Abstract
Signal transducer and activator of transcription 3 (STAT3) mediates signals of various growth factors and cytokines, including interleukin-6 (IL-6). In certain IL-6-responsive cell lines, the stat3 gene is autoregulated by STAT3 through a composite IL-6 response element in its promoter that contains a STAT3-binding element (SBE) and a cyclic AMP-responsive element. To reveal the nature and roles of the stat3 autoregulation in vivo, we generated mice that harbor a mutation in the SBE (stat3mSBE). The intact SBE was crucial for IL-6-induced stat3 gene activation in the spleen, especially in the red pulp region, the kidney, and both mature and immature T lymphocytes. The SBE was not required, however, for IL-6-induced stat3 gene activation in hepatocytes. T lymphocytes from the stat3mSBE/mSBE mice were more susceptible to apoptosis despite the presence of IL-6 than those from wild-type mice. Consistent with this, IL-6-dependent activation of the Pim-1 and junB genes, direct target genes for STAT3, was attenuated in T lymphocytes of the stat3mSBE/mSBE mice. Thus, the tissue-specific autoregulation of the stat3 gene operates in vivo and plays a role in IL-6-induced antiapoptotic signaling in T cells.
Signal transducers and activators of transcription (STATs) have been shown to play key roles in transmitting growth factor and cytokine signals (8, 14, 18; see also reviews in Oncogene [19]). Upon stimulation of the receptors for a variety of cytokines and some growth factors, members of the STAT family, which are present in the cytoplasm in latent form, are recruited to the tyrosine-phosphorylated receptors through their SH2 domains, where they can be activated by the receptor-associated Janus kinases (JAKs), receptor tyrosine kinases, and, in some cases, cytoplasmic tyrosine kinases (3). Tyrosine-phosphorylated STATs form homo- and heterodimers, enter the nucleus, and activate the transcription of target genes by binding to the specific DNA element TTN5AA (9, 35).
STAT3 is involved in various biological responses elicited by the interleukin-6 (IL-6) family of cytokines (14, 15), some growth factors, including epidermal growth factor (EGF), and v-Src (3). STAT3 plays a critical role in IL-6/gp130-induced cell growth and differentiation and in the survival of cultured cells (4, 12, 13, 21, 24, 26, 36, 43). STAT3 is also required for the leukemia inhibitory factor-mediated maintenance of the pluripotency of mouse embryonic stem (ES) cells (2, 28) and for ciliary neurotrophic factor-mediated astrocyte differentiation (44). The stat3 gene has been disrupted in mice by conventional and conditional gene knockout methods. These studies reveal a variety of roles for STAT3 in vivo, including roles in early embryogenesis, IL-6-mediated antiapoptosis in T cells, IL-10-induced repression of inflammatory responses in macrophages, wound healing, development of secondary hair follicles in the skin, and apoptosis of mammary gland epithelial cells (5, 32, 37–39).
Several negative regulatory mechanisms have been postulated, especially at the level of STAT3 activation. A mutation in the SHP2-binding motif, pYSTV, in gp130 in mice causes prolonged activation of STAT3, suggesting that SHP2-mediated signals negatively regulate STAT3 (29). The expression of the mRNA for SOCS3 (suppressor of cytokine signaling 3), a member of the SOCS/JAB/SSI family, was recently shown to inhibit STAT3 activation by binding to the phosphorylated YSTV motif in gp130, causing negative feedback (27, 34). One of the PIAS (protein inhibitor of activated STAT) family proteins, PIAS3, inhibits STAT3's function by binding to dimerized STAT3, thus blocking STAT3's DNA-binding activity (6). Pretreatment with tetradecanoyl phorbol acetate (TPA), which activates extracellular signal-regulated kinase and protein kinase C (PKC), or nerve growth factor (NGF) inhibits the IL-6-induced STAT3 tyrosine phosphorylation (7, 17).
In contrast, a positive regulatory mechanism has been reported only at the level of stat3 gene expression. Treatment of mice with IL-6 increases the level of stat3 mRNA in the liver (1). We have reported that IL-6 induces stat3 mRNA in cell lines through an IL-6 response element in the promoter containing both a low-affinity STAT3-binding element (SBE) and a cyclic AMP-responsive element (CRE) (16). This result suggested that STAT3 is likely to be involved in maintaining the duration and strength of STAT3-mediated signals by activating its own gene expression.
In this study, we addressed the question of whether the autoregulation of stat3 gene activation through the low-affinity SBE could be demonstrated in vivo. We generated a line of mice that harbor a mutation in the low-affinity SBE in the stat3 gene promoter (mSBE) by homologous recombination. The intact SBE was required for IL-6-induced stat3 gene activation in the spleen, particularly in the red pulp region, and in the kidney and T cells, but not in hepatocytes. Furthermore, the autoregulatory activation of the stat3 gene was involved in IL-6-induced T-cell survival.
MATERIALS AND METHODS
Generation of knockin mice.
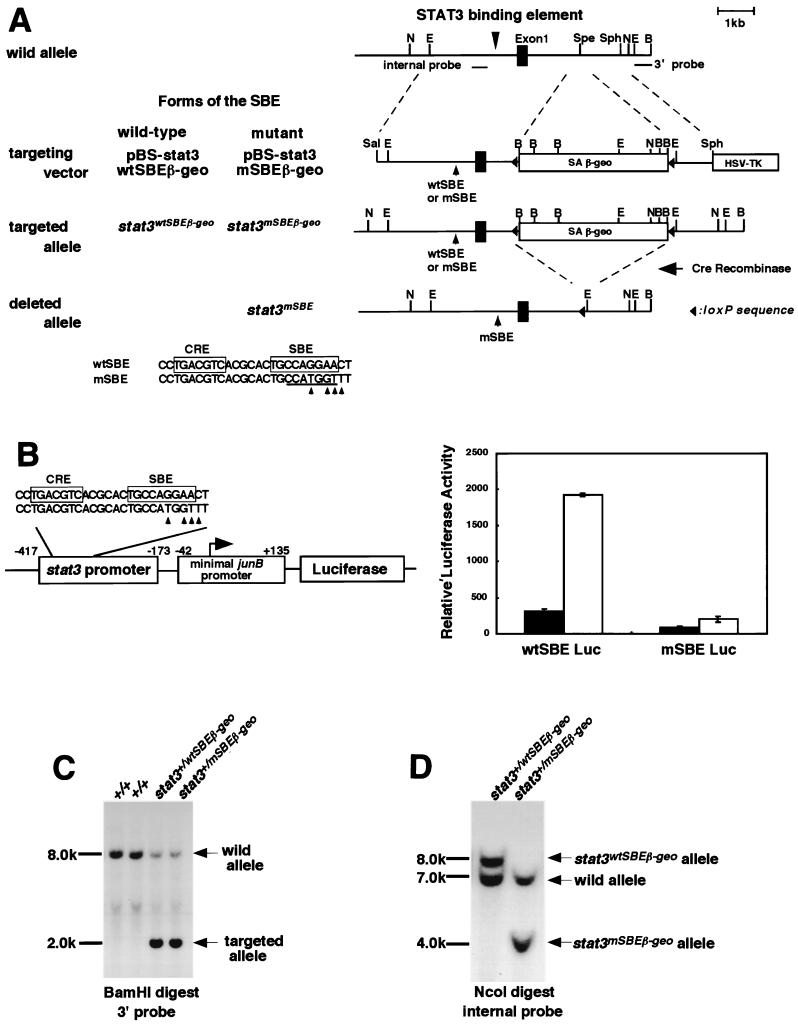
A genomic DNA fragment of the murine stat3 gene was isolated from a λ fix II 129/sv mouse genomic library (Stratagene) using a 2.2-kb genomic fragment of the stat3 promoter as a probe (16). A 7-kb fragment containing the 3 kb of the 5′ region, the first exon, and a part of the first intron was subcloned into the SalI and BamHI sites of pBluescriptII SK+ (Stratagene) to make pBS-stat3. pBS-wtSBE was made by subcloning the 0.25-kb SacI fragment of pBS-stat3, which contains the IL-6 response element, into the SacI site of pBluescriptII SK+. The IL-6 response element is composed of an SBE, located at −338 to −331 upstream of the transcriptional initiation site, and a typical CRE (16). To make pBS-mSBE, a mutation was introduced into the SBE of pBS-wtSBE by PCR-mediated mutagenesis using a QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol; the resulting mutant SBE has an NcoI site. To make pBS-stat3mSBE, the SacI fragment in the stat3 promoter in pBS-stat3 was replaced by the SacI fragment from pBS-mSBE. The primers used for the mutagenesis were 5′-CAC GCA CTG CCA TGG TTT CAG CTG AG-3′ and 5′-CTC AGC TGA-AAC CAT GGC AGT GCG TG-3′.
To generate targeting vectors, a cassette named floxed SAβ-geo, consisting of a splice acceptor (SA), β-galactosidase-neomycin resistance gene (β-geo) (10), and two loxP sites at the 5′ and 3′ ends, was inserted into the SpeI sites in the first intron and a herpes simplex virus thymidine kinase gene was inserted into the SphI and BamHI sites of pBS-stat3 and of pBS-stat3mSBE, resulting in pBS-stat3wtSBEβ-geo and pBS-stat3mSBEβ-geo, respectively (Fig. 1A). R1 ES cells were transfected with pBS-stat3wtβ-geo and pBS-stat3mSBEβ-geo to create the stat3wtSBEβ-geo and stat3mSBEβ-geo alleles, respectively. The transfected cells were selected with G418 at 400 μg/ml and ganciclovir at 2 μM. Homologous recombination in the ES cells was confirmed by Southern blot analysis of BamHI-digested genomic DNA with a 0.5-kb EcoRI-BamHI fragment of pBS-stat3 as a 3′ probe. In this analysis, a 2.0-kb hybridized fragment was detected in DNA samples from ES cells that underwent homologous recombination and an 8.0-kb hybridized fragment was detected from the DNA of ES cells that underwent random integration (Fig. 1C). To detect the stat3mSBEβ-geo allele, NcoI-digested genomic DNA from ES cells with homologous recombination was further subjected to Southern blot analysis using the 0.4-kb SpeI fragment of pBS-stat3 as an internal probe. In this analysis, digestion of the stat3wtSBEβ-geo, stat3mSBEβ-geo, and wild-type alleles created 8.0-, 4.0-, and 7.0-kb hybridized fragments, respectively (Fig. 1D).
FIG. 1.
Generation of knockin mice. (A) Schematic diagram of the endogenous locus for the stat3 gene, knockin vectors, and targeted allele. The knockin vectors have a splice acceptor (SA)-β-geo gene flanked with two loxP sequences in the first intron (pBS-stat3wtβ-geo). The mutant promoter has a mutated STAT3-binding site with an NcoI site (underlined, left panel, at bottom). Arrowheads indicate the bases that were altered. The alleles for the targeting vectors were designated stat3wtSBEβ-geo and stat3mSBEβ-geo, respectively. The SA and β-geo cassette were removed by crossing stat3mSBEβ-geo mice with CAG-cre transgenic mice, resulting in the generation of the stat3mSBE allele (right panel, at bottom). E, EcoRI; N, NcoI; Spe, SpeI; B, BamHI; Sph, SphI; TK, thymidine kinase. (B) Loss of IL-6 responsiveness of the mutated STAT3-binding site. We made reporter constructs containing the minimal junB promoter and the luciferase gene (Luc) with either the wild-type STAT3-binding site from the 5′ region of the stat3 gene, wtSBE Luc, or the mutated binding site, mSBE Luc (left). These were transiently transfected into HepG2 cells and stimulated with IL-6 (open bars) for 5 h or left unstimulated (solid bars). Luciferase activity was normalized to β-galactosidase activity, and averages from triplicate experiments are shown (right). (C) Southern blot analysis of targeted ES clones. BamHI-digested DNA from wild-type ES clones (+/+), targeted clones with the wild-type promoter (stat3+/wtSBEβ-geo), and the mutated promoter (stat3+/mSBEβ-geo) were hybridized with the 3′ probe shown in A (right panel, at top). (D) Detection of the mutated STAT3-binding site by Southern blot. NcoI-digested DNA from stat3+/wtSBEβ-geo and stat3+/mSBEβ-geo ES clones was hybridized with the internal probe shown in A (right panel, at top).
Targeted ES cell clones were injected into C57BL/6 mouse blastocysts to create chimeric male founders. Chimeric mice were mated to C57BL/6 to generate F1 heterozygous mice that harbored a stat3wtSBEβ-geo or stat3mSBEβ-geo allele. F1 stat3+/mSBEβ-geo heterozygous mice were mated with CAG-cre transgenic mice that ubiquitously expressed Cre recombinase (31) so that the floxed SAβ-geo fragment was deleted from the stat3mSBEβ-geo allele, generating heterozygous mice with the stat3+/mSBE genotype. The presence of the stat3mSBE allele was determined by Southern blot analysis using genomic DNA digested with EcoRI or NcoI and the internal probe. The wild-type, stat3mSBEβ-geo, and stat3mSBE alleles generated, respectively, a 6.0-, 9.2-, and 4.0-kb hybridized fragment in the EcoRI digestion. The wild-type and stat3mSBE alleles generated a 7.0- and a 4.0-kb fragment in the NcoI digestion, respectively. F2 stat3+/mSBE mice were intercrossed to generate F3 homozygous mice with a stat3mSBE/mSBE genotype.
To test the ability of the SacI fragments bearing either the mSBE or the wild-type SBE to respond to IL-6 in HepG2 cells, each 0.25-kb SacI fragment containing wtSBE or mSBE was inserted into pSP-Luc upstream of the minimal junB promoter linked with the luciferase gene (22). HepG2 cells were transfected with DNA mixtures by the standard calcium phosphate precipitation method. Typically, 1.2 μg of one of the reporter plasmids, 1 μg of pEFLacZ, a pEF-BOS expression vector containing the lacZ gene encoding β-galactosidase as an internal control for transfection efficiency, and 3 μg of pEF-BOS, as carrier DNA, were used. Forty-two hours after the transfection, cells were stimulated with IL-6 (100 ng/ml) for 6 h, harvested, and subjected to assays for luciferase and β-galactosidase activity as described previously (22).
β-Galactosidase staining, measurement of β-galactosidase activity, and immunohistochemistry.
For β-galactosidase staining, tissue samples were fixed in 2% formaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline (PBS) containing 0.1% NP-40 for 30 min and frozen in OCT compound (Sakura Finetechnical). Ten-micrometer-thick frozen sections were prepared and stained with 0.1% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) in PBS solution containing 2 mM MgCl2, 5 mM K3Fe(CN6), and 5 mM K4Fe(CN6) at room temperature for the indicated period of time. Counterstaining was done with Nuclear Fast Red (Vector).
For immunohistochemistry, 7-μm-thick frozen sections were air dried and subsequently fixed with 4% paraformaldehyde in PBS. The sections were blocked with 20% goat and donkey serum and incubated with 1:200 diluted rat anti-CD31 antibody (clone 390; PharMingen), allophycocyanin (APC)-conjugated rat anti-CD11b antibody (clone M1/70; PharMingen), APC-conjugated rat anti-CD45R antibody (clone RA3-6B2; PharMingen), biotinylated rat anti-CD4 antibody (clone H129.19; PharMingen), or rabbit anti-β-galactosidase antibody (Cappel). After washing, the sections that had been incubated with primary antibody against β-galactosidase were stained with Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G) (IgG; Molecular Probes), and the sections incubated with primary antibody against CD31 were reacted with biotinylated donkey anti-rat IgG (Jackson ImmunoResearch). Sections were then washed with 0.05% Triton X-100 in PBS and stained with rhodamine-conjugated avidin for biotinylated antibody.
The β-galactosidase activity of each tissue homogenate (30 μg of protein) was measured in triplicate, and relative activity was obtained by dividing the average activity of +/stat3mSBEβ-geo mice with that of +/stat3wtSBEβ-geo mice without IL-6 stimulation.
Northern blot analysis.
Total RNA was extracted using the Sepazol I reagent (Nakarai-tesque). Twenty micrograms of total RNA was fractionated on 1% agarose gels containing formaldehyde and transferred to Hybond N+ membranes (Amersham Pharmacia Biotech). The membranes were hybridized with 32P-labeled cDNA fragments overnight, washed three times with 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 56°C and subjected to autoradiography. The amounts of RNA loaded were verified by ethidium bromide staining. The probes used were the 2.5-kb SalI-BamHI fragment of pBSHA-stat3 containing the stat3 cDNA (24), a 1.0-kb EcoRI-XhoI fragment of Pim-1 cDNA (36), and a 1.5-kb EcoRI fragment of junB cDNA.
Preparation of lymph node and splenic T cells.
Splenocytes were treated with 0.165 M NH4Cl to lyse erythrocytes. Splenic T and lymph node T cells were enriched with anti-CD90 antibody-conjugated magnetic beads (Miltenyi biotec) and a Mini MACS column (Miltenyi biotec). The purity of the isolated T cells was determined by staining cells with a phycoerythrin-conjugated anti-CD3 monoclonal antibody (145-2C11; PharMingen), and more than 95% of the cells were CD3 positive.
T-cell proliferation.
Cells were cultured with RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2-mercaptoethanol (2-ME, 50 μM), penicillin (50 μg/ml), and streptomycin (50 μg/ml). Thymocytes (5 × 105 cells), splenic T cells, and lymph node T cells (105 cells) were cultured in 96-well plates and stimulated with human recombinant IL-6 (10 ng/ml) and an anti-CD3 monoclonal antibody (145-2C11, 100 ng/ml) for 72 h. The cultured cells were pulsed for the last 6 h with 0.5 μCi of 3H-labeled thymidine per well, followed by scintillation counting.
Cell death analysis.
Splenic T or lymph node T cells (2 × 105 cells) were cultured in 96-well plates and stimulated with human recombinant IL-6 (10 ng/ml) and the anti-CD3 antibody (100 ng/ml) for 24 h. The cells were stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) (ApoAlert annexin V-FITC apoptosis kit; Clontech) according to the manufacturer's protocol. Flow cytometric analysis was performed with a FACScalibur flow cytometer and Cell Quest software (Becton Dickinson).
RESULTS
Generation of mice harboring a stat3 gene with a wild-type or mutant SBE in the stat3 gene promoter and a β-galactosidase-expressing cassette in the first intron.
To examine whether the autoregulatory mechanism of the stat3 gene operates in vivo and to demonstrate its biological role, we designed two targeting constructs, one with a wild-type stat3 promoter and one with the stat3 promoter containing a mutant SBE. In the first intron, the constructs carried the β-galactosidase-neomycin resistance fusion gene with a polyadenylation signal (β-geo) linked with a splice acceptor site (SAβ-geo) at the 5′ end and flanked with two loxP sequences (Fig. 1A). When correctly targeted by homologous recombination, the promoter activity could be monitored by assaying the β-galactosidase activity. For the mutant SBE, we selected a point mutation generating an NcoI site (mSBE, Fig. 1A and 1B) in the SBE. The effectiveness of the mutation was verified by a transient-transfection assay using HepG2 cells and the reporter gene constructs with or without the mutated SBE. As shown in Fig. 1B, the mutant SBE effectively lost its IL-6 responsiveness. ES cells with the correctly targeted gene were selected by both Southern blot analysis (Fig. 1C and 1D) and reverse transcription (RT)-PCR analysis, which allowed us to detect the mRNA containing the first exon and the correctly spliced sequence for β-galactosidase (data not shown). Using these ES clones, we generated hetero-stat3wtSBEβ-geo and -stat3mSBEβ-geo mice. Heterozygous stat3wtSBEβ-geo and stat3mSBEβ-geo mice were viable and fertile. Embryos with homozygous stat3wtSBEβ-geo alleles died in utero (data not shown), and their phenotypes were similar to those of the stat3-deficient mice reported previously (39), suggesting that the introduction of the SAβ-geo cassette disrupted the stat3 gene.
Promoter activity of wild-type and mutant stat3 promoters in mouse tissues.
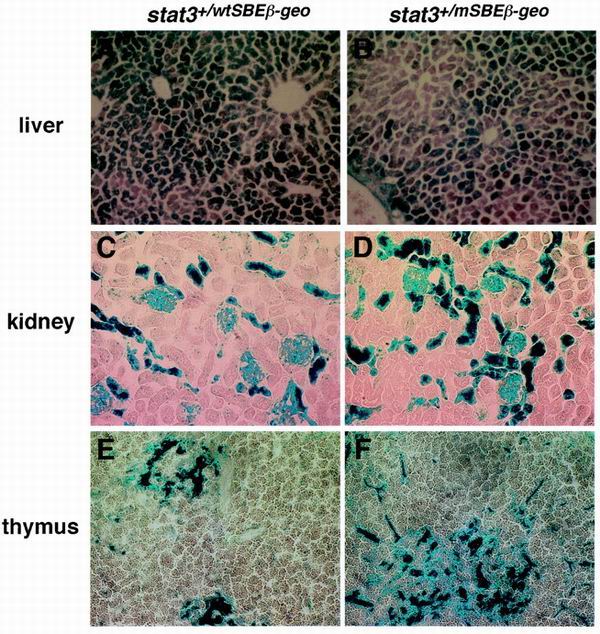
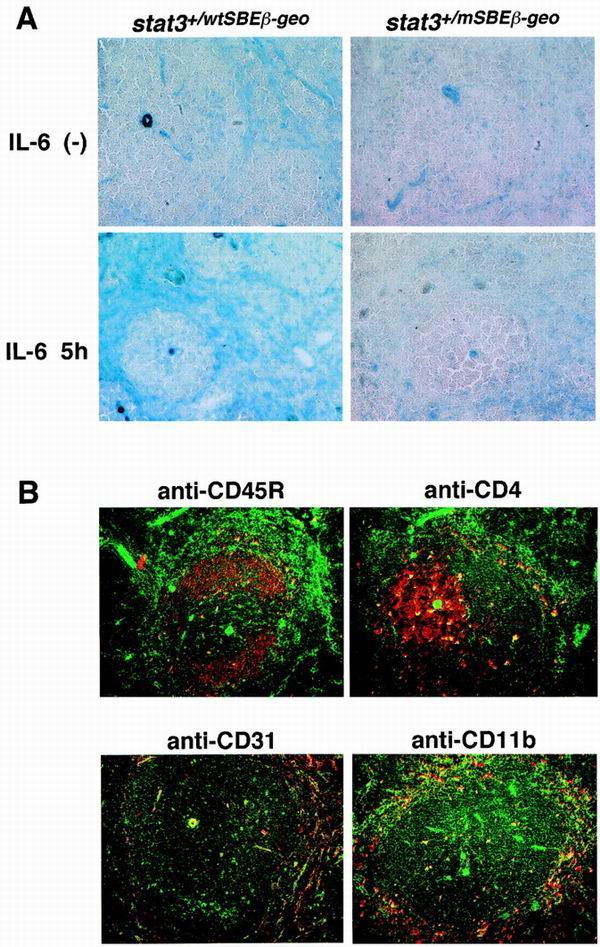
To examine in vivo the promoter activities of the wild-type and mutant mSBE stat3 promoters, histological sections of various tissues from the stat3+/wtSBEβ-geo and stat3+/mSBEβ-geo mice were subjected to β-galactosidase staining. Positive staining for β-galactosidase was easily detected in hepatocytes and in the kidney. In the kidney, the cells having β-galactosidase activity were some renal tubules and glomeruli (Fig. 2C and D). Arterial walls were also positive for β-galactosidase (data not shown). In the thymus, the cells containing detectable levels of enzymatic activity were located mainly in the medulla regions, and most of them appeared to be nonlymphoid cells, although we do not neglect that thymocytes expressed undetectable levels of enzymatic activities (Fig. 2E and F). There was no apparent difference in the expression patterns between the stat3+/wtSBEβ-geo and stat3+/mSBEβ-geo mice (Fig. 2), although we observed some quantitative difference in β-galactosidase activity of whole tissues between the stat3+/wtSBEβ-geo and stat3+/mSBEβ-geo mice: the basal activities of β-galactosidase in the lysates of the liver and kidney from stat3+/mSBEβ-geo mice were 74.2 and 65.5% of the average activity of stat3+/wtSBEβ-geo, respectively. We did not observe a difference in the basal activity in the lysate of the thymus between the stat3+/wtSBEβ-geo mice and stat3+/mSBEβ-geo mice. These results indicate that the mutation in the SBE affects the basal stat3 promoter activity slightly in liver and kidney, but not thymus (Fig. 2A to 2F). In the spleen, the cells having detectable levels of β-galactosidase activity were found mainly in the red pulp region (Fig. 3A) and the perifollicular tissue of the lymph nodes (data not shown). In the stat3+/mSBEβ-geo mice, the cells having detectable levels of β-galactosidase were rare (Fig. 3A). The β-galactosidase activity of the whole spleen lysate was also reduced in the stat3+/mSBEβ-geo mice (40.1% of that of stat3+/wtSBEβ-geo mice). Furthermore, intravenous injection of IL-6 enhanced the β-galactosidase activity in the red pulp region of the spleen of stat3+/wtSBEβ-geo mice but not of the stat3+/mSBEβ-geo mice (Fig. 3A). These results indicate that the autoregulation of the stat3 gene through the SBE is important in cells of the red pulp region for both basal and IL-6-induced expression. To identify these cells, we performed double staining of spleen sections with an anti-β-galactosidase antibody and antibodies recognizing cell type-specific markers. As shown in Fig. 3B, the cells expressing detectable levels of β-galactosidase colocalized with CD31- or CD11b-positive cells but not CD45R- or CD4-positive cells. These data suggest that the SBE-dependent autoregulation of the stat3 gene is operating in endothelial cells or macrophages of peripheral lymphoid organs.
FIG. 2.
Promoter activities of the stat3 gene in vivo. β-Galactosidase activities were determined by X-Gal staining. Frozen sections were obtained from stat3+/wtSBEβ-geo (left panel) and stat3+/mSBEβ-geo (right panel) mice. X-Gal staining was performed with sections from the liver (A and B), kidney (C and D), and thymus (E and F) by incubation with a 0.1% X-Gal solution overnight at room temperature.
FIG. 3.
Promoter activity of the stat3 gene in the spleen. (A) Expression of β-galactosidase driven by the stat3 promoter in the spleen. IL-6 (30 μg) was injected intravenously into 6-week-old stat3+/wtSBEβ-geo and stat3+/mSBEβ-geo mice (lower panels), or mice were left untreated (upper panels). Five hours after the injection, the mice were sacrificed and their spleens were removed and sectioned. The expression of β-galactosidase was detected by X-Gal staining for 5 h.(B) Detection of stat3 promoter activity in CD31- and CD11b-positive cells. A section of the spleen from stat3+/wtSBEβ-geo mice was doubly stained with anti-β-galactosidase (green) and antibodies recognizing cell surface markers for CD4, CD45R, CD31, and CD11b (red). The fluorescence images are shown superimposed.
Tissue-specific role for the SBE in basal and IL-6-induced stat3 gene expression in vivo
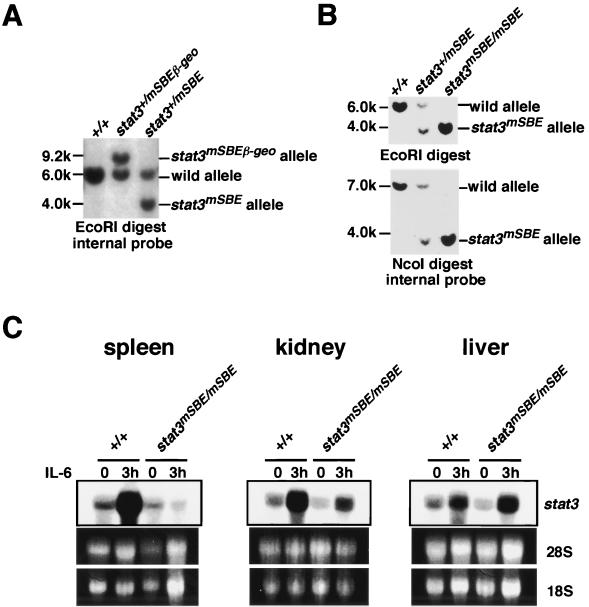
To examine the effect of the SBE mutation in endogenous stat3 expression in vivo, we generated mice with a mutant stat3 promoter in both alleles. To do so, the floxed SAβ-geo cassette was deleted by Cre recombinase by crossing the stat3+/mSBEβ-geo mice with CAG-cre transgenic mice (31), generating mice carrying the stat3mSBE allele heterozygously (Fig. 1A, 4A, and 4B). Homozygous stat3mSBE mice (stat3mSBE/mSBE) were generated by crossing the heterozygous mice with each other. One-quarter of the mice born were stat3mSBE/mSBE, in accord with Mendelian expectations, and grew normally, suggesting that autoregulation of the stat3 gene through the SBE is not essential for development.
FIG. 4.
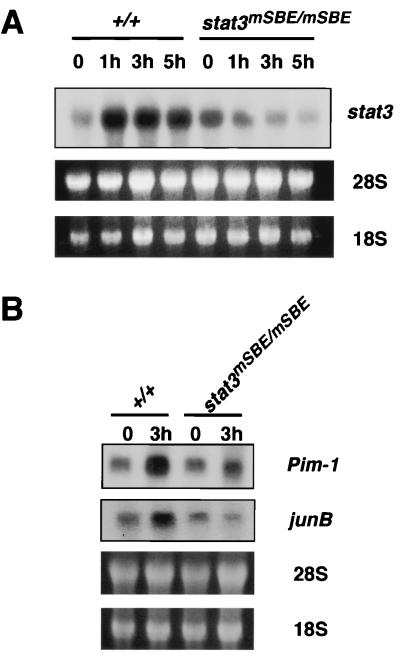
Generation of stat3mSBE/mSBE mice. (A and B) Southern blot analysis of the stat3mSBE allele. The stat3+/mSBE mice were generated by mating stat3+/mSBEβ-geo mice with CAG-cre transgenic mice (A), and stat3mSBE/mSBE mice were generated by intercrossing the stat3+/mSBE mice (B). Genomic DNA from wild-type and mutant mice was digested with either EcoRI (A and B, upper panel) or NcoI (B, lower panel) and subjected to Southern blot analysis with the internal probe (see Fig. 1A, right panel at top). (C) Induction of stat3 mRNA in stat3mSBE/mSBE mice. IL-6 was injected intravenously into wild-type and stat3mSBE/mSBE mice. Three hours after the injection, the mice were sacrificed, and the spleen, kidney, and liver were removed to analyze the levels of stat3 mRNA by Northern blot analysis. The amounts of RNA loaded were evaluated with ethidium bromide staining of 28S and 18S rRNAs.
We first compared the expression levels of stat3 mRNA in various tissues of wild-type and stat3mSBE/mSBE mice with and without stimulation with IL-6. The stat3 mRNA levels increased 3 h after injection of IL-6 in the spleen, kidney, and liver of the wild-type littermates (Fig. 4C). The stat3 mRNA levels were also upregulated in thymocytes in response to IL-6 in vitro (Fig. 5A). The IL-6-dependent stat3 mRNA induction was severely perturbed in the spleen, kidney, and thymocytes from the stat3mSBE/mSBE mice, but was preserved in the liver (Fig. 4C and 5A). These data indicate that IL-6 regulation of the stat3 gene through the SBE takes place in a variety of organs, tissues, and cells. In some organs or tissues such as the liver, however, the SBE is not critically involved in IL-6-induced stat3 gene activation, suggesting the presence of an alternative mechanism that regulates stat3 expression.
FIG. 5.
Low IL-6 responses of stat3 gene and target genes for STAT3, Pim-1, and junB in thymocytes from stat3mSBE/mSBE mice. (A) stat3 mRNA expression in thymocytes of stat3mSBE/mSBE mice. Thymocytes from wild-type and stat3mSBE/mSBE mice were stimulated in vitro with IL-6 (100 ng/ml) for the indicated periods. Total RNA was examined for stat3 mRNA expression levels by Northern blot analysis. Even loading was evaluated with ethidium bromide staining of 28S and 18S rRNAs. (B) Expression of Pim-1 and junB mRNAs, two target genes for STAT3. Total RNA was prepared from thymocytes that were obtained from wild-type and stat3mSBE/mSBE mice, without (lanes 0) or with stimulation of IL-6 in vitro for 3 h. The RNA samples were examined for Pim-1 and junB mRNA expression by Northern blot analysis.
Antiapoptosis role for stat3 autoregulation in T cells.
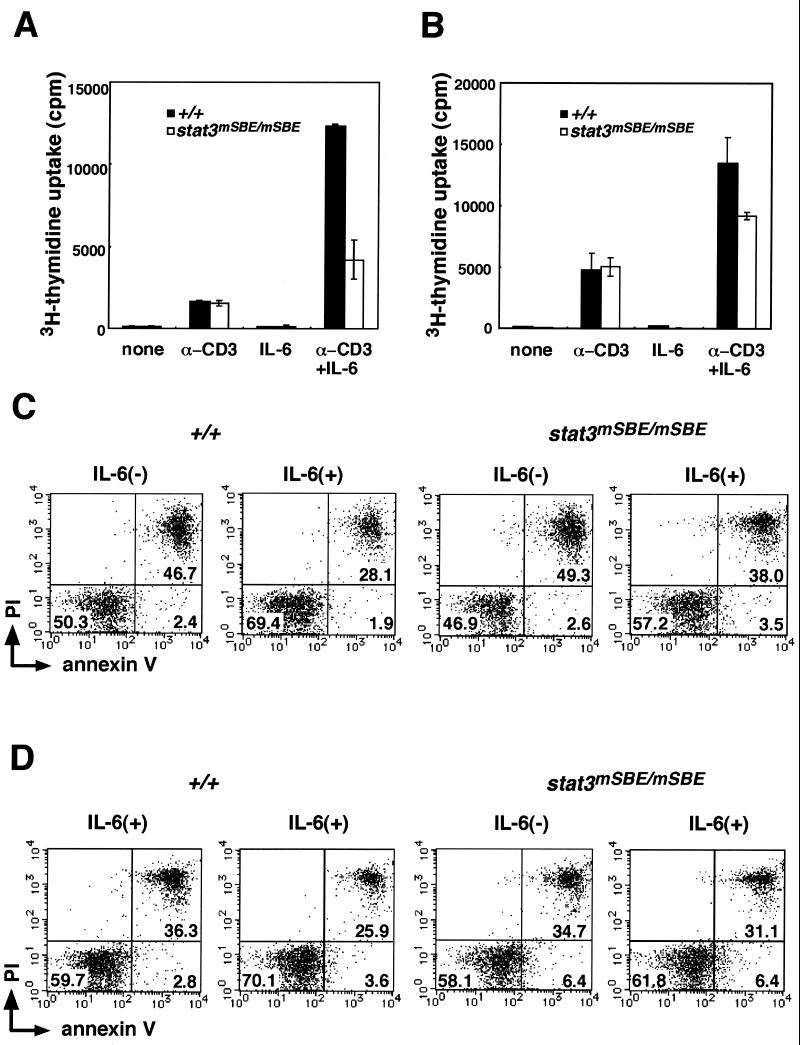
Because the IL-6-induced stat3 mRNA expression in thymocytes was dependent on the intact SBE (Fig. 5A), we next examined the role played by stat3 gene autoregulation in T cells, where we evaluated the IL-6-induced mRNA expression profile, DNA synthesis, and cell survival. T cells were obtained from the thymus, lymph nodes, and spleen of wild-type and stat3mSBE/mSBE mice. We first analyzed the mRNA levels of the junB and Pim-1 genes, which act immediately downstream of the gp130-mediated STAT3 signal (11, 22, 23, 36). Pim-1 has been reported to be involved in gp130-mediated cell proliferation and survival (36). As shown in Fig. 5B, after 3 h of IL-6 stimulation in vitro, the junB and Pim-1 mRNAs were elevated in the thymocytes from wild-type mice, whereas their induction was markedly reduced in the thymocytes from the stat3mSBE/mSBE mice (Fig. 5B). We next analyzed the effects of disrupting stat3 gene autoregulation on IL-6-induced DNA synthesis and cell survival. As shown in Fig. 6A and B, the anti-CD3 antibody given alone elicited similar DNA synthesis responses in the thymocytes and splenic T cells from both wild-type and stat3mSBE/mSBE mice. In contrast, costimulation with the anti-CD3 antibody and IL-6 consistently elicited less DNA synthesis in the thymocytes and splenic T cells from the stat3mSBE/mSBE mice than in those from wild-type mice.
FIG. 6.
SBE-mediated stat3 autoregulation is involved in IL-6-dependent proliferation and survival of T cells. (A and B) Proliferation of T cells from stat3mSBE/mSBE mice. Thymocytes (A) and splenic T cells (B) from wild-type (solid bars) and stat3mSBE/mSBE mice (open bars) were stimulated with anti-CD3 antibody and/or IL-6 for 72 h or left unstimulated (none). Proliferation of these cells was analyzed by labeling them with [3H]thymidine and measuring the incorporated [3H]thymidine with a liquid scintillation counter. (C and D) IL-6-dependent survival of T cells from stat3mSBE/mSBE mice. Splenic T cells (C) and LN T cells (D) were isolated and cultured with or without IL-6 for 24 h. The cells were stained with PI and FITC-conjugated annexin V and subjected to flow cytometric analysis. The data are divided into quadrants, and the percentage of the entire cell population of the following fractions is indicated in the following quadrants: PI high and annexin V high, PI low and annexin V low, and PI low and annexin V high. There were very few cells that were PI high and annexin V low, and no percentage is given for that quadrant. Representative data from three independent experiments are shown.
To examine the IL-6-induced protection of T cells from apoptosis, we cultured splenic (Fig. 6C) or lymph node (LN) T cells (Fig. 6D) for 24 h in the presence or absence of IL-6, then stained them with annexin V and PI to detect apoptotic cells. Consistent with previous reports (40, 41), IL-6 reduced the number of apoptotic T cells (i.e., those that stained strongly with both annexin V and PI) from the spleen and LNs of wild-type mice. In contrast, the IL-6-induced protection of T cells from apoptosis was diminished in T cells from the stat3mSBE/mSBE mice. The decrease in the frequency of apoptotic cells by IL-6 was significantly higher in the wild-type mice (splenic T, 15.8% ± 2.1%; LN T, 14.7% ± 2.5%) than in the SBE mutant mice (splenic T, 9.1% ± 2.4%; LN T, 6.9% ± 3.3% [P < 0.05]), indicating that the IL-6-induced antiapoptotic function requires autoregulatory stat3 gene activation.
Taken together, these observations indicate that the IL-6-dependent gene expression, DNA synthesis, and protection from apoptosis in T cells require, at least in part, the autoregulation mechanism which is mediated through the low-affinity SBE in the stat3 gene promoter.
DISCUSSION
In this report, we provide the first evidence that the autoregulatory mechanism for stat3 gene activation through the low-affinity SBE in the promoter is critically required for IL-6-induced stat3 gene activation in a tissue-specific manner. The tissues and cells that required the autoregulatory mechanism for the IL-6-induced stat3 gene activation through the SBE were the kidney and spleen, especially the CD31- or CD11b-positive cells that are abundant in the spleen red pulp and the immature and mature T cells from the thymus, spleen, and LNs. Moreover, we showed that the autoregulatory loop of the stat3 gene activation is involved in the IL-6-induced protection from apoptosis and proliferation in both mature and immature T cells and for the maintenance in thymocytes of the mRNA expression of two STAT3 target genes, junB and Pim-1. In contrast, the SBE was not involved in IL-6-induced stat3 gene activation in hepatocytes. This result is not consistent with previous findings that the SBE is required for IL-6-induced activation of the stat3 gene promoter in HepG2 human hepatoma cells (16) (see also Fig. 1B). We do not yet understand the cause of this discrepancy. IL-6 generates various signals through gp130, including the STAT3-mediated signal and Ras-ERK mitogen-activated protein kinase (MAP kinase) cascade (15), and the latter may be involved in IL-6-induced stat3 gene activation in the liver. It is also possible that STAT3 may activate the stat3 gene in hepatocytes through an unidentified STAT3-binding site(s) in the stat3 promoter or some other region. STAT3 has been reported to interact with other transcription factors directly or indirectly, such as c-Jun, p300, and Smad1 (25, 33), and such STAT3 complexes may be involved in regulation of stat3 in the liver. It is also not known what determines tissue specificity in the autoregulatory mechanism. Elucidation of the IL-6-responsive element for stat3 expression in the liver will reveal this point. In any case, our data provide evidence that the stat3 gene is autoregulated by a tissue-specific mechanism.
The cells expressing detectable levels of β-galactosidase in the red pulp of the spleen colocalized with CD31- and CD11b-positive cells. Because these cells were abundant in the red pulp and the shapes of the stained cells were streaky in many cases, they were likely to be endothelial cells and macrophages. The SBE mutation reduced the basal level of stat3 expression as well as the IL-6-activated promoter activity in these cells (Fig. 2A). These cells may therefore function as a sensor, detecting low levels of circulating IL-6 in the blood. Alternatively, considering that splenic endothelial cells produce IL-6 in vitro, they may respond to IL-6 that is produced in an autocrine manner (40). It has been reported that IL-6 acts on endothelial cells to increase their ability to adhere to lymphocytes by enhancing their expression of ICAM-1, VCAM-1, and E-selectin (30, 42), and a combination of IL-6 and soluble IL-6 receptor has been shown to stimulate endothelial cells to activate STAT3, induce MCP-1 expression, and augment ICAM-1 expression (30). Therefore, STAT3-mediated signals are likely to be involved in enhancing the adhesion for and transendothelial migration of lymphocytes by increasing the expression levels of certain adhesion molecules and chemokines. It has also been reported that STAT3-deficient macrophages showed high sensitivity to lipopolysaccharide (LPS) (37). It may be that stat3 autoregulation is also involved in the regulation of activation of macrophages.
Although the mutation in the SBE inhibited the autoregulation of the stat3 gene in several tissues, as described above, the development and function of those tissues in the stat3mSBE/mSBE mice were not significantly affected (data not shown). These results suggest that although the autoregulation of the stat3 gene can modify some of the IL-6-induced responses, it is not essential for the development and maintenance of most tissues.
Some transcription factors other than STAT3 have been shown to autoregulate their own genes. c-Jun together with ATF-2 activates the c-jun gene (20). MyoD1 activates the MyoD1 gene promoter through two proximal E-boxes located close to the MyoD1 core promoter (45). The autoregulation of genes by their own products provides a positive feedback regulation mechanism that may amplify the intensity and prolong the duration of signals.
When and in what situation is the autoregulation of stat3 required? To elucidate these, we have examined the LPS-induced production of nitric oxide and cytokines using peritoneal macrophages. We have not yet found significant differences in those assays between the wild-type and mutant mice (unpublished observation). However, in other conditions and/or in other assays, we might see differences. Next, we examined the status of T cells of stat3mSBE/mSBE mice in an IL-6-stimulated condition. Proliferation of thymocytes and splenic T cells in response to costimulation with anti-CD3 and IL-6 was significantly reduced (Fig. 6A), and IL-6-mediated prevention of the apoptosis of splenic and lymph node T cells was perturbed in stat3mSBE/mSBE mice. This situation is similar to that of T cells defective in stat3 expression, in which IL-6-induced cell proliferation is severely impaired (38). These data indicate that the proliferation of T cells in response to IL-6 requires the SBE-mediated autoregulation of the stat3 gene. Correlated with this, the IL-6-dependent expression of Pim-1 and junB was attenuated in T cells from the stat3mSBE/mSBE mice (Fig. 5B). In summary, we provide evidence that stat3 is regulated by the autoregulatory mechanism in vivo. It seems likely that this mechanism is involved in other biological responses to IL-6 or other factors that activate STAT3 in a variety of tissues.
ACKNOWLEDGMENTS
We thank R. Masuda and A. Kubota for secretarial assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan and the Osaka Foundation for Promotion of Clinical Immunology. M.N. and K.N. are Research Fellows of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Akira S, Nishio Y, Inoue M, Wang X J, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 2.Boeuf H, Hauss C, Graeve F D, Baran N, Kedinger C. Leukemia inhibitory factor-dependent transcriptional activation in embryonic stem cells. J Cell Biol. 1997;138:1207–1217. doi: 10.1083/jcb.138.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 4.Catlett-Falcone R, Landowski T H, Oshiro M M, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna J L, Nunez G, Dalton W S, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 5.Chapman R S, Lourenco P C, Tonner E, Flint D J, Selbert S, Takeda K, Akira S, Clarke A R, Watson C J. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13:2604–2616. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung C D, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 7.Chung J, Uchida E, Grammer T C, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 9.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 11.Fujitani Y, Nakajima K, Kojima H, Nakae K, Takeda T, Hirano T. Transcriptional activation of the IL-6 response element in the junB promoter is mediated by multiple Stat family proteins. Biochem Biophys Res Commun. 1994;202:1181–1187. doi: 10.1006/bbrc.1994.2053. [DOI] [PubMed] [Google Scholar]
- 12.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 13.Fukada T, Ohtani T, Yoshida Y, Shirogane T, Nishida K, Nakajima K, Hibi M, Hirano T. STAT3 orchestrates contradictory signals in cytokine-induced G1 to S cell-cycle transition. EMBO J. 1998;17:6670–6677. doi: 10.1093/emboj/17.22.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 15.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 16.Ichiba M, Nakajima K, Yamanaka Y, Kiuchi N, Hirano T. Autoregulation of the Stat3 gene through cooperation with a cAMP-responsive element-binding protein. J Biol Chem. 1998;273:6132–6138. doi: 10.1074/jbc.273.11.6132. [DOI] [PubMed] [Google Scholar]
- 17.Ihara S, Nakajima K, Fukada T, Hibi M, Nagata S, Hirano T, Fukui Y. Dual control of neurite outgrowth by STAT3 and MAP kinase in PC12 cells stimulated with interleukin-6. EMBO J. 1997;16:5345–5352. doi: 10.1093/emboj/16.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 19.Jove R. STAT signaling. Oncogene. 2000;19:2415–2616. doi: 10.1038/sj.onc.1203549. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 21.Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima H, Nakajima K, Hirano T. IL-6-inducible complexes on an IL-6 response element of the junB promoter contain Stat3 and 36 kDa CRE-like site binding protein(s) Oncogene. 1996;12:547–554. [PubMed] [Google Scholar]
- 23.Nakajima K, Kusafuka T, Takeda T, Fujitani Y, Nakae K, Hirano T. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol Cell Biol. 1993;13:3027–3041. doi: 10.1128/mcb.13.5.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 26.Narimatsu M, Nakajima K, Ichiba M, Hirano T. Association of Stat3-dependent transcriptional activation of p19INK4D with IL-6-induced growth arrest. Biochem Biophys Res Commun. 1997;238:764–768. doi: 10.1006/bbrc.1997.7387. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson S E, De Souza D, Fabri L J, Corbin J, Willson T A, Zhang J G, Silva A, Asimakis M, Farley A, Nash A D, Metcalf D, Hilton D J, Nicola N A, Baca M. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, Itoh S, Narimatsu M, Maeda H, Fukada T, Itoh M, Okano H, Hibi M, Hirano T. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 30.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 31.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 32.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer T S, Sanders L K, Nathans D. Cooperative transcriptional activity of Jun and Stat3 beta, a short form of Stat3. Proc Natl Acad Sci USA. 1995;92:9097–9101. doi: 10.1073/pnas.92.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz J, Weissenbach M, Haan S, Heinrich P C, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J Biol Chem. 2000;275:12848–12856. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 35.Seidel H M, Milocco L H, Lamb P, Darnell J E, Jr, Stein R B, Rosen J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc Natl Acad Sci USA. 1995;92:3041–3045. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirogane T, Fukada T, Muller J M, Shima D T, Hibi M, Hirano T. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999;11:709–719. doi: 10.1016/s1074-7613(00)80145-4. [DOI] [PubMed] [Google Scholar]
- 37.Takeda K, Clausen B E, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 38.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 39.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teague T K, Marrack P, Kappler J W, Vella A T. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–5796. [PubMed] [Google Scholar]
- 41.Teague T K, Schaefer B C, Hildeman D, Bender J, Mitchell T, Kappler J W, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson C, Whittaker S, Smith N, Vora A J, Dumonde D C, Brown K A. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 1996;105:112–119. doi: 10.1046/j.1365-2249.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamanaka Y, Nakajima K, Fukada T, Hibi M, Hirano T. Differentiation and growth arrest signals are generated through the cytoplasmic region of gp130 that is essential for Stat3 activation. EMBO J. 1996;15:1557–1565. [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagisawa M, Nakashima K, Taga T. STAT3-mediated astrocyte differentiation from mouse fetal neuroepithelial cells by mouse oncostatin M. Neurosci Lett. 1999;269:169–172. doi: 10.1016/s0304-3940(99)00447-4. [DOI] [PubMed] [Google Scholar]
- 45.Zingg J M, Pedraza-Alva G, Jost J P. MyoD1 promoter autoregulation is mediated by two proximal E-boxes. Nucleic Acids Res. 1994;22:2234–2241. doi: 10.1093/nar/22.12.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]