Abstract
Multisystem inflammatory syndrome in children (MIS-C) is a rare progressive inflammatory process temporally associated with exposure to SARS-CoV-2 (COVID-19) in patients 20 years of age and younger. At this time, much of MIS-C is not well understood, including the pathogenesis, long-term implications, and how each variant of the COVID-19 virus affects the progression and severity. We present the unusual case of a 19-year-old man with a history of homozygous sickle cell disease who developed a vaso-occlusive pain crisis and cerebral fat embolism syndrome as a complication of MIS-C secondary to the Omicron variant of COVID-19.
Keywords: Cerebral fat embolism syndrome, COVID-19, MIS-C, Omicron, sickle cell disease
Multisystem inflammatory syndrome in children (MIS-C) is typically characterized by fever, a hyperinflammatory state, and recent COVID-19 exposure or infection.1 The effect of this syndrome on the sickle cell disease population has not been well studied to date. Fat embolism syndrome occurs when globules of fat are released from the bone marrow into the circulatory system, resulting in occlusion. Nontraumatic fat embolism syndrome is known to be a rare complication of sickle cell disease.2 In this report, we describe the first case in the literature of a patient with a history of homozygous sickle cell disease who developed a vaso-occlusive pain crisis and cerebral fat embolism syndrome as a complication of MIS-C secondary to the Omicron variant.
CASE DESCRIPTION
A 19-year-old man with a history of homozygous sickle cell disease was emergently evaluated for symptoms of a vaso-occlusive pain crisis after presenting with fever, tachycardia, tachypnea, hypertension, altered mental status, and a 1-day history of pain in his chest, shoulders, and bilateral knees. Due to concern for acute chest syndrome, the patient was immediately started on an aggressive pain management regimen and empiric antibiotics with minimal improvement. Despite escalation of treatment, the patient continued to decompensate and was subsequently transferred to the pediatric intensive care unit for management of acute encephalopathy, cardiogenic shock, sepsis, acute hypoxic respiratory failure, coagulopathy, thrombocytopenia, acute kidney injury, elevated troponins, transaminitis, and metabolic acidosis.
Upon arrival to the intensive care unit, his respiratory status deteriorated and echocardiography showed evidence of decreased cardiac function, resulting in intubation and vasopressor support. Infectious evaluation excluded acute viral etiology, including COVID-19 and human parvovirus B19. Additional laboratory results were significant for severe anemia; positive SARS-CoV-2 IgG antibody; platelet count of 26 × 109/L; coagulopathy; leukocytosis; acute kidney injury; and elevation in ferritin (above the reportable limit of 40,000 ng/mL), IL-6 (565.5 pg/mL), C-reactive protein (260.0 mg/L), D-dimer (>20.00 μg/mL), and troponin I (1.15 ng/mL). Imaging via computed tomography exhibited no signs of cerebrovascular accident, hemorrhage, pulmonary embolism, or deep vein thrombosis.
Treatment of MIS-C was initiated using intravenous immunoglobulin and steroids with a positive response. However, despite his physical improvement, the patient’s neurological status continued to decline, necessitating further evaluation, with hypertonia, sustained clonus, brisk reflexes, and a positive Babinski sign noted on exam. Subsequent magnetic resonance imaging (MRI) of the brain showed diffuse scattered punctate ischemic changes in both cerebral hemispheres and throughout the supratentorial and infratentorial brain, consistent with the classic “star-field” pattern of cerebral fat embolism syndrome (Figure 1).2 Bone marrow biopsy revealed necrosis.
Figure 1.
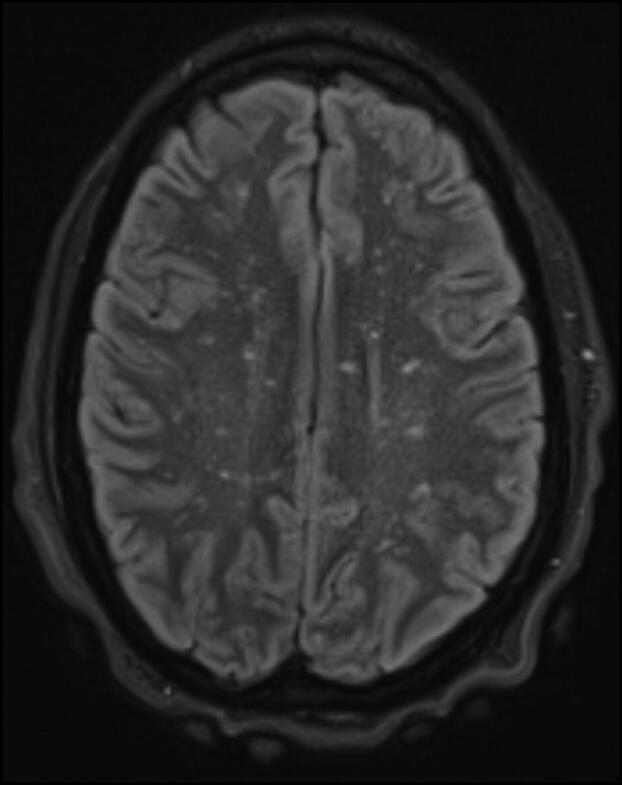
Brain MRI shows diffuse scattered punctuate ischemic changes consistent with the classic “star-field” pattern found in cerebral fat embolism syndrome.
Multiple rounds of therapeutic plasma exchange were added to the patient’s treatment plan, resulting in marked improvement. Over a period of 10 days, his altered mental status resolved, though he never regained memory of the first week. The patient returned fully to baseline and was discharged home after a 14-day admission with no complications. Close outpatient follow-up throughout the next month revealed no lasting effects of either syndrome.
DISCUSSION
This case demonstrates the rare occurrence of an adolescent male with homozygous sickle cell disease who suffered from cerebral fat embolism syndrome as a complication of MIS-C due to the Omicron variant of SARS-CoV-2. Currently, limited data are available regarding the incidence of either fat embolism syndrome or MIS-C secondary to the Omicron variant in the sickle cell disease population; however, the likelihood of developing either syndrome alone is very low.1,3
To be classified as MIS-C, a person 20 years of age or younger should display fever, laboratory evidence of multisystem inflammation, clinically severe illness requiring hospitalization not attributed to another source, and exposure to SARS-CoV-2 within the weeks prior to the onset of symptoms.4 Our patient exhibited fever, inflammation, and severe illness with multisystem involvement and had a positive IgG without prior symptomatic infection. Though he denied any known sick contacts, he had extensive nosocomial exposures in high-level transmission counties and cross-country travel in the 6 weeks preceding onset. The pathogenesis of MIS-C is not well-defined, but it is thought to occur secondary to an antibody-related immune response, with treatment typically involving intravenous immunoglobulin, steroids, and supportive care.1 While our patient responded well to these treatments, he required additional therapeutic plasma exchange due to the development of fat emboli.
COVID-19 has been shown to affect multiple types of cells and can cause adipocytes to undergo necrosis, predisposing those with a higher proportion of adipose tissue to fat embolism syndrome. While there have been reports of fat embolism syndrome in COVID-19 patients with obesity, there is no documentation to date of this syndrome occurring simultaneously with MIS-C.5 Though the patient in our case was not obese, because of his comorbid homozygous sickle cell disease and resulting necrosis, his risk of developing a cerebral fat embolism was likely significantly higher than that of the general population.
Fat embolism syndrome is typically elicited by trauma, such as with long bone fractures or following surgery; when nontraumatic fat embolism syndrome occurs in the sickle cell population, it is most often secondary to extensive bone marrow necrosis, as seen in this patient. Unlike our case, current literature indicates that patients who develop a fat embolism most often exhibit milder genotypes of sickle cell disease and are associated with parvovirus infection.1 Because of its heterogeneous presentation and lack of pathognomonic attributes, there is no consensus on the diagnostic criteria or exact pathogenesis of fat embolism syndrome, making it challenging to diagnose. Of the more commonly accepted criteria (such as those proposed by Schonfeld, Gurd, and Wilson), a focus is placed on the “classic triad” of symptoms—respiratory distress, altered mental status, and petechiae—though some authors recommend the addition of intercellular fat in pulmonary macrophages found on bronchial alveolar lavage and multiple cerebral white matter lesions on MRI.3,6
The pathophysiological changes associated with sickle cell disease (such as anemia and pulmonary hypertension) coupled with brief spikes in right-heart pressure caused by the precipitating event are believed to create conditions favorable for a temporary right-to-left cardiac shunt; this transient pathway potentially allows emboli to pass directly from the heart to the brain through otherwise innocuous cardiac malformations.7 While our patient’s initial imaging did not show any circulatory abnormalities, an agitated saline transthoracic contrast echocardiograph performed on hospital day 6 revealed a very small left-to-right shunt elicited only by Valsalva maneuver, consistent with patent foramen ovale. This case illustrates the need for change in the management of suspected MIS-C in patients with sickle cell disease, as extensive interventions and close monitoring may be required.
Disclosure statement/Funding
The authors report no funding or conflicts of interest. The patient and family have consented to the publication of this case.
References
- 1.Bastug A, Aslaner H, Aybar Bilir Y, et al. Multiple system inflammatory syndrome associated with SARS-CoV-2 infection in an adult and an adolescent. Rheumatol Int. 2021;41(5):993–1008. doi: 10.1007/s00296-021-04843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsitsikas DA, Bristowe J, Abukar J.. Fat embolism syndrome in sickle cell disease. J Clin Med. 2020;9(11):3601. doi: 10.3390/jcm9113601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vichinsky E, Williams R, Das M, et al. Pulmonary fat embolism: a distinct cause of severe acute chest syndrome in sickle cell anemia. Blood. 1994;83(11):3107–3112. [PubMed] [Google Scholar]
- 4.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72(11):1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinti S, Graciotti L, Giordano A, Valerio A, Nisoli E.. COVID-19 and fat embolism: a hypothesis to explain the severe clinical outcome in people with obesity. Int J Obes (Lond). 2020;44(8):1800–1802. doi: 10.1038/s41366-020-0624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatt ME, Seamon MJ.. Fat embolism syndrome. Int J Crit Illn Inj Sci. 2013;3(1):64–68. doi: 10.4103/2229-5151.109426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling MM, Quinn CT, Ramaciotti C, et al. Increased prevalence of potential right-to-left shunting in children with sickle cell anaemia and stroke. Br J Haematol. 2017;176(2):300–308. doi: 10.1111/bjh.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]


