Abstract
Medicinal plants contain valuable compounds that have attracted worldwide interest for their use in the production of natural drugs. The presence of compounds such as rosmarinic acid, carnosic acid, and carnosol in Rosmarinus officinalis has made it a plant with unique therapeutic effects. The identification and regulation of the biosynthetic pathways and genes will enable the large-scale production of these compounds. Hence, we studied the correlation between the genes involved in biosynthesis of the secondary metabolites in R. officinalis using proteomics and metabolomics data by WGCNA. We identified three modules as having the highest potential for the metabolite engineering. Moreover, the hub genes highly connected to particular modules, TFs, PKs, and transporters were identified. The TFs of MYB, C3H, HB, and C2H2 were the most likely candidates associated with the target metabolic pathways. The results indicated that the hub genes including Copalyl diphosphate synthase (CDS), Phenylalanine ammonia lyase (PAL), Cineole synthase (CIN), Rosmarinic acid synthase (RAS), Tyrosine aminotransferase (TAT), Cinnamate 4-hydroxylase (C4H), and MYB58 are responsible for biosynthesis of important secondary metabolites. Thus, we confirmed these results using qRT-PCR after treating R. officinalis seedlings with methyl jasmonate. These candidate genes may be employed for genetic and metabolic engineering research to increase R. officinalis metabolite production.
Introduction
Global interest in the use of medicinal plants is growing and their beneficial effects on the production of drugs are being identified. It is estimated that only 10% of the 250,000 plant species important to human health have been studied [1]. On the other hand, essential oils are in high demand globally with a compound annual growth rate of 9.3%, which is expected to reach 16 million dollars by 2026 [2]. It is therefore necessary for researchers to find a way to increase the production of essential oils and derived compounds.
Due to its flavoring and therapeutic properties, Rosmarinus officinalis is the most commonly used medicinal herb among spices in food, pharmaceuticals, and cosmetics [3]. Previous studies have shown that R. officinalis has showed very high therapeutic effects due to the presence of bioactive compounds such as 1,8-cineol (15–55%), verbenone (2.2–11.1%), borneol (1.5–5.0%), camphor (5–31%), limonene (1.5–5.0%), pinene (2.0–9.0%), pinene (9–26%), caryophyllene (1.8–5.1%), camphene (2.5–12.0%), and myrcene (0.9–4.5%) [4, 5]. These metabolites have been applied for the prevention and treatment of various diseases such as colds, rheumatoid arthritis, and musculoskeletal discomforts [4, 5]. In addition, this plant presents a wide range of biological properties including antibacterial [6], antitumor [7], antidiabetic [8], antioxidant [9], and anti-inflammatory [10]. Accordingly, the rosemary extract was approved as food additives by EU Food Standards Agency (E392) [4]. Generally, medicinal plants are a renewable and almost unlimited source for new and complex chemical compounds [11–13]. According to these properties, research on this plant will be very interesting.
Although the secondary metabolites show diverse functions in the plants, including regulating growth [14], interacting with the environment [15], and ameliorating abiotic and biotic stresses [16, 17], the structure and metabolic pathways of secondary metabolites are still unknown. It is therefore crucial to identify key genes involved in these pathways in order to expand large-scale production and to maximize its potential in the fields of biotechnology, horticulture, and pharmacy. In recent years, the approach of gene co-expression analysis has become a powerful tool for predicting the function and role of genes. This vision provide a deeper understanding of metabolic pathways in plants and increase the potential for genome-scale pathways and systems metabolic engineering to produce more bioactive compounds [18]. In gene co-expression study, those genes that have a similar expression pattern among different samples are identified [19]. Genes involved in the biosynthesis of specific metabolites have a common regulatory network and most of these genes have closely related to each other (modules) in the co-expression network, which facilitate their identification [20]. However, the aim of this study is the identification of transcription factors, protein kinases, transporters, and hub genes involved in the biosynthesis pathway of rosmarinic acid using co-expression analysis and then evaluating the expression profile of hub genes in methyl jasmonate (MeJA)-treated R. officinalis seedlings.
Materials and methods
Data collection and processing
Metabolomics and transcriptomics data of R. officinalis were retrieved from the Plant/Eukaryotic and Microbial Systems Resource database (http://metnetweb.gdcb.iastate.edu/P-MR/) and Medicinal Plant Genomics Resource database (http://medicinalplantgenomics.msu.edu-/), respectively. First, the transcript expression level and the quantity of secondary metabolites within the tissues of transcriptomic and metabolomic data were classified correspondently. In addition, transcripts with low expression values observed in the transcriptomic data were also filtered out using the genefilter package based on the variance filtering through varFilter function. Then transcriptomics data is related to 32402 genes in 15 different samples i.e., young and mature leaf, secondary stem, root, callus, and flower. Transcriptomics and metabolomics data were integrated to identify the modules correlated with the biosynthesis of secondary metabolites by calculating module eigengenes (ME) and estimating Pearson’s correlations between the MEs and selected metabolites to perform a comparative analysis using R Package Weighted Gene Co-expression Network Analysis (WGCNA). In this study, the expression value of Fragments Per Kilobase of exon per Million reads (FPKM) and liquid chromatography/time-of-flight/mass spectrometry (LC/TOF/MS) of the metabolome profiles were used for the transcriptome and proteome data, respectively.
Co-expression analysis
All samples were used to construct the co-expression modules related to the biosynthesis pathway of secondary metabolites. The co-expression network was constructed using the WGCNA [21]. The gene clusters were analyzed in the transcriptome datasets by measuring Pearson correlation coefficient using the power adjacency function [22]. Topological Overlap Matrix (TOM) and a hierarchical cluster tree were set up according to setting soft thresholding power β = 16 and transforming the Pearson correlation matrix into a weighted matrix. Therefore, a appreciate power value was measured to construct the network, which obtained by measuring the scale free fit index in a group of powers with a minimum of 1 to maximum of 30. The identification of gene co-expression modules was conducted by the blockwiseModules function and a dynamics tree cut algorithm. Finally, the correlation of the modules with the major secondary metabolites was investigated.
Gene ontology and pathway analysis
The modules were obtained by BLASTX of transcript data in Swissport database, followed by submitted to the STRING database (https://string-db.org/) [23] to perform functional enrichment analysis. Afterwards, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed using by the DAVID web tool (http://david.abcc.ncifcrf.gov/) with an FDR ≤ 0.05. Transcription factors (TFs) and protein kinases (PKs) were identified by BLASTX of transcript data in the iTAK database (http://bioinfo.bti.cornell.edu/cgibin/itak/index.cgi) [24]. In addition, the TCDB database (http://www.tcdb.org/) [25] was used to predict the transporters.
Identification of hub genes
The network was visualized in Cytoscape software in the cytohubba plug-in. After calculating the connectivity scores using the Maximal Clique Centrality (MCC) function, the genes with the highest connectivity score in a module were considered as hub genes. The number of hub genes were selected based on the number of genes in each module.
Plant selection and treatment
Many plant species treated with MeJA are able to produce compounds such as alkaloids, terpenoids, coumarins, and phenolic compounds [26]. On the other hand, the accumulation of rosmarinic acid in cell culture of MeJA-treated Mentha balsamea has been proven [27]. Recent studies have found that MeJA stimulates the synthesis of active compounds in rosemary suspension cells over a broad concentration range using concentration-dependent differential expression patterns. MeJA also reduced the peroxidative damage to the rosemary suspension cells over a broad concentration range using these same techniques [28, 29]. Therefore, MeJA was selected to elicit the expression of genes involved in the biosynthesis pathway of secondary metabolites in R. officinalis.
The seedlings were cultivated under optimum conditions in pot and then treated with MeJA in the concentration of 100 mM for 12 and 24 h in three biological replicates [30, 31]. After applying treatment, the 15 leaves were collected for each sample and frizzed in liquid nitrogen, followed by transferred to -80 °C for more analysis. Finally, we used just 100 mg leaf powder for RNA extraction for each sample. RNA extraction was carried out using the RNX-PlusTM kit of Sinagene Co. (S-1020-1) according to the manufacturer’s instructions. The cDNA synthesis kit of Thermo Fisher Scientific Co. (K1622) was used to synthesize the first strand cDNA.
Gene expression analysis using qRT-PCR
Gene expression analysis was performed using Real-time PCR (Bio-Rad, Hercules, CA) using specific primers of hub genes (Table 1).
Table 1. The sequences of specific primers used to the Real-time PCR.
| Gene | Sequence (5ʹ to 3ʹ) |
|---|---|
| Copalyl diphosphate synthase (CPS) | F: GTTGGTGAAGTTAGTGCT |
| R: GTCTCATCATCGTGGTAG | |
| Phenylalanine amonia-lyase (PAL) | F: CTGGTCACTGCCTTCTAA |
| R: CTGAGGTAGGGTATGGTC | |
| MYB 4 | F: TGGTAAGTGGTTGAGAATC |
| R: AGACAGTGATGAGTAGCA | |
| Alpha tubulin (αTUB) | F: GGAAATGCTTGCTGGGAG |
| R: GAAGAAGGTGTTGAAGGC | |
| Cinenol synthase (CIN1) | F: TGTATGACGAGTGATGGATGAG |
| R: ACTTGCGTGGATGCTATCTT | |
| Rosmarinic acid synthase (RAS) | F: ATGAAGATCGATATCACAGAC |
| R: GTAGAAATGCACGCTGAG | |
| Tyrosine aminotransferase (TAT) | F: TGCTTCCACGCTAGTAAT |
| R: GATTGCCTCTCTGGTTTG | |
| Cinnamate 4-hydroxylase (C4H) | F: TCGTGTTCGACATCTTCAC |
| R: TACTGCTGCACCACCTTG |
All primers were designed by Allele ID software. The diluted cDNA samples used as the template for qRT-PCR. The achieved data were analyzed using a thermocycler Line GeneK with the software Line GeneK Fluorescent Quantitative Detection system (BIOER Technology, Hangzhou, China). The mean of Ct values of the housekeeping gene of alpha tubulin was used as the internal control. The relative expression level was calculated using ΔΔCt method.
Statistical analysis
Three technical replicates were applied for each sample in qRT-PCR. The data were analyzed using the SAS 9.4 software (SAS Institute Inc., USA) at a P-value of ≤ 0.05. A mean comparison analysis was performed using the Tukey test. The graphs were plotted using GraphPad prism software v8 (GraphPad Software Inc., San Diego, CA).
Results
Evaluation of co-expression modules
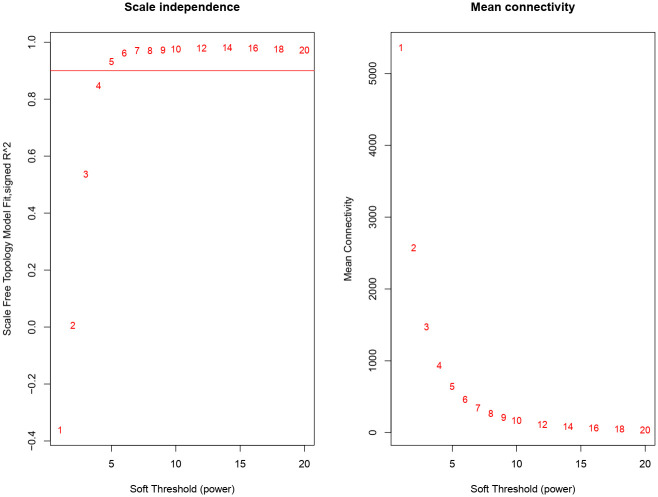
This study presents the data collection and analysis process to identify some central genes involved in the biosynthesis of secondary metabolites in R. officinalis. The correlation among differentially expressed genes (DEGs) was evaluated using WGCNA R-package to identify these genes. The hierarchical clustering tree was produced with a power value of 16 and a scale independence of up to 0.9 through network topology analysis to determine the average degree of connectivity and the independence within co-expression modules (Fig 1).
Fig 1. Analysis of soft-thresholding power in the WGCNA.
The left picture displays determination of scale-free fit index (y-axis) for various soft-thresholding powers. The right picture shows determination of mean connectivity (degree, y-axis) for various soft-thresholding powers (x-axis).
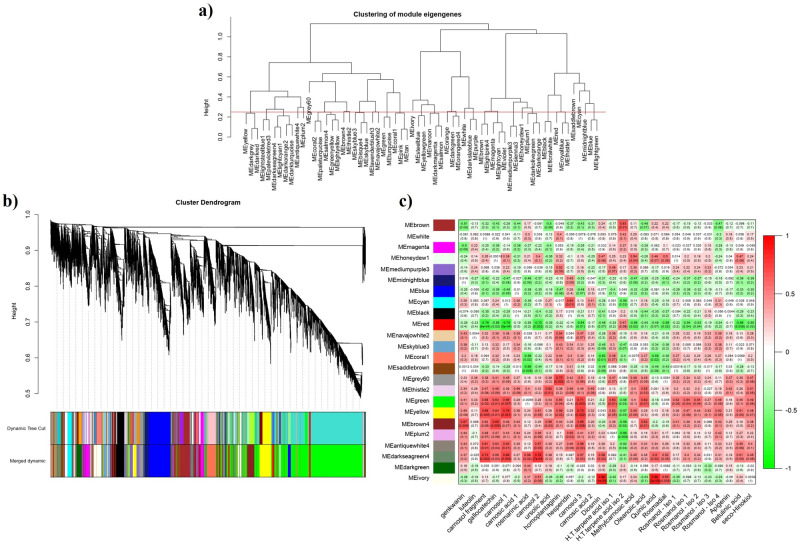
Based on the eigengenes, an analysis of cluster for the modules of R. officinalis was performed. Results showed that modules were divided into 28 clades. Closed modules were combined by setting up the height of branch merge cut by 0.25 and showed in distinct colors (Fig 2a). Totally 24 co-expression modules were identified based on the dynamic tree cutting algorithm (Fig 2b). The modules related to important secondary metabolites were detected by the correlation between secondary metabolites and co-expression modules (Fig 2c).
Fig 2. The gene network analysis of the biosynthesis pathway of secondary metabolites in R. officinalis.
a) Clustering dendrogram of the modules, whereas the horizontal line showing merge cut height of 0.25. b) Co-expression modules constructed. Dendrograms produced by average linkage of hierarchical clustering of genes, which is based on a topological overlap matrix (TOM). The modules were assigned colors as indicated in the horizontal bar beneath the dendrogram. c) Heat map of correlation between eigengene modules and secondary metabolites. Each row shows a module eigengene, each column shows a secondary metabolite. Each cell contains the corresponding correlation and P-value. The correlation between modules and secondary metabolites in the cells has a ratio ranged from -1 to +1. The more this ratio closer to +1, the color changes from green to red.
Accordingly, the three major modules including brown4, green, and yellow, each containing 5567, 880, and 818 genes, respectively, showed higher levels of correlation with desirable secondary metabolites. These modules with the greatest potential for metabolic engineering were selected for further investigations.
Related modules to secondary metabolites
According to our results, secondary metabolites have a significant correlation with co-expression modules, therefore we identified the modules with the most compounds. Intensive research has been conducted on rosemary and its components carnosol, carnosic acid, ursolic acid, rosmarinic acid, and caffeic acid over the past decade [32]. The results showed that the selected modules have the highest correlation with carnosol, carcinoic acid, and ursolic acid. Results indicate that rosmarinic acid and genkwanin, respectively, exhibited low and high correlations with all three modules, whereas genkwanin exhibited a remarkable correlation with brown4 module.
Gene ontology and KEGG analysis
Gene Ontology (GO) and KEGG enrichment analysis were performed on the selected modules (Tables 2 & 3).
Table 2. GO enrichment analysis of genes in the selected modules.
| Module | GO No. | Term | Count | FDR |
|---|---|---|---|---|
| Brown4 | GO:0009651 | Response to salt stress | 24 | 0.0092 |
| GO:0009408 | Response to heat | 12 | 0.0350 | |
| Green | GO:0055114 | Oxidation-reduction process | 295 | 2.59E-13 |
| GO:0046686 | Response to cadmium ion | 81 | 8.18E-04 | |
| GO:0006810 | Transport | 78 | 0.0064 | |
| GO:0009409 | Response to cold | 71 | 0.0022 | |
| GO:0015979 | Photosynthesis | 66 | 1.25E-17 | |
| Yellow | GO:0055114 | Oxidation-reduction process | 56 | 0.0022 |
| GO:0006468 | Protein phosphorylation | 37 | 0.0354 | |
| GO:0006633 | Fatty acid biosynthetic process | 18 | 4.41E-06 | |
| GO:0080167 | Response to karrikin | 16 | 5.45E-05 | |
| GO:0007169 | Transmembrane receptor protein | 16 | 5.45E-05 | |
| Tyrosine kinase signaling pathway |
Table 3. KEGG pathway enrichment analysis of genes in the selected modules.
| Module | KEGG No. | Pathway | Count | FDR |
|---|---|---|---|---|
| Brown4 | - | - | - | - |
| Green | ath01100 | Metabolic pathways | 378 | 5.16E-14 |
| ath01110 | Biosynthesis of secondary metabolites | 237 | 7.33E-13 | |
| ath01130 | Biosynthesis of antibiotics | 101 | 9.50E-05 | |
| ath01200 | Carbon metabolism | 72 | 5.22E-06 | |
| ath00710 | Carbon fixation in photosynthetic organisms | 32 | 8.32E-08 | |
| Yellow | ath01110 | Biosynthesis of secondary metabolites | 45 | 1.67E-05 |
| ath01212 | Fatty acid metabolism | 10 | 2.80E-04 | |
| ath00061 | Fatty acid biosynthesis | 8 | 2.80E-04 | |
| ath00073 | Cutin, suberine and wax biosynthesis | 7 | 2.80E-04 | |
| ath00062 | Fatty acid elongation | 6 | 0.0092 |
Functional enrichment showed that genes in the module brown4 were mainly enriched in response to salt stress (GO:0009651) and response to heat (GO:0009408). Genes in the module green were mainly enriched in oxidation-reduction process (GO:0055114), response to cadmium ion (GO:0046686), transport (GO:0006810), response to cold (GO:0009409), and photosynthesis (GO:0015979). Genes in the module yellow were mainly enriched in oxidation-reduction process (GO:0055114), protein phosphorylation (GO:0006468), fatty acid biosynthetic process (GO:0006633), response to karrikin (GO:0080167), and transmembrane receptor protein tyrosine kinase signaling pathway (GO:0007169).
Identification of transcription factors
Since the TFs regulate the genes involved in the biosynthesis of secondary metabolites [33], the identification of TFs-related genes in the modules was performed by alignment of transcript sequences against the iTAK database. We identified 132 TFs in selected modules that are associated with metabolic pathways, among which MYB, Cysteine3Histidine (C3H), homebox (HB), and Cys2-His2 (C2H2) are the most likely candidates (Table 4).
Table 4. TF families in the selected modules.
| Brown4 | Green | Yellow | |||
|---|---|---|---|---|---|
| B3 | 2 | bHLH | 28 | MYB | 8 |
| GARP-G2-like | 2 | WRKY | 21 | HB-HD-ZIP | 6 |
| MADS-M-type | 2 | NAC | 17 | WRKY | 5 |
| Trihelix | 2 | C2H2 | 14 | SBP | 5 |
| bZIP | 2 | AP2/ERF-ERF | 13 | OFP | 4 |
| GRAS | 2 | bZIP | 13 | bHLH | 4 |
| MYB | 2 | MYB-related | 12 | bZIP | 3 |
| TUB | 2 | C2C2-Dof | 11 | C2C2-YABBY | 3 |
| C2H2 | 2 | GARP-G2-like | 11 | MYB-related | 3 |
| C3H | 2 | MYB | 10 | GARP-G2-like | 2 |
| DDT | 1 | zf-HD | 9 | GRAS | 2 |
| MADS-MIKC | 1 | LOB | 9 | C2C2-GATA | 2 |
| TCP | 1 | GRAS | 9 | HB-other | 2 |
| bHLH | 1 | TCP | 8 | B3-ARF | 2 |
| HB-HD-ZIP | 1 | C3H | 7 | C2H2 | 2 |
| NAC | 1 | C2C2-GATA | 6 | HB-WOX | 2 |
| WRKY | 1 | Trihelix | 5 | NAC | 2 |
| HSF | 1 | HSF | 4 | AP2/ERF-RAV | 1 |
| SBP | 1 | OFP | 4 | NF-YA | 1 |
| C2C2-YABBY | 4 | Trihelix | 1 | ||
| C2C2-CO-like | 3 | AP2/ERF-AP2 | 1 | ||
| SRS | 3 | LOB | 1 | ||
| AP2/ERF-AP2 | 3 | AP2/ERF-ERF | 1 | ||
| PLATZ | 3 | PLATZ | 1 | ||
| B3 | 3 | zf-HD | 1 | ||
| B3-ARF | 3 | HB-BELL | 1 | ||
| HB-HD-ZIP | 3 | C3H | 1 | ||
| E2F-DP | 3 | SRS | 1 | ||
| MADS-M-type | 3 | ||||
| DBB | 2 | ||||
| HB-BELL | 2 | ||||
| TUB | 2 | ||||
| HB-other | 2 | ||||
| CPP | 1 | ||||
| EIL | 1 | ||||
| GRF | 1 | ||||
| CSD | 1 | ||||
| GARP-ARR-B | 1 | ||||
| HB-WOX | 1 | ||||
| LIM | 1 | ||||
| RWP-RK | 1 | ||||
| C2C2-LSD | 1 | ||||
| DBP | 1 | ||||
| GeBP | 1 | ||||
| MADS-MIKC | 1 | ||||
| NF-YA | 1 | ||||
| S1Fa-like | 1 | ||||
| BES1 | 1 | ||||
| CAMTA | 1 | ||||
| NF-YB | 1 | ||||
| SBP | 1 | ||||
| VOZ | 1 |
MYB is a very large TF family that was detected in every selected module except green. Here, C3H has been detected in all selected modules. GAI-RGA- and -SCR (GRAS), SQUAMOSA promoter binding protein (SBP), Cys2Cys2 (C2C2), and homebox homeodomain leucine zipper (HB-HD-ZIP) play a major role in all modules, as can be seen in the results (Table 4). In addition, MCM1, AG, DEF and SRF (MADS) is an important family of proteins that was identified in all modules except for yellow. According to the results, the yellow module includes a higher number of TFs than the other modules.
Identification of protein kinases
Recognition of PKs as regulators of critical signaling in response to environmental stresses will be very effective in improving the biosynthesis pathway of secondary metabolites [34]. In the selected modules, 220 genes encoding PKs were identified and classified into (PKA, PKG, PKC (AGC)), calmodulin-dependent protein kinase (CAMK), cytokeratin 1 (CK1), (CDK, MAPK, GSK3, CLK kinases (CMGC)), receptor-likekinases (RLK)-Pelle, homologs of yeast sterile (STE), and tyrosine kinase-like (TKL) families (Table 5).
Table 5. PK families in the selected modules.
| Brown4 | Green | Yellow | |||
|---|---|---|---|---|---|
| TKL | 5 | RLK-Pelle | 101 | RLK-Pelle | 36 |
| CMGC | 4 | CAMK | 18 | AGC | 3 |
| CAMK | 3 | TKL | 13 | TKL | 2 |
| RLK-Pelle | 3 | AGC | 12 | Others | 1 |
| Plant-specific | 2 | CMGC | 6 | STE | 1 |
| CK1 | 1 | Others | 5 | ||
| Plant-specific | 2 | ||||
| STE | 2 |
The CMGC family was the largest among those present in the brown4 module. The RLK-Pelle_DLSV subfamily of RLK-Pelle and TKL families were the only family that was commonly identified in all the selected modules (S1 Table). Based on the results, the green module showed the most variable PKs in the selected modules.
Identification of transporters
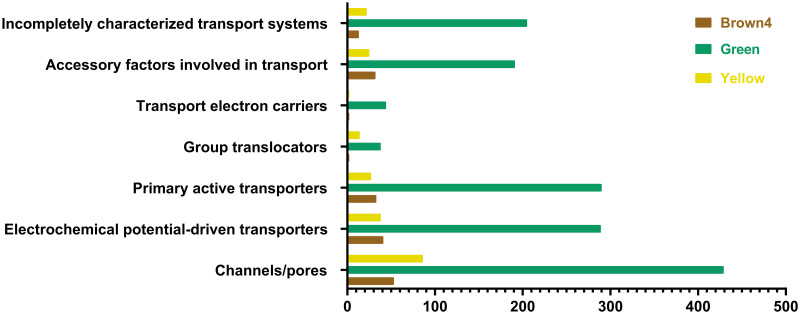
According to the key role of transporters in transport of plant secondary metabolites [35], we examined 7,265 unigenes in selected modules that were identified in the TCDB database. The TCDB classified transporters into 7 systems (Fig 3, S2 Table).
Fig 3. Transporter systems in selected modules of R. officinalis.
Y-axis shows the transporter systems and x-axis shows the number of unigenes.
This database contains 7 main systems with subsystems. The main systems are listed as follows: channels/pores, electrochemical potential-driven transporters, primary active transporters, group translocators, transmembrane electron carriers, accessory factors involved in transport, and incompletely characterized transport systems [36]. According to the results, 25.7% of transporters were classified in incompletely characterized transport systems, which means that many transporters of R. officinalis have potential for exploring their structures. Based on the results, most transporters including Voltage-gated Ion Channel (VIC) superfamily, Gap Junction-forming Innexin family, MCA family, nuclear pore complex (NPC) family, and membrane-limited channels, belonged to the channel/pore family, where the share of channel types is higher. The VIC superfamily was identified in 6.4%, 7.7% and 9.7% of transporters in the brown4, green and yellow modules, respectively. Electrochemical potential-driven transporters, which part of the Major Facilitator Superfamily (MFS) [25], were identified in all selected modules in 3.9%, 3.4% and 2.8% in the brown4, green and yellow modules, respectively. Primary active transporters were also identified in all selected modules as 0.2%, 0.7% and 0.2% in the brown4, green, and yellow modules, respectively (Fig 3). Study of the transporters shows that the green module was more active than the other selected modules in secondary metabolites transformation, as it provides a higher expression level of major transporters.
Identification of hub genes
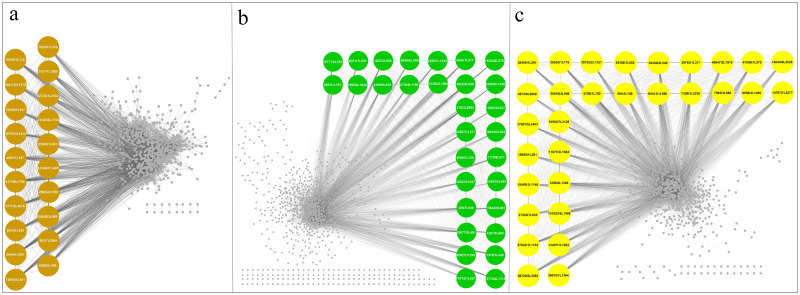
Hub genes were identified using Cytoscape software (Cytohubba plugin) and grouped by the MCC method (Fig 4).
Fig 4. Identification of hub genes involved in metabolite biosynthesis pathway of R. officinalis.
Diagrams show the identified hub genes in the brown4 (a), green (b), and yellow (c) modules. Nodes represent genes and brown, green, and yellow bigger nodes indicate the hub genes. The gray line connecting two nodes indicates the correlation between them.
Among the 80 hub genes completely screened out in the current study, some genes with unknown functions may be worth exploring further. Following their strongest connections, the top 20 genes were selected as hubs for brown4 module and the 30 top genes were selected for both green and yellow modules. Among hub genes identified in the selected modules, Cineole synthase (CIN1), Copalyl diphosphate synthase (CPS), Rosmarinic acid synthase (RAS), and Tyrosine aminotransferase (TAT) were placed in yellow modules. In addition, Phenylalanine ammonia-lyase 2 (PAL) and Cinnamate 4-hydroxylase (C4H) were placed in brown4 module, as well as MYB58 (MYB) were placed in green module (Table 6). Seven hub genes were selected for the qRT-PCR and their expression profile was confirmed in MeJA-treated R. officinalis seedlings.
Table 6. The identified hub genes in correlation with rosmarinic and carnosic acid pathways.
| Genes | Module | Correlation |
|---|---|---|
| Cineole synthase (CIN1) | Yellow | 74–89% |
| Copalyl diphosphate synthase (CPS) | Yellow | 74–89% |
| Rosmarinic acid synthase (RAS) | Yellow | 74–89% |
| Gernylgeranyl reductase | Green | 72–86% |
| MYB 4 | Green | 72–86% |
| R2r3-myb58 transcription factor | Green | 72–86% |
| Cytochrome P450 (CYP76C4) | Yellow | 74–86% |
| Prephenate aminotransferase (PAT) | Brown4 | 72–89% |
| Tyrosine aminotransferase (TAT) | Yellow | 74–86% |
| Gernylgeranyl diphosphate synthase (GGPS1) | Yellow | 74–86% |
| Phenylalanine ammonia-lyase 2 (PAL2) | Brown4 | 72–89% |
| Cinnamate 4-hydroxylase (C4H) | Brown4 | 72–89% |
| Phenylalanine ammonia-lyase 1 (PAL1) | Green | 72–85% |
| Hydroxycinnamoyl-CoA shikimate: quinate hydroxycinnamoyl transferase (HCT) | Green | 72–85% |
| Cytochrome P450 (CYP98A3) | Brown4 | 72–89% |
| Limonene synthase | Yellow | 74–86% |
| Chorismate synthase | Brown4 | 72–89% |
qRT-PCR of hub genes
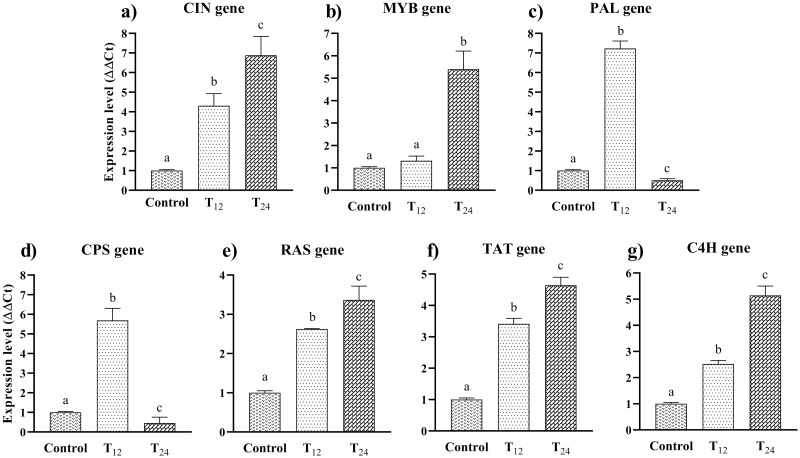
The relative expression analysis of seven hub genes, including PAL, MYB, CPS, CIN1, TAT, C4H, and RAS, showed that all of the genes increasingly expressed after MeJA treatment in comparison with control after 12 h. After 12 h of treatment, the highest and lowest of relative expression level were respectively related to PAL and MYB (Fig 5a & 5b). Accordingly, the expression level of the PAL, CPS, and MYB was about 7.6-, 5.7-, and 1.27-fold in comparison with the control after 12 h, respectively (Fig 5a–5c). After 24 h, the expression level of CPS and PAL decreased sharply by 0.46- and 0.5-fold, respectively, whereas MYB expression was increased by 3-fold (Fig 5a–5c). As the results showed, the CIN1 significantly up-regulated at 12 and 24 h (4.3 and 6.9-fold in comparison with the control, respectively) (Fig 5d). In addition, TAT and C4H showed significantly up-regulated at 12 and 24 h (3.41 and 4.64-fold for TAT and 2.52 and 5.14-fold for C4H, respectively) (Fig 5e & 5f). Similarly, the relative expression of RAS increased in 12 and 24 h by 2.62 and 3.36-fold, respectively (Fig 5g).
Fig 5. Relative expression of hub genes under MeJA treatment using qRT-PCR.
The graphs show the expression level of the PAL (a), MYB (b), CPS (c), CIN (d), TAT (e), C4H (f), and RAS (g) after exposure times of 12 and 24 h in comparison with the control. Data were analyzed using the GraphPad prism at P-value of < 0.01 and expressed as mean ± SD (standard deviation).
Interestingly, the expression level of CIN, MYB, RAS, TAT, and C4H increased over the time. It can be suggested that the MeJA plays an elicitor role in more production of secondary metabolites related to these genes.
Discussion
Functional enrichment analysis of modules
There are a variety of secondary metabolites in R. officinalis, each of which is useful in treating disease [5]. Identifying the hub genes involved in the biosynthesis of each secondary metabolite is crucial for the development of pharmaceuticals and plant breeding programs, as well as industrial applications. In rosemary, the most important biological activities are associated with polyphenols such as carnosol and carnosic acid [37, 38]. Unigenes in brown4, green, and yellow were functionally classified into various categories by GO term enrichment analysis. In the biological process, GO terms of cellular process, transmembrane transport, metabolite process, and responses to metabolites and elements were more significant than other terms. Among the modules, the genes classified in the green module are most associated with the production of secondary metabolites (Table 2). Based on the KEGG enrichment analysis, biosynthesis of secondary metabolites was found as a noteworthy significant pathway for the green and yellow modules (Table 3).
In addition, results show that some of the co-expression genes are responsible for vital cellular functions. In brown4 and green modules, PAL2 and PAL1 were respectively identified as key genes in the rosmarinic acid biosynthesis pathway. Studies on Satureja spicigera showed that PAL is an essential enzyme in the biosynthesis of rosmarinic acid and phenolic compounds because of the expression of PAL and the total amount of rosmarinic acid is significantly correlated [39]. In addition, TAT, RAS, and Cytochrome P450, which identified in yellow module, are key genes in the rosmarinic acid biosynthesis pathway. Based on the presence of these genes, it is a key module in the pathway of rosmarinic acid biosynthesis [40]. The carnosic acid biosynthesis pathway starts with Granylgranyl diphosphate, which synthesized by Granylgranyl diphosphate synthase (GGPS), followed by copalyl diphosphate, synthesized by CPS, and finished by contribution of Cytochrome P450 [41]. According to identification of GGPS, CPS and Cytochrome P450 in the yellow module, this module has a huge role in the carnosic acid biosynthesis.
TFs, key regulatory proteins in metabolic pathways
In understanding how gene networks are regulated, it is important to understand how TFs control the biosynthesis of metabolites. Based on the identification of TFs in different rosemary modules, 132 TFs, belonging to 34 TF families, are associated with metabolic pathways, but MYB (v-myb myeloblastosis viral oncogene homolog), C3H, HB, and C2H2 are the most likely ones (Table 4). Hence, MYB is a large TF family and was identified in all selected modules except green. The MYB TFs are the most abundant, regulating the biosynthesis of secondary metabolites [42]. In addition to regulating secondary metabolism, responses to hormones and environments, and cell differentiation and morphogenesis, MYB TFs also play a crucial role in resistance to drought and other abiotic stresses [43]. In Arabidopsis overexpressing OsMYB3R-2, the expression of dehydration-responsive element-binding protein 2A, COR15a, and RCI2A was significantly increased, leading to enhanced abiotic stress tolerance [44]. C3H were detected in all selected modules. C3H is zinc finger proteins involved in pre-mRNA processing, transcriptional regulation, and stress tolerance. C2H2 identified in all selected modules and C2C2 identified in green and yellow modules, both known as zinc finger TFs. In Catharanthus roseus, protein complexes containing C2H2 zinc fingers bind to the monoterpenoid indole alkaloid biosynthesis gene promoter and control the promotor activity [45]. In Arabidopsis, OBP2 designated TF, which is a C2C2-Dof (DNA-binding One Zinc Finger), showed an increase in the regulation of indole glucosinolates biosynthesis [45].
Studies on C. roseus under MeJA treatment showed that CrMYC2, an MYC family TF of bHLH, is contribution in the regulation of terpenoid indole alkaloids biosynthesis genes [28]. Studies showed that WRKYs could regulate the expression of genes that contribution in terpenes biosynthesis pathways through control the promoters of related genes [46]. Among bioactive compounds of R. officinalis, verbenone and camphor known as terpene biocompounds, in the other hand WRKY is identified in all selected modules. In addition, studies on Cinnamomum camphora showed that WRKY, MYB, AP2/ERF, bZIP and bHLH are regulate borneol biosynthesis pathway genes [47]. HB-HD-ZIP also plays a crucial role in all selected modules. Aliakbari and coworkers found that the expression of the protein group HB-HD-ZIP is effective in increasing secondary metabolites [48]. One of the protein families that play an important role in the modules is GRAS, which was identified in all modules. Homologs of GRAS proteins can be found in Arabidopsis, tomato, petunia, lily, rice and barley. GRAS family members are essential for physiological processes such as gibberellic acid (GA) signal transduction, stem cell maintenance, axillary meristem initiation, light signaling, photochromic signaling, male gametogenesis, and detoxification [49, 50]. According to the findings, this protein family plays a very important role in metabolism, especially under stressful conditions, and is thought to be involved in the production of secondary metabolites. In all plants, SBP protein family plays an important role in the process of flowering and reproduction, as well as regulating the ripening of fruits and ovarian function [51, 52], so that it found in all modules in this study. GA and anthocyanins are synthesized by this protein family, and they play a crucial role during abiotic stresses [53, 54]. MADS, found in all modules except yellow, is another important family of proteins. MADS boxes regulate the activity of topoisomerase PA, which is involved in DNA replication [55], temperature responses and flowering process regulation and also play a part in protein-protein interactions [56, 57].
Therefore, based on our results and previous researches, this protein family is likely caused by temperature-dependent environmental conditions in the regulation of secondary metabolites. Totally, TF analysis of R. officinalis enlighten that bHLH and WRKY are major protein families that are potentially contribute in synthesis pathways of biocompounds such as verbenone, camphor and borneol, also have noteworthy quantity in the selected modules.
PKs involved in biosynthesis of secondary pathways
We have investigated potential PKs to find out how signal transduction occurs in rosemary. PKs play a pivotal role in regulating eukaryotic cell cycle and division [58]. From the iTAK database, 220 PKs were identified in 11 families. RLK-Pelle_DLSV (Receptor-Like Kinase/Pelle) subfamily is one of the PKs, which was identified in all three modules. According to the previous studies, this subfamily is involved in biotic and abiotic stresses [59, 60]. Leucine-rich repeats (LRRs) are a subfamily of RLK-Pelle identified in all modules. In a study, it has shown that LRR-RLKs play important roles in defense-related responses [61]. Receptor-like cytoplasmic kinase (RLCK) is another RLK subfamily that has 17 and 4 genes encoding in brown4 and yellow modules, respectively. The other hand, RLCKs are involved as mediators of cellular signaling in response to developmental and environmental signals [62]. In Marchantia polymorpha, an early-divergent land plant lineage, RLKs and SA pathway genes have been found [63]. In fact, a conserved biochemical defense response has been found in M. polymorpha, namely the role of PP-associated metabolites in mitigating pathogen infection [64]. LRR-RLKs have been shown to be involved in the cotton fiber development as well as cell wall biosynthesis in cotton. RLK1 was induced in developing cotton fibers and was predicted to be involved in the secondary cell wall synthesis in cotton fiber [65].
In addition, tyrosine kinase like (TKL) is another group of PKs that was identified in all selected modules. In this family, biological roles in development, stress response and infection process were demonstrated using the gene silencing method [66]. AGC (protein kinase A/protein kinase G/protein kinase C-family) is another group of PKs identified in green and yellow modules, which has a direct effect on auto-phosphorylation [67]. Studies have shown that AGC protein kinase plays an important role in growth control in a regulatory cycle [68]. Some of the best-studied plant AGC kinases mediate auxin signaling and are thereby involved in the regulation of growth and morphogenesis. Furthermore, certain members are regulated by lipid-derived signals via the 3-phosphoinositide-dependent kinase 1 (PDK1) and the kinase target of rapamycin (TOR), similar to its animal counterparts [69]. Ca2+/calmodulin-dependent protein kinase) CAMK (group contains a total of 21 encoding genes in brown4 and green modules. CAMK is directly and indirectly regulated by Ca2+ and is involved in the response to hormone signaling, various biotic and abiotic stresses [70, 71]. It may be suggested that the mentioned gene families are part of the necessary enzymes for the production of secondary metabolites in R. officinalis.
Therefore, these gene families either can act as signal transmitters, like LRRs and RLCKs, which are the main building blocks of the metabolite production system, or participate in defense pathways, like TKL and RLK-Pelle, which are thought to be engaged in metabolite products with defense system activation. Here, there are significant protein families, like CAMK, which are crucial for controlling the cell cycle when metabolite production is taking place.
Transporters involved in biosynthesis of secondary pathways
Since the key role of transporters in accumulation of secondary metabolites in specific tissues is undeniable [72], in this study, 7,265 unigenes from selected modules were analyzed in the TCDB database to identify the transporters involved in the biosynthesis pathway of secondary metabolites in R. officinalis. Therefore, due to the distinction between the location of biosynthesis and storage of secondary metabolites in plants, identifying their transport pathways for accumulation in specific tissues or subcellular compartments has attracted more attention [73]. In most cases, the transferring of secondary metabolites is carried out intracellularly, intracellularly, or in an intra-tissue manner with the help of transporters [73]. One of the most important families of transporters is ABC (ATP-binding cassette) [74]. ABC transporters are membrane proteins that carry metabolites in two lipid layers using energy provided by ATP hydrolysis [75]. Hence, two issues highlight the importance of ABC transporters in complementing our results: (i) since most plant secondary metabolites are used as medicines, ABC transporters are associated with drug resistance [76], and (ii) ABC transporters have characterized among the MeJA up-regulated transcripts [77].
It has been shown that ABC transporters play a role in the transport of plant secondary metabolites including alkaloids, terpenoids and phenols [78, 79]. This role has been proven in several studies through the artemisinin yield in Artemisia annua [80], the expression of PDR-type ABC transporter gene PgPDR3 in Panax ginseng [81], and the expression of a large proportion of ABC transporters in Lycoris aurea [77], under MeJA treatment. As well, it has demonstrated that the major facilitator superfamily (MFS) is necessary for plant tolerance against Fusarium oxysporum in Populus trichocarpa [82] and strong light in Arabidopsis [83], while their role has not assessed against MeJA treatment yet. In addition to these results, a large proportion of ABC transporters, which are subfamily of primary active transporters system based on the TCDB, were identified in MeJA-treated R. officinalis by analyzing the DEGs (Fig 3). Therefore, these results provide a significant understanding of the ABC transporters involved in the transport of plant secondary metabolites, especially in R. officinalis.
Validation of hub genes correlated with secondary metabolites
The identification of hub genes involved in metabolites biosynthesis help to find an avenue for their large-scale production. Hence, visualization of the selected modules was performed to identify the hub genes that had the greatest number of connections with the other genes. R. officinalis may contain these hub genes that increase the number of secondary metabolites more efficiently. Some studies in the field of metabolomics coupled with RNA sequencing analysis in Salvia sclarea [84], S. miltiorrhiza [85], and S. fruticosa [86] have shown the effective role of CPS enzyme in the biosynthesis of abietane diterpenes, such as carnosic acid and other phenolic diterpenes. These studies showed that the accumulation of abietane diterpenes is increased by MeJA elicitor, which is in line with our results. However, there are many reports regarding the use of high concentrations of MeJA (≥ 100 μM) to stimulate the biosynthetic pathway of secondary metabolites [87, 88]. Therefore, our data showed that the expression level of CPS was increased after 12 h and then decreased after 24 h. This decrease in expression may be due to another role of CPS enzyme, i.e., gibberellin biosynthesis, to suspend plant growth and focus on the production of abietane diterpenes to enhance the plant’s immune system against MeJA. In addition, it has demonstrated that MeJA can increase the expression of PAL, TAT, C4H and RAS involved in the biosynthesis pathway of rosmarinic acid [89].
Hence, in the phenylpropanoid pathway, L-phenylalanine converts to t-Cinnamic acid by PAL enzyme, followed by t-Cinnamic acid converts to 4-Coumaric acid by C4H enzyme. In addition, in the Tyrosine-derived pathway, L-tyrosine converts to 4-Hydroxyphenylpyruvic acid by TAT enzyme. Consequently, the final products of two pathways are combined by RAS enzyme and converted into rosmarinic acid through several reactions. Also, there is a positive relation between PvTAT expression and rosmarinic acid accumulation in Prunella vulgaris [90]. Same results were obtained in Ocimum basilicum [91] and Agastache rugosa [92]. However, our results showed that the expression level of TAT, C4H, and RAS increased over time, while the expression level of PAL increased after 12 h and then decreased after 24 h. It is possible that the plant was able to strengthen the defense system in the first 12 h. In this way, MYB58 and MYB63 are two TFs that positively regulate the general phenylpropanoid pathway as well as the monolignol-specific pathway. Accordingly, in this study, the expression level of MYB increased over time under MeJA treatment. This strongly suggests that the biosynthetic pathway of rosmarinic acid is activated by MeJA.
The studies have demonstrated that CIN involves in the biosynthesis pathway of terpenes, especially 1, 8-Cineole, in Melissa offiinalis, [93], Melaleuca alternifolia [94], Salvia officinalis [95], and Salvia fruticosa [86]. By comparing to our results, the expression level of CIN increased over time under MeJA treatment, which was in the line with previous studies. Therefore, acquiring this information can give a deeper understanding of the genes involved in the biosynthesis of various secondary metabolites in R. officinalis and subsequently increase their production. Generally, the present study’s findings by identifying the genes involved in the secondary metabolites biosynthesis of R. officinalis may open a way to produce those metabolites on a large scale.
Conclusions
Most of R. officinalis applications are related to medicine and pharmacy. Consequently, breeding R. officinalis is aimed at increasing the biosynthesis of metabolites through metabolic engineering. On the basis of the computational systems biology analysis of the transcriptome and metabolome data, R. officinalis demonstrates that the co-expression modules correlate with secondary metabolite synthesis. Functional annotators and enrichment analyses have been conducted on the modules and most unigenes that are involved in metabolite biosynthesis pathways. In addition, TFs, PKs, transporters and hub genes were identified in the selected modules. These results indicate that the identified hub genes, such as Copalyl diphosphate synthase, Phenylalanine ammonia lyase, Cineole synthase, Rosmarinic acid synthase, Tyrosine aminotransferase, Cinnamate 4-hydroxylase and MYB58, may play an important role in R. officinalis metabolism. After bioinformatics analysis, qRT-PCR was conducted to confirm the results. It may be possible to use these genes in the future as candidate genes for genetic and metabolic engineering research in order to increase R. officinalis biosynthesis of secondary metabolites.
Supporting information
(DOCX)
(XLSX)
Acknowledgments
The authors would like to thank the Institute of Biotechnology and the Bioinformatics Research Group in the College of Agriculture.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Heinrich M, Williamson EM, Gibbons S, Barnes J, Prieto-Garcia J. Fundamentals of Pharmacognosy and Phytotherapy E-Book: Elsevier Health Sciences; 2017.
- 2.Rathore S, Mukhia S, Kapoor S, Bhatt V, Kumar R, Kumar R. Seasonal variability in essential oil composition and biological activity of Rosmarinus officinalis L. accessions in the western Himalaya. Scientific Reports. 2022;12(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lešnik S, Furlan V, Bren U. Rosemary (Rosmarinus officinalis L.): extraction techniques, analytical methods and health-promoting biological effects. Phytochemistry Reviews. 2021;20(6):1273–328. [Google Scholar]
- 4.Andrade JM, Faustino C, Garcia C, Ladeiras D, Reis CP, Rijo P. Rosmarinus officinalis L.: an update review of its phytochemistry and biological activity. Future science OA. 2018;4(4):FSO283. doi: 10.4155/fsoa-2017-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begum A, Sandhya S, Vinod KR, Reddy S, Banji D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta scientiarum polonorum Technologia alimentaria. 2013;12(1):61–74. [PubMed] [Google Scholar]
- 6.Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. Journal of agricultural and food chemistry. 2007;55(19):7879–85. doi: 10.1021/jf0715323 [DOI] [PubMed] [Google Scholar]
- 7.Tai J, Cheung S, Wu M, Hasman D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine. 2012;19(5):436–43. doi: 10.1016/j.phymed.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 8.Bakırel T, Bakırel U, Keleş OÜ, Ülgen SG, Yardibi H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. Journal of ethnopharmacology. 2008;116(1):64–73. doi: 10.1016/j.jep.2007.10.039 [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Fons L, GarzÓn MaT, Micol V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. Journal of agricultural and food chemistry. 2010;58(1):161–71. doi: 10.1021/jf9026487 [DOI] [PubMed] [Google Scholar]
- 10.Yu M-H, Choi J-H, Chae I-G, Im H-G, Yang S-A, More K, et al. Suppression of LPS-induced inflammatory activities by Rosmarinus officinalis L. Food Chemistry. 2013;136(2):1047–54. doi: 10.1016/j.foodchem.2012.08.085 [DOI] [PubMed] [Google Scholar]
- 11.Taghizadeh MS, Niazi A, Moghadam A, Afsharifar A. Experimental, molecular docking and molecular dynamic studies of natural products targeting overexpressed receptors in breast cancer. PloS one. 2022;17(5):e0267961. doi: 10.1371/journal.pone.0267961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taghizadeh MS, Retzl B, Muratspahić E, Trenk C, Casanova E, Moghadam A, et al. Discovery of the cyclotide caripe 11 as a ligand of the cholecystokinin-2 receptor. Scientific Reports. 2022;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moghadam A, Niazi A, Afsharifar A, Taghavi SM. Expression of a recombinant anti-HIV and anti-tumor protein, MAP30, in nicotiana tobacum hairy roots: A pH-stable and thermophilic antimicrobial protein. PloS one. 2016;11(7):e0159653. doi: 10.1371/journal.pone.0159653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L, et al. Linking plant secondary metabolites and plant microbiomes: a review. Frontiers in Plant Science. 2021;12:621276. doi: 10.3389/fpls.2021.621276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23(4):762. doi: 10.3390/molecules23040762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosová K, Vítámvás P, Urban MO, Prášil IT, Renaut J. Plant abiotic stress proteomics: the major factors determining alterations in cellular proteome. Frontiers in plant science. 2018;9:122. doi: 10.3389/fpls.2018.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghadam A, Tahmasebi A, Taghizadeh MS, Bolhassani M, Ebrahimie E. Identification of LncRNAs involved in secondary metabolites biosynthesis in Echinacea purpurea. Scientific reports. 2022;Under review. [Google Scholar]
- 18.Bolhassani M, Niazi A, Tahmasebi A, Moghadam A. Identification of key genes associated with secondary metabolites biosynthesis by system network analysis in Valeriana officinalis. Journal of plant research. 2021;134(3):625–39. doi: 10.1007/s10265-021-01277-5 [DOI] [PubMed] [Google Scholar]
- 19.Usadel B, Obayashi T, Mutwil M, Giorgi FM, Bassel GW, Tanimoto M, et al. Co-expression tools for plant biology: opportunities for hypothesis generation and caveats. Plant, cell & environment. 2009;32(12):1633–51. doi: 10.1111/j.1365-3040.2009.02040.x [DOI] [PubMed] [Google Scholar]
- 20.Wisecaver JH, Borowsky AT, Tzin V, Jander G, Kliebenstein DJ, Rokas A. A global coexpression network approach for connecting genes to specialized metabolic pathways in plants. The Plant Cell. 2017;29(5):944–59. doi: 10.1105/tpc.17.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics. 2008;9(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Statistical applications in genetics and molecular biology. 2005;4(1). doi: 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
- 23.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic acids research. 2016:gkw937. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Jiao C, Sun H, Rosli H, Pombo MA, Zhang P, et al. iTAK: a program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Molecular plant. 2016;9. [DOI] [PubMed] [Google Scholar]
- 25.Saier MH Jr, Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic acids research. 2006;34(suppl_1):D181–-D6. doi: 10.1093/nar/gkj001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akula R, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant signaling & behavior. 2011;6(11):1720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krzyzanowska J, Czubacka A, Pecio L, Przybys M, Doroszewska T, Stochmal A, et al. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha× piperita cell suspension cultures. Plant cell, tissue and organ culture (pctoc). 2012;108(1):73–81. [Google Scholar]
- 28.Yao D, Chen Y, Xu X, Lin Y, Lai Z. Exploring the Effect of Methyl Jasmonate on the Expression of microRNAs Involved in Biosynthesis of Active Compounds of Rosemary Cell Suspension Cultures through RNA-Sequencing. International journal of molecular sciences. 2022;23(7):3704. doi: 10.3390/ijms23073704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao D, Zhang Z, Chen Y, Lin Y, Xu X, Lai Z. Transcriptome Analysis Reveals Differentially Expressed Genes That Regulate Biosynthesis of the Active Compounds with Methyl Jasmonate in Rosemary Suspension Cells. Genes. 2021;13(1):67. doi: 10.3390/genes13010067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aminfar Z, Rabiei B, Tohidfar M, Mirjalili MH. Selection and validation of reference genes for quantitative real-time PCR in Rosmarinus officinalis L. in various tissues and under elicitation. Biocatalysis and Agricultural Biotechnology. 2019;20:101246. [Google Scholar]
- 31.Payriz A, Nejadsadeghi L, Nabati Ahmadi D. Identification of miRNAs and their target genes in Trachyspermum ammi. Crop Biotechnology. 2022;11(36):1–15. [Google Scholar]
- 32.Slameňová D, Kubošková Kn, Horváthová E, Robichová S. Rosemary-stimulated reduction of DNA strand breaks and FPG-sensitive sites in mammalian cells treated with H2O2 or visible light-excited Methylene Blue. Cancer letters. 2002;177(2):145–53. doi: 10.1016/s0304-3835(01)00784-4 [DOI] [PubMed] [Google Scholar]
- 33.Yang CQ, Fang X, Wu XM, Mao YB, Wang LJ, Chen XY. Transcriptional regulation of plant secondary metabolism F. Journal of integrative plant biology. 2012;54(10):703–12. [DOI] [PubMed] [Google Scholar]
- 34.Tahmasebi A, Ashrafi-Dehkordi E, Shahriari AG, Mazloomi SM, Ebrahimie E. Integrative meta-analysis of transcriptomic responses to abiotic stress in cotton. Progress in biophysics and molecular biology. 2019;146:112–22. doi: 10.1016/j.pbiomolbio.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 35.Do THT, Martinoia E, Lee Y. Functions of ABC transporters in plant growth and development. Current opinion in plant biology. 2018;41:32–8. doi: 10.1016/j.pbi.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 36.Saier MH Jr, Yen MR, Noto K, Tamang DG, Elkan C. The transporter classification database: recent advances. Nucleic acids research. 2009;37(suppl_1):D274–D8. doi: 10.1093/nar/gkn862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer letters. 2011;305(1):1–7. doi: 10.1016/j.canlet.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer letters. 2006;237(1):130–6. doi: 10.1016/j.canlet.2005.05.045 [DOI] [PubMed] [Google Scholar]
- 39.Bektaş E. Changes in essential oil composition, phenylalanine ammonia lyase gene expression and rosmarinic acid content during shoot organogenesis in cytokinin-treated Satureja spicigera (C. Koch) boiss. shoots. Journal of Plant Biochemistry and Biotechnology. 2020;29(3):450–60. [Google Scholar]
- 40.Babaei M, Borja Zamfir GM, Chen X, Christensen HB, Kristensen M, Nielsen J, et al. Metabolic engineering of Saccharomyces cerevisiae for rosmarinic acid production. ACS synthetic biology. 2020;9(8):1978–88. doi: 10.1021/acssynbio.0c00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mafu S, Zerbe P. Plant diterpenoid metabolism for manufacturing the biopharmaceuticals of tomorrow: prospects and challenges. Phytochemistry Reviews. 2018;17(1):113–30. [Google Scholar]
- 42.Wang D, Song Y, Chen Y, Yao W, Li Z, Liu W, et al. Metabolic pools of phenolic acids in Salvia miltiorrhiza are enhanced by co-expression of Antirrhinum majus Delila and Rosea1 transcription factors. Biochemical engineering journal. 2013;74:115–20. [Google Scholar]
- 43.Liu J, Osbourn A, Ma P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Molecular plant. 2015;8(5):689–708. doi: 10.1016/j.molp.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 44.Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, et al. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant physiology. 2007;143(4):1739–51. doi: 10.1104/pp.106.094532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou M, Memelink J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnology advances. 2016;34(4):441–9. doi: 10.1016/j.biotechadv.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 46.Ding W, Ouyang Q, Li Y, Shi T, Li L, Yang X, et al. Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiology. 2020;40(4):557–72. doi: 10.1093/treephys/tpz129 [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Xie C, Huang Y, An W, Liu S, Huang S, et al. Metabolism and transcriptome profiling provides insight into the genes and transcription factors involved in monoterpene biosynthesis of borneol chemotype of Cinnamomum camphora induced by mechanical damage. PeerJ. 2021;9:e11465. doi: 10.7717/peerj.11465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aliakbari M, Razi H, Alemzadeh A, Tavakol E. RNA-seq Transcriptome Profiling of the Halophyte Salicornia persica in Response to Salinity. Journal of Plant Growth Regulation. 2021;40(2):707–21. [Google Scholar]
- 49.Dill A, Jung H-S, Sun T-p. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proceedings of the National Academy of Sciences. 2001;98(24):14162–7. doi: 10.1073/pnas.251534098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuurman J, Jäggi F, Kuhlemeier C. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes & development. 2002;16(17):2213–8. doi: 10.1101/gad.230702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandikota M, Birkenbihl RP, Höhmann S, Cardon GH, Saedler H, Huijser P. The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. The Plant Journal. 2007;49(4):683–93. doi: 10.1111/j.1365-313X.2006.02983.x [DOI] [PubMed] [Google Scholar]
- 52.Klein J, Saedler H, Huijser P. A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Molecular and General Genetics MGG. 1996;250(1):7–16. doi: 10.1007/BF02191820 [DOI] [PubMed] [Google Scholar]
- 53.Yu S, Galvão VC, Zhang Y-C, Horrer D, Zhang T-Q, Hao Y-H, et al. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA PROMOTER BINDING–LIKE transcription factors. The Plant Cell. 2012;24(8):3320–32. doi: 10.1105/tpc.112.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Schwarz S, Saedler H, Huijser P. SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant molecular biology. 2007;63(3):429–39. doi: 10.1007/s11103-006-9099-6 [DOI] [PubMed] [Google Scholar]
- 55.Gramzow L, Ritz MS, Theißen G. On the origin of MADS-domain transcription factors. Trends in Genetics. 2010;26(4):149–53. doi: 10.1016/j.tig.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 56.Honma T, Goto K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001;409(6819):525–9. doi: 10.1038/35054083 [DOI] [PubMed] [Google Scholar]
- 57.Thei G, Saedler H. Plant biology: floral quartets. Nature. 2001;409(6819):469–72. [DOI] [PubMed] [Google Scholar]
- 58.Peter M, Nakagawa J, Doree M, Labbe J, Nigg E. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p [DOI] [PubMed] [Google Scholar]
- 59.Vaid N, Pandey PK, Tuteja N. Genome-wide analysis of lectin receptor-like kinase family from Arabidopsis and rice. Plant molecular biology. 2012;80(4):365–88. doi: 10.1007/s11103-012-9952-8 [DOI] [PubMed] [Google Scholar]
- 60.Amiri F, Moghadam A, Tahmasebi A, Niazi A. Identification of key genes involved in secondary metabolite biosynthesis in Digitalis purpurea. Plos One. 2022;Under review. doi: 10.1371/journal.pone.0277293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu T, Tian Z, Liu J, Xie C. A novel leucine-rich repeat receptor-like kinase gene in potato, StLRPK1, is involved in response to diverse stresses. Molecular biology reports. 2009;36(8):2365–74. doi: 10.1007/s11033-009-9459-9 [DOI] [PubMed] [Google Scholar]
- 62.Liang X, Zhou J-M. Receptor-like cytoplasmic kinases: central players in plant receptor kinase–mediated signaling. Annual review of plant biology. 2018;69:267–99. doi: 10.1146/annurev-arplant-042817-040540 [DOI] [PubMed] [Google Scholar]
- 63.Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell. 2017;171(2):287–304. e15. doi: 10.1016/j.cell.2017.09.030 [DOI] [PubMed] [Google Scholar]
- 64.Carella P, Gogleva A, Hoey DJ, Bridgen AJ, Stolze SC, Nakagami H, et al. Conserved biochemical defenses underpin host responses to oomycete infection in an early-divergent land plant lineage. Current Biology. 2019;29(14):2282–94. e5. doi: 10.1016/j.cub.2019.05.078 [DOI] [PubMed] [Google Scholar]
- 65.Li Y-L, Sun J, Xia G-X. Cloning and characterization of a gene for an LRR receptor-like protein kinase associated with cotton fiber development. Molecular Genetics and Genomics. 2005;273(3):217–24. doi: 10.1007/s00438-005-1115-z [DOI] [PubMed] [Google Scholar]
- 66.Shen D, Dong Y, Wei Y, Zhang M, Wang J, Tang Z, et al. Genome-wide and functional analyses of tyrosine kinase-like family genes reveal potential roles in development and virulence in mosquito pathogen Pythium guiyangense. Fungal Genetics and Biology. 2019;130:11–8. doi: 10.1016/j.fgb.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 67.Enugutti B, Kirchhelle C, Oelschner M, Ruiz RAT, Schliebner I, Leister D, et al. Regulation of planar growth by the Arabidopsis AGC protein kinase UNICORN. Proceedings of the National Academy of Sciences. 2012;109(37):15060–5. doi: 10.1073/pnas.1205089109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leroux AE, Schulze JO, Biondi RM, editors. AGC kinases, mechanisms of regulation and innovative drug development. Seminars in cancer biology; 2018: Elsevier. [DOI] [PubMed] [Google Scholar]
- 69.Garcia AV, Al-Yousif M, Hirt H. Role of AGC kinases in plant growth and stress responses. Cellular and Molecular Life Sciences. 2012;69(19):3259–67. doi: 10.1007/s00018-012-1093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ray SD. Ca2+, The miracle molecule in plant hormone signaling during abiotic stress. Mechanism of plant hormone signaling under stress. 2017;2:45–90. [Google Scholar]
- 71.Soltani Z, Moghadam A, Tahmasebi A, Niazi A. Integrative systems biology analysis of barley transcriptome ─ hormonal signaling against biotic stress. Plos One. 2022;Under review. doi: 10.1101/2021.10.19.464927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gunawardena AH, Greenwood JS, Dengler NG. Programmed cell death remodels lace plant leaf shape during development. The Plant Cell. 2004;16(1):60–73. doi: 10.1105/tpc.016188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nour-Eldin HH, Halkier BA. The emerging field of transport engineering of plant specialized metabolites. Current opinion in biotechnology. 2013;24(2):263–70. doi: 10.1016/j.copbio.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 74.Shoji T. ATP-binding cassette and multidrug and toxic compound extrusion transporters in plants: a common theme among diverse detoxification mechanisms. International review of cell and molecular biology. 2014;309:303–46. doi: 10.1016/B978-0-12-800255-1.00006-5 [DOI] [PubMed] [Google Scholar]
- 75.Holland IB, Cole SP, Kuchler K, Higgins CF. ABC proteins: from bacteria to man: Elsevier; 2003.
- 76.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature Reviews Cancer. 2010;10(2):147–56. doi: 10.1038/nrc2789 [DOI] [PubMed] [Google Scholar]
- 77.Wang R, Xu S, Wang N, Xia B, Jiang Y, Wang R. Transcriptome analysis of secondary metabolism pathway, transcription factors, and transporters in response to methyl jasmonate in Lycoris aurea. Frontiers in plant science. 2017;7:1971. doi: 10.3389/fpls.2016.01971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv H, Li J, Wu Y, Garyali S, Wang Y. Transporter and its engineering for secondary metabolites. Applied microbiology and biotechnology. 2016;100(14):6119–30. doi: 10.1007/s00253-016-7605-6 [DOI] [PubMed] [Google Scholar]
- 79.Yazaki K. ABC transporters involved in the transport of plant secondary metabolites. FEBS letters. 2006;580(4):1183–91. doi: 10.1016/j.febslet.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Lu X, Shen Q, Chen Y, Wang T, Zhang F, et al. Identification of putative Artemisia annua ABCG transporter unigenes related to artemisinin yield following expression analysis in different plant tissues and in response to methyl jasmonate and abscisic acid treatments. Plant Molecular Biology Reporter. 2012;30(4):838–47. [Google Scholar]
- 81.Zhang R, Huang J, Zhu J, Xie X, Tang Q, Chen X, et al. Isolation and characterization of a novel PDR-type ABC transporter gene PgPDR3 from Panax ginseng CA Meyer induced by methyl jasmonate. Molecular biology reports. 2013;40(11):6195–204. [DOI] [PubMed] [Google Scholar]
- 82.Diao J, Li S, Ma L, Zhang P, Bai J, Wang J, et al. Genome-Wide Analysis of Major Facilitator Superfamily and Its Expression in Response of Poplar to Fusarium oxysporum. Frontiers in genetics. 2021;12. doi: 10.3389/fgene.2021.769888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyaji T, Kuromori T, Takeuchi Y, Yamaji N, Yokosho K, Shimazawa A, et al. AtPHT4; 4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nature communications. 2015;6(1):1–11. doi: 10.1038/ncomms6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaccaro M, Mariaevelina A, Malafronte N, De Tommasi N, Leone A. Increasing the synthesis of bioactive abietane diterpenes in Salvia sclarea hairy roots by elicited transcriptional reprogramming. Plant cell reports. 2017;36(2):375–86. doi: 10.1007/s00299-016-2076-x [DOI] [PubMed] [Google Scholar]
- 85.Hao X, Shi M, Cui L, Xu C, Zhang Y, Kai G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic S alvia miltiorrhiza hairy roots. Biotechnology and applied biochemistry. 2015;62(1):24–31. doi: 10.1002/bab.1236 [DOI] [PubMed] [Google Scholar]
- 86.Chatzopoulou FM, Makris AM, Argiriou A, Degenhardt J, Kanellis AK. EST analysis and annotation of transcripts derived from a trichome-specific cDNA library from Salvia fruticosa. Plant cell reports. 2010;29(5):523–34. doi: 10.1007/s00299-010-0841-9 [DOI] [PubMed] [Google Scholar]
- 87.Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, et al. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21(2):182. doi: 10.3390/molecules21020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geng X, Jin L, Shimada M, Kim MG, Mackey D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta. 2014;240(6):1149–65. doi: 10.1007/s00425-014-2151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fatemi F, Abdollahi MR, Mirzaie-asl A, Dastan D, Garagounis C, Papadopoulou K. Identification and expression profiling of rosmarinic acid biosynthetic genes from Satureja khuzistanica under carbon nanotubes and methyl jasmonate elicitation. Plant Cell, Tissue and Organ Culture (PCTOC). 2019;136(3):561–73. [Google Scholar]
- 90.Ru M, Wang K, Bai Z, Peng L, He S, Wang Y, et al. A tyrosine aminotransferase involved in rosmarinic acid biosynthesis in Prunella vulgaris L. Scientific reports. 2017;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Kim JK, Park SU. Molecular cloning and characterization of rosmarinic acid biosynthetic genes and rosmarinic acid accumulation in Ocimum basilicum L. Saudi journal of biological sciences. 2019;26(3):469–72. doi: 10.1016/j.sjbs.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park WT, Arasu MV, Al-Dhabi NA, Yeo SK, Jeon J, Park JS, et al. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agastache rugosa cell culture. Molecules. 2016;21(4):426. doi: 10.3390/molecules21040426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mansouri M, Mohammadi F. Transcriptome analysis to identify key genes involved in terpenoid and rosmarinic acid biosynthesis in lemon balm (Melissa officinalis). Gene. 2021;773:145417. doi: 10.1016/j.gene.2021.145417 [DOI] [PubMed] [Google Scholar]
- 94.Bustos-Segura C, Padovan A, Kainer D, Foley WJ, Külheim C. Transcriptome analysis of terpene chemotypes of Melaleuca alternifolia across different tissues. Plant, Cell & Environment. 2017;40(10):2406–25. doi: 10.1111/pce.13048 [DOI] [PubMed] [Google Scholar]
- 95.Ali M, Li P, She G, Chen D, Wan X, Zhao J. Transcriptome and metabolite analyses reveal the complex metabolic genes involved in volatile terpenoid biosynthesis in garden sage (Salvia officinalis). Scientific reports. 2017;7(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]