Abstract
We report a patient with Ebstein’s anomaly, on chronic milrinone therapy for right ventricular failure, who underwent palliative percutaneous closure of her atrial septal defect (ASD) due to recurring strokes. Repeated evaluation of right-sided pressures was performed prior to ASD closure to determine if our patient could tolerate the intervention. Definitive ASD closure was performed under fluoroscopic and transesophageal echocardiogram guidance.
KEYWORDS: Atrial septal defect, Ebstein anomaly, right ventricular failure, stroke, tricuspid regurgitation
Ebstein’s anomaly (EA) is a rare congenital heart defect that accounts for <1% of congenital heart disease and commonly involves apical displacement of the tricuspid valve into the right ventricle (RV) with atrialization of the RV and ventriculization of the septal leaflet. EA is also associated with an atrial septal defect (ASD) and patent foramen ovale in 80% to 90% of cases.1 There is limited data regarding percutaneous closure of ASDs and patent foramen ovales in adults in patients with EA and after cryptogenic stroke.
CASE DESCRIPTION
A 72-year-old woman had a medical history of uncorrected EA, severe tricuspid regurgitation with RV dilation and failure (recently started on home milrinone therapy, 0.125 mcg/kg/min), hypertension, and multiple prior cerebrovascular accidents (CVAs). She continued to have recurrent strokes despite previous treatment with aspirin and statin therapy as well as three different anticoagulants. A Holter monitor previously did not detect any atrial arrhythmias. She was referred for percutaneous ASD closure. Four months prior to evaluation of ASD closure, she was diagnosed with endometrial cancer and underwent hysterectomy and chemotherapy with chest wall port placement. A month later, she suffered a CVA that was attributed to new onset atrial fibrillation and was started on apixaban. Despite compliance with anticoagulation, she suffered another CVA a month later. At the time of her last CVA, she presented with a facial droop and dysarthria and with a new left parietal convexity CVA as well as evidence of several other prior CVAs on computed tomography. Her anticoagulation was switched to dabigatran at this time. Previous transthoracic echocardiogram demonstrated an enlarged, atrialized, and severely dilated RV with severely reduced systolic function, severe tricuspid regurgitation, and an ASD with bidirectional shunting. Left heart catheterization after her most recent CVA demonstrated minimally obstructive coronary artery disease and a left ventricular end-diastolic pressure of 14 mm Hg. Right heart catheterization demonstrated normal pulmonary pressures with moderately elevated RV filling pressures and severely reduced Fick and thermodilution cardiac output/index consistent with RV cardiogenic shock. Pulmonary vascular resistance was 3.4 Wood units with a Qp/Qs 0.91 consistent with a right to left shunt.
A multispecialty plan for ASD closure was determined involving neurology, adult congenital cardiology, heart failure, and interventional cardiology. Given recurrent CVAs despite anticoagulation, our patient elected to undergo percutaneous ASD closure. Transesophageal echocardiogram (TEE) at the time of intervention confirmed severe RV dilation with severely reduced systolic function, severe tricuspid regurgitation, and a 1.8 × 1.0 cm ASD with a right to left shunt (Figure 1). She was transitioned to heparin from oral anticoagulation prior to the procedure. Under general anesthesia, the patient underwent TEE-guided ASD closure. TEE demonstrated a thrombus on the distal tip of her chest wall port (Figure 2). The initial procedure was aborted and her port was removed by interventional radiology. She was brought back to the lab 3 days later, afebrile leading up to the procedure. Repeat TEE was performed for measurement of the ASD (Figure 3a). Right heart catheterization was performed at the start of the procedure to assess hemodynamic status (Table 1). Balloon occlusion of her ASD with a 24 mm Amplatzer sizing balloon (Abbott, Santa Clara, CA) was performed (Figure 3b–3d). Right heart catheterization was repeated 5 and 10 minutes after balloon occlusion of the ASD (Table 1). Hemodynamic data continued to improve with ASD occlusion. Under TEE guidance, definitive closure was performed by placing a 25 mm Cribriform Amplatzer ASD occluder device (Abbott, Santa Clara, CA) across the ASD (Figure 4). There was no change in RV function and no residual interatrial shunt noted on TEE after the procedure. The patient did well postprocedure was discharged on rivaroxaban and clopidogrel for 6 months before being transitioned to rivaroxaban and aspirin. She remained on milrinone therapy on discharge but was weaned off after 6 months.
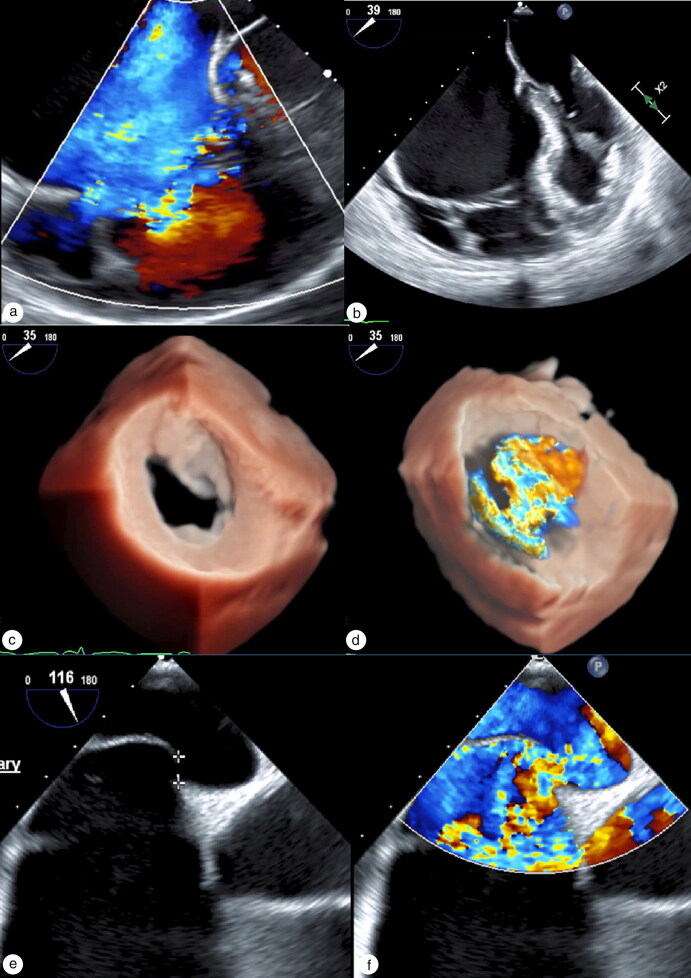
Figure 1.
TEE showing (a) severe tricuspid regurgitation with color Doppler and (b) severely dilated and arterialized right ventricle. (c) 3D TEE showing ASD with right to left shunt with (d) color Doppler across ASD. (e) 3D TEE showing ASD and (f) color Doppler of right to left shunt through ASD.
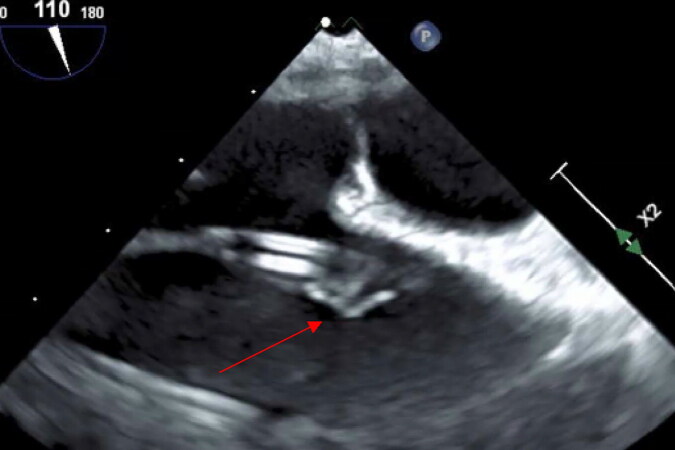
Figure 2.
TEE demonstrating mobile thrombus on the tip of the chemo port (arrow) in the right atrium.
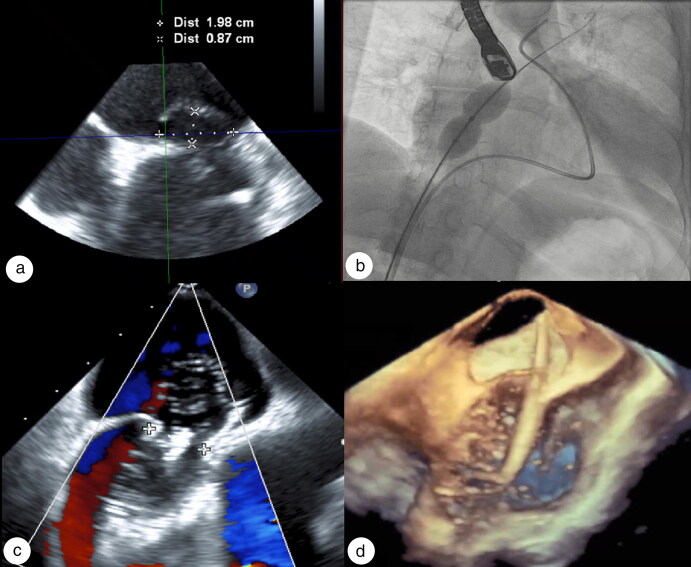
Figure 3.
(a) TEE demonstrating a 1.9 cm ASD. (b, c) Balloon occlusion of ASD before deployment and during hemodynamic assessment by right heart catheterization on fluoroscopy and echocardiogram, respectively. (d) 3D TEE showing sizing balloon across ASD.
Table 1.
Hemodynamics during balloon occlusion of ASD
| Time | PA pressure (mm Hg) | PA saturation (% O2) | Aorta saturation (% O2) | CO Fick (L/Min) | CI Fick (L/min/m2) |
|---|---|---|---|---|---|
| Pre–ASD occlusion | 26/4/13* | 58.0 | 91.6 | 4.1 | 2.1 |
| 5 min after balloon occlusion of ASD | 32/6 | 64.0 | 96.3 | 4.4 | 2.3 |
| 10 min after balloon occlusion of ASD | 35/6 | 64.7 | 96.4 | 4.9 | 2.5 |
| After ASD device deployment | 36/8 | 63.0 | 94.0 | 4.9 | 2.5 |
*Pulmonary capillary wedge pressure 9 mm Hg.
ASD indicates atrial septal defect; CI, cardiac index; CO, cardiac output; PA, pulmonary artery.
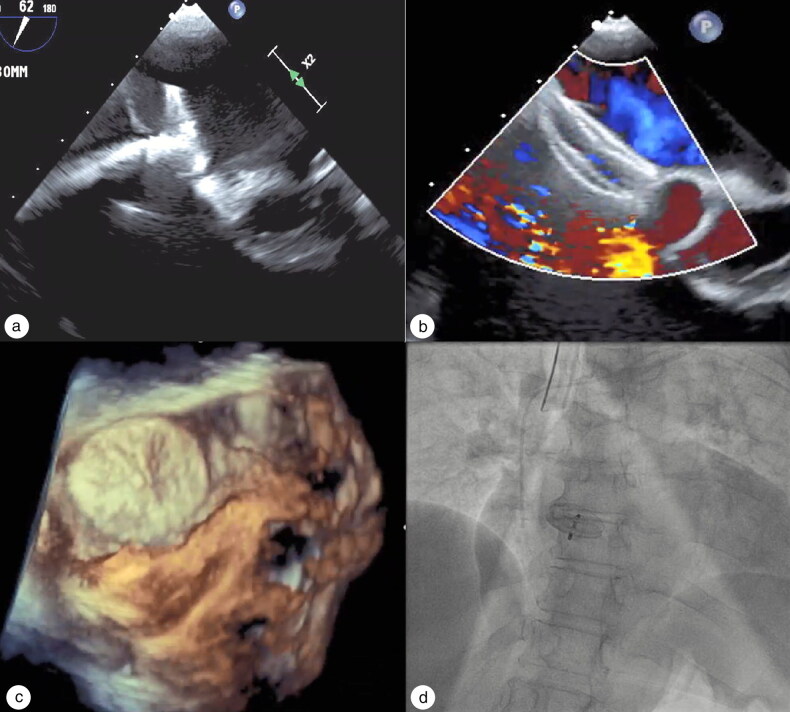
Figure 4.
(a) 2D TEE: Predeployment of the cribriform ASD occluder. (b) Deployed ASD occluder with color Doppler demonstrating no residual right to left shunt across the atrial septum. (c) 3D TEE showing final placement of ASD occluder. (d) Final fluoroscopy after deployment of cribriform ASD occluder device.
DISCUSSION
EA is a rare congenital heart defect associated with an ASD. Despite the tricuspid valve being the most affected structure in EA, an ASD can play a significant role. While many patients with EA undergo surgical correction, the 1-year mortality rate remains high. There is very little literature of patients who survive past 70 years of age with EA without surgical correction.1 Our patient was previously referred for surgery but refused. Patients with EA have a significantly increased risk of CVA from paradoxical emboli due to right to left shunting.2,3 We performed balloon occlusion, which mimics closure, under TEE guidance to visually assess the function of her RV and performed right heart catheterization measurements with pulmonary artery saturations to assess cardiac output and index before definitive closure. Had the patient’s RV function worsened during the balloon occlusion of her ASD, the procedure would have been aborted. This case demonstrates that percutaneous ASD closure can be safely performed in carefully selected patients with EA with RV failure who have a right to left shunt after cryptogenic strokes. Repeated assessment of cardiac output as well as monitoring for worsening hemodynamics and RV function can assist in determining if a patient with EA can tolerate ASD closure.
Disclosure statement/Funding
The authors report no funding. Dr. Al-Azizi reports consultant work with Philips Volcano and Edwards Life Science; the other authors have no potential conflicts to disclose. The patient provided permission for this case to be published.
References
- 1.Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK.. Ebstein’s anomaly. Circulation. 2007;115(2):277–285. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 2.Attenhofer Jost CH, Connolly HM, Scott CG, Burkhart HM, Ammash NM, Dearani JA.. Increased risk of possible paradoxical embolic events in adults with Ebstein anomaly and severe tricuspid regurgitation. Congenit Heart Dis. 2014;9(1):30–37. doi: 10.1111/chd.12068. [DOI] [PubMed] [Google Scholar]
- 3.Tan NY, Attenhofer Jost CH, Polkinghorne MD, et al. Cerebrovascular accidents in Ebstein’s anomaly. Congenit Heart Dis. 2019;14(6):1157–1165. doi: 10.1111/chd.12841. [DOI] [PubMed] [Google Scholar]