunosuppressive potential of PMN-MDSCs (Figure 1), indicating the involvement of other molecules in PMN-MDSCs immunosuppression.
Furthermore, lipid species containing esterified PGE2 were found to have an immunosuppressive effect. In particular, these PGE2-containing lipids extracted from RSL3-treated PMN-MDSCs significantly reduced T-cell proliferation, highlighting their role in immunosuppression. The importance of oxidized lipids in ferroptosis is undeniable, but their role in immunomodulation during ferroptosis is not completely clear.4
It has already been shown that hypoxia induces potent immunosuppression mediated by ferroptosis in PMN-MDSCs. Indeed, it has been demonstrated that hypoxia can sensitize cancer cells to ferroptosis via acidosis.9 During acidosis, n-3 and n-6 poly-unsaturated fatty acids (PUFAs) accumulate in lipid droplets, where they can undergo peroxidation leading to cytotoxic effects via ferroptosis, but this has not been linked to immunosuppression. Though little is known about the effect of hypoxia in the induction of ferroptosis in PMN-MDSC, taking these data into account, one can assume that in the hypoxic and acidic tumor microenvironment, PUFAs in PMN-MDSC also accumulate in lipid droplets and undergo peroxidation, which might lead to ferroptosis-associated immunosuppression.
Finally, it has been reported that ferroptosis inhibition can also synergize with anti-PD-1 therapy.8 When tumor-bearing mice were treated with liproxstatin-1 and the immune checkpoint inhibitor against PD-1, the tumors regressed, even in PD-1 resistant cancer types. Increased levels of CD8+ and effector memory T-cells were observed in the lymph nodes, indicating anti-tumor memory formation. Thus, inhibiting ferroptosis of cells of the tumor microenvironment might be an interesting strategy for cancer immunotherapy. These data8 were mainly obtained from murine models. Nevertheless, a negative correlation was observed between high ferroptosis gene levels in PMN-MDSCs and survival, indicating that ferroptosis in PMN-MDSCs decreases patient survival.
The groups led by Dmitry Gabrilovitch and Valerian Kagan8 unraveled the immunosuppressive part of ferroptosis in immune cells. In this context, it has been shown that ferroptotic Jurkat cells (an immortalized line of human T lymphocytes) suppressed the expression of genes associated with the adaptive immune response in dendritic cells, pointing to an immunosuppressive role of ferroptotic immune cells.10 In this work, immunosuppression of ferroptotic cancer cells was also observed, as these cells decreased cross-presentation in dendritic cells.10 A strong increase in lipid peroxidation occurred in the early phase of ferroptosis, which might lead to the oxidation of antigens11 and DAMPs, including HMGB1,12 changing their antigenic and adjuvant potential, respectively, and thus eventually affecting the immunogenicity of ferroptosis.
The data presented by Kim and colleagues suggest a much more complicated view of ferroptosis than previously thought (Figure 1). It is now clear that the immunogenicity of ferroptosis depends not only on the cell death stage5 and experimental conditions (i.e., drug-induced inhibition of GPX45 versus genetic ablation of GPX410), but also on the cell type. It is also conceivable that the immunosuppressive nature of ferroptotic cancer cells10 can be due to a rapid modification of the oxidative state of DAMPs12 and antigens11 leading to modulation of ferroptosis immunogenicity. Moreover, ferroptosis-derived PGE28 may function as an inhibitory DAMP (iDAMP)13counteracting the immunostimulatory DAMPs in the context of ferroptosis. Obviously, knowledge of the precise interplay and balance between immunostimulatory and inhibitory DAMPs during ferroptosis and their functional consequences is limited, and more work is required to understand their role in the immune modulation dictated by ferroptosis in cancer therapy. Before targeted or cell type-specific ferroptosis induction or inhibition might be used in the clinic, it will be interesting to see how ferroptosis affects the other cells in the tumor microenvironment and how they interact with each other. The tight balance between the immunostimulatory (friend) and the immunosuppressive (foe) sides eventually determines the translational potential of ferroptosis for cancer immunotherapy.
Acknowledgments
The Cell Death Investigation and Therapy (CDIT) Laboratory led by DVK is supported by FWO-Flanders (G016221N; G043219N), Ghent University BOF (Special Research Fund; BOF/IOP01/O3618, BOF/IOP/2022/033, and BOF23/GOA/029). This project (40007488) has received funding from the FWO and F.R.S.-FNRS under the Excellence of Science (EOS) program. Robin Demuynck (11E3123N) and Iuliia Efimova (11F7723N) are Ph.D. students in the CDIT Laboratory, and they both hold Ph.D. fellowships from FWO-Flanders.
Funding Statement
This work was supported by the Research Foundation Flanders [11F7721N]; Research Foundation Flanders [11E3121N]; Research Foundation Flanders [The Excellence of Science (EOS) program 40007488]; Research Foundation Flanders [G016221N, G043219N]; Special Research Fund (BOF) of Ghent University [BOF/IOP01/O3618, BOF/IOP/2022/033, BOF23/GOA/029].
Data availability statement
All data that led to the conclusions in this manuscript have been referenced and all sources have been described.
Disclosure statement
Authors declare no conflict of interests.
References
- 1.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–3. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon SJ, Lemberg K, Lamprecht M, Skouta R, Zaitsev E, Gleason C, Patel D, Bauer A, Cantley A, Yang W, et al. Ferroptosis: An Iron-Dependent Form of Non-Apoptotic Cell Death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedmann Angeli JP, Krysko DV, Conrad M.. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 4.Demuynck R, Efimova I, Naessens F, Krysko DV. Immunogenic ferroptosis and where to find it? J Immunother Cancer. 2021;9(e003430):e003430. doi: 10.1136/jitc-2021-003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8(e001369):e001369. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022;23(4):487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Zhu S, Zeng L, Li J, Klionsky DJ, Kroemer G, Jiang J, Tang D, Kang R, et al. DCN released from ferroptotic cells ignites AGER-dependent immune responses. Autophagy. 2021;1–14. doi: 10.1080/15548627.2021.2008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G, Fu S, Sehgal M, Garcia-Gerique L, Kossenkov A, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612(7939):338–346. doi: 10.1038/s41586-022-05443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L, Bastien E, Dessy C, Larondelle Y, Feron O, et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021;33(8):1701–1715.e5. doi: 10.1016/J.CMET.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Wiernicki B, Maschalidi S, Pinney J, Adjemian S, Vanden Berghe T, Ravichandran KS, Vandenabeele P. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun. 2022;13(3676). doi: 10.1038/s41467-022-31218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement CC, Nanaware PP, Yamazaki T, Negroni MP, Ramesh K, Morozova K, Thangaswamy S, Graves A, Kim HJ, Li TW, et al. Pleiotropic consequences of metabolic stress on the major histocompatibility complex class II molecule antigen processing and presentation machinery. Immunity. 2021;S1074-7613(21) 00085–6. doi: 10.1016/j.immuni.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang D, Billiar TR, Lotze MT. Tale of Two Active High Mobility Group Box 1 (HMGB1) Redox States. Mol. Med. 2012;18(10):1360–1362. doi: 10.2119/molmed.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi K, Nikolos F, Lee YC, Jain A, Tsouko E, Gao H, Kasabyan A, Leung HE, Osipov A, Jung SY, et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat. Commun. 2020;11(6299). doi: 10.1038/s41467-020-19970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that led to the conclusions in this manuscript have been referenced and all sources have been described.


