Abstract
Objective:
To assess the prevalence of CMV viremia in HIV-positive patients starting antiretroviral therapy (ART) and to evaluate its impact on clinical outcomes.
Design:
Retrospective analysis of four clinical trials (INSIGHT FIRST, SMART, START, and ANRS REFLATE TB).
Methods:
Stored plasma samples from participants were used to measure CMV viremia at baseline prior to initiating ART and at visits through one year of follow-up after ART initiation. CMV viremia was measured centrally using a quantitative PCR assay. Within FIRST, associations of CMV viremia at baseline and through eight months of ART were examined with a composite clinical outcome of AIDS, serious non-AIDS events, or death using Cox proportional hazards regression.
Results:
Samples from a total of 3176 participants, 1169 from FIRST, 137 from ANRS REFLATE TB, 54 from SMART, and 1816 from START were available with baseline CMV viremia prevalence of 17%, 26%, 0% and 1%, respectively. Pooled across trials, baseline CMV viremia was associated with low CD4+ T-cell counts and high HIV RNA levels. In FIRST, CMV viremia was detected in only 5% of participants between baseline and month 8. After adjustment for CD4+ T-cell count and HIV RNA levels, hazard ratios (HR) for risk of clinical outcomes was 1.15 (0.86–1.54) and 2.58 (1.68 – 3.98) in FIRST participants with baseline and follow-up CMV viremia, respectively.
Conclusion:
Baseline CMV viremia in HIV-positive patients starting ART is associated with advanced infection and only persistent CMV viremia after ART initiation is associated with a higher risk of morbidity and mortality.
Keywords: CMV, HIV, AIDS, mortality, antiretroviral therapy, CD4+ T-cell count
Introduction
Morbidity and mortality in advanced HIV-infection remains high despite the use of antiretroviral therapy (ART) and prophylaxis for opportunistic infections [1]. In the Reality trial, enhanced prophylaxis for opportunistic infections other than cytomegalovirus (CMV) reduced the risk of adverse clinical outcomes in antiretroviral naïve participants starting ART, but the death rate at week 48 was still 11% [2].
CMV infection and reactivation are frequent in patients with advanced HIV-infection and may also significantly contribute to disease progression and death [3–8]. CMV prophylaxis or preemptive therapy is not recommended, however, in patients starting ART [9]. Before the ART era, CMV prophylaxis with ganciclovir was shown to reduce the incidence of CMV disease without affecting overall mortality[10]. However, subsequent trials of CMV prophylaxis using valganciclovir conducted in persons starting ART were underpowered to assess their impact on CMV disease or other clinical outcomes due to the small sample size and the low incidence of CMV disease following ART-induced immune reconstitution [10,11]. In addition, drugs available for CMV prophylaxis were associated with significant hematologic toxicity [10,12].
Newer anti-CMV drugs for prophylaxis with a better safety profile, such as letermovir (a drug already approved for CMV prophylaxis in stem cell transplant recipients) prompted us to examine the role of CMV infection on disease progression and death in patients living with HIV starting ART [13]. Indeed, the deleterious effect of CMV replication on clinical outcomes is not only linked to the development of CMV disease, but is also likely related to persistent immune activation and exacerbated inflammatory responses which have been associated with serious non-AIDS clinical events and death [14].
We therefore assessed the prevalence of CMV viremia before ART initiation in a large number of participants living with HIV, and explored the association between CMV replication, before and after ART initiation, with future risk of clinical outcomes. For that purpose, we used stored plasma samples from individuals with HIV-infection, undergoing initiation of ART and enrolled in four different clinical trials. Participants in these trials had a wide range of CD4+ T-cell counts at study entry, a long follow-up and a prospective collection of centrally adjudicated clinical events including AIDS, serious non-AIDS events and all-cause death [15–18].
Methods
Study population
The study population included treatment-naïve HIV-positive participants starting ART after randomization in one of four clinical trials: ANRS REFLATE TB, FIRST (CPCRA 058), SMART (INSIGHT/CPCRA 065) and START (INSIGHT 001), with available stored plasma specimens at baseline and at least one visit during the first year of follow-up (post-ART initiation). Details for each clinical trial have been reported elsewhere [15–18]. In brief, ANRS REFLATE TB participants were enrolled in France and Brazil and had tuberculosis co-infection. FIRST participants were enrolled in the USA were randomized to one of three antiretroviral treatment strategies as initial therapy for HIV-infection. SMART participants were enrolled in 33 countries, but only treatment-naive participants randomized to the viral suppression group were eligible for this analysis. START participants were enrolled in 35 countries, but only those randomized to the immediate ART arm were eligible for this study. All participants provided written informed consent for stored specimens and institutional review board approval was obtained by each study site.
CMV assays
Baseline CMV-specific IgG measurements were performed on plasma using the Immuno-Biological Laboratories (IBL-America, Minneapolis, MN, USA) IgG ELISA assay, following the manufacturer’s specifications [19]. Plasma that demonstrated an equivocal signal were resolved with the Diamedix CMV IgG Enzyme Immunoassay Test Kit (ERBA Diagnostics, Miam Lake, FL, USA) following the manufacturer’s specifications [20].
CMV viremia was measured at baseline and during the first year of follow-up (post-ART initiation) using available stored plasma specimens. For FIRST, SMART and START available time points were baseline, months 4, 8 and 12. For ANRS REFLATE TB available time points were baseline, weeks 2, 4, 8, 16 (month 4), 20, 24 (month 6) and 48.
DNA was extracted from 200 μL of each plasma sample, and CMV viremia was measured centrally, using a quantitative PCR assay with TaqMan probes to detect human CMV DNA with a lower limit of detection of 88.5 IU/mL [21]. All samples were run in duplicate and CMV viremia (CMV+) was defined as the presence of quantifiable CMV DNA in plasma in at least one run.
A participant was considered CMV viremic (CMV+) during follow-up if they had detectable CMV viremia at any of the follow-up visits. Using baseline (BL) and follow-up (F/U) CMV viremia status, participants were classified into 4 subgroups. These were Group 1: BL CMV+, F/U CMV+ (detectable viremia at baseline and during follow-up); Group 2: BL CMV-, F/U CMV+ (detectable viremia during follow-up only); Group 3: BL CMV+, F/U CMV- (detectable viremia at baseline only); and Group 4: BL CMV-, F/U CMV- (no viremia at baseline and during follow-up).
Statistical analysis
Data from the 4 trials were pooled to assess the prevalence of baseline CMV viremia according to baseline CD4+ T-cell counts. Based on these findings, we then pooled only the REFLATE TB and FIRST studies to assess the prevalence of CMV viremia according to baseline CD4+ T-cell counts and plasma HIV RNA levels.
We restricted the analysis of the association of CMV viremia with HIV disease progression to the FIRST trial since this trial had a large number of participants, a long follow-up, a high number of clinical endpoints, and a high prevalence of CMV viremia at baseline. Clinical endpoints collected prospectively during the trial included AIDS and all-cause death. Serious non-AIDS events (cardiovascular diseases, non-AIDS cancer, end stage renal disease, liver cirrhosis) were defined as previously reported [17,18].
Cox proportional hazards regression was used to assess associations of a composite clinical outcome of AIDS including CMV disease, serious non-AIDS events, or death with baseline CMV viremia, with and without adjustment for baseline CD4+ T-cell count and HIV RNA levels, using all follow-up accrued. CD4+ T-cell count and HIV RNA level (log) were included in the model as continuous variables. Hazard ratios (HRs) and 95% confidence intervals (CIs) are cited. We also examined associations of the composite clinical outcome after 8 months of follow-up across the 4 subgroups defined by baseline and month 8 follow-up CMV viremic status. A participant was considered viremic through month 8 if they had detectable CMV viremia in either the 4 or 8 month plasma sample. Only participants still alive and under follow-up after month 8 were included in these analyses. Participants who experienced an event before month 8 (other than death) were included in the time-to-event analyses and time ‘0’ was re-set to start from month 8. Models are presented with and without adjustment for month 8 CD4+ T-cell count and HIV RNA levels, using follow-up accrued after month 8. For these analyses, CD4+ T-cell count was included in the models as a continuous variable and HIV RNA as a 3 level categorical variable (≤400 copies/mL, 400–10,000 copies/mL, and >10,000 copies/mL) to account for viral suppression post ART initiation. This period of eight months was chosen as a “burn-in” period to allow enough time to achieve HIV RNA suppression in plasma after initiation of anti-HIV therapy.
Finally, we explored the association between high vs. low CMV viremia at baseline with clinical outcomes by subdividing the baseline CMV viremic group into those with CMV viremia levels above and below the baseline median level for the cohort. For the month 8 associations, we similarly subdivided those with CMV during follow-up into those with high (at least 1 follow-up CMV viral load above the baseline median) and low levels (all follow-up values below the median). Analysis were performed using SAS Version 9.3 (SAS Institute, Cary, NC). A 2-sided p-value <0.05 was considered significant.
Results
Prevalence of CMV viremia at baseline and during the first year of follow-up
Samples from a total of 3176 participants, 1169 from FIRST, 137 from ANRS REFLATE TB, 54 from SMART, and 1816 from START were available at baseline and at least one follow-up visit after ART initiation (Fig 1).
Figure 1:
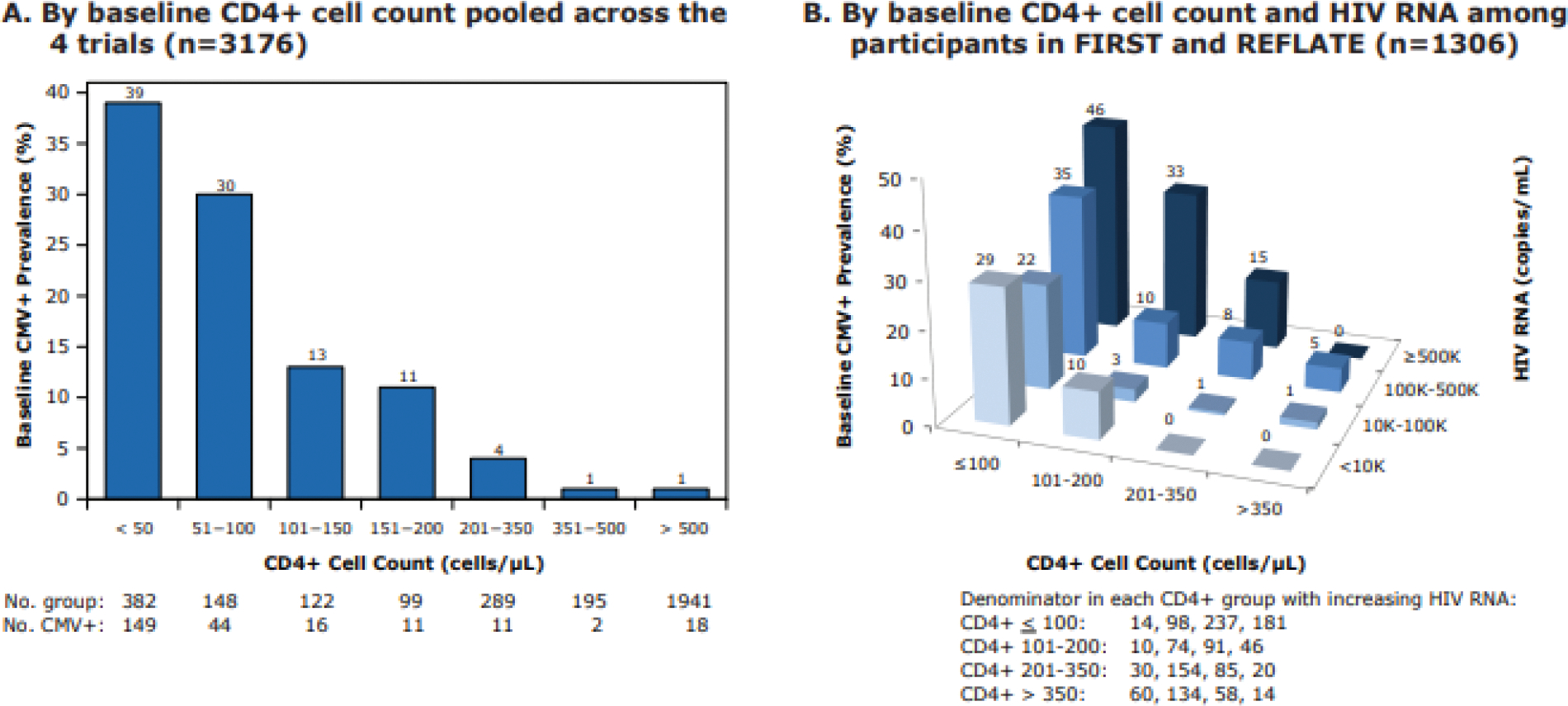
Baseline prevalence of CMV viremia (A) by baseline CD4+ T-cell counts pooled across all trials and (B) by baseline HIV viral load among participants in FIRST and REFLATE
Baseline characteristics of participants in each trial are shown in Table 1. Median baseline CD4+ T-cell counts varied across trials from 140, 153, 429 to 648/μL in ANRS REFLATE TB, FIRST, SMART and START respectively. Median plasma HIV RNA levels were higher in ANRS RELFATE TB and FIRST (Table 1). CMV-specific IgG seropositivity at baseline was 94% or more across all trials. Prevalence of CMV viremia at baseline was 26%, 17%, 0% and 1%, in ANRS REFLATE TB, FIRST, SMART and START respectively (Table 1). Among those with CMV viremia at baseline, the median CMV viral load was 361 IU/mL (IQR: 159–2381) in ANRS REFLATE TB, 543 IU/mL (IQR: 184–2124) in FIRST, and 88.5 IU/mL (IQR: 88.5– 740) in START.
Table 1:
Baseline characteristics of participants included from each trial.
| Characteristic | ANRS REFLATE TB (n=137) |
FIRST (n=1169) |
SMART (n=54) |
START (n=1816) |
|---|---|---|---|---|
|
| ||||
|
| ||||
| Age, years | 38 (30,44) | 38 (31, 43) | 36 (31, 41) | 36 (29, 44) |
|
| ||||
| Female, n (%) | 37 (27.0) | 252 (21.5) | 21 (38.9) | 485 (26.7) |
|
| ||||
| Black race, n (%) | 46 (33.6) | 614 (52.5) | 9 (16.7) | 545 (30.1) |
|
| ||||
| CD4+ T-cells/μL | 140 (58, 302) | 153 (34, 318) | 429 (380, 535) | 648 (585, 759) |
|
| ||||
| HIV RNA, copies/mL | 87000 (27022, 294479) |
143843 (38654, 400147) |
17012 (4040, 61200) |
13480 (3440, 43759) |
|
| ||||
| CMV-specific IgG*, n (%) | ||||
| Positive | 136 (99.3) | 1102 (94.4) | 51 (94.4) | 1752 (96.5) |
| Negative | 1 (0.7) | 66 (5.6) | 3 (5.6) | 64 (3.5) |
|
| ||||
| CMV viremia prevalence¤, n (%) | ||||
| Positive | 35(25.6) | 201 (17.1) | 0 (0) | 15 (0.8) |
| IU/L (median, IQR) | 361 (159–2381) | 543 (184–2124) | - | 88.5 (88.5–740) |
| Negative | 102 (74.4) | 968 (82.9) | 54 (100) | 1801 (99.2) |
IQR=interquartile range
IgG was not available for 1 participant in FIRST
CMV DNAemia defined as quantifiable CMV DNA (assay lower limit of detection 88.5 IU/mL)
Using pooled data across the four trials, CMV viremia at baseline was associated with lower CD4+ T-cell counts (Fig. 1A). Using pooled data from FIRST and ANRS REFLATE TB, a higher percentage of CMV viremia at baseline was associated with lower CD4+ T-cell counts and higher plasma HIV RNA levels (Fig. 1B).
During the first year of follow-up after ART initiation, the percentage with CMV viremia declined over time. In START and SMART, only 1.2% (n=22) and 2% (n=1) of participants, respectively, had CMV viremia detected during follow-up. In FIRST, 5% of participants had CMV viremia during follow-up: 3% at both baseline and follow-up (n=34), and 2% during follow-up only (n=28). In ANRS REFLATE TB, 52% of participants had CMV viremia detected during follow-up, which was mainly observed during the first 8 weeks after ART initiation. When using comparable time points in ANRS REFLATE TB (months 4 and 6; n=128) and FIRST (months 4 and 8; n=1151), detectable CMV viremia during follow-up was similar in both trials (7% (n=9) of ANRS REFLATE TB and 5% (56) of FIRST, supplementary Fig. 2).
Associations between CMV viremia and clinical endpoints in FIRST.
Participants in FIRST with baseline CMV viremia had lower CD4+ T-cell counts and higher HIV viral load compared to those without baseline CMV viremia (49 vs 156 CD4+ T-cells/μL and 456,808 vs 100,344 copies/mL for HIV RNA levels). After a median follow-up time of 4.8 years, baseline CMV viremia was significantly associated with the composite clinical outcome of AIDS, serious non-AIDS events, or death (Fig. 2A and Table 2) in unadjusted comparisons. Rates of this composite clinical outcome were 9.4 vs. 4.7 per 100 person-years (PY) in participants with and without baseline CMV viremia (log rank p-value < 0.001). However, after adjusting for baseline CD4+ T-cell counts and plasma HIV RNA levels, this association was no longer significant with a HR of 1.15 (95% CI: 0.86–1.54, p=0.36). Rates of the composite outcome in participants with high vs. low baseline CMV viremia (above vs. at or below the median of 543 IU/mL) were 10.1 and 8.8 per 100 PY respectively. These rates were not significantly differently from each other and did not differ from the group without baseline CMV viremia in the model that adjusted for baseline CD4+ T-cell counts and HIV RNA (data not shown). Among participants who experienced the composite outcome, 8 out of the 71 (11.3%) participants with baseline CMV viremia experienced CMV-end organ disease as their first event compared to 1/193 (0.5%) in those without CMV viremia. The most common events defining the composite outcome were death (19.7% in CMV viremic participants at baseline, 26.4% in non-viremic participants), candidiasis (18.3% and 12.4%) and pneumocystis (11.3% and 8.3%) (Suppl. Table 1).
Figure 2:
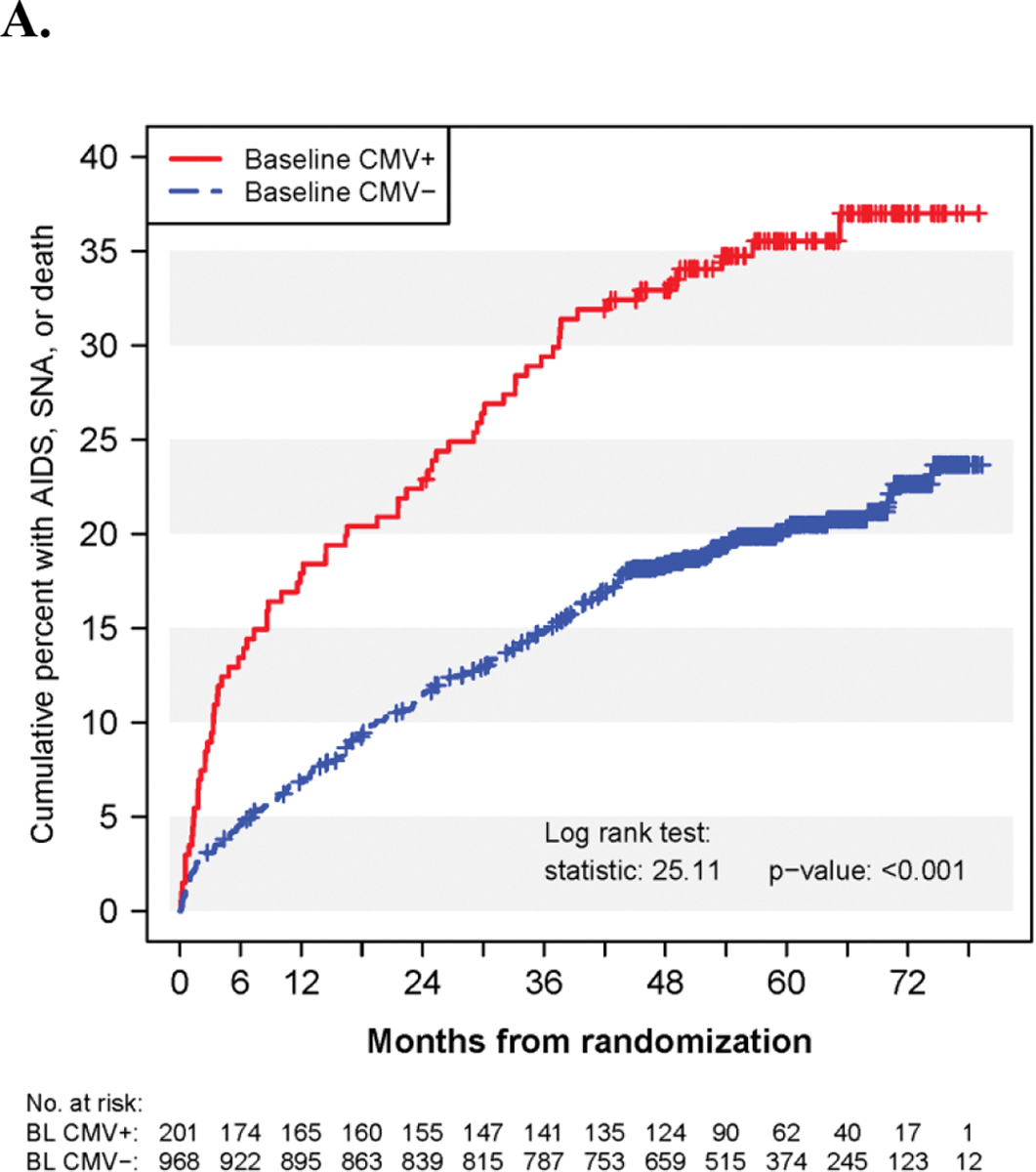
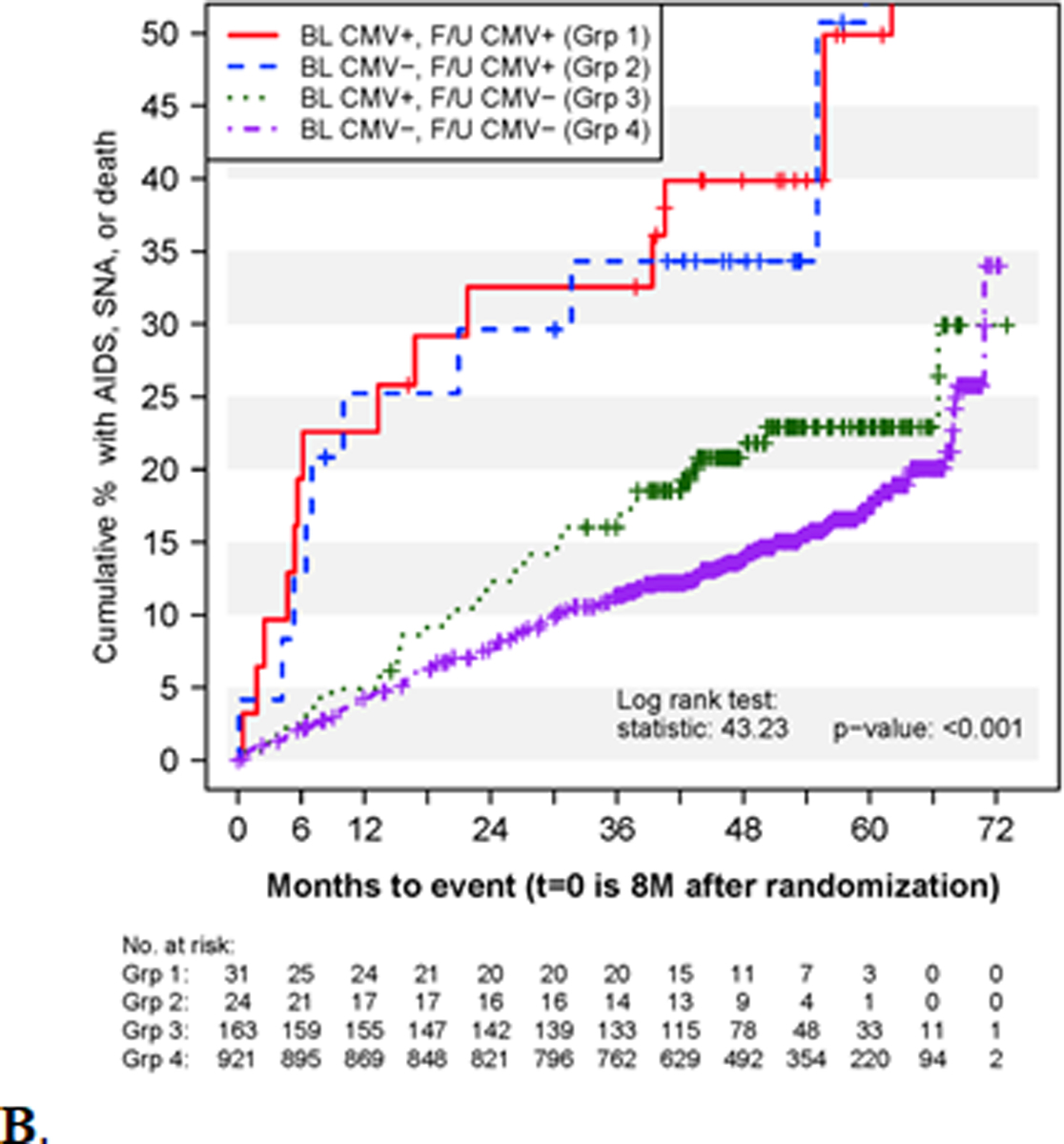
Kaplan-Meier curves for the cumulative percent of participants in FIRST experiencing the composite outcome of AIDS, SNA, or death according to A) subgroups defined by baseline CMV+/CMV- status for events during all follow-up and B) the 4 subgroups defined by baseline and follow-up CMV+/CMV- status through month 8 for events occurring after eight months of follow-up.
Table 2:
Hazard ratios (HRs) for the risk of the composite outcome of death, AIDS, or serious non-AIDS events during follow-up in FIRST.
| Unadjusted | Adjusting for BL CD4 | Adjusting for BL CD4 and HIV RNA | ||||
|---|---|---|---|---|---|---|
| Subgroup | N in Grp. | N. Evt. | Rate (100 PY) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Using all follow-up for time-to-first-event | ||||||
| BL CMV+ | 201 | 71 | 9.4 | 1.98 (1.51, 2.60) p= <0.001 |
1.22 (0.92, 1.64) p=0.17 |
1.15 (0.86, 1.54) p=0.36 |
| BL CMV− | 968 | 193 | 4.7 | -- ref -- | -- ref -- | -- ref -- |
| Unadjusted | Adjusting for Month 8 CD4 | Adjusting for Month 8 CD4 and HIV RNA category | ||||
| Using follow-up after eight months of ART use for time-to-first-event after 8 months | ||||||
| BL CMV+,F/U CMV+ | 31 | 14 | 15.4 | 3.68 (2.12, 6.37) p=<0.001 |
2.42 (1.39, 4.23) p=0.002 |
2.29 (1.31, 4.00) p=0.004 |
| BL CMV−, F/U CMV+ | 24 | 10 | 14.7 | 3.50 (1.85, 6.65) p=<0.001 |
3.37 (1.77, 6.41) p=<0.001 |
3.18 (1.67, 6.04) p=<0.001 |
| BL CMV+, F/U CMV− | 163 | 36 | 5.9 | 1.44 (1.00, 2.07) p=0.051 |
0.95 (0.65, 1.38) p=0.78 |
1.05 (0.72, 1.52) p=0.81 |
| BL CMV−, F/U CMV− | 921 | 145 | 4.1 | -- ref -- | -- ref -- | -- ref -- |
| Using follow-up after eight months of ART use for time-to-first-event after 8 months, pooled groups | ||||||
| F/U CMV+ | 55 | 24 | 15.1 | 3.38 (2.21, 5.18) p=<0.001 |
2.78 (1.81, 4.28) p=<0.001 |
2.58 (1.68, 3.98) p=< 0.001 |
| F/U CMV− | 1084 | 181 | 4.3 | -- ref -- | -- ref -- | -- ref -- |
BL: baseline; CI: confidence interval; CMV− : not CMV viremic ; CMV+ : CMV viremic ; F/U: follow-up; SNA : serious non-AIDS (cancer, CVD, renal, liver disease)
Incidence of CMV end-organ disease was 1.1 per 100 PY (10 events) in those with CMV viremia and 0.1 per 100 PY (5 events) in those without CMV viremia; the HR after adjustment for baseline CD4+ T-cell counts and plasma HIV RNA levels was HR=3.27 (95% CI: 1.11–9.6, p=0.031). Of note, the incidence of CMV end-organ disease was higher during the first six months after ART initiation, with incidence rates of 7.1 per 100 PY (7 events) in those with CMV viremia and 0.2 per 100 PY (1 event) in those without CMV viremia, with a HR of 11.97 (95% CI: 1.46–98.26, p=0.021) after adjustment for baseline CD4+ T-cell counts and plasma HIV RNA levels (data not shown).
We then explored the association of CMV viremia post-ART initiation at month 4 or 8 (irrespective of baseline status) with the composite clinical outcome after month 8 in 1,151 FIRST participants. Baseline and month 8 CD4+ T-cell counts and HIV RNA levels for the 4 subgroups are presented in Supplemental Table 2. Participants without detectable CMV viremia during the first 8 months had higher HIV RNA suppression rates by the month 8 visit. Participants with CMV viremia during the first 8 months of follow-up (irrespective of baseline status) had significantly higher rates of the composite clinical outcome compared to those without CMV viremia (16.1 vs. 4.3 per 100 PY) even after adjustment for month 8 CD4+ T-cell count and plasma HIV RNA level (HR: 2.58, 95%CI: 1.68–3.98, p< 0.001; Fig. 2B and Table 2). Of note, only two participants experienced CMV end-organ disease after month 8. Both participants had baseline CMV viremia but only one experienced persistent CMV viremia. Also, only three of the 24 participants with follow-up viremia experienced serious non-AIDS events (Suppl. Table 3). The event rates after month 8 for the groups with high (n=15) and low (n=40) CMV viremia during follow-up were 29.3 and 11.7 per 100 PY respectively. Both of these rates were significantly higher than the rate for those without CMV viremia during follow-up (HRs: 3.95, 95%CI: 1.99–7.81, p <0.001 and 2.13, 95% CI 1.25–3.62, p=0.005) after adjustment for month 8 CD4+ T-cell count and HIV RNA levels. The event rates between those with high and low follow-up CMV viremic levels were not significantly different (data not shown).
Discussion
In this large, pooled analysis of four clinical trials of treatment naïve HIV-positive patients starting ART with a very high rate (>94%) of CMV co-infection as demonstrated by specific anti-CMV IgG, the prevalence of CMV viremia at baseline was strongly associated with low CD4+ T-cell counts and high plasma HIV RNA levels. Indeed, among participants with less than 100 CD4+ T-cells/μL, prevalence of baseline CMV viremia was 30% or more, compared to 1% or less in those with CD4+ T-cell counts above 350 CD4+ T-cells/μL (Fig 1A). These results are consistent with previously published studies in participants with low CD4+ T-cell counts and are in agreement with guidelines recommending monitoring CMV replication in blood mainly among participants with <100 CD4 T-cells/μL [4,8,11,14,22].
We assessed the association between CMV viremia and a composite clinical outcome that included AIDS, serious non-AIDS and deaths using data from the FIRST trial only.
We observed that participants with baseline CMV viremia had a higher rate of the composite clinical outcome than participants without baseline CMV viremia, although this association was not significant after adjustment on baseline CD4+ T-cell count and plasma HIV RNA level with an HR of 1.15 (95%CI: 0.86–1.54) (Table 2).
This observation is in contrast with studies conducted in the pre-ART era where baseline CMV viremia was independently associated with HIV disease progression and death, but consistent with more recent studies in patients with CMV viremia on ART [5,6]. This difference can be explained in part by the impact of ART on CMV replication due to the rapid immune reconstitution following ART initiation, with an increase in memory CD4+ and CD8+ T-cells, especially CMV-specific memory T-cells in patients with CMV co-infection [23,24]. This is also consistent with the high clearance rate of CMV viremia in these participants within months of starting ART. Indeed, 17% of participants in FIRST had CMV viremia at baseline, but only 5% during follow-up at months 4 or 8. Similarly in ANRS REFLATE TB, most of the post-baseline CMV viremia occurred before 8 weeks and later cleared as previously reported [25].
However, the incidence of CMV disease during follow-up remained higher in participants with baseline CMV viremia in FIRST (1.1 vs 0.1 per 100 PY). The HR after adjustment for baseline CD4+ T-cell counts and plasma HIV RNA levels was significantly greater than 1.0 ( HR: 3.27,95% CI: 1.11–9.60). Most CMV disease occurred during the first six months after ART initiation, before anti-CMV immune defenses could be restored. Still, the incidence of CMV disease may remain too low to warrant CMV prophylaxis or even preemptive therapy for these patients, as reported previously [11].
Interestingly, participants with CMV viremia during follow-up on ART, irrespective of whether or not baseline CMV viremia was detected, had significantly higher rates of the composite clinical outcome of AIDS, serious non-AIDS events or death as compared to those without CMV viremia during follow-up, even after adjustment for month 8 CD4+ T-cell count and plasma HIV RNA levels (HR: 2.38 (95% CI: 1.54–3.68), p< 0.001). Only one person, a participant with persistent CMV viremia (baseline and during follow-up), experienced a CMV disease event after month 8, so CMV disease was not the main factor contributing to this higher rate of clinical events. These findings are consistent with those of Deayton et al., showing that persistent detection of CMV in blood by PCR identified participants with a poor prognosis and was associated with increased relative rates of progression to AIDS and death [4]. Similar findings were reported in the Swiss HIV Cohort Study [7].
CMV replication could be to the consequence of impaired T-cell reconstitution in patients on ART and thus be associated with worse outcomes. But CMV replication could also be on the causal pathway to clinical events.
The mechanisms by which persistent CMV replication might cause an increased risk of disease progression and death remains to be elucidated. It is plausible that persistent CMV replication during ART could be associated with immune activation, and exacerbate inflammatory-related diseases that have been associated with AIDS and death [26,27]. CMV replication has been associated with CD8+ T-cell expansion and inflammation [28], and lower CD4/CD8 ratio during ART [29]. Moreover, increased T-cell activation and exhaustion linked to CMV replication has been suggested to contribute to the pathogenesis of HIV-associated atherosclerosis [30]. CMV replication has also been previously associated with serious non-AIDS events. Gianella et al., in their case-control study of patients starting ART enrolled in different ACTG trials, showed that detection of CMV DNA in peripheral blood mononuclear cells was associated with an increased risk of non-AIDS clinical events, with odds ratios ranging from 1.4 to 1.8. In this study, higher levels of CMV DNA correlated with elevated levels of multiple inflammatory markers and lower CD4/CD8 ratio [14]. However, in our study very few non-AIDS events were recorded after month 8, and we did not have the power to look at the association between persistent CMV replication and non-AIDS events only. Alternatively, CMV replication could contribute to impaired T-cell reconstitution in patients on ART without a direct role in disease progression.
CMV preemptive therapy might be therefore considered in these patients with persistent CMV viremia to improve clinical outcomes but it remains to be shown whether anti-CMV drugs could have an impact on non-CMV AIDS events, serious non-AIDS events and death. Of interest, Hunt et al have shown that valganciclovir could reduce T-cell activation in patients living with HIV with incomplete CD4+ T-cell recovery on ART [31]. However, according to the low rate of persistent CMV viremia on ART, even in patients with low CD4+ T-cell counts, a large prophylaxis study would be required. Additional studies are therefore warranted to better identify factors associated with persistent CMV viremia under ART and to investigate whether CMV replication is a marker or a determinant of HIV disease progression.
Our study has a number of limitations. Associations between CMV viremia and clinical outcome were limited to a single clinical trial. As it is a purely observational study, no causal relationships could be established between persistent CMV replication and clinical outcome. We also could not assess the role of CMV infection on clinical outcome since the vast majority of our participants were CMV seropositive [3]. Finally, we did evaluate the potential role of high versus low CMV viremia as suggested in previous studies on the risk of clinical outcome and did not find a difference, however our analysis are limited due to small numbers in the groups with CMV viremia and should be interpreted with caution [7].
In conclusion, our study showed that in treatment naïve HIV-positive participants starting ART, baseline CMV viremia is associated with low CD4+ T-cell counts and high HIV RNA levels, but not with overall disease progression or death. However, persistent CMV viremia on ART was associated with a higher rate of clinical outcomes. Further investigations are needed to identify patients who might be eligible for CMV preemptive therapy with the goal of reducing HIV-related morbidity and mortality.
Supplementary Material
Acknowledgements
We thank all the participants without whom our work would not be possible.
This CMV project was funded via Subcontract 13XS134 under Leidos Biomed’s Prime Contract HHSN261200800001E and HHSN261201500003I, NCI/NIAID. Funding for the respective trials is noted in the primary publications; see N Engl J Med 2015; 373:795–807 for the complete list of START investigators; N Engl J Med 2006; 355:2283–96 for the complete list of SMART investigators; and Lancet 2006; 368:2125–35 for the complete list of FIRST investigators.
Footnotes
This work was presented in part at the 2020 Conference on Retroviruses and Opportunistic Infections, Boston, March 8–11, 2020
References
- 1.Gupta A, Nadkarni G, Yang W-T, Chandrasekhar A, Gupte N, Bisson GP, et al. Early Mortality in Adults Initiating Antiretroviral Therapy (ART) in Low- and Middle-Income Countries (LMIC): A Systematic Review and Meta-Analysis. Zhang C, editor. PLoS ONE. 2011;6: e28691. doi: 10.1371/journal.pone.0028691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N Engl J Med. 2017;377: 233–245. doi: 10.1056/NEJMoa1615822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Lo Caputo S, et al. Cytomegalovirus Coinfection Is Associated With an Increased Risk of Severe Non–AIDS-Defining Events in a Large Cohort of HIV-Infected Patients. J Infect Dis. 2015;211: 178–186. doi: 10.1093/infdis/jiu417 [DOI] [PubMed] [Google Scholar]
- 4.Deayton JR, Prof Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet Lond Engl. 2004;363: 2116–2121. doi: 10.1016/S0140-6736(04)16500-8 [DOI] [PubMed] [Google Scholar]
- 5.Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest. 1998;101: 497–502. doi: 10.1172/JCI1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabs DA, Holbrook JT, Van Natta ML, Clark R, Jacobson MA, Kempen JH, et al. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2005;112: 771–779. doi: 10.1016/j.ophtha.2004.10.049 [DOI] [PubMed] [Google Scholar]
- 7.El Amari EB, Combescure C, Yerly S, Calmy A, Kaiser L, Hasse B, et al. Clinical relevance of cytomegalovirus viraemia*,†: Clinical relevance of cytomegalovirus viraemia. HIV Med. 2011;12: 394–402. doi: 10.1111/j.1468-1293.2010.00900.x [DOI] [PubMed] [Google Scholar]
- 8.Durier N, Ananworanich J, Apornpong T, Ubolyam S, Kerr SJ, Mahanontharit A, et al. Cytomegalovirus Viremia in Thai HIV-Infected Patients on Antiretroviral Therapy: Prevalence and Associated Mortality. Clin Infect Dis. 2013;57: 147–155. doi: 10.1093/cid/cit173 [DOI] [PubMed] [Google Scholar]
- 9.DHHS Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Reviewed June 26, 2019. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/cytomegalovirus-disease. Accessed December 26, 2020.
- 10.Spector SA, McKinley GF, Lalezari JP, Samo T, Andruczk R, Follansbee S, et al. Oral Ganciclovir for the Prevention of Cytomegalovirus Disease in Persons with AIDS. N Engl J Med. 1996;334: 1491–1497. doi: 10.1056/NEJM199606063342302 [DOI] [PubMed] [Google Scholar]
- 11.Wohl DA, Kendall MA, Andersen J, Crumpacker C, Spector SA, Feinberg J, et al. Low Rate of CMV End-Organ Disease in HIV-Infected Patients Despite Low CD4+ Cell Counts and CMV Viremia: Results of ACTG Protocol A5030. HIV Clin Trials. 2009;10: 143–152. doi: 10.1310/hct1003-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizushima D, Nishijima T, Gatanaga H, Tsukada K, Teruya K, Kikuchi Y, et al. Preemptive Therapy Prevents Cytomegalovirus End-Organ Disease in Treatment-Naïve Patients with Advanced HIV-1 Infection in the HAART Era. Nevels M, editor. PLoS ONE. 2013;8: e65348. doi: 10.1371/journal.pone.0065348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377: 2433–2444. doi: 10.1056/NEJMoa1706640 [DOI] [PubMed] [Google Scholar]
- 14.Gianella S, Moser C, Vitomirov A, McKhann A, Layman L, Scott B, et al. Presence of asymptomatic cytomegalovirus and Epstein--Barr virus DNA in blood of persons with HIV starting antiretroviral therapy is associated with non-AIDS clinical events: AIDS. 2020;34: 849–857. doi: 10.1097/QAD.0000000000002484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinsztejn B, De Castro N, Arnold V, Veloso VG, Morgado M, Pilotto JH, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14: 459–467. doi: 10.1016/S1473-3099(14)70711-X [DOI] [PubMed] [Google Scholar]
- 16.MacArthur RD, Novak RM, Peng G, Chen L, Xiang Y, Hullsiek KH, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. The Lancet. 2006;368: 2125–2135. doi: 10.1016/S0140-6736(06)69861-9 [DOI] [PubMed] [Google Scholar]
- 17.Lundgren JD, Babiker A, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373: 795–807. doi: 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Sadr WM, Lundgren JD, Neaton JD, Abrams D, Arduino RC, Babiker A, et al. CD4+ Count–Guided Interruption of Antiretroviral Treatment. N Engl J Med. 2006;355: 2283–2296. doi: 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- 19.Gupta CK, Leszczynski J, Gupta RK, Siber GR. An Enzyme Immunoassay Based Micro-neutralization Test for Titration of Antibodies to Human Cytomegalovirus (CMV) and its Correlation with Direct ELISA Measuring CMV IgG Antibodies. Biologicals. 1996;24: 41–49. doi: 10.1006/biol.1996.0004 [DOI] [PubMed] [Google Scholar]
- 20.Doern GV, Robbie L, Marrama L. Comparison of two enzyme immunoassays and two latex agglutination assays for detection of cytomegalovirus antibody. Diagn Microbiol Infect Dis. 1994;20: 109–112. doi: 10.1016/0732-8893(94)90101-5 [DOI] [PubMed] [Google Scholar]
- 21.Skipper C, Schleiss MR, Bangdiwala AS, Hernandez-Alvarado N, Taseera K, Nabeta HW, et al. Cytomegalovirus Viremia Associated With Increased Mortality in Cryptococcal Meningitis in Sub-Saharan Africa. Clin Infect Dis. 2020;71: 525–531. doi: 10.1093/cid/ciz864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery VC, Sabin C, Feinberg JE, Grywacz M, Knight S, Griffiths PD. Quantitative effects of valacyclovir on the replication of cytomegalovirus (CMV) in persons with advanced human immunodeficiency virus disease: baseline CMV load dictates time to disease and survival. The AIDS Clinical Trials Group 204/Glaxo Wellcome 123–014 International CMV Prophylaxis Study Group. J Infect Dis. 1999;180: 695–701. doi: 10.1086/314936 [DOI] [PubMed] [Google Scholar]
- 23.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive Effects of Combined Antiretroviral Therapy on CD4 + T Cell Homeostasis and Function in Advanced HIV Disease. Science. 1997;277: 112–116. doi: 10.1126/science.277.5322.112 [DOI] [PubMed] [Google Scholar]
- 24.Hsu DC, Kerr SJ, Iampornsin T, Pett SL, Avihingsanon A, Thongpaeng P, et al. Restoration of CMV-Specific-CD4 T Cells with ART Occurs Early and Is Greater in Those with More Advanced Immunodeficiency. Sandberg JK, editor. PLoS ONE. 2013;8: e77479. doi: 10.1371/journal.pone.0077479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deayton J, Mocroft A, Wilson P, Emery VC, Johnson MA, Griffiths PD. Loss of cytomegalovirus (CMV) viraemia following highly active antiretroviral therapy in the absence of specific anti-CMV therapy: AIDS. 1999;13: 1203–1206. doi: 10.1097/00002030-199907090-00008 [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, Wit SD, Drummond F, Lane HC, et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients with HIV Infection. Deeks S, editor. PLoS Med. 2008;5: e203. doi: 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW. HIV and Inflammation: Mechanisms and Consequences. Curr HIV/AIDS Rep. 2012;9: 139–147. doi: 10.1007/s11904-012-0118-8 [DOI] [PubMed] [Google Scholar]
- 28.Freeman ML, Mudd JC, Shive CL, Younes S-A, Panigrahi S, Sieg SF, et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clin Infect Dis. 2016;62: 392–396. doi: 10.1093/cid/civ840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith DM, Nakazawa M, Freeman ML, Anderson CM, Oliveira MF, Little SJ, et al. Asymptomatic CMV Replication During Early Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower CD4/CD8 Ratio During HIV Treatment. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63: 1517–1524. doi: 10.1093/cid/ciw612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacre K, Hunt PW, Hsue PY, Maidji E, Martin JN, Deeks SG, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis: AIDS. 2012;26: 805–814. doi: 10.1097/QAD.0b013e328351f780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203: 1474–1483. doi: 10.1093/infdis/jir060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


