Abstract
To investigate the transcriptional program underlying thyroid hormone (T3)-induced cell proliferation, cDNA microarrays were used to survey the temporal expression profiles of 4,400 genes. Of 358 responsive genes identified, 88% had not previously been reported to be transcriptionally or functionally modulated by T3. Partitioning the genes into functional classes revealed the activation of multiple pathways, including glucose metabolism, biosynthesis, transcriptional regulation, protein degradation, and detoxification in T3-induced cell proliferation. Clustering the genes by temporal expression patterns provided further insight into the dynamics of T3 response pathways. Of particular significance was the finding that T3 rapidly repressed the expression of key regulators of the Wnt signaling pathway and suppressed the transcriptional downstream elements of the β-catenin–T-cell factor complex. This was confirmed biochemically, as β-catenin protein levels also decreased, leading to a decrease in the transcriptional activity of a β-catenin-responsive promoter. These results indicate that T3-induced cell proliferation is accompanied by a complex coordinated transcriptional reprogramming of many genes in different pathways and that early silencing of the Wnt pathway may be critical to this event.
Thyroid hormone (3,3′,5-triiodo-l-thyronine [T3]) receptors (TRs) are ligand-dependent transcription factors which are members of the steroid hormone/retinoic acid (RA) receptor superfamily. Two TR genes, TRα and TRβ, located on chromosomes 17 and 3, respectively, give rise, by alternative splicing, to three T3-binding TR isoforms, α1, β1, and β2 (reviewed by Cheng [12]). TRs mediate the biological activities of T3 by binding to specific DNA sequences known as the T3 response elements present in the promoter regions of T3 target genes (reviewed by Cheng [12]). The transcriptional regulatory activity of TRs depends not only on T3 and the types of T3 response elements but also on a host of coregulatory proteins, including corepressors, coactivators, and the tumor suppressor p53 (3, 38).
The growth-stimulatory effect of T3 has long been recognized. In humans, lack of T3 during development leads to growth retardation and cretinism. Growth retardation is also evident in patients with resistance to T3, a genetic disease due to mutations in the TRβ gene (54). TRβ1 mutants act in a dominant negative fashion to cause growth retardation and delayed bone maturation (54). Moreover, mutant mice harboring a potent dominant negative mutant TRβ1 also exhibit a similar phenotype (25, 60).
GC is a rat pituitary cell line that expresses functional TRs and has long been used as a model cell line to understand the mechanisms of T3 action. Previously we have shown that GC cells are induced to proliferate by T3 in cultured medium containing 10% T3-depleted (Td) serum (2, 27). This induction is T3 specific, because the inactive T3 analogs, l-thyronine and reverse T3, failed to stimulate proliferation of GC cells in the same culture medium (27). Recently, we showed that the growth-stimulatory effect of T3 was mainly due to a shortening of G0/G1 phase of the cell cycle (2). T3 induces G1 progression by increasing the mRNA and protein levels of two key regulators of G1 progression, cyclins D1 and E, as well as those of cdk2. These increases lead to hyperphosphorylation of the retinoblastoma proteins, resulting in transcriptional activation of growth-promoting genes (2). Thus, by both direct and indirect means, TRs control specific transcriptional cassettes associated with a host of cellular functions.
cDNA microarrays are capable of profiling gene expression patterns of thousands of genes in a single experiment. Microarray analysis in time course studies has been particularly fruitful in elucidating underlying biological and biochemical mechanisms in cellular processes and responses such as the diauxic shift in yeast (14), sporulation in budding yeast (13), serum stimulation of human fibroblasts (24), and phytohemagglutinin stimulation of human peripheral blood mononuclear cells (58). Despite the diversity in biological investigations that have utilized microarray strategies, few have sought to study the effects of hormone action on cells and tissues. In the present study, we used cDNA microarrays consisting of 4,400 rat cDNAs to identify genes associated with T3-induced cell proliferation in a time-dependent fashion. Approximately 88% of the genes were not previously known to be T3 responsive, and 36% are uncharacterized genes. We demonstrate that T3-induced cell proliferation is associated with the activation of various metabolic pathways and the immediate silencing of the Wnt signaling pathway.
MATERIALS AND METHODS
GC cells and T3 treatment.
GC cells were plated at a density of 107/superdish (600 cm2) in Dulbecco's modified Eagle medium (DMEM) containing 10% calf serum. After 2 days, the medium was changed to DMEM containing 10% Td calf serum (27). Forty-eight hours later, cells were incubated without or with T3 (100 nM) for 1, 3, 6, 12, 24, and 72 h. Addition of T3 to plated cells was staggered so that all plates in the time course were harvested simultaneously to prevent gene expression artifacts owing to length of time in culture conditions and differences in cell density. In some experiments, after cells were cultured for 2 days in DMEM containing 10% calf serum, the medium was changed to DMEM containing 0.1% Td calf serum. Cells were treated without or with T3 (100 nM) for 1, 6, and 12 h. At each time point, cells were harvested for total RNA preparation for microarray analyses as shown below.
RNA preparation and labeling.
Total RNA from GC cells with or without T3 treatment was prepared using RNeasy Midi kit (Qiagen Inc., Valencia, Calif.). The RNA was further purified using TRIzol (Life Technologies, Rockville, Md.). For fluorescence labeling of cDNAs, 30 μg of total RNA from untreated cells and 50 μg of total RNA from T3-treated cells were reverse transcribed in the presence of Cy3-dUTP and Cy5-dUTP (Amersham Inc., Piscataway, N.J.), respectively. Labeled cDNAs were combined, concentrated, and resuspended in microarray hybridization buffer as described previously (17).
Microarray manufacture.
Rat cDNAs were assembled at The Institute for Genomic Research (Rockville, Md.) and arrayed at the NCI Microarray Facility, Advanced Technology Center (Gaithersburg, Md.). Briefly, rat 3′ expressed sequence tags (3′-ESTs) were derived from single-pass sequencing of the 3′ ends of randomly selected, directionally cloned cDNA clones from 12 libraries (10 normalized and 2 nonnormalized) representing 10 different tissues and 2 developmental states (fetus and adult). The normalized libraries were from rat fetus, placenta, ovary, heart, lung, liver, spleen, kidney, skeletal muscle, and adult brain. The nonnormalized libraries were from a clonal cell line treated with and without growth factor (30). The 3′-ESTs were assembled into approximately 22,000 distinct tentative consensus sequences (i.e., overlapping consensus sequences representing nominally unique genes), and a minimal clone set was sequenced at the 5′ end to provide clone verification and gene identification. A subset of the minimal clone set was selected for inclusion on the 4,400-element rat cDNA microarray and can be viewed at http://www.tigr.org/tdb/ratarrays/. The arrays were printed using an OmniGrid Microarrayer (GeneMachines, San Carlos, Calif.) on poly-l-lysine-coated glass slides prepared essentially as previously described (17).
Hybridization, scanning, and analysis.
Labeled cDNAs were hybridized to the arrays overnight at 70°C. The arrays were washed as previously described (17), dried by centrifugation, and scanned on a GenePix 4000A microarray scanner (Axon Instruments, Foster City, Calif.) to generate 16-bit TIFF images of Cy3 and Cy5 signal intensities. The images were analyzed using GenePix Pro 3.0 microarray analysis software (Axon Instruments) to measure fluorescence signals and format data for database deposition. All of the array data were deposited in the NCI-CIT microarray database (http://nciarray.nci.nih.gov), where Cy3 and Cy5 signals were normalized and expression ratios (Cy5/Cy3) were calculated. Multiarray analytical tools available on the database were used to apply the selection criteria. Agglomerative clustering by Euclidean distance measurement was accomplished using S-PLUS 5.1 software (MathSoft, Inc., Cambridge, Mass.), and the clustered data were visualized using Eisen Treeview software (18)
Selection of T3-responsive genes.
The time course expression data were initially distilled to the set of array features having signal-to-background ratios of ≥2.0 in both channels at at least five of the six time points between 1 and 72 h. Within this data set, T3-responsive genes were identified as those with temporal expression profiles demonstrating the greatest magnitude and consistency of change. This set of outliers was composed of genes demonstrating a twofold or greater change at two or more time points and showing a ≥1.8-fold change at at least two consecutive time points. To eliminate outliers potentially owing to experimental technique, a control screen was performed in which two reference RNA samples (i.e., RNA from cells grown in Td medium) were isolated in parallel from separate tissue culture plates, used to generate labeled cDNA, and hybridized against each other on three separate microarrays. Genes identified in this control screen with expression ratios of ≥2.0 in any one experiment or ≥1.7 in two of three experiments were excluded from the final set of T3-responsive genes. Five array features were excluded based on these criteria. The complete list of T3-responsive genes, GenBank accession numbers, and corresponding time course expression ratios can be downloaded at http://nciarray.nci.nih.gov/publications.
Northern blot analysis.
GC cells were plated (3.5 × 106/15-cm dish) and cultured as described above. Total RNA was isolated from cells treated with T3 for 3, 12, and 24 h and from cells grown in Td medium using the RNeasy Midi kit (Qiagen Inc.) according to the manufacturer's protocol. Total RNA (10 μg) from each time point was separated on a 1% agarose-formaldehyde gel and transferred to a Hybond membrane (Amersham Inc.). The blots were probed with purified [α-32P]dCTP-labeled cDNA fragments of adenine nucleotide translocator, Na+/K+ ATPase β subunit, transforming growth factor β2, regulator of G-protein signaling 2, GADD45, and the unknown EST AW144553 derived from PCRs. The same blots were stripped and rehybridized with [α-32P]dCTP-labeled cDNA encoding glyceraldehyde-3-phosphate dehydrogenase. The intensities of the bands were quantified by Eagle Eye II (Stratagene, La Jolla, Calif.) and were normalized to the glyceraldehyde-3-phosphate dehydrogenase internal control. Expression ratios were determined at each time point by dividing the band intensities of mRNA derived from T3-treated cells by those from cells without T3 treatment in the same experiments.
Western blot analysis.
Western blot analyses of β-catenin and axin were carried out as described previously (5). Briefly, GC cells treated with or without T3 in medium containing 10% Td calf serum were lysed, and the proteins were separated by sodium dodecyl sulfate-gel electrophoresis. The proteins were transferred to blots, which were blocked, washed, and reacted with primary antibodies. The concentrations of the primary antibodies for the detection of β-catenin (Transduction Lab, Lexington, Ky.) and axin (R-20; Santa Cruz Biotechnology, Inc., San Diego, Calif.) were 0.5 and 1 μg/ml, respectively. Signals were developed using an enhanced chemiluminescence detection kit.
Transient-transfection assay.
GC cells were plated at a density of 3.5 × 105 cells/35-mm dish and cultured overnight. Cells were transfected with the T-cell factor (TCF) reporter plasmid (pGL3-OT; 1 μg) and β-galactosidase expression plasmid (pCH110; 0.5 μg) using FuGene6 (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's protocol. Briefly, 3.8 μl of FuGene6 was mixed with 1.5 μg of DNA in 1 ml of Opti-Mem1 (GIBCO-BRL, Rockville, Md.) and added to cells which had been washed twice with phosphate-buffered saline. After 24 h, cells were cultured in medium containing 10% Td calf serum with or without 100 nM T3 for 24 or 48 h. The luciferase and β-galactosidase activities of cell lysates were determined according to the manufacturer's procedures (PharMingen, Santa Cruz, Calif., and Roche, Indianapolis, Ind., respectively). The luciferase activity was normalized against the β-galactosidase activity for transfection efficiency.
RESULTS
Microarray analysis identifies genes responsive to T3-induced cell proliferation.
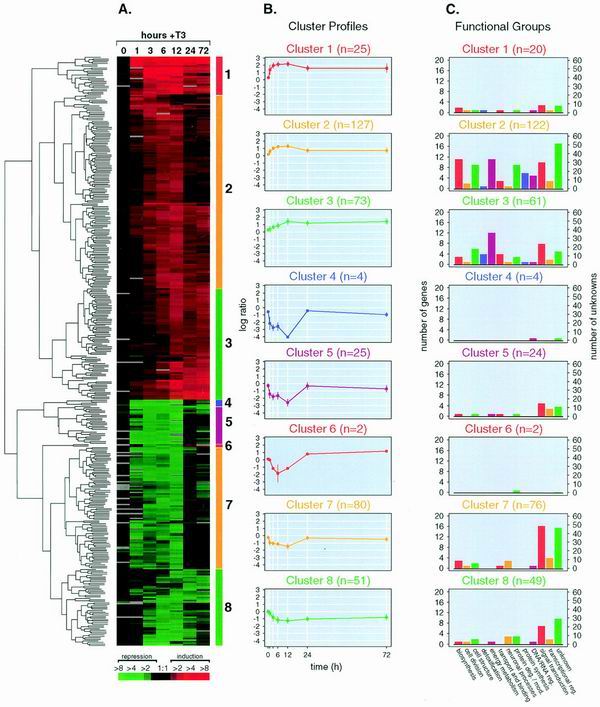
Because GC cells are induced to proliferate specifically by T3 (2, 27), we used this cell line to profile T3-induced changes in gene expression. Rat-derived cDNA microarrays were used to monitor the temporal changes in mRNA levels of 4,400 genes at 1, 3, 6, 12, 24, and 72 h after T3 treatment and time zero (in medium containing Td medium only). At each time point, total RNA was isolated and reverse transcribed into Cy5-labeled cDNA. This cDNA was hybridized to the microarrays against Cy3-labeled cDNA derived from the total RNA of cells that received no T3 treatment (Td medium only). Using rigorous selection criteria (see above), we identified 358 distinct genes (outliers) demonstrating time-dependent changes in mRNA level during T3-induced cell proliferation (Fig. 1A). The majority of genes were either only induced or only repressed; only a few genes showed biphasic expression patterns (Fig. 1B, cluster 6). One-hundred thirty genes (36%) were represented by unnamed ESTs, and 228 were named genes. In total, 203 genes (57%) were up-regulated and the remainder (155 genes; 43%) were down-regulated by T3.
FIG. 1.
(A) Expression dynamics of T3-responsive genes. Temporal expression profiles of 387 arrayed features exhibiting change over time in response to T3 were hierarchically clustered by Euclidean distance measurement and visualized in a clustergram using Treeview software (see Materials and Methods). Microarray experiments for each time point are in columns; individual genes are in rows. Red indicates increasing mRNA levels and green denotes decreasing mRNA levels. The degree of color saturation reflects the magnitude of the ratio (see color key at the bottom). (B) Cluster profiles. Gene expression profiles were algorithmically subdivided into eight clusters (shown to the right of the clustergram by colored bars). The average expression profile of each gene cluster is shown in a line graph and is colored according to the matching bars. n, number of array features within each cluster. Error bars show standard deviations. (C) Distribution of functional groups across clusters. The number of individual genes comprising each functional group is indicated for each cluster profile. The cluster sizes differ from those in panel B because single genes having multiple expression profiles owing to redundant spotting on the microarrays were counted only once per functional group to prevent overrepresentation bias. For 5 of 19 redundant genes, two or more expression profiles fell into more than one cluster. In three cases, the gene was counted in the cluster that contained two of the three same-gene profiles, and in two cases where two profiles fell into two clusters, the gene was placed in the cluster whose mean had the smallest Euclidean distance from the two profiles.
To confirm that the T3-responsive genes detected were indeed associated with T3-induced cell proliferation, we uncoupled T3 signaling not associated with proliferation from the proliferative response. Minimal serum (0.1% Td calf serum) was used to keep cells viable, but without proliferation. Cells treated with or without T3 for 1, 6, and 12 h remained quiescent throughout the time course with no measurable increase in proliferation (data not shown). Microarray analysis revealed very little T3-induced expression response under these culture conditions. For example, at the 12-h time point, microarray analysis of T3-stimulated proliferating cells in normal culture conditions demonstrated 171 genes with expression ratios greater than threefold. In contrast, T3-treated quiescent cells showed no changes of threefold or greater at the same time point in replicate array experiments (data not shown). Only 2 of the 358 outlier genes identified in T3-induced cell proliferation (encoding cytochrome c oxidase subunit 1 and ATP synthase alpha) were also detected as reproducible outliers in T3-treated quiescent cells at 12 h (each was induced by T3 with average expression ratios between 2.1 and 2.7, the ratio range observed at 12 h in the proliferating cells). These results indicate that the genes identified under proliferating conditions are associated with T3-induced growth response.
Microarray analysis identifies previously reported T3-responsive genes.
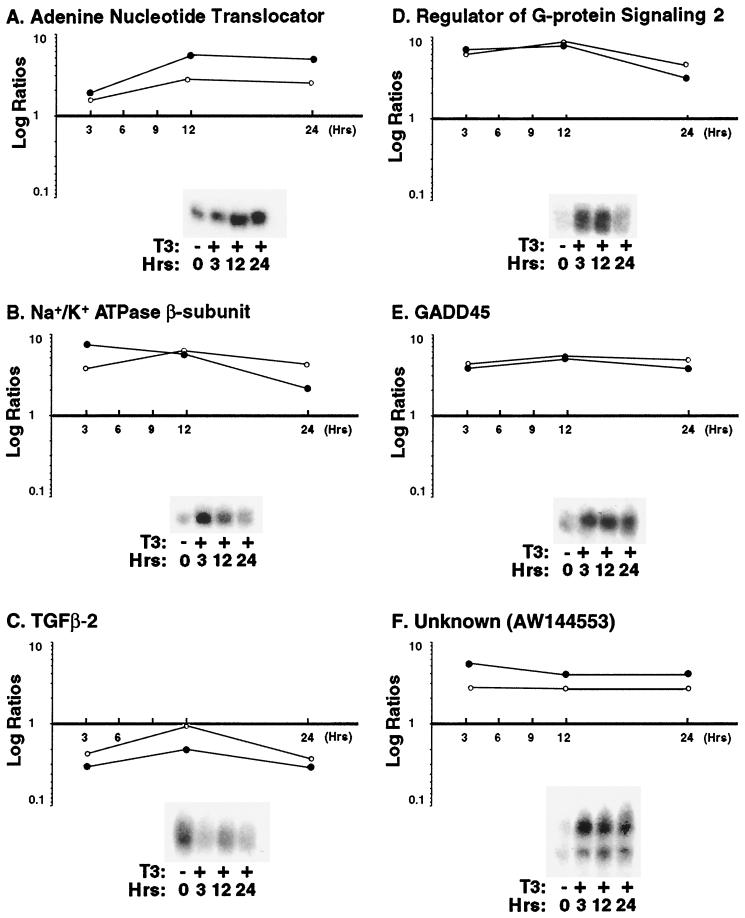
To test the validity of the microarray results, six clones were selected for Northern analysis across three time points of T3 treatment (3, 12, and 24 h). Three of these (encoding adenine nucleotide translocator, Na+/K+ ATPase β subunit, and GADD45) had been previously implicated in T3 action (Fig. 2A, B, and E, respectively), while three (those for transforming growth factor β2 and regulator of G-protein signaling 2 and the unknown EST AW144553) had no previous association (Fig. 2C, D, and F, respectively). The intensities of mRNA bands on the Northern blots were quantified (Fig. 2). The time-dependent expression profiles of these six genes were consistent with the temporal changes detected by the arrays. The six clones were also resequenced, and their identities were confirmed.
FIG. 2.
Concordance of gene expression patterns determined by microarray and Northern blotting. Expression ratios of six genes identified as outliers by microarray were determined by Northern blot analysis at three time points following T3 exposure. Intensities of the Northern bands were quantified (as described in Materials and Methods) and used to calculate expression ratios, with the Td reference intensity in the denominator. Ratios derived from Northern blots (○) are compared to the microarray results (●). Representative mRNA bands are shown.
We further assessed the ability of our microarrays to detect the coordinate expression of known T3-responsive genes represented on the arrays (Fig. 3). In total, 44 of the 219 named gene outliers (20%) were previously implicated in T3 action. Twenty-five were known to be transcriptionally regulated, 10 were known to be modulated by T3 at the protein level, and the gene products of 9 were known to be associated with T3 processing or downstream signaling pathways (Fig. 3). Several of these genes (e.g., those encoding GADD45, cytochrome c, thioredoxin, hemoglobin alpha, lactate dehydrogenase, c-jun, p53, and cathepsin L) were represented multiple times on the microarray and showed concordant expression profiles, further validating the technique (Fig. 3).
FIG. 3.

Clustergram of genes previously implicated in T3 action. Each of the named outlier genes identified in our screen was searched via PubMed for a reported association with T3. A clustergram of the T3-associated genes is shown. T, gene shown to be regulated transcriptionally by T3; P, gene whose product is reportedly modulated by T3 (e.g., protein levels, activity, specific activity, and posttranslational modifications); A, gene whose product is known to be associated with T3 processing or signaling.
Complex coordination of cellular pathways is associated with T3-induced cell proliferation.
Of the 228 named outlier genes, 196 had known biological roles. These genes were therefore classified by broad biological function to assess the involvement of cellular pathways in T3-induced proliferation. Twelve functional groups were identified, and each gene was assigned to a single group (Table 1). The other 32 genes that were named but without known functions are shown in Table 2. One of the largest functional groups shown in Table 1 was that involved in energy metabolism. This set contained 25 genes, most of which are involved in various aspects of glucose metabolism and oxidative phosphorylation (Fig. 4A and B). Induction of these cellular pathways is consistent with known biochemical and clinical manifestations of T3 effect. T3 increases respiration, heat production, and carbohydrate metabolism (40). Several key enzymes involved in the metabolism of ATP, gluconeogenesis, and glucose production, such as ATP synthase, adenine nucleotide translocator, and Na+/K+ ATPase, have been shown to be stimulated by T3 (40, 41). We found that these and other genes involved in glucose metabolism and oxidative phosphorylation were transcriptionally activated by T3 (Fig. 4A and B). Thus, the putative activation of these cellular pathways may be in response to increased physiologic need during cell proliferation.
TABLE 1.
Functional classification and expression characteristics of T3-responsive genesa
| Function | Protein | Specific role | Assignmentb | Peak expression ratioc |
|---|---|---|---|---|
| Biosynthesis | Glutamate decarboxylase | Amino acids | 2E | 2.21 |
| Cystathionine gamma-lyase | Amino acids | 3L | 3.28 | |
| Acyl-CoA synthetase | Cholesterol | 2E | 2.51 | |
| Fatty acid synthase | Fatty acids | 2E | 2.59 | |
| Stearyl-CoA desaturase 2 | Fatty acids | 2E | 3.01 | |
| Stearyl-CoA desaturase | Fatty acids | 1E | 3.77 | |
| δ-Aminolevulinate synthase | Heme, porphyrin, cobalamin | 2E | 3.11 | |
| Adenylate kinase isozyme 1 | Nucleoside, nucleotide metabolism | 7I | 0.25 | |
| Nicotinamide N-methyltransferase | Nucleoside, nucleotide metabolism | 7I | 0.33 | |
| Inosine 5′-monophosphate dehydrogenase | Nucleoside, nucleotide metabolism | 2I | 2.56 | |
| Nucleoside diphosphate kinase | Nucleoside, nucleotide metabolism | 3I | 2.59 | |
| IMP-synthetase | Nucleoside, nucleotide metabolism | 2I | 3.14 | |
| Orotidine-5′-phosphate decarboxylase | Nucleoside, nucleotide metabolism | 2I | 3.30 | |
| CAD carbamoyl phosphate synthetase 2 | Nucleoside, nucleotide metabolism | 2E | 3.42 | |
| GalNAc-α2,6-sialyltransferase | Oligosaccharides | 7I | 0.21 | |
| GDP-L-fucose pyrophosphorylase | Oligosaccharides | 5I | 0.21 | |
| β-1,3-Galactosyltransferase III | Oligosaccharides | 8E | 0.40 | |
| β-1,4-Galactosyltransferase | Oligosaccharides | 2E | 2.86 | |
| Fucose operon protein | Oligosaccharides | 1I | 5.92 | |
| Spermidine synthase | Polyamines | 2I | 2.98 | |
| Ornithine decarboxylase | Polyamines | 3I | 3.41 | |
| Cell division | PC3 NGF-inducible protein | Cell cycle arrest | 8I | 0.34 |
| GADD45 | Cell cycle arrest | 1E | 5.20 | |
| cdc25a | Cell cycle regulation | 7E | 0.34 | |
| PCNA (proliferating cell nuclear antigen) | Cell cycle regulation | 3I | 2.28 | |
| Cdk-4 (cyclin dependent kinase 4) | Cell cycle regulation | 2I | 2.48 | |
| Homo sapiens CINP (CDK2-interacting protein) | Unknown | 2E | 2.05 | |
| Cell structure | Cytohesin binding protein HE | Cytoskeleton | 7I | 0.29 |
| MAP-1B | Cytoskeleton | 8I | 0.36 | |
| Cytoplasmic linker protein CLIP-115 | Cytoskeleton | 2E | 2.18 | |
| Profilin | Cytoskeleton | 2I | 2.45 | |
| Peripherin | Cytoskeleton | 2I | 2.56 | |
| Tubulin alpha | Cytoskeleton | 3I | 2.61 | |
| Actinin alpha | Cytoskeleton | 2E | 3.04 | |
| Ribophorin I | ER microsomal membranes | 2I | 2.70 | |
| Tenascin | Extracellular matrix | 2I | 3.64 | |
| Alpha fibrinogen | Extracellular matrix | 2I | 4.06 | |
| Mitochondrial outer membrane protein | Mitochondria | 2I | 2.70 | |
| Creatine kinase sarcomeric mitochondrial | Mitochondria | 2I | 3.37 | |
| Nucleosome assembly protein 1-like 2 | Nucleus | 8E | 0.25 | |
| Lamin B2 | Nucleus | 3I | 2.24 | |
| Nucleosome assembly protein | Nucleus | 3I | 2.47 | |
| Nucleolar phosphoprotein B23 | Nucleus | 3I | 3.27 | |
| Nucleolin | Nucleus | 3I | 3.31 | |
| Nucleolar phosphoprotein B23.2 | Nucleus | 3I | 4.01 | |
| Treacher Collins syndrome protein | Nucleus | 1I | 5.35 | |
| Pex14 peroxisomal membrane anchor protein | Peroxisome | 7I | 0.32 | |
| Detoxification | Mer5 | Antioxidant, oxidative stress | 3L | 2.20 |
| SOD (Cu-Zn) (superoxide dismutase) | Antioxidant, oxidative stress | 3L | 2.26 | |
| Thioredoxin | Antioxidant, oxidative stress | 3I | 2.74 | |
| Microsomal glutathione S-transferase 3 | Antioxidant, oxidative stress | 2I | 2.99 | |
| Thioredoxin-dependent peroxide reductase | Antioxidant, oxidative stress | 3I | 3.46 | |
| PTPase (oxidative stress-inducible protein Tyr phosphatase) | Antioxidant, oxidative stress | 1E | 5.57 | |
| Energy metabolism | Ornithine aminotransferase | Amino acids and amines | 2I | 2.90 |
| Lactate dehydrogenase | Anaerobic glycolysis | 3I | 3.66 | |
| Alcohol dehydrogenase type III | Ethanol oxidation | 8I | 0.29 | |
| Pyruvate dehydrogenase E1 component β subunit | Glycolysis, gluconeogenesis | 2E | 2.28 | |
| Triose isomerase | Glycolysis, gluconeogenesis | 3L | 2.39 | |
| Glucose-6-phosphate dehydrogenase | Glycolysis, gluconeogenesis | 3I | 2.59 | |
| Pyruvate dehydrogenase phosphatase isoenzyme 2 | Glycolysis, gluconeogenesis | 2E | 2.89 | |
| PEP carboxykinase | Glycolysis, gluconeogenesis | 2I | 2.92 | |
| Phosphoglycerate kinase | Glycolysis, gluconeogenesis | 2I | 3.09 | |
| Pyruvate kinase | Glycolysis, gluconeogenesis | 3L | 3.44 | |
| Cytochrome c oxidase Va | Oxidative phosphorylation | 3L | 2.11 | |
| NADH-ubiquinone oxidoreductase MLRQ subunit | Oxidative phosphorylation | 3I | 2.19 | |
| ATP synthase α subunit | Oxidative phosphorylation | 2I | 2.51 | |
| Cytochrome c oxidase subunit I | Oxidative phosphorylation | 3L | 2.58 | |
| Cytochrome c 1 | Oxidative phosphorylation | 3I | 2.60 | |
| ATP synthase β subunit | Oxidative phosphorylation | 2I | 2.83 | |
| NADH-ubiquinone oxidoreductase B17 subunit | Oxidative phosphorylation | 2I | 2.85 | |
| IF1 ATPase inhibitor protein | Oxidative phosphorylation | 2I | 3.22 | |
| Enolase alpha | Oxidative phosphorylation | 3I | 3.63 | |
| Aldose reductase | Oxidative phosphorylation | 3I | 4.58 | |
| Cytochrome c | Oxidative phosphorylation | 3I | 5.08 | |
| Transketolase | Pentose phosphate pathway | 2I | 2.50 | |
| ATP-specific succinyl-CoA synthetase β subunit | TCA cycle | 5I | 0.14 | |
| Malate dehydrogenase | TCA cycle | 3L | 2.25 | |
| Fumarase | TCA cycle | 2I | 3.50 | |
| Transport and binding | Glutamate aspartate transporter protein | Amino acids | 2E | 2.22 |
| ATP2B1 (calcium ATPase isoform 1) | Cations | 3L | 2.09 | |
| Na+/K+ ATPase α-1 subunit | Cations | 2I | 2.55 | |
| Na-K-Cl cotransporter | Cations | 2I | 3.05 | |
| Na+/K+ ATPase β subunit | Cations | 1I | 5.28 | |
| Fatty acid transport protein 3 | Fatty acids | 5I | 0.18 | |
| Adenine nucleotide translocator | Nucleotides | 3L | 3.13 | |
| Hemoglobin alpha chain | Oxygen | 3L | 5.59 | |
| Metabolite transport protein hemolog | Unknown | 7I | 0.26 | |
| Acyl-CoA-binding protein/chazepam binding inhibitor | Unknown | 3L | 2.84 | |
| Neuronal processes | Hippocalcin | Calcium binding | 8I | 0.35 |
| Neuropilin | Receptor, axon growth | 2L | 3.06 | |
| Dendrin | Synaptic plasticity | 7I | 0.32 | |
| Synaptophysin | Synaptic vesicle protein | 8L | 0.40 | |
| Synapsin IIb | Synaptic vesicle protein | 3L | 2.53 | |
| NAC-1 protein | Unknown | 7E | 0.31 | |
| Neurotransmitter-induced early gene 2 | Unknown | 8L | 0.43 | |
| Sixbp4 (syntaxin binding protein 4) | Unknown, synapse | 7I | 0.46 |
The named outlier genes were separated into functional groups and are further described by a more specific biological role where applicable. A complete list of all genes, accession numbers, and corresponding temporal expression data can be accessed at http://nciarray.nci.nih.gov/publications. Abbreviations: CoA, coenzyme A; NGF, nerve growth factor; PEP, phosphoenolpyruvate; ER, endoplasmic reticulum; TCA, tricarboxylic acid.
Each designation consists of a number indicating the cluster profile the gene belongs to (Fig. 1B) and a letter (E, I, or L) indicating the temporal group the gene belongs to (Fig. 5A and B).
Ratios greater than 1.0 reflect fold induction, and those less than 1.0 indicate the reciprocal of fold repression.
TABLE 2.
Named T3-responsive genes with unknown functionsa
| Protein | Assignment | Peak expression ratio |
|---|---|---|
| 0–44 protein | 3I | 2.04 |
| Arabidopsis thaliana hypothetical protein F13C5.220 | 7E | 0.38 |
| Adapter protein APPL | 5I | 0.21 |
| AW140801 putative secreted protein | 2E | 2.34 |
| Caenorhabditis elegans hypothetical protein F09E5.2 | 4I | 0.07 |
| C. elegans hypothetical protein F29B9.10 | 2I | 2.05 |
| C. elegans hypothetical protein R13F6.10 | 7I | 0.40 |
| C. elegans hypothetical protein RGIAC52 | 7I | 0.35 |
| C184L protein | 3L | 2.92 |
| Cell growth regulator | 2E | 3.75 |
| Clone E536 estrogen-induced gene | 1E | 6.96 |
| Clone N27 | 7E | 0.45 |
| Clusterin | 8I | 0.39 |
| E 1B 19K/Bcl-2-binding protein homolog | 3L | 3.74 |
| Factor VIII intron 22 protein | 7I | 0.28 |
| H. sapiens adrenal gland protein AD-002 | 1E | 4.77 |
| H. sapiens hypothetical protein KIAA0148 | 5I | 0.20 |
| H. sapiens KIAA1250 protein | 4E | 0.10 |
| H. sapiens hypothetical protein KIAA0099 | 4I | 0.05 |
| H. sapiens hypothetical protein KIAA0536 | 5I | 0.13 |
| H. sapiens hypothetical protein KIAA0876 | 7I | 0.29 |
| H. sapiens protein KIAA0668 | 7I | 0.40 |
| Immediate early response 5 (IER5) | 2E | 4.01 |
| Intestinal epithelium proliferating cell-associated mRNA | 2I | 3.65 |
| JTV-1 protein | 1I | 4.38 |
| Leucine-rich protein | 2I | 2.90 |
| Mus musculus B6D2F1 clone 2C1IB | 2I | 3.08 |
| mak 11 protein | 1I | 4.48 |
| Rattus norvegicus development-related protein | 8I | 0.44 |
| Saccharomyces cerevisiae hypothetical protein, 13.4 kDa | 3I | 3.05 |
| Suppressor of cytokine signaling 1 | 7E | 0.42 |
| Surfeit locus surfeit 4 protein | 7I | 0.49 |
| Tmp21-I integral membrane protein | 7E | 0.33 |
Named outlier genes lacking known biological roles are listed. Cluster number, temporal group assignment, and expression ratio are reported as in Table 1. Unnamed outlier genes (ESTs) described by GenBank accession numbers can be downloaded at http://nciarray.nci.nih.gov/publications.
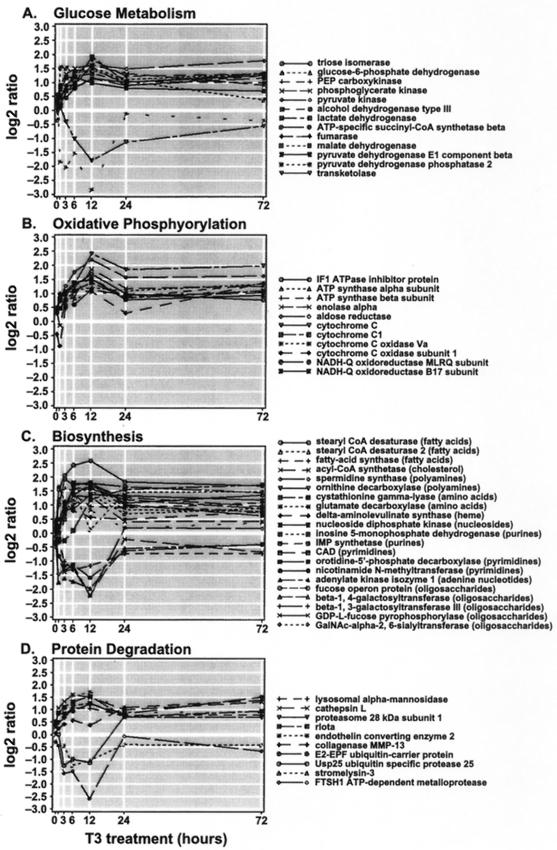
FIG. 4.
Temporal profiles of genes involved in glucose metabolism, oxidative phosphorylation, biosynthesis, and protein degradation. Shown are the expression trajectories of genes comprising the functional subgroups glucose metabolism (A), oxidative phosphorylation (B), and protein degradation (D) and the functional group biosynthesis (C). Each gene expression profile is the log-transformed expression ratio (y axis) plotted against hours post-T3 treatment (x axis).
Figure 4C and D show that T3-induced cell proliferation is associated with the transcriptional activation of genes involved in biosynthesis and protein degradation (a subgroup of the functional protein degradation and modification group). That T3 increases the activity of biosynthetic pathways is not unexpected. The role of T3 in amino acid incorporation and biosynthesis of DNA and protein constituents has been well studied (reviewed by Schwartz and Oppenheimer [52]). T3 has also been shown to increase protein turnover in both cardiac and skeletal muscle (1, 10, 11). In rats, skeletal muscle protein content following T3 administration is reduced due to increased proteolysis (1). Thus, our data are consistent with these earlier biochemical findings in that mRNAs of genes involved in protein degradation (e.g., lysosomal proteinases and proteasome subunits) and stabilization (heat shock proteins) were increased.
When a group of genes involved in cellular detoxification were examined, we found six antioxidant genes up-regulated after T3 exposure. These included genes encoding thioredoxin, thioredoxin-dependent peroxide reductase, glutathione S-transferase, superoxide dismutase, Mer5 antioxidant protein, and the oxidative stress-inducible protein tyrosine phosphatase (Table 1). The role of T3 in oxidative stress is not well studied. However, it has been reported that in rats, T3 administration enhances the production of superoxide radicals and hydrogen peroxides (56). Furthermore, T3 treatment of rats has reportedly led to augmentation of lipid and protein peroxidation and increased susceptibility to oxidative stress (53, 55). These observations suggest that the up-regulation of antioxidant genes is necessary to counteract the deleterious effect of oxidative stress induced by T3 exposure.
Another functional group we uncovered was composed of eight genes involved almost exclusively in neuronal processes (Table 1). The products of these genes have known roles in neuronal signaling (hippocalcin) and growth (neuropilin) and synaptic processes (synaptophysin, synapsin IIb, and dendrin). T3 has long been studied as an important physiological regulator of brain development and is known to play key roles in neuronal growth, differentiation, and migration (44). Specifically, T3 is known to control transcription of nerve growth factor in the rat pituitary and forebrain (8, 9) and brain-derived neurotropic factor in the developing rat cerebellum (28), and T3 deficiency is known to impair development of the rat cerebellar cortex (42). Thus, the discovery of this T3-regulated neuronal cassette may provide insight into the mechanism by which T3 and its downstream effectors modulate mammalian brain development (44; reviewed by Bernal and Nunez [4]).
Recent studies have shown that grouping of genes with similar expression profiles may reveal the function of a coordinately controlled gene cluster (18). We therefore assessed the temporal pattern of gene expression by unsupervised (unbiased) agglomerative hierarchical clustering (Fig. 1A). Agglomerative clustering begins with single-object clusters that are recursively merged into larger clusters. In this analysis, the dendrogram of the T3 data was partitioned into eight gene clusters with distinct mean expression profiles, three with different patterns of up-regulation, four with patterns of down-regulation, and one two-gene cluster with biphasic expression (Fig. 1B). We next examined the content of each gene cluster with respect to the functional categories we previously identified (Fig. 1C). As defined above, genes involved in biosynthesis, energy metabolism, and protein fate were largely up-regulated, falling into clusters 1, 2, and 3. In addition, we found that genes involved in protein synthesis, transport and binding, cell structure, and DNA and RNA regulation were also primarily up-regulated (Fig. 1C). In contrast, slightly more than half of the genes in the outlier list with primary roles in signal transduction were repressed (clusters 4 to 8). This finding is provocative in that it suggests that T3 activates cellular biosynthesis and remodeling yet may act to block other incoming signals.
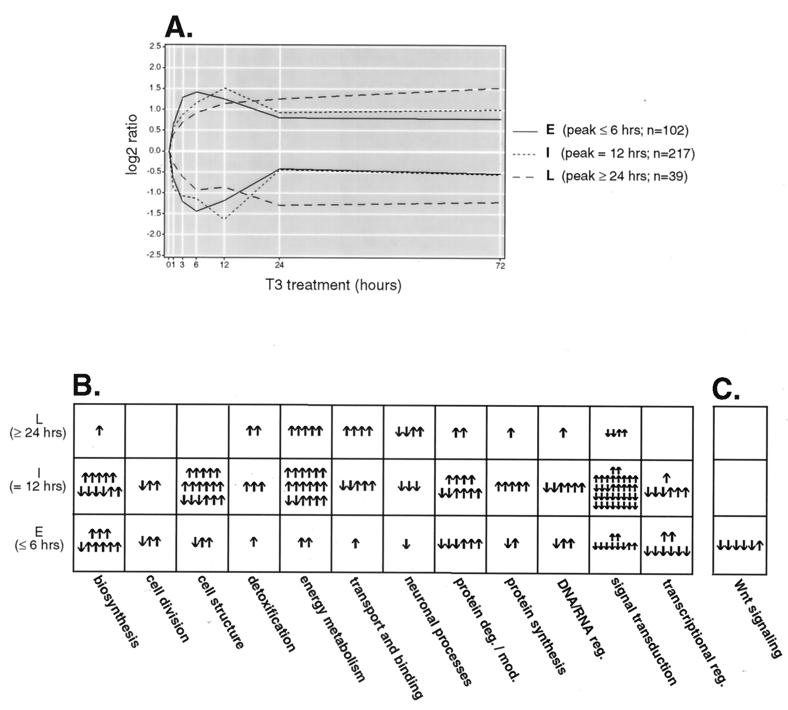
Genes were also segregated according to the timing of their peak expression levels. This has been the manner by which viral genes have been historically classified. We grouped the gene outliers into early (peak at ≤6 h), intermediate (peak at 12 h), and late (peak at ≥24 h) temporal expression groups (Fig. 5A). One hundred two genes (28%) responded rapidly, with peak responses at 6 h or earlier. The majority of the genes (217 genes; 61%) reached the peak response at 12 h, and 39 genes (11%) reached the peak response at 24 or 72 h. For Fig. 5B, the genes comprising each functional group were separated into their temporal groups. In this way we were able to approximate and compare the relative timing of the functional gene sets. This analysis revealed that genes involved in transcription, biosynthesis, cell structure, and protein degradation and modification made up the majority of the early gene responses, whereas genes associated with energy metabolism and intracellular transport and binding reached peak ratios primarily at the intermediate and late time points. This finding is consistent with the view that transcription, synthesis, and remodeling of cellular constituents precede expenditure of energy in a proliferative response.
FIG. 5.
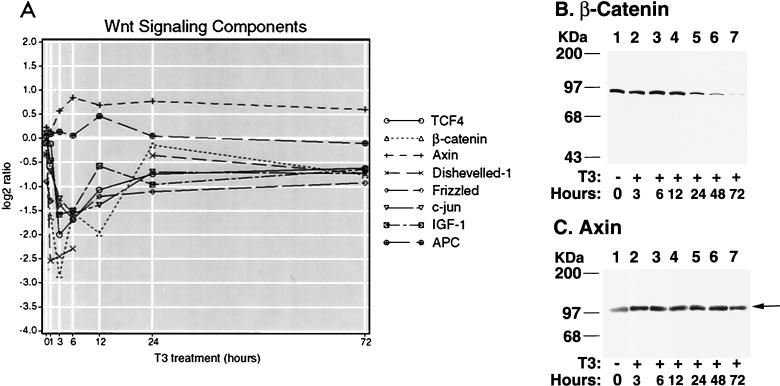
(A) Trend lines for early (E), intermediate (I), and late (L) T3 response genes. Outlier genes were segregated into temporal expression groups based on the timing (in parentheses) of their peak ratios. The median expression ratio at each time point in each group is plotted. n, number of genes in each expression group. (B) Kinetics of T3 response pathways. Annotated genes were grouped according to cellular function (Table 1) and partitioned into temporal expression groups to visualize the T3-induced activation of cellular pathways as a function of time. The pathways are in columns, and the temporal expression groups are in rows. Each arrow represents a single gene, and its direction reflects up- or down-regulation. (C) Early repression of T3-responsive genes of the Wnt pathway. The kinetics of Wnt pathway-associated genes (encoding β-catenin, TCF4, Dishevelled-1, Frizzled, axin, and APC) are shown.
To gain a more detailed understanding of the molecular events downstream of T3, we scanned the broad functional groups for components of specific signaling pathways. Intriguingly, several members of the Wnt signaling pathway were observed. Upon close inspection of the comprehensive array data set, we found six key components of Wnt signaling with expression profiles consistent with inhibition of the pathway. This response was quite rapid in that five of six expression patterns exhibited the early temporal profile (Fig. 5C). The expression of β-catenin, TCF4, Dishevelled-1, and Frizzled was rapidly repressed within 1 h upon T3 treatment, with maximal inhibition occurring by 6 h (Fig. 6A). The expression of axin was steadily increased to about twofold by 6 h and remained above the baseline during the course of T3 treatment (Fig. 6A). The expression of adenomatous polyposis coli (APC) was increased ∼1.4-fold after treatment with T3 for 12 h and returned to basal level after 24 h (Fig. 6A). β-Catenin, TCF4, Dishevelled-1, and Frizzled are all agonists of Wnt signaling (reviewed by Peifer and Polakis [46]), while axin and APC suppress the pathway by promoting degradation of β-catenin (61). Thus, the expression profiles of these genes suggest that T3 acts to silence the Wnt signaling pathway. This notion was further supported by the observation that the expression of the c-jun transcription factor, which is induced subsequent to activation of Wnt signaling (36), was also down-regulated by T3 (Fig. 6A).
FIG. 6.
(A) Response of Wnt signaling genes to T3. Temporal expression profiles of the six canonical Wnt signaling components present on the microarray, a putative regulator of the pathway (IGF-1), and one transcriptional target (c-jun) are shown. β-Catenin, TCF4, Dishevelled-1, IGF-1 and c-jun were selected in the initial high-stringency screening procedure for T3-responsive genes. Axin, APC, and Frizzled were not uncovered by the initial screen. Axin and APC did not demonstrate expression ratios of twofold or higher at at least two time points, and the Frizzled array feature failed to achieve a signal-to-background ratio of ≥2.0 at more than one time point. Despite these selection criterion discrepancies, both axin and Frizzled displayed expression trajectories consistent with time-dependent differential expression and further support the repression hypothesis. APC showed a small increase but did not demonstrate a profile consistent with sustained differential expression. c-jun, though not a specific component of Wnt signaling, is a transcriptional target of β-catenin–TCF and is down-regulated in a fashion consistent with repression of this pathway. The 12-h expression ratio for Dishevelled-1 was filter excluded in the initial screen due to a low signal-to-background ratio and was therefore omitted from the line graph. (B and C) Western blot analysis of β-catenin and axin. After cells were treated with T3 for the time indicated, 50 μg of cell lysates were analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis. Western blot analysis was carried out using anti-β-catenin or antiaxin antibodies as described in Materials and Methods.
T3 suppresses Wnt signaling.
β-Catenin is the major effector of Wnt downstream transcription, and the stability of β-catenin protein regulates Wnt signaling (26, 46). We therefore evaluated the effect of T3 on the expression of β-catenin at the protein level. Western blot analysis (Fig. 6B) showed that treatment of cells with T3 led to a significant reduction of β-catenin protein, with a half-life of ∼24 h. The repression of the β-catenin protein persisted, while β-catenin mRNA recovered. At the 72-h time point, β-catenin protein was barely detectable under these experimental conditions (Fig. 6B), whereas the β-catenin mRNA had nearly recovered by 24 h (Fig. 6A). This suggests that translational and posttranslational mechanisms may act coordinately to down-regulate the expression of the β-catenin gene during T3 treatment of GC cells. Axin is a negative regulator of the Wnt signaling pathway (26) that forms a complex with APC and glycogen synthase kinase 3β (GSK-3β), facilitating the phosphorylation and degradation of β-catenin (26, 46). Western blot analysis showed that axin was increased about twofold by T3 treatment (Fig. 6C), similar to that detected by the microarrays (Fig. 6A). This finding suggests that another mechanism by which T3 may decrease the stability of β-catenin is enhancement of the expression of axin.
Recently, other signaling pathways have been shown to modulate β-catenin levels. Insulin-like growth factor 1 (IGF-1) is thought to stabilize β-catenin protein through tyrosine phosphorylation (47) and therefore will also augment Wnt signaling by a posttranscriptional mechanism. Our array results confirm that IGF-1 transcripts are reduced within 1 h after T3 exposure (Fig. 6A). Thus, this T3-associated reduction in IGF-1 mRNA may further contribute to the observed attenuation of β-catenin protein levels.
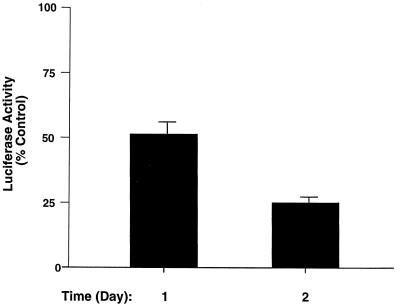
We further carried out functional assays to confirm that T3 suppresses the transcriptional activity of β-catenin and TCF4. To this end, we used a β-catenin–TCF reporter assay to examine whether treatment of cells with T3 results in the repression of the transcriptional activity of the β-catenin–TCF complex. GC cells were transfected with pGL3-OT, a luciferase reporter previously shown to be β-catenin–TCF4 responsive (22, 29), which contains the binding sequence for the TCF transcription factor derived from the c-myc regulatory domain. Figure 7 shows that T3 administration led to a time-dependent repression of its transactivation activity. After 24 h of T3 treatment, TCF-mediated transactivation was consistently reduced by ∼50%, and after 48 h, the reduction was ∼75%. The repression in the transcriptional activity of the β-catenin–TCF complex is consistent with the T3-induced reduction in the abundance of β-catenin protein (Fig. 6B). These results further support the hypothesis that T3 represses Wnt signaling activity.
FIG. 7.
Repression of β-catenin–TCF transactivation activity by T3. GC cells transfected with TCF reporter plasmid (pGL3-OT; 1 μg) and β-galactosidase expression plasmid (pCH110; 0.5 μg) were treated with or without T3 (100 nM) as described in Materials and Methods. Cell lysates were prepared, and the luciferase activities were determined at the times indicated. The luciferase activities were normalized against the β-galactosidase activities. Control, Td activity. Error bars reflect the standard deviations in triplicate experiments.
DISCUSSION
Previously, the activity of T3 and its mechanisms of action have been studied with much emphasis on its role in organismal development, its physiologic effects on specific tissues, and its pathological role in human disease. However, comparatively little is known about the function of T3 in promoting cell growth and proliferation. With the availability of cDNA microarrays, it has become possible to explore the global changes in transcriptional programs during T3-induced cell proliferation. Using a rat microarray consisting of 4,400 genes, we identified 358 T3-responsive genes that underwent defined temporal changes in expression associated with cell proliferation. About 43% of these genes were repressed by T3, indicating that gene repression may be as important as the activation of genes in cell proliferation induced by T3. About 46% of these genes had unknown functions, underscoring the utility of the microarray approach in discovering novel biological components of T3 action. Of the 219 known genes identified, only 20% were previously reported to be transcriptionally and/or functionally regulated by T3. Therefore, we have significantly expanded the list of T3-regulated genes.
Close examination of the biological functions of the named responsive genes revealed that many cellular processes contribute to T3-induced cell growth. The involvement of gene groups functioning in energy metabolism, biosynthesis, protein degradation and modification, detoxification, and neuronal processes concurs with historical findings regarding T3 action. Analysis of pattern clustering and temporal dynamics of gene expression provided a unique opportunity to assess the biological coordination of cellular pathways in response to T3-induced growth. Feng and coworkers recently identified 55 T3-responsive hepatic genes following treatment of hypothyroid mice with T3 for 6 h (19). A comparison with our data set showed an overlap with the differentially expressed genes belonging to each functional group identified by Feng and coworkers (i.e., glucose and fatty acid metabolism, insulin action, cell proliferation, signal transduction, glycoprotein synthesis, cellular immunity, and cytoskeleton). More specifically, our two studies identified in common the down-regulation of Akt-2 and β-galactoside α-2,6-sialyltransferase in the presence of T3. Additionally, where Feng and coworkers identified T-complex protein 1 δ subunit and endothelin-converting enzyme 1 as T3 responsive, we identified T-complex protein 1 α subunit and endothelin-converting enzyme 2 as T3 responsive. That two data sets derived from highly disparate sources (i.e., rat pituitary and in vitro compared to mouse liver and in vivo and using unrelated microarrays) yielded overlapping results suggests that common pathways are engaged after T3 stimulation that is independent of organ site.
The findings that the transcripts of key regulators in the Wnt signaling pathway responded rapidly to T3 treatment prompted us to explore further the role of Wnt signaling in T3-induced cell proliferation. Wnt signaling is initiated by the binding of Wnt ligands to the transmembrane receptors of the Frizzled family (59). Signaling of Frizzleds through Dishevelled inhibits the kinase activity of a complex containing GSK-3β, APC, and axin which binds and phosphorylates β-catenin, thus targeting the protein for ubiquitination (7, 59). Hypophosphorylation enhances the stability of β-catenin. The accumulated β-catenin is translocated to the nucleus and interacts with the TCF/LEF family of transcription factors to regulate the expression of Wnt target genes. In this model, central to the control of Wnt signaling is the stability of the key signal transducer, β-catenin. During T3-induced cell proliferation, we found both a temporal decrease in β-catenin protein level and an increase in axin protein level which were accompanied by a reduction in the TCF transactivation activity. Though these data do not preclude the possibility that T3 evokes a more general transcriptional repression than that limited to the TCF targets such as the c-myc promoter, it is clear that the proximate components of the Wnt/TCF/β-catenin pathway are affected by T3.
The present study indicates that silencing of Wnt is accompanied by T3-induced cell proliferation. Paradoxically, activation of Wnt signaling is known to stimulate cell proliferation in oncogenic systems (reviewed by Peifer and Polakis [46]). That the same signaling pathway induces diametrically opposite phenotypes has precedence. Oncogenic forms of ras and myc can either transform or immortalize cells or induce cell death (6; reviewed by Fuhrmann et al. [20] and Olson and Marais [45]). Thus, depending on the profile of concurrent signals activating other pathways, the cellular outcome may diverge.
It is possible that silencing of Wnt signaling could allow T3-induced cell proliferation by turning down the expression or activity of inhibitors for T3-induced growth. Alternatively, silencing of the Wnt pathway may lead to the activation of genes and signaling events required for T3-induced cell proliferation. The latter possibility is supported by a recent report which shows that differentiation of preadipocytes into adipocytes occurs when Wnt signaling is silenced (50). The evidence suggests that Wnt signaling maintains preadipoctyes in an undifferentiated state through inhibition of the adipogenic transcription factors CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ (50).
It is possible that T3 suppression of the Wnt pathway has important biological ramifications not directly related to cell growth and proliferation. Both T3 and Wnt signaling factors play a critical role in brain development. Specifically, T3 is known to promote neuronal differentiation and migration (57; reviewed by Nunez et al. [43]), and Wnt signaling induces axon and growth cone remodeling (21). Recent studies suggest that both signaling pathways play a role in synapse formation (35, 51). This hypothesis is supported by the observation that both T3 and Wnt7a (a Wnt family member that, like Wnt1, can inhibit GSK-3β activity) are capable of inducing expression of synapsin I, a presynaptic protein involved in synapse formation and neurotransmitter release (15, 33). In the present study, we identified T3-induced expression of the synapsin IIb gene and the altered expression of others involved in synaptic processes (synaptophysin and dendrin) consistent with the hypothesized role for T3 in synaptogenesis. Wnt7a, which induces axonal branching and spreading (33), has been proposed to exert its effect through the inhibition of GSK-3β, which in turn leads to decreased phosphorylation of the GSK-3β substrate, microtubule-associated protein 1B (MAP-1B), thereby decreasing the stability of axonal microtubules (34). That both T3 and Wnt pathways modulate synapsin I levels and that our study revealed the T3-induced down-regulation of the MAP-1B gene strengthen the possibility that the two pathways have intersecting roles in brain development.
Wnt signaling plays a critical role in carcinogenesis (48). For example, in most human colon cancers, the Wnt pathway is aberrantly activated. In most cases, mutated APC fails to down-regulate β-catenin, leading to overabundance and mislocalization of β-catenin (23, 29, 39). The finding that treatment of cells with T3 results in silencing of Wnt signaling by lowering β-catenin level raises the possibility that T3, via its receptors, may interfere directly and/or indirectly with the carcinogenesis mediated by β-catenin. This possibility is not without precedent. RA is a regulator of embryogenesis, cell proliferation, and carcinogenesis. Its action is mediated by the RA receptor, a member of the steroid/TR superfamily. It has recently been shown that RA also decreases the activity of the β-catenin–LEF/TCF signaling pathway, and this regulation may influence cell differentiation and cancer development (16). At present, how T3, via TR, negatively regulates the expression of β-catenin and other key regulators of the Wnt signaling pathway is unknown. However, the possibility that T3 may interfere with β-catenin associated carcinogenesis is supported by reports of loss of TRβ gene expression in human colon carcinomas compared to normal colon mucosa (37), and patients with hepatocellular carcinoma and kidney cancers have mutations of TRα and TRβ that interfere with T3 and DNA binding and transactivation activities (31, 32, 49). Such abnormalities of TRs could abolish the negative regulation of Wnt signaling by T3, thereby contributing to the carcinogenesis of liver and kidney cancers. This possibility will need to be addressed in future studies.
ACKNOWLEDGMENTS
We thank Bert Vogelstein for the generous gift of the expression plasmid pGL3-OT.
This work was supported in part by National Heart Lung and Blood Institute grant HL59781 (to N.H.L.).
L.D.M. and K.S.P. contributed equally to this work.
REFERENCES
- 1.Angeras U, Hasselgren P O. Protein turnover in different types of skeletal muscle during experimental hyperthyroidism in rats. Acta Endocrinol (Copenhagen) 1985;109:90–95. [PubMed] [Google Scholar]
- 2.Barrera-Hernandez G, Park K S, Dace A, Zhan A, Cheng S-Y. Thyroid hormone-induced cell proliferation in GC cells is mediated by changes in G1 cyclin/cyclin-dependent kinase levels and activity. Endocrinology. 1999;140:5267–5274. doi: 10.1210/endo.140.11.7145. [DOI] [PubMed] [Google Scholar]
- 3.Barrera-Hernandez G, Zhan Q, Wong R, Cheng S-Y. Thyroid hormone receptor is a negative regulator in p53-mediated signaling pathways. DNA Cell Biol. 1998;17:743–750. doi: 10.1089/dna.1998.17.743. [DOI] [PubMed] [Google Scholar]
- 4.Bernal J, Nunez J. Thyroid hormones and brain development. Eur J Endocrinol. 1995;133:390–398. doi: 10.1530/eje.0.1330390. [DOI] [PubMed] [Google Scholar]
- 5.Bhat M K, Dace A, Cheng S-Y. Tissue-specific differential repression of gene expression by a dominant negative mutant of thyroid hormone β1 receptor. Thyroid. 1999;9:411–418. doi: 10.1089/thy.1999.9.411. [DOI] [PubMed] [Google Scholar]
- 6.Birchenall-Roberts M C, Engelmann G L, Keller J R, Lohrey N, Ruscetti F W. Retroviral v-myc infection of primary fetal liver cells: transformation of monocytes in vitro. Oncogene. 1989;4:731–735. [PubMed] [Google Scholar]
- 7.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 8.Calza L, Giardino L, Aloe L. NGF content and expression in the rat pituitary gland and regulation by thyroid hormone. Brain Res Mol Brain Res. 1997;51:60–68. doi: 10.1016/s0169-328x(97)00213-1. [DOI] [PubMed] [Google Scholar]
- 9.Calza L, Giardino L, Aloe L. Thyroid hormone regulates NGF content and p75LNGFR expression in the basal forebrain of adult rats. Exp Neurol. 1997;143:196–206. doi: 10.1006/exnr.1996.6361. [DOI] [PubMed] [Google Scholar]
- 10.Carter W J, Van Der Weijden B W S, Faas F H. Effect of experimental hyperthyroidism on protein turnover in skeletal and cardiac muscle. Metabolism. 1980;10:910–915. doi: 10.1016/0026-0495(80)90032-3. [DOI] [PubMed] [Google Scholar]
- 11.Carter W J, Van Der Weijden B W S, Faas F H. Effect of thyroid hormone on protein turnover in cultured cardiac myocytes. J Mol Cell Cardiol. 1985;17:897–905. doi: 10.1016/s0022-2828(85)80103-6. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S-Y. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocrine Metab disorders. 2000;1:9–18. doi: 10.1023/a:1010052101214. [DOI] [PubMed] [Google Scholar]
- 13.Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown P O, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 14.DeRisi J L, Iyer V R, Brown P O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 15.Di Liegro I, Savettieri G, Coppolino M, Scaturro M, Monte M, Nastasi T, Salemi G, Castiglia D, Cestelli A. Expression of synapsin I gene in primary cultures of differentiating rat cortical neurons. Neurochem Res. 1995;20:239–243. doi: 10.1007/BF00970550. [DOI] [PubMed] [Google Scholar]
- 16.Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 17.Eisen M B, Brown P O. DNA arrays for analysis of gene expression. Methods Enzymol. 1999;303:179–205. doi: 10.1016/s0076-6879(99)03014-1. [DOI] [PubMed] [Google Scholar]
- 18.Eisen M B, Spellman P T, Brown P O, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng X, Jiang Y, Meltzer P, Yen P M. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol. 2000;14:947–955. doi: 10.1210/mend.14.7.0470. [DOI] [PubMed] [Google Scholar]
- 20.Fuhrmann G, Rosenberger G, Grusch M, Klein N, Hofmann J, Krupitza G. The MYC dualism in growth and death. Mutat Res. 1999;437:205–217. doi: 10.1016/s1383-5742(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 21.Hall A C, Lucas F R, Salinas P C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 22.He T C, Sparks A B, Rago C, Hermeking H, Zawel L, da Costa L T, Morin P J, Vogelstein B, Kinzler K W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 23.Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 1996;56:2213–2217. [PubMed] [Google Scholar]
- 24.Iyer V R, Eisen M B, Ross D T, Schuler G, Moore T, Lee J C F, Trent J M, Staudt L M, Hudson J, Jr, Boguski M S, Lashkari D, Shalon D, Botstein D, Brown P O. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 25.Kaneshige M, Kaneshige K, Zhu X-G, Dace A, Garrett L, Carter T A, Kazlauskaite R, Pankratz D G, Wynshaw-Boris A, Weintraub B, Willingham M C, Barlow C, Cheng S-Y. Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi A. Roles of axin in the Wnt signalling pathway. Cell Signal. 1999;11:777–788. doi: 10.1016/s0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa S, Obata T, Willingham M C, Cheng S-Y. Thyroid hormone action. Induction of morphological changes and stimulation of cell growth in rat pituitary tumor GH3 cells. Endocrinology. 1987;120:2591–2596. doi: 10.1210/endo-120-6-2591. [DOI] [PubMed] [Google Scholar]
- 28.Koibuchi N, Fukuda H, Chin W W. Promoter-specific regulation of the brain-derived neurotropic factor gene by thyroid hormone in the developing rat cerebellum. Endocrinology. 1999;140:3955–3961. doi: 10.1210/endo.140.9.6997. [DOI] [PubMed] [Google Scholar]
- 29.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 30.Lee N H, Weinstock K G, Kirkness E F, Earle-Hughes J A, Fuldner R A, Marmaros S, Glodek A, Gocayne J D, Adams M D, Kerlavage S R, Fraser C M, Venter J C. Comparative expressed-sequence-tag analysis of differential gene expression profiles in PC-12 cells before and after nerve growth factor treatment. Proc Natl Acad Sci USA. 1995;92:8303–8307. doi: 10.1073/pnas.92.18.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin K-H, Zhu X-G, Shieh H Y, Hsu S-T, McPhie P, Cheng S-Y. Identification of naturally occurring dominant negative mutants of thyroid hormone α1 and β1 receptors in a human hepatocellular carcinoma cell line. Endocrinology. 1996;137:4073–4081. doi: 10.1210/endo.137.10.8828459. [DOI] [PubMed] [Google Scholar]
- 32.Lin K-H, Zhu X-G, Hsu H-C, Chen S L, Shieh H-Y, Chen S-T, McPhie P, Cheng S-Y. Dominant negative activity of mutant thyroid hormone α1 receptors from patients with hepatocellular carcinoma. Endocrinology. 1997;138:5308–5315. doi: 10.1210/endo.138.12.5625. [DOI] [PubMed] [Google Scholar]
- 33.Lucas F R, Salinas P C. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- 34.Lucas F R, Goold R G, Gordon-Weeks P R, Salinas P C. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 35.Madeira M D, Paula-Barbosa M M. Reorganization of mossy fiber synapses in male and female hypothyroid rats: a stereological study. J Comp Neurol. 1993;337:334–352. doi: 10.1002/cne.903370213. [DOI] [PubMed] [Google Scholar]
- 36.Mann M, Gelos M, Siedow A, Hanski M L, Gratchev A, Ilyas M, Bodmer W F, Moyer M P, Riecken E O, Buhr H J, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markowitz S, Haut M, Stellato T, Gerbic C, Molkentin K. Expression of the ErbA-beta class of thyroid hormone receptors is selectively lost in human colon carcinoma. J Clin Investig. 1989;84:1683–1687. doi: 10.1172/JCI114349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKenna N J, Lanz R B, O'Malley B W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 39.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 40.Muller M J, Seitz H J. Thyroid hormone action on intermediary metabolism. Part I: respiration, thermogenesis and carbohydrate metabolism. Klin Wochenschr. 1984;62:11–18. doi: 10.1007/BF01725187. [DOI] [PubMed] [Google Scholar]
- 41.Muller M J, Seitz H J. Interrelation between thyroid state and the effect of glucagon on gluconeogenesis in perfused rat livers. Biochem Pharmacol. 1987;36:1623–1627. doi: 10.1016/0006-2952(87)90046-3. [DOI] [PubMed] [Google Scholar]
- 42.Neveu I, Arenas E. Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J Cell Biol. 1996;133:631–646. doi: 10.1083/jcb.133.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunez J, Couchie D, Aniello F, Bridoux A M. Regulation by thyroid hormone of microtubule assembly and neuronal differentiation. Neurochem Res. 1991;16:975–982. doi: 10.1007/BF00965840. [DOI] [PubMed] [Google Scholar]
- 44.Nunez J, Couchie D, Aniello F, Bridoux A M. Thyroid hormone effects on neuronal differentiation during brain development. Acta Med Austriaca. 1992;19:36–39. [PubMed] [Google Scholar]
- 45.Olson M F, Marais R. Ras protein signalling. Semin Immunol. 2000;12:63–73. doi: 10.1006/smim.2000.0208. [DOI] [PubMed] [Google Scholar]
- 46.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 47.Playford M P, Bicknell D, Bodmer W F, Macaulay V M. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of beta-catenin. Proc Natl Acad Sci USA. 2000;97:12103–12108. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 49.Puzianowska-Kuznick M, Nauman A, Madej A, Tanski Z, Cheng S-Y, Nauman J. Expression of thyroid hormone receptor is deeply impaired in human renal clear cell carcinoma. Cancer Lett. 2000;155:145–152. doi: 10.1016/s0304-3835(00)00416-x. [DOI] [PubMed] [Google Scholar]
- 50.Ross S E, Hemati N, Longo K A, Bennett C N, Lucas P C, Erickson R L, MacDougal O A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 51.Salinas P C. Wnt factors in axonal remodelling and synaptogenesis. Biochem Soc Symp. 1999;65:101–109. [PubMed] [Google Scholar]
- 52.Schwartz L H, Oppenheimer J H. Physiologic and biochemical actions of thyroid hormone. In: Hershman J M, Bray G A, editors. The thyroid. New York, N.Y: Pergamon Press; 1979. pp. 139–166. [Google Scholar]
- 53.Tapia G, Cornejo P, Fernandez V, Videla L A. Protein oxidation in thyroid hormone-induced liver oxidative stress: relation to lipid peroxidation. Toxicol Lett. 1999;106:209–214. doi: 10.1016/s0378-4274(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 54.Usala S J. New developments in clinical and genetic-aspects of thyroid hormone resistance syndromes. Endocrinologist. 1995;5:68–76. [Google Scholar]
- 55.Venditti P, Daniele M C, Masullo P, Di Meo S. Antioxidant-sensitive shortening of ventricular action potential in hyperthyroid rats is independent of lipid peroxidation. Mol Cell Endocrinol. 1998;142:15–23. doi: 10.1016/s0303-7207(98)00123-3. [DOI] [PubMed] [Google Scholar]
- 56.Videla L A, Fernandez V. Thyroid calorigenesis and oxidative stress: modification of the respiratory burst activity in polymorphonuclear leukocytes. Braz J Med Biol Res. 1994;27:2331–2342. [PubMed] [Google Scholar]
- 57.Vincent J, Legrand C, Rabie A, Legrand J. Effects of thyroid hormone on synaptogenesis in the molecular layer of the developing rat cerebellum. J Physiol (Paris) 1982;78:729–738. [PubMed] [Google Scholar]
- 58.Walker J, Rigley K. Gene expression profiling in human peripheral blood mononuclear cells using high-density filter-based cDNA microarrays. J Immunol Methods. 2000;239:167–179. doi: 10.1016/s0022-1759(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 59.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 60.Wong R, Vasilyev V V, Ting Y T, Kutler D I, Willingham M, Cheng S-Y, Weintraub B D. Transgenic mice bearing human mutant thyroid β receptor a model of resistance to thyroid hormone, fat loss and hyperactivity. Mol Med. 1997;3:303–314. [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry III W L, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]