Abstract
Malaria remains a significant threat to global health, and despite concerted efforts to curb the disease, malaria-related morbidity and mortality increased in recent years. Malaria is caused by unicellular eukaryotes of the genus Plasmodium, and all clinical manifestations occur during asexual proliferation of the parasite inside host erythrocytes. In the blood stage, Plasmodium proliferates through an unusual cell cycle mode called schizogony. Contrary to most studied eukaryotes, which divide by binary fission, the parasite undergoes several rounds of DNA replication and nuclear division that are not directly followed by cytokinesis, resulting in multinucleated cells. Moreover, despite sharing a common cytoplasm, these nuclei multiply asynchronously. Schizogony challenges our current models of cell cycle regulation and, at the same time, offers targets for therapeutic interventions. Over the recent years, the adaptation of advanced molecular and cell biological techniques have given us deeper insight how DNA replication, nuclear division, and cytokinesis are coordinated. Here, we review our current understanding of the chronological events that characterize the unusual cell division cycle of P. falciparum in the clinically relevant blood stage of infection.
Introduction
Cell division is a precisely orchestrated sequence of molecular events and has been studied in great detail in many eukaryotic model organisms. Typically, cells proliferate by duplicating their genome and subsequently dividing in two daughter cells, but deviations from this scheme are commonly found on many branches of the eukaryotic tree of life. Unicellular apicomplexan parasites, such as Toxoplasma or Plasmodium, represent one of those divergent branches, where the cell cycle and its regulation remain enigmatic [1]. Although these parasites use some conserved cell cycle factors [2], the cytoskeletal elements, membrane topologies, and regulatory pathways are organized in a substantially different manner [3,4]. Here, we focus on the malaria-causing parasites of the genus Plasmodium, which still cause the death of over 600,000 people per year [5]. Extensive Plasmodium proliferation inside erythrocytes causes all malaria-related pathology and generates high numbers of progeny by an unconventional cell cycle mode, termed schizogony.
Among the most important differences to the cell cycles of model eukaryotes are multinucleated cells with asynchronous nuclear divisions, the atypical structure of the centrosome, a specialized daughter cell segmentation mechanism, and the apparent absence of most canonical cell cycle checkpoints. In this review, we lay out our current understanding and knowledge gaps about the chronology of events, starting from the initiation of the first DNA replication during S–phase to the completion of cellularization during cytokinesis.
Defining entry into the schizont stage
The proliferative cycle begins when a Plasmodium merozoite invades a host erythrocyte. Inside, the merozoite transforms into a ring–stage parasite and subsequently into a trophozoite. During this time, the parasite substantially remodels the host cell and consumes its cytoplasm [6–8]. In analogy to the canonical cell cycle (consisting of a gap phase, G1; S–phase, during which DNA is replicated; a second gap, G2; and M–phase, when mitosis occurs), both ring and trophozoite resemble cells in the G1 phase. The trophozoite stage is followed by the schizont stage, during which nuclei multiply. Schizogony is concluded with cellularization and release of the daughter cells. Integrating external stimuli [9], a fraction of asexually proliferating parasites commits to sexual development and form gametocytes, which can continue life cycle progression when taken up by a mosquito. This commitment occurs 34 to 38 hours post invasion (hpi) and is regulated by the transcription factor PfAP2–G [9–11]. Conditional expression of PfAP2–G results in approximately 90% conversion to sexual stages [12], which cease to proliferate.
Classically, the distinction between the trophozoite stage and the schizont stage has been made morphologically, and a schizont is often referred to as a multinucleated cell, i.e., a cell with more than two nuclei. While this classification seems intuitive, it appears artificial from a cell cycle point of view. According to the morphological definition, DNA replication and nuclear division occur in both the trophozoite and the schizont stage. Moreover, intrinsic and extrinsic perturbations appear to demarcate a major cell cycle transition at the beginning of the first S–phase (Box 1) [13–16]. While the classic, morphology-based staging is experimentally easy to accomplish and sufficient for many research questions, it bears limitations as it pools stages that resemble cells in G1 with stages in which S–phase or nuclear division already occurred. Therefore, we encourage, if possible, the use of an alternative, cell cycle–based staging, in particular when investigating the cell cycle of P. falciparum. Thus, in the context of this review, we consider a schizont as all developmental stages from the onset of the first S–phase to the conclusion of merozoite formation.
Box 1. Cell cycle checkpoints
Canonical cell cycle checkpoints are opportunities for the cell to induce a controlled arrest of cell cycle progression until all prerequisites for the next step are met. An example is the spindle attachment checkpoint during M–phase, which ensures that all kinetochores are properly attached to the spindle microtubules, so sister chromatids can be faithfully segregated [109]. Importantly, cell cycle checkpoints share certain characteristics. A checkpoint must be reproducibly induced by a defined error at a defined time but should also be reversible once problems have been resolved. To conclusively demonstrate the existence of a checkpoint, one must further demonstrate that a deletion of a candidate protein prevents checkpoint activation.
Even though recently a first cell cycle checkpoint protein candidate, a potential homologue of Mad1, has been identified in the P. berghei genome [56], experimental data still argue against the existence of a spindle attachment checkpoint. Indeed, schizonts continue to replicate their DNA in the presence of drugs that interfere with microtubule formation, which causes erroneous nuclear division [110–112]. In contrast, inhibition of DNA replication appears to induce an arrest without readily detectable attempts to divide the nucleus [113].
In line with this, more evidence starts pointing towards a controlled entry into the schizont stage. Deprivation of the essential amino acid isoleucine prior to the onset of S–phase dramatically affects cell cycle progression [13,16]. As the only amino acid absent from human hemoglobin, P. falciparum largely depends on import of isoleucine from the serum or medium. In the absence of exogenous isoleucine, P. falciparum development arrests at the trophozoite/schizont transition, which is reminiscent of a controlled entry into S–phase via a G1/S checkpoint. More recent results suggest that the arrest is not complete and that parasites progress through the cell cycle extremely slowly and eventually start DNA replication [16]. Importantly, isoleucine deprivation after S–phase onset does not stall the cell cycle [16], which agrees with a potential checkpoint. Moreover, the phosphorylation status of PfeIF2α depends on isoleucine availability and, although this does not seem to be involved in the developmental delay, it demonstrates the parasite’s responsiveness to amino acid starvation [13]. Similarly, disruption of polyamine synthesis via DL–α–difluoromethylornithine resulted in a reversible arrest at the trophozoite to schizont transition [15], but it is unclear if both responses are rooted in the same regulatory pathway. Together, these findings indicate that a G1/S checkpoint may exist, which controls entry into the schizont stage.
In contrast, several studies have shown that proper nuclear multiplication or orderly segmentation are neither required for organellar development nor for the initiation of egress [14,43,61,90,97]. Thus, there may not be a cytokinesis checkpoint, which would prevent cell cycle progression even if nonviable daughter cells are formed.
Conclusive evidence for cell cycle checkpoints in asexual Plasmodium blood stages will require a combination of cell cycle perturbation, time-resolved data acquisition, and genetic deletions. In addition, how the cell cycle and potential checkpoints may be affected by the parasite’s intrinsic circadian clock and the daily host rhythms remains elusive [114].
The first round of DNA replication
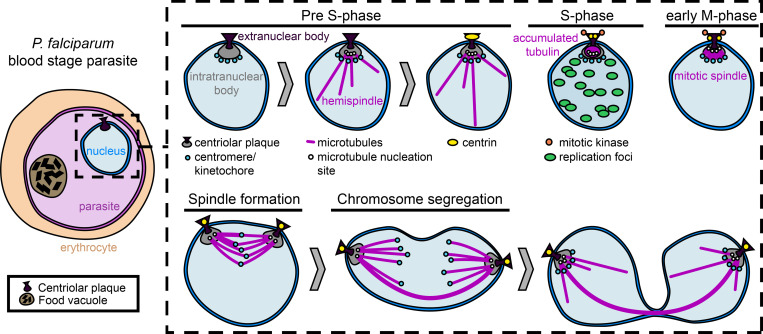
Currently, the first cellular feature indicating that a parasite with a single nucleus prepares to enter S–phase and nuclear multiplication is the so-called hemispindle, which consists of intranuclear microtubules that radiate from the parasite’s centrosome, called centriolar plaque, into the nucleoplasm (Fig 1) [17,18]. While it is unclear if the hemispindle serves a specific cellular function or whether it forms due to high local concentrations of tubulin [18–20], its presence likely marks the transition from trophozoite to schizont stage and heralds the impending first round of DNA replication [14–16].
Fig 1. Progression of nuclear cycle stages in mononucleated parasites.
Nuclear division is organized around the centriolar plaque, which spans the nuclear envelope and consists of an extranuclear compartment and an intranuclear compartment acting as the microtubule organizing center. Prior to S–phase initiation and the formation of a mitotic spindle, large, dynamic hemispindles can be observed in the nucleus. Centrins are recruited to the extranuclear compartment of the centriolar plaque during the later hemispindle stages before S–phase occurs. Nuclear division is closed, with the duplicated centriolar plaques eventually moving to opposite sides of the dividing nucleus during chromosome segregation, before nuclei are separated.
In P. falciparum, various techniques have been used to determine the timing of the first S–phase relative to merozoite invasion. Doubling of fluorescent signals from telomeric repeats stained by in situ hybridization was used as proxy for DNA replication and reported it to start approximately 24 to 26 hpi [21]. However, other studies used flow cytometry with nucleic acid dyes and reported increasing DNA contents approximately 30 to 32 hpi [14,22]. Concordantly, labelling nascent DNA in synchronized parasite cultures reported that the first S–phase commences approximately 31 hpi [23] and incorporation of radioactively labeled nucleotides suggested an onset of DNA replication at approximately 30 hpi [24]. Collectively, this indicates that in P. falciparum asexual blood stages the first round of DNA replication commences at approximately 30 hpi.
During the preceding G1 phase of canonical cell cycles, the origins of replications are licensed for DNA replication. This licensing occurs by the sequential binding of the origin of replication complex (ORC) and two minichromosome maintenance (MCM) complexes along with other factors at the DNA. At the transition from G1 to S–phase, cyclin-dependent kinase (CDK) phosphorylates components of the ORC and MCM complexes, more proteins assemble (including the DNA polymerases), and DNA replication commences. At the same time, CDK activity inhibits the licensing of origins, as, e.g., phosphorylated ORC does not bind to DNA. This temporal separation of licensing of origins and origin firing during DNA replication ensures that the DNA is replicated once and only once per cycle [25,26].
Only some of the components needed to reconstitute origin licensing and firing in vitro were identified in the Plasmodium genome by sequence homology, but key components like ORC and MCM are conserved [14,27–29]. Kinases related to canonical CDK have been shown to play a pivotal role for DNA replication in the blood stage and in activated male gametocytes [14,30,31]. Depletion of the essential cdc2-related kinase (PfCRK) 4 led to a reversible arrest just prior to the onset of DNA replication [14]. In absence of PfCRK4, several components of the DNA replication machinery are less phosphorylated, including components of ORC and MCM complex, DNA polymerase alpha and epsilon, as well as topoisomerase 2. PfCRK4 activity is required for the initial and subsequent rounds of DNA replication. The serine/arginine–rich protein kinase (PfSRPK) 1 likely acts downstream of PfCRK4, and deletion of PfSRPK1 led to a reduced number of daughter cells and defective male gamete development in the mosquito vector [14,32]. A potential role for the related kinase PfCRK5 for DNA replication in schizonts was recently questioned [31,33], but CRK5 appears to fill the role of PfCRK4 during DNA replication in activated male gametocytes, in both P. falciparum and P. berghei [30,31]. These regulators offer a starting point to identify other molecular determinants and characterize the underlying regulatory networks that govern the entry into the schizont stage.
The dynamics of the active DNA replication fork were recently determined by pulse-labeling of nascent DNA. This was made possible by ectopic expression of a viral thymidine kinase that phosphorylates nucleoside analogues into nucleotides, which can be incorporated into nascent DNA and later be detected by immunofluorescence or click chemistry [34]. In early schizonts of both P. falciparum and P. knowlesi, DNA is replicated at approximately 1.5 kb per minute and this rate decreased over time to approximately 1 kb per minute in late schizonts. At the same time, the spacing of two origins of replication also decreases. The reasons for this slowdown are not known, but nucleotide availability or a changing chromatin state may play a role [23,35].
Coordination of DNA replication and nuclear division
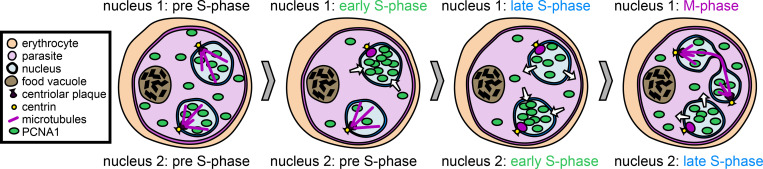
Once DNA replication is initiated, it takes approximately 15 hours in P. falciparum until the next generation of parasites are produced by repeated rounds of DNA replication and nuclear division [23,36]. Two different models for the coordination of DNA replication and nuclear division in a Plasmodium schizont have been proposed. One model assumed that several rounds of DNA replication occur prior to nuclear divisions, thus forming a polyploid nucleus [27,37]. This proposed mode is similar to what has been described for activated male gametocyte, which undergoes three rounds of DNA replication. Thereby, the DNA content of the single nucleus increases from 1 to 8 C, and, subsequently, this nucleus is divided and male gametes emerge [38–40]. The other model proposed consecutive and alternating rounds of DNA replication and nuclear division [21,41,42]. This model assumes that the DNA content of a given nucleus does not exceed the diploid level and that the DNA content and the number of nuclei in a given parasite increase gradually. Experimental data strongly support the latter mode, i.e., consecutive rounds of DNA replication and nuclear division (Fig 2) [23,36], and, to our knowledge, polyploid nuclei have only been described in mutant parasites, such as the PfMCMPB knockdown [43].
Fig 2. Nuclei cycle asynchronously in multinucleated parasites.
After nuclear division, hemispindle structures remain briefly within the separated nuclei. In parasites with two nuclei, one may enter S–phase earlier than the other; S–phase entry is marked by the rapid accumulation of the replication marker PCNA1 within a nucleus. Asynchrony is visible in both S–phase and nuclear division, as nuclei may show nuclear accumulation of PCNA1 as well as different microtubule structures at different times.
The first nuclear division
A central regulatory hub for nuclear division is the centrosome as it organizes microtubules into a mitotic spindle that drives segregation of the replicated chromosomes. Unlike in mammalian cells or in the related apicomplexan parasite Toxoplasma, the centriolar plaque of asexual Plasmodium spp. blood stages does not contain centrioles [44]. It was previously observed merely as an amorphous electron dense mass embedded in the nuclear envelope [45]. Recent work showed that the centriolar plaque of asexual blood stages is a protein-dense structure consisting of an extra- and an intranuclear compartment. The latter is devoid of chromatin, but microtubule minus ends are nucleated here [18]. Both compartments of the centriolar plaque are connected via an opening in the nuclear envelope, which is densely filled with protein and may be a derivative of a nuclear pore (Fig 1) [18,46]. This overall organization of the centriolar plaque is also found in sexual stages, although they contain an extranuclear centriole-like structure [47,48].
Four centrins are among the few canonical centrosomal proteins that are conserved in Plasmodium spp. [18,48–50]. Centrins accumulate at the centriolar plaque when the hemispindle is already present, and although being essential for blood stage parasites, their precise function remains unknown (Fig 1, Table 1) [50,51]. Another protein of the centriolar plaque is the essential anaphase promoting complex subunit 3 (APC3) of the related parasite P. berghei [52]. The canonical anaphase promoting complex/cyclosome is an E3 ubiquitin ligase that promotes cell cycle progression [53]. In activated male gametocytes, PbAPC3 is involved in chromosome condensation and cytokinesis, but its function for P. falciparum schizogony remains unknown [52].
Table 1. Selected marker for live–cell imaging of P. falciparum schizogony in blood stages.
| Structure | Target | Fluorophore/Dye | Source |
|---|---|---|---|
| Centriolar plaque (extranuclear compartment) | Cen3, Cen4, Cen1 | GFP | [18,48,50,93] |
| Centriolar plaque (intranuclear compartment) | γ–Tubulin | GFP | [52] |
| Centromere | CenH3 | GFP | [54] |
| Kinetochore | NDC80, NUF2, SKA2 | mCherry, GFP | [48,55,56] |
| DNA | 5–SiR–Hoechst dye | SiR–Hoechst dye | [18] |
| Nucleoplasm | NLS–mCherry | mCherry | [36,140] |
| Heterochromatin/Telomeres | HP1 | GFP | [141] |
| Active DNA replication | PCNA1 | GFP | [36] |
| Nucleopores | Nup313/138/221 | GFP | [18,142] |
| Microtubules | SPY555–Tubulin dye, | SPY555 dye | [18] |
| Microtubule | Kinesin8X, Kinesin–5, EB1 | GFP | [48,117,143] |
| Membrane (e.g., nuclear envelope) | Lipids | BODIPY TR Ceramide | [46,116] |
| IMC | GAP45, MAL13P1.130, PFE1285w, GAP50, ALV5 | GFP, mCherry | [92,93,144] |
| Basal complex | BTP1, MORN1, HAD2, | GFP | [94,145] |
| (Merozoite) plasma membrane | SMS1 | mCherry | [94] |
| Rhoptry | ARO, RON12, AIP | mCherry | [144] |
The centromeres, as marked by histone PfCenH3, cluster at the nuclear periphery and are positioned at the intersection between the centriolar plaque and chromatin (Fig 1) [18,54]. In P. falciparum and P. berghei, the kinetochore marker NDC80 is recruited to the centromeres during the trophozoite stage, potentially reflecting the assembly of the kinetochore [48,55]. Our understanding of the Plasmodium kinetochore composition was recently expanded through the discovery of previously unknown kinetochore proteins and their organization in the multilayered kinetochore, with four individual compartments [56]. In addition, the phosphatase PP1 of P. berghei partially colocalizes with kinetochores from the trophozoite stage until egress. Inducible depletion of PfPP1 at the ring stage led to decreased DNA replication dynamics, which most likely translate into fewer daughter cells. Later depletion did not affect DNA replication dynamics, but when the phosphatase was absent from the trophozoite stage onward, schizonts had a lower number of nuclei. In addition, depletion of PfPP1 at any stage of the intraerythrocytic development revealed a critical role for this phosphatase during egress. The molecular mechanism through which PfPP1 affects cell cycle progression remains, however, elusive [57,58].
Likely at the onset of S–phase, the hemispindle rearranges into an immature mitotic spindle (Fig 1) [18,36]. At the same time, the centrin signal duplicates [18], which coincides with the accumulation of the essential mitotic aurora kinase PfArk1 at the centriolar plaque (Fig 1) [59]. Treatment with the aurora kinase inhibitor hesperadin suggested a role for PfArk1 in spindle formation and nuclear division, potentially through impaired centriolar plaque function [60]. After S–phase has concluded, there is a lag period before the nucleus divides [36]. Likely during this time, the mitotic spindle completes its bipolar arrangement and kinetochore microtubules attach to the duplicated chromosomes (Fig 1). The Plasmodium spindle is initially highly compact with an average length of approximately 550 nm until it rapidly extends to segregate both chromosome sets (Fig 1) [18,46]. How the sister chromatids are released, what generates the necessary forces to drive them apart, and how kinetochore and interpolar microtubules are reorganized are only some of the open questions around anaphase in P. falciparum. Ultimately, nuclear division is completed by karyofission [18,46].
Few factors have been functionally associated with nuclear division, but depletion of PfMCMBP led to uneven or incomplete karyofission, potentially through defective chromosome segregation [43,46]. Similarly, deletion of schizont egress antigen–1 (PfSEA–1), which localizes close to the centromeres, led to severe defects in DNA segregation and nuclear division [61].
The intermediate steps of mitotic spindle assembly and nuclear division and the regulatory proteins, such as the spindle microtubule associated PfEB1 [48], remain to be characterized in more detail. Recent advances in imaging techniques now permit to resolve nuclear division with sufficient spatial and temporal resolution (Box 2).
Box 2. Advances in microscopy promoting the study of Plasmodium proliferation
Time lapse microscopy. The difficulty to tightly synchronize blood stage malaria parasites, partly due to missing checkpoints, is significantly limiting the study of schizogony using bulk population analysis. Understanding the dynamic progression through schizogony and following the fate of individual nuclei requires temporally resolved single cell data. Time-lapse fluorescence microscopy has been pioneered in the liver stage development [115] and was used to follow protein transport in asexual blood stages [116]. 4D live–cell imaging has only been more recently applied to study nuclear multiplication [36] and to follow the dynamics of the microtubule associated protein Pbkinesin–5 [117]. Time-lapse imaging of microtubules has been achieved through live–cell compatible fluorogenic microtubule dyes and image deconvolution [18]. The list of live–cell markers for schizogony is, however, steadily growing (Table 1). As the generation of transgenic parasite lines is still a rate-limiting step, the use of state-of-the-art live–cell dyes is an important tool [118]. Due to their excellent background-to-noise ratio, fluorogenic dyes have significantly contributed to this progress [119,120], while classical dyes like the membrane label BODIPY still have their utility [46,116]. The observation that, e.g., Hoechst-based dyes can impact mitotic progression, however, calls for careful monitoring of their effect on cell physiology [18]. As recently demonstrated, 4D multicolor live–cell microscopy generates large quantitative data sets that can inform mathematical modelling to generate insightful predictions and hypotheses about schizogony [36]. The key challenge of time lapse imaging remains to balance spatial and temporal resolution with phototoxic effects. In this respect, the development of lattice light–sheet microscopy [121] has been a milestone that has showcased its exceptional potential in the analysis of merozoite invasion [122], and we await its application in the study of schizogony.
Super-resolution microscopy. The second important challenge in studying schizogony is the small size of the nucleus and associated mitotic structures. Structure–illumination microscopy (SIM) was the first super-resolution microscopy technique adapted to parasites to investigate merozoite invasion [123,124]. It offers a 2-fold improved resolution in 3D and can, despite stronger illumination, be used on live parasites [125]. A significantly higher resolution can be achieved by Stimulated Emission Depletion (STED) nanoscopy [126], which has been used in liver stage parasites [127]. Two approaches, namely RescueSTED and Guided STED, have made STED available for blood stages by avoiding the destructive hemozoin illumination by the STED laser [128,129] and thereby contributed to building the current working model of the centriolar plaque (Fig 1) [18].
Although STochastic Optical Reconstruction Microscopy (STORM) has been successfully used to characterize the subdiffraction organization of the adhesion structures that parasites assemble on the surface of red blood cells [130,131], its application remains technically challenging and has limitations for live, 3D, and multicolor applications.
Lately, a new type of super-resolution microscopy based on the isotropic expansion of labelled cells in a gel, called expansion microscopy (ExM), has gained more attention [132]. The cellular expansion by a factor of 4 to 5 allows multicolor imaging of full 3D–stacks of fixed cells while reaching a resolution similar to STED. A later optimization, named ultrastructure expansion microscopy (U–ExM) [133], has been applied in multiple parasite life cycle stages to reveal organization of cytoskeletal as well as intranuclear microtubules with stunning detail [18,100]. Combination of U–ExM with NHS–Ester staining (pan–ExM) reveals a protein density map of the entire cell [46,100].
Nevertheless, maximal spatial resolution is still achieved by electron microscopy (EM), which has contributed the key initial insight into schizogony in early transmission EM studies [45,134–136]. In later years, 3D electron EM technologies have become more important, such as the highly resolving tomography [18,137] and focus ion beam scanning electron microscopy (FIB–SEM), which can produce 3D reconstruction of entire cells, albeit with lower resolution [95,97,138].
The benefits of fluorescence and electron microscopy have now been combined in Plasmodium using correlative light and electron microscopy (CLEM) approaches that can put specific protein labelling in the ultrastructural context acquired in EM [18,36,139]. CLEM approaches are, however, still in their infancy in the Plasmodium field, likely related to their challenging implementation. Ultimately, all aforementioned techniques rely on consistent cellular markers, antibodies, and sample preparation [128]. While for most relevant division structures reliable markers are known (Table 1), a genetically encoded nuclear envelope marker is still lacking [46].
Asynchronous nuclear cycles in multinucleated schizonts
In contrast to many other multinucleated cells, such as the early Drosophila embryo [62] or the unicellular marine eukaryote Sphaeroforma arctica [63], the successive rounds of DNA replication and nuclear division in Plasmodium schizonts occur asynchronously (Fig 2) [18,23,36,64].
Asynchronous nuclear divisions can also be observed in the hyphae of the multinucleated fungi Ashbya gossypii [65]. Here, asynchrony is dependent on cytoplasmic territories that are established by the nuclei and which limit the diffusion of cytoplasmic factors that regulate nuclear cycle progression. In addition, nucleus-intrinsic factors, such as different transcriptional activity, contribute to the asynchronous nuclear division [66–69]. Compared to Ashbya, where nuclei are on average approximately 5 μm apart, P. falciparum nuclei are typically in much closer proximity, sometimes less than 100 nm apart [36,69]. This suggest that nucleus intrinsic mechanisms probably contribute more to asynchrony than cytoplasmic ones. However, the underlying molecular mechanisms that establish asynchronous nuclear cycles in Plasmodium spp. remain elusive.
To obtain a molecular understanding of the underlying mechanisms, two recent studies investigated the dynamics of nuclear multiplication in the schizont stage. One employed pulse labelling of nascent DNA in thymidine kinase–expressing P. falciparum and P. knowlesi parasites [23]. The other study used live–cell imaging of a P. falciparum nuclear cycle sensor line, which expresses the red fluorescent protein mCherry in all nuclei and a component of the DNA replication fork, the proliferating cell nuclear antigen (PfPCNA) 1 fused to GFP [36]. Since PfPCNA1 accumulates only in S–phase nuclei that replicate their DNA, this dual-color line allows tracking of nuclear cycles in analogy to the fluorescence ubiquitination cell cycle indicator (FUCCI) for mammalian cells (Fig 2, Table 1) [70].
Both studies report a high cell-to-cell variability and directly showed that not only nuclear division occurs asynchronously, but also DNA replication. Live–cell imaging also showed that the asynchrony between sister nuclei is predominantly introduced during the time from nuclear division to the ensuing S–phase, which may be similar to the G1 phase of the canonical cell cycle (Fig 2) [36].
While both studies report similar dynamics in early schizonts (e.g., approximately 50 minutes for the initial round of DNA replication), the datasets are less coherent for late schizonts. From different regimens of pulse labelling, it was concluded that the time between consecutive DNA replications increases as nuclear multiplication progresses, while the time needed for DNA replication decreases. In contrast, live–cell imaging suggested that S–phases in late schizonts, where more nuclei are in S–phase at the same time, become longer due to competition for shared limited resources.
Mathematical modelling showed that schizonts need to slow down their nuclear cycle dynamics over time to produce the experimentally determined final number of nuclei [36]. This overall slowing can be achieved in different ways. While live–cell imaging suggests slower DNA replication as more nuclei replicate simultaneously [36], nucleotide analogue labelling indicated an extended (≥5 hours) replicative arrest of one to two nuclei per cell [23]. Another open question concerns the end of nuclear multiplication and whether or not nuclei undergo a final synchronous round of DNA replication. While pulse labelling supports a final synchronous round [23], live–cell imaging and mathematical modelling suggest that nuclei cease to multiply without a final synchronous round of DNA replication [36].
When comparing P. falciparum and P. knowlesi, the overall pattern of nuclear multiplication and replication fork velocity was highly similar. But although both species spent approximately 30% of their blood stage cycle multiplying their nuclei and have a comparable genome size, the dynamics of nuclear multiplication differed and the gap phases between successive S–phases were shorter in P. knowlesi than in P. falciparum [23].
Tracking nuclei until the end of schizogony to see if the final round of DNA replication is distinct from previous rounds and to see if some nuclei undergo replicative arrest will help resolve some of these conflicting data and further inform on how nuclear cycles are organized during nuclear multiplication. In any case, asynchronous nuclear cycles appear to support rapid parasite proliferation in an environment with limited resources [36]. An open question is how transcriptional homeostasis is maintained as nuclei multiply and the cellular DNA content increases [71].
Concluding nuclear multiplication
An essential decision for the parasite is when to stop nuclear multiplication and transition to cell division—in other words, how does the parasite know when the desired number of daughter nuclei is reached? In general terms, this decision may be accomplished by two different mechanisms [72,73]: A timer mechanism would stop further nuclear multiplication after a predefined time period elapsed. Conversely, a counter mechanism would stop further multiplication once a predefined number of nuclei is reached. Timer and counter mechanisms can be experimentally distinguished by correlating parameters such as the length of the initial cycles, the length of the overall nuclear multiplication time, and the final number of nuclei. Correlating the duration of the initial nuclear cycle with the overall time spend for nuclear multiplication strongly supports a counter mechanism [36], but how this counter could be set remains elusive.
Parasites cultured in the same conditions display a remarkable variability in progeny numbers, ranging from less than 10 to more than 30 [73–77]. This suggests that factors intrinsic to the host–parasite unit may play an important role. Indeed, when comparing normal to hemoglobinopathic host erythrocytes, the number of daughter cells correlated positively with the mean erythrocyte volume and hemoglobin content [77]. However, whether this correlation is entirely due to cell size or is also influenced by the molecular characteristics of each specific type of haemoglobinopathy remains to be seen.
To better understand the counter mechanism, it will also be critical to investigate how environmental factors that act on the entire parasite population affect the number of daughter cells, such as nutrient levels in cell culture media or patient blood plasma [78–81].
Organellar genome replication
Aside from the chromosomal DNA contained within the nucleus, Plasmodium harbors two additional organelles, which carry their own genome: the apicoplast, which is specific for Apicomplexa, and the mitochondrion. Both must be distributed to the daughter cells during schizogony, which requires replication of their genomes. The apicoplast originated from a secondary endosymbiosis event and is involved in several essential metabolic processes, such as isoprenoid and fatty acid synthesis [82]. The AT-rich 35-kB circular apicoplast genome is present in no more than three copies per parasite in early stages, and its replication was estimated to start shortly before the onset of chromosomal replication and then continues in parallel to DNA replication in the nucleus [83]. Replication is dependent on the essential multifunctional plastidic DNA replication/repair enzyme complex (PREX). This complex is encoded in the nuclear genome and carries primase, helicase, and polymerase domains [84]. Other replication factors such as gyrases and single-strand-binding proteins (SSB) are likewise encoded in the nuclear genome and are imported into the apicoplast for replication and repair [84].
In contrast, the highly conserved 6 kB mitochondrial genome is present in approximately 20 copies per mitochondrion in P. falciparum [85]. In the mitochondrion, the genomes are arranged into highly complex linear concatemer structures, which are replicated at approximately the same time as the chromosomal DNA [85]. Like for the apicoplast genome, replication of the mitochondrial genome depends on nuclear-encoded proteins, such as the P. falciparum topoisomerase III [86].
A P. falciparum mutant, in which nuclear DNA replication is profoundly suppressed, shows seemingly well-developed apicoplasts and mitochondria [14]. Thus, organelle development and potentially organellar DNA replication may be independent of chromosomal replication. Quantification of the organellar DNA content under these circumstances will inform on if and how apicoplast and mitochondrial DNA replication are coupled to cell cycle regulation and chromosomal replication.
Preparing for daughter cell formation
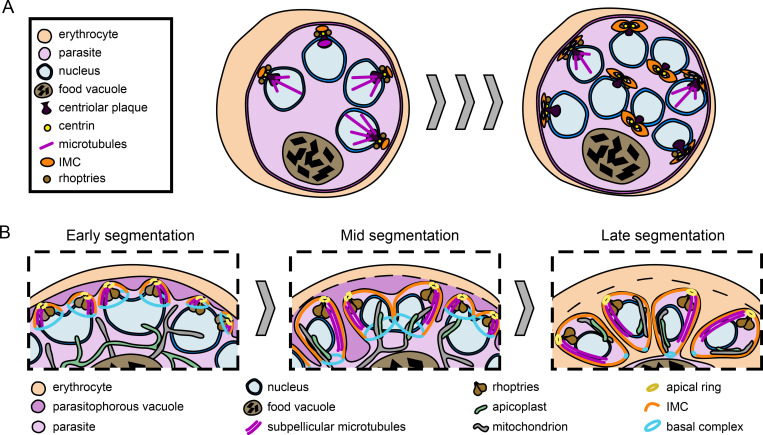
While the nuclei multiply, many organelles are already being primed for daughter cell formation [87,88]. The apicoplast and mitochondrion grow into large, branched structures as their genomes are replicated, and the ER becomes a complex structure that extends throughout the cell [89]. Already in schizonts with as little as three nuclei, first components of the inner membrane complex (IMC) can be detected [90]. The mature IMC is a system of large, flattened vesicles that reside directly underneath the plasma membrane of merozoites and is a crucial part of the parasites’ pellicle [91]. In schizonts, the developing IMC initially has a cramp-like shape, and in later schizonts, the IMC is an ellipsoid disk with two small holes developing adjacent to the centriolar plaque. Subsequently, the IMC is pulled around the nascent daughter cell by the basal complex, which acts as a contractile ring [92–95]. Similar to the IMC, rhoptries also start developing adjacent to the centriolar plaque in schizonts with as few as four nuclei [49]. Thus, the centriolar plaque may direct the positioning of the apical complex and structures that are critical for cytokinesis and invasion, which begin their development early during nuclear multiplication. Being important for both processes, the centriolar plaque may connect nuclear multiplication and organization of cytokinesis (Fig 3A).
Fig 3. The end of nuclear multiplication and formation of daughter cells.
(A) Structures and organelles involved in cytokinesis start forming during early nuclear cycles. Proteins of the IMC and the rhoptries start organizing around the centriolar plaque in cells with nuclei numbers as low as four and develop into larger structures as nuclear multiplication progresses. (B) Daughter cells are formed during segmentation. Concomitant to daughter cell formation, the nuclei are divided in a final, semisynchronous round of nuclear division. One pair of rhoptries associates with the apical end of the developing daughter cells. Segmentation commences when the plasma membrane (PM) starts invaginating. During segmentation, the IMC, which sits below the PM and originates from the apical end, starts enveloping the emerging daughter cell alongside the PM, with the basal complex localizing to the leading edge of the IMC. Concurrently to the expansion of the IMC, subpellicular microtubules start developing from the apical end of the emerging daughter cells. At the end of segmentation, the daughter cells now containing all necessary organelles are pinched off a remnant body containing the food vacuole.
Initiation and progression of segmentation
Daughter cell formation, cellularization, or segmentation are terms that describe the same overall process, i.e., the transition from a single multinucleated cell into multiple daughter cells with a single nucleus each (Fig 3). After the final DNA replication, it is assumed that nuclei arrest in the diploid stage until the final nuclear division occurs concomitantly to segmentation. Early in this process, the parasite plasma membrane (PM) begins to invaginate [95], and the basal complex localizes to the leading edge of the PM, pulling the IMC along. PM and IMC encapsulate the emerging daughter cells starting from the apical end until the basal complex comes to rest at the posterior end. Then, the daughter cells are pinched off the residual body, which contains the food vacuole of the mother cell (Fig 3B) [94–97]. With the expansion of the IMC, subpellicular microtubules assemble from the apical ring, supporting the IMC [98–100]. Why P. falciparum merozoites contain only one or two subpellicular microtubules while P. berghei merozoites contain approximately nine microtubules is unclear [100].
Contrary to previous hypotheses [1], nuclear division during segmentation does not seem to occur in perfect synchrony (Fig 3B) [95]. At early stages, most nuclei associate with two sets of rhoptries instead of one. Simultaneously, a small number of nuclei may associate with four sets of rhoptries, indicating a certain degree of asynchrony or imprecision. In each set of rhoptries, one rhoptry appears more mature than the other. All rhoptry necks reach into the apical ring, which is not yet well defined during early stages of cellularization. At this point, the apicoplast and the mitochondrion are still large, branched structures that wind independently around the nuclei (Fig 3) [95]. During mid-segmentation, the parasites’ PM is strongly invaginated at some places, and about half of the nuclei have one set of rhoptries, while the other half is still associated with two sets, again indicating a certain degree of asynchrony. At mid-segmentation, the apicoplast is already divided, while the single mitochondrion has still a branched structure. At this stage, both organelles are now closely associated with each other [89,95]. Only during the final steps of segmentation, the mitochondrion is also divided and, along with the nucleus and an apicoplast, packaged into the emerging daughter merozoite [89,95]. At some point before daughter cells are completely separated, the ER fragments and forms crescent organelles that associate with each daughter cell [89].
The distribution of the apicoplast to the daughter cells depends on Pfactin 1, which is also involved in apicoplast branching [101,102], but the molecular basis for mitochondrial fission and how it is coordinated with cytokinesis are not well understood [87]. The only investigated mitochondrial fission protein PfFis1 is not essential, although the P. falciparum protein kinase 7 may be involved in regulation of mitochondrial genes involved in fission [103,104].
Pfactin 1 and the actin nucleator Pfformin–2 are also required for proper and efficient segmentation [101,102] as is the cyclin H homolog PfCyc1, which potentially acts in concert with the CDK-activating kinase assembly factor PfMAT1 and the Cdk7 homologue PfMRK [105]. Cells lacking PfCyc1 fail to form merozoites, although nuclei and apical organelles appear normal [105]. Additionally, parasites with a defective IMC, e.g., in absence of the apical protein PfMOP, fail to form daughter cells properly [90]. Similarly, depletion of PfMCMBP or deletion of PfSEA–1 led to defective nuclear division, which translated into abnormal merozoite formation. Still, segmentation and egress were induced in absence of PfMCMBP or PfSEA–1, and a fraction of the respective mutant merozoites lacked the nucleus entirely [43,61].
The basal complex and, specifically, the protein PfCINCH have been shown to facilitate separation of merozoites from the residual body [97]. Another basal complex protein, PfMORN, is dispensable for blood stage development, including cytokinesis [106]. At the time of their release from the residual body, merozoites contain a single pair of rhoptries, and egress from the host erythrocyte is already initiated, as the parasitophorous vacuolar membrane is being disintegrated (Fig 3) [95]. Egress concludes by rupture of the host erythrocyte plasma membrane, and mature merozoites are released into the blood stream to start a proliferative cycle anew [107,108].
Daughter cell segmentation is a highly complex process that should be precisely controlled to allow for the efficient formation of viable daughter cells. Further improvements in live–cell imaging will help elucidate the regulatory cascades that initiate apical complex formation and organelle distribution, and how they relate to nuclear multiplication.
Conclusions
Although studying the cell cycle of Plasmodium (re)gained momentum in the last years, it remains enigmatic. Resolving conflicting data, employing modern biology approaches, and asking fundamental questions will help us to gain more insight into the molecular and cellular mechanisms that orchestrate Plasmodium proliferation. As we are just beginning to understand proliferation of Plasmodium in the blood stage, more challenges and discoveries await when proliferation in the oocyst and the liver stage will be in focus. These insights are a prerequisite to unlock the therapeutic potential of the parasite’s unusual cell cycle. In addition, understanding Plasmodium proliferation will also inform on the diversity of cell cycle modes and on the fundamental biology of an early branching eukaryote.
Acknowledgments
We apologize to all authors whose work we could not discuss due to space limitations. We thank Franziska Hentzschel for critical comments on the manuscript. The Plasmodium database PlasmoDB facilitated this work.
Funding Statement
This work was supported through funding from the German Research Foundation (DFG) (349355339), the Human Frontiers Science Program (CDA00013/2018–C), and the Daimler and Benz Foundation to J.G., the Studienstiftung des Deutschen Volkes to Y.V., as well as the German Research Foundation (DFG) – Project number 240245660 – SFB 1129 and the Baden–Württemberg Foundation (1.16101.17) to M.G. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Francia ME, Striepen B. Cell division in apicomplexan parasites. Nat Rev Microbiol. 2014;12:125–136. doi: 10.1038/nrmicro3184 [DOI] [PubMed] [Google Scholar]
- 2.White MW, Suvorova ES. Apicomplexa Cell Cycles: Something Old, Borrowed, Lost, and New. Trends Parasitol. 2018;34:759–771. doi: 10.1016/j.pt.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubbels M-J, Keroack CD, Dangoudoubiyam S, Worliczek HL, Paul AS, Bauwens C, et al. Fussing About Fission: Defining Variety Among Mainstream and Exotic Apicomplexan Cell Division Modes. Front Cell Infect Mi. 2020;10:269. doi: 10.3389/fcimb.2020.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubbels M-J, Coppens I, Zarringhalam K, Duraisingh MT, Engelberg K. The Modular Circuitry of Apicomplexan Cell Division Plasticity. Front Cell Infect Mi. 2021;11:670049. doi: 10.3389/fcimb.2021.670049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World malaria report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
- 6.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110 [DOI] [PubMed] [Google Scholar]
- 7.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and Disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055 [DOI] [PubMed] [Google Scholar]
- 8.Beck JR, Ho C-M. Transport mechanisms at the malaria parasite-host cell interface. PLoS Pathog. 2021;17:e1009394. doi: 10.1371/journal.ppat.1009394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brancucci NMB, Gerdt JP, Wang C, Niz MD, Philip N, Adapa SR, et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell. 2017;171:1532–1544.e15. doi: 10.1016/j.cell.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kafsack BFC, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llorà-Batlle O, Michel-Todó L, Witmer K, Toda H, Fernández-Becerra C, Baum J, et al. Conditional expression of PfAP2-G for controlled massive sexual conversion in Plasmodium falciparum. Sci Adv. 2020;6:eaaz5057. doi: 10.1126/sciadv.aaz5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babbitt SE, Altenhofen L, Cobbold SA, Istvan ES, Fennell C, Doerig C, et al. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc National Acad Sci. 2012;109:E3278–E3287. doi: 10.1073/pnas.1209823109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganter M, Goldberg JM, Dvorin JD, Paulo JA, King JG, Tripathi AK, et al. Plasmodium falciparum CRK4 directs continuous rounds of DNA replication during schizogony. Nat Microbiol. 2017;2:17017. doi: 10.1038/nmicrobiol.2017.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Biljon R, Niemand J, van Wyk R, Clark K, Verlinden B, Abrie C, et al. Inducing controlled cell cycle arrest and re-entry during asexual proliferation of Plasmodium falciparum malaria parasites. Sci Rep 2018;8: 16581. doi: 10.1038/s41598-018-34964-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLean KJ, Jacobs-Lorena M. The response of Plasmodium falciparum to isoleucine withdrawal is dependent on the stage of progression through the intraerythrocytic cell cycle. Malar J. 2020;19:147. doi: 10.1186/s12936-020-03220-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerald N, Mahajan B, Kumar S. Mitosis in the Human Malaria Parasite Plasmodium falciparum. Eukaryot Cell. 2011;10:474–482. doi: 10.1128/EC.00314-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon CS, Funaya C, Bauer J, Voβ Y, Machado M, Penning A, et al. An extended DNA-free intranuclear compartment organizes centrosome microtubules in malaria parasites. Life Sci Alliance. 2021;4:e202101199. doi: 10.26508/lsa.202101199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker RA, O’Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, et al. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988;107:1437–1448. doi: 10.1083/jcb.107.4.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spreng B, Fleckenstein H, Kübler P, Biagio CD, Benz M, Patra P, et al. Microtubule number and length determine cellular shape and function in Plasmodium. EMBO J. 2019;38:e100984. doi: 10.15252/embj.2018100984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnot DE, Ronander E, Bengtsson DC. The progression of the intra-erythrocytic cell cycle of Plasmodium falciparum and the role of the centriolar plaques in asynchronous mitotic division during schizogony. Int J Parasitol. 2011;41:71–80. doi: 10.1016/j.ijpara.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 22.Russo I, Oksman A, Vaupel B, Goldberg DE. A calpain unique to alveolates is essential in Plasmodium falciparum and its knockdown reveals an involvement in pre-S-phase development. Proc National Acad Sci. 2009;106:1554–1559. doi: 10.1073/pnas.0806926106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald J, Merrick CJ. DNA replication dynamics during erythrocytic schizogony in the malaria parasites Plasmodium falciparum and Plasmodium knowlesi. PLoS Pathog. 2022;18:e1010595. doi: 10.1371/journal.ppat.1010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inselburg J, Banyal HS. Synthesis of DNA during the asexual cycle of Plasmodium falciparum in culture. Mol Biochem Parasitol. 1984;10:79–87. doi: 10.1016/0166-6851(84)90020-3 [DOI] [PubMed] [Google Scholar]
- 25.Zegerman P. Evolutionary conservation of the CDK targets in eukaryotic DNA replication initiation. Chromosoma. 2015;124:309–321. doi: 10.1007/s00412-014-0500-y [DOI] [PubMed] [Google Scholar]
- 26.Boos D, Ferreira P. Origin Firing Regulations to Control Genome Replication Timing. Genesis. 2019;10:199. doi: 10.3390/genes10030199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, Mehra P, Dhar SK. Plasmodium falciparum origin recognition complex subunit 5: functional characterization and role in DNA replication foci formation. Mol Microbiol. 2008;69:646–665. doi: 10.1111/j.1365-2958.2008.06316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeeles JTP, Deegan TD, Janska A, Early A, Diffley JFX. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature. 2015;519:431–435. doi: 10.1038/nature14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews H, Duffy CW, Merrick CJ. Checks and balances? DNA replication and the cell cycle in Plasmodium. Parasite Vector. 2018;11:216. doi: 10.1186/s13071-018-2800-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balestra AC, Zeeshan M, Rea E, Pasquarello C, Brusini L, Mourier T, et al. A divergent cyclin/cyclin-dependent kinase complex controls the atypical replication of a malaria parasite during gametogony and transmission. Elife. 2020;9:e56474. doi: 10.7554/eLife.56474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Gargaro OR, Kappe SHI. Plasmodium falciparum CRK5 Is Critical for Male Gametogenesis and Infection of the Mosquito. MBio. 2022:e02227–e02222. doi: 10.1128/mbio.02227-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Baranwal VK, Leeb AS, Haile MT, Oualim KMZ, Hertoghs N, et al. PfSRPK1 Regulates Asexual Blood Stage Schizogony and Is Essential for Male Gamete Formation. Microbiol Spectr. 2022:e02141–e02122. doi: 10.1128/spectrum.02141-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorin-Semblat D, Carvalho TG, Nivez M-P, Halbert J, Poullet P, Semblat J-P, et al. An atypical cyclin-dependent kinase controls Plasmodium falciparum proliferation rate. Kinome. 2013;1:4–16. doi: 10.2478/kinome-2013-0001 [DOI] [Google Scholar]
- 34.Merrick CJ. Transfection with thymidine kinase permits bromodeoxyuridine labelling of DNA replication in the human malaria parasite Plasmodium falciparum. Malar J. 2015;14:490. doi: 10.1186/s12936-015-1014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanojcic S, Kuk N, Ullah I, Sterkers Y, Merrick CJ. Single-molecule analysis reveals that DNA replication dynamics vary across the course of schizogony in the malaria parasite Plasmodium falciparum. Sci Rep. 2017;7:4003. doi: 10.1038/s41598-017-04407-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klaus S, Binder P, Kim J, Machado M, Funaya C, Schaaf V, et al. Asynchronous nuclear cycles in multinucleated Plasmodium falciparum facilitate rapid proliferation. Sci Adv. 2022:8. doi: 10.1126/sciadv.abj5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnot DE, Gull K. The Plasmodium cell cycle: facts and questions. Ann Trop Med Parasitol. 1998;92:361–365. doi: 10.1080/00034989859357 [DOI] [PubMed] [Google Scholar]
- 38.Sinden RE, Canning EU, Spain B. Gametogenesis and fertilization in Plasmodium yoelii nigeriensis: a transmission electron microscope study. Proc R Soc Lond B Biol Sci. 1976;193:55–76. doi: 10.1098/rspb.1976.0031 [DOI] [PubMed] [Google Scholar]
- 39.Sinden RE, Canning EU, Bray RS, Smalley ME. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci. 1978;201:375–399. doi: 10.1098/rspb.1978.0051 [DOI] [PubMed] [Google Scholar]
- 40.Janse CJ, der Klooster PFJV, der Kaay HJV, der Ploeg MV, Overdulve JP. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei. T Roy Soc Trop Med H. 1986;80: 154–157. doi: 10.1016/0035-9203(86)90219-1 [DOI] [PubMed] [Google Scholar]
- 41.Janse CJ, van der Klooster PFJ, van der Kaay HJ, van der Ploeg M, Overdulve JP. DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol Biochem Parasitol. 1986;20:173–182. doi: 10.1016/0166-6851(86)90029-0 [DOI] [PubMed] [Google Scholar]
- 42.Leete TH, Rubin H. Malaria and the cell cycle. Parasitol Today. 1996;12:442–444. doi: 10.1016/0169-4758(96)10068-5 [DOI] [PubMed] [Google Scholar]
- 43.Absalon S, Dvorin JD. Depletion of the mini-chromosome maintenance complex binding protein allows the progression of cytokinesis despite abnormal karyokinesis during the asexual development of Plasmodium falciparum. Cell Microbiol. 2021;23:e13284. doi: 10.1111/cmi.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasina R, González FC, Francia ME. Structural and Functional Insights into the Microtubule Organizing Centers of Toxoplasma gondii and Plasmodium spp. Microorg. 2021;9:2503. doi: 10.3390/microorganisms9122503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aikawa M, Beaudoin RL. Studies on nuclear division of a malarial parasite under pyrimethamine treatment. J Cell Biol. 1968;39:749–754. doi: 10.1083/jcb.39.3.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liffner B, Absalon S. Expansion Microscopy Reveals Plasmodium falciparum Blood-Stage Parasites Undergo Anaphase with A Chromatin Bridge in the Absence of Mini-Chromosome Maintenance Complex Binding Protein. Microorg. 2021;9:2306. doi: 10.3390/microorganisms9112306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rashpa R, Brochet M. Expansion microscopy of Plasmodium gametocytes reveals the molecular architecture of a bipartite microtubule organisation centre coordinating mitosis with axoneme assembly. PLoS Pathog. 2022;18:e1010223. doi: 10.1371/journal.ppat.1010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Shami GJ, Cho E, Liu B, Hanssen E, Dixon MWA, et al. Repurposing the mitotic machinery to drive cellular elongation and chromatin reorganisation in Plasmodium falciparum gametocytes. Nat Commun. 2022;13:5054. doi: 10.1038/s41467-022-32579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahajan B, Selvapandiyan A, Gerald NJ, Majam V, Zheng H, Wickramarachchi T, et al. Centrins, Cell Cycle Regulation Proteins in Human Malaria Parasite Plasmodium falciparum. J Biol Chem. 2008;283:31871–31883. doi: 10.1074/jbc.M800028200 [DOI] [PubMed] [Google Scholar]
- 50.Roques M, Stanway RR, Rea EI, Markus R, Brady D, Holder AA, et al. Plasmodium centrin PbCEN-4 localizes to the putative MTOC and is dispensable for malaria parasite proliferation. Biol Open. 2018;8:bio.036822. doi: 10.1242/bio.036822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dantas TJ, Daly OM, Morrison CG. Such small hands: the roles of centrins/caltractins in the centriole and in genome maintenance. Cell Mol Life Sci. 2012;69:2979–2997. doi: 10.1007/s00018-012-0961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wall RJ, Ferguson DJP, Freville A, Franke-Fayard B, Brady D, Zeeshan M, et al. Plasmodium APC3 mediates chromosome condensation and cytokinesis during atypical mitosis in male gametogenesis. Sci Rep. 2018;8:5610. doi: 10.1038/s41598-018-23871-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters J-M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988 [DOI] [PubMed] [Google Scholar]
- 54.Hoeijmakers WAM, Flueck C, Françoijs K, Smits AH, Wetzel J, Volz JC, et al. Plasmodium falciparum centromeres display a unique epigenetic makeup and cluster prior to and during schizogony. Cell Microbiol. 2012;14:1391–1401. doi: 10.1111/j.1462-5822.2012.01803.x [DOI] [PubMed] [Google Scholar]
- 55.Zeeshan M, Pandey R, Ferguson DJP, Tromer EC, Markus R, Abel S, et al. Real-time dynamics of Plasmodium NDC80 reveals unusual modes of chromosome segregation during parasite proliferation. J Cell Sci. 2020;134:jcs245753. doi: 10.1242/jcs.245753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brusini L, Pacheco NDS, Tromer EC, Soldati-Favre D, Brochet M. Composition and organization of kinetochores show plasticity in apicomplexan chromosome segregation. J Cell Biol. 2022;221:e202111084. doi: 10.1083/jcb.202111084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paul AS, Miliu A, Paulo JA, Goldberg JM, Bonilla AM, Berry L, et al. Co-option of Plasmodium falciparum PP1 for egress from host erythrocytes. Nat Commun. 2020;11:3532. doi: 10.1038/s41467-020-17306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeeshan M, Pandey R, Subudhi AK, Ferguson DJP, Kaur G, Rashpa R, et al. Protein phosphatase 1 regulates atypical mitotic and meiotic division in Plasmodium sexual stages. Commun Biology. 2021;4:760. doi: 10.1038/s42003-021-02273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reininger L, Wilkes JM, Bourgade H, Miranda-Saavedra D, Doerig C. An essential Aurora-related kinase transiently associates with spindle pole bodies during Plasmodium falciparum erythrocytic schizogony. Mol Microbiol. 2011;79:205–221. doi: 10.1111/j.1365-2958.2010.07442.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morahan BJ, Abrie C, Al-Hasani K, Batty MB, Corey V, Cowell AN, et al. Human Aurora kinase inhibitor Hesperadin reveals epistatic interaction between Plasmodium falciparum PfArk1 and PfNek1 kinases. Commun Biol. 2020;3:701. doi: 10.1038/s42003-020-01424-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perrin AJ, Bisson C, Faull PA, Renshaw MJ, Lees RA, Fleck RA, et al. Malaria Parasite Schizont Egress Antigen-1 Plays an Essential Role in Nuclear Segregation during Schizogony. MBio. 2021:12. doi: 10.1128/mBio.03377-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farrell JA, O’Farrell PH. From Egg to Gastrula: How the Cell Cycle Is Remodeled During the Drosophila Mid-Blastula Transition. Annu Rev Genet. 2014;48:1–26. doi: 10.1146/annurev-genet-111212-133531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ondracka A, Dudin O, Ruiz-Trillo I. Decoupling of Nuclear Division Cycles and Cell Size during the Coenocytic Growth of the Ichthyosporean Sphaeroforma arctica. Curr Biol. 2018;28:1964–1969.e2. doi: 10.1016/j.cub.2018.04.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Read M, Sherwin T, Holloway SP, Gull K, Hyde JE. Microtubular organization visualized by immunofluorescence microscopy during erythrocytic schizogony in Plasmodium falciparum and investigation of post-translational modifications of parasite tubulin. Parasitology. 1993;106:223–232. doi: 10.1017/s0031182000075041 [DOI] [PubMed] [Google Scholar]
- 65.Gladfelter AS, Hungerbuehler AK, Philippsen P. Asynchronous nuclear division cycles in multinucleated cells. J Cell Biol. 2006;172:347–362. doi: 10.1083/jcb.200507003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee C, Zhang H, Baker AE, Occhipinti P, Borsuk ME, Gladfelter AS. Protein Aggregation Behavior Regulates Cyclin Transcript Localization and Cell-Cycle Control. Dev Cell. 2013;25:572–584. doi: 10.1016/j.devcel.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson CA, Eser U, Korndorf T, Borsuk ME, Skotheim JM, Gladfelter AS. Nuclear Repulsion Enables Division Autonomy in a Single Cytoplasm. Curr Biol. 2013;23:1999–2010. doi: 10.1016/j.cub.2013.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, et al. RNA Controls PolyQ Protein Phase Transitions. Mol Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dundon SER, Chang S-S, Kumar A, Occhipinti P, Shroff H, Roper M, et al. Clustered nuclei maintain autonomy and nucleocytoplasmic ratio control in a syncytium. Mol Biol Cell. 2016;27:2000–2007. doi: 10.1091/mbc.E16-02-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, et al. Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 71.Machado M, Steinke S, Ganter M. Plasmodium Reproduction, Cell Size, and Transcription: How to Cope With Increasing DNA Content? Front Cell Infect Mi. 2021;11:660679. doi: 10.3389/fcimb.2021.660679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Facchetti G, Chang F, Howard M. Controlling cell size through sizer mechanisms. Curr Opin Syst Biol. 2017;5:86–92. doi: 10.1016/j.coisb.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon CS, Stürmer VS, Guizetti J. How Many Is Enough?—Challenges of Multinucleated Cell Division in Malaria Parasites. Front Cell Infect Mi. 2021;11:658616. doi: 10.3389/fcimb.2021.658616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reilly HB, Wang H, Steuter JA, Marx AM, Ferdig MT. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int J Parasitol. 2007;37:1599–1607. doi: 10.1016/j.ijpara.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dorin-Semblat D, Sicard A, Doerig C, Ranford-Cartwright L, Doerig C. Disruption of the PfPK7 Gene Impairs Schizogony and Sporogony in the Human Malaria Parasite Plasmodium falciparum. Eukaryot Cell. 2008;7:279–285. doi: 10.1128/EC.00245-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garg S, Agarwal S, Dabral S, Kumar N, Sehrawat S, Singh S. Visualization and quantification of Plasmodium falciparum intraerythrocytic merozoites. Syst Synth Biol. 2015;9:23–26. doi: 10.1007/s11693-015-9167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glushakova S, Balaban A, McQueen PG, Coutinho R, Miller JL, Nossal R, et al. Hemoglobinopathic Erythrocytes Affect the Intraerythrocytic Multiplication of Plasmodium falciparum In Vitro. J Infect Dis. 2014;210:1100–1109. doi: 10.1093/infdis/jiu203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc National Acad Sci. 2006;103:8840–8845. doi: 10.1073/pnas.0601876103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mancio-Silva L, Slavic K, Ruivo MTG, Grosso AR, Modrzynska KK, Vera IM, et al. Nutrient sensing modulates malaria parasite virulence. Nature. 2017;547:213–216. doi: 10.1038/nature23009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ch’ng J-H, Moll K, Wyss K, Hammar U, Rydén M, Kämpe O, et al. Enhanced virulence of Plasmodium falciparum in blood of diabetic patients. PloS ONE. 2021;16:e0249666. doi: 10.1371/journal.pone.0249666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar M, Skillman K, Duraisingh MT. Linking nutrient sensing and gene expression in Plasmodium falciparum blood-stage parasites. Mol Microbiol. 2021;115:891–900. doi: 10.1111/mmi.14652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Dooren GG, Striepen B. The Algal Past and Parasite Present of the Apicoplast. Annu Rev Microbiol. 2012;67: 271–289. doi: 10.1146/annurev-micro-092412-155741 [DOI] [PubMed] [Google Scholar]
- 83.Williamson DH, Preiser PR, Moore PW, McCready S, Strath M, Wilson RJM, et al. The plastid DNA of the malaria parasite Plasmodium falciparum is replicated by two mechanisms. Mol Microbiol. 2002;45:533–542. doi: 10.1046/j.1365-2958.2002.03033.x [DOI] [PubMed] [Google Scholar]
- 84.Milton ME, Nelson SW. Replication and maintenance of the Plasmodium falciparum apicoplast genome. Mol Biochem Parasitol. 2016;208:56–64. doi: 10.1016/j.molbiopara.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 85.Preiser PR, Wilson RJ, Moore PW, McCready S, Hajibagheri MA, Blight KJ, et al. Recombination associated with replication of malarial mitochondrial DNA. EMBO J. 1996;15:684–693. doi: 10.1002/j.1460-2075.1996.tb00401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bansod S, Bung N, Singh P, Suthram N, Choudhury H, Roy A, et al. Elucidation of an essential function of the unique charged domain of Plasmodium topoisomerase III. Biochem J. 2020;477:4745–4767. doi: 10.1042/BCJ20200318 [DOI] [PubMed] [Google Scholar]
- 87.Verhoef JMJ, Meissner M, Kooij TWA. Organelle Dynamics in Apicomplexan Parasites. MBio. 2021;12:e01409–e01421. doi: 10.1128/mBio.01409-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elaagip A, Absalon S, Florentin A. Apicoplast Dynamics During Plasmodium Cell Cycle. Front Cell Infect Mi. 2022;12:864819. doi: 10.3389/fcimb.2022.864819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol. 2005;57: 405–419. doi: 10.1111/j.1365-2958.2005.04699.x [DOI] [PubMed] [Google Scholar]
- 90.Absalon S, Robbins JA, Dvorin JD. An essential malaria protein defines the architecture of blood-stage and transmission-stage parasites. Nat Commun. 2016;7:11449. doi: 10.1038/ncomms11449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harding CR, Meissner M. The IMC in Plasmodium and Toxoplasma. Cell Microbiol. 2014;16:632–641. doi: 10.1111/cmi.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeoman JA, Hanssen E, Maier AG, Klonis N, Maco B, Baum J, et al. Tracking Glideosome-Associated Protein 50 Reveals the Development and Organization of the Inner Membrane Complex of Plasmodium falciparum. Eukaryot Cell. 2011;10:556–564. doi: 10.1128/EC.00244-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kono M, Herrmann S, Loughran NB, Cabrera A, Engelberg K, Lehmann C, et al. Evolution and Architecture of the Inner Membrane Complex in Asexual and Sexual Stages of the Malaria Parasite. Mol Biol Evol. 2012;29:2113–2132. doi: 10.1093/molbev/mss081 [DOI] [PubMed] [Google Scholar]
- 94.Kono M, Heincke D, Wilcke L, Wong TWY, Bruns C, Herrmann S, et al. Pellicle formation in the malaria parasite. J Cell Sci. 2016;129:673–680. doi: 10.1242/jcs.181230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudlaff RM, Kraemer S, Marshman J, Dvorin JD. Three-dimensional ultrastructure of Plasmodium falciparum throughout cytokinesis. PLoS Pathog. 2020;16:e1008587. doi: 10.1371/journal.ppat.1008587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson DJP, Sahoo N, Pinches RA, Bumstead JM, Tomley FM, Gubbels M-J. MORN1 Has a Conserved Role in Asexual and Sexual Development across the Apicomplexa. Eukaryot Cell. 2008;7:698–711. doi: 10.1128/EC.00021-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rudlaff RM, Kraemer S, Streva VA, Dvorin JD. An essential contractile ring protein controls cell division in Plasmodium falciparum. Nat Commun. 2019;10:2181. doi: 10.1038/s41467-019-10214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. Ultrastructure of rhoptry development in Plasmodium falciparum erythrocytic schizonts. Parasitology. 2000;121:273–287. doi: 10.1017/s0031182099006320 [DOI] [PubMed] [Google Scholar]
- 99.Hanssen E, Dekiwadia C, Riglar DT, Rug M, Lemgruber L, Cowman AF, et al. Ultrastructure of the invading malaria parasite. Cell Microbiol. 2013;15:1457–1472. doi: 10.1111/cmi.12132 [DOI] [PubMed] [Google Scholar]
- 100.Bertiaux E, Balestra AC, Bournonville L, Louvel V, Maco B, Soldati-Favre D, et al. Expansion microscopy provides new insights into the cytoskeleton of malaria parasites including the conservation of a conoid. PLoS Biol. 2021;19:e3001020. doi: 10.1371/journal.pbio.3001020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017;15:70. doi: 10.1186/s12915-017-0406-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stortz JF, Rosario MD, Singer M, Wilkes JM, Meissner M, Das S. Formin-2 drives polymerisation of actin filaments enabling segregation of apicoplasts and cytokinesis in Plasmodium falciparum. Elife. 2019;8:e49030. doi: 10.7554/eLife.49030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scarpelli PH, Tessarin-Almeida G, Viçoso KL, Lima WR, Borges-Pereira L, Meissner KA, et al. Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related-genes for mitochondria fission: Mitoemerald-GFP as a tool to visualize mitochondria structure. J Pineal Res. 2019;66:e12484. doi: 10.1111/jpi.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maruthi M, Ling L, Zhou J, Ke H. Dispensable Role of Mitochondrial Fission Protein 1 (Fis1) in the Erythrocytic Development of Plasmodium falciparum. Msphere. 2020;5:e00579–e00520. doi: 10.1128/msphere.00579-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins JA, Absalon S, Streva VA, Dvorin JD. The Malaria Parasite Cyclin H Homolog PfCyc1 Is Required for Efficient Cytokinesis in Blood-Stage Plasmodium falciparum. MBio. 2017;8:e00605–e00617. doi: 10.1128/mBio.00605-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moran CJ, Dvorin JD. The Basal Complex Protein PfMORN1 Is Not Required for Asexual Replication of Plasmodium falciparum. Msphere. 2021;6:e00895–e00821. doi: 10.1128/msphere.00895-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan MSY, Blackman MJ. Malaria parasite egress at a glance. J Cell Sci. 2021;134:jcs257345. doi: 10.1242/jcs.257345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dvorin JD, Goldberg DE. Plasmodium Egress Across the Parasite Life Cycle. Annu Rev Microbiol. 2022;76:67–90. doi: 10.1146/annurev-micro-041320-020659 [DOI] [PubMed] [Google Scholar]
- 109.Musacchio A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr Biol. 2015;25:R1002–R1018. doi: 10.1016/j.cub.2015.08.051 [DOI] [PubMed] [Google Scholar]
- 110.Sinou V, Boulard Y, Grellier P, Schrevel J. Host Cell and Malarial Targets for Docetaxel (TaxotereTM) during the Erythrocytic Development of Plasmodium falciparum. J Eukaryot Microbiol. 1998;45:171–183. doi: 10.1111/j.1550-7408.1998.tb04522.x [DOI] [PubMed] [Google Scholar]
- 111.Fennell BJ, Naughton JA, Dempsey E, Bell A. Cellular and molecular actions of dinitroaniline and phosphorothioamidate herbicides on Plasmodium falciparum: Tubulin as a specific antimalarial target. Mol Biochem Parasitol. 2006;145:226–238. doi: 10.1016/j.molbiopara.2005.08.020 [DOI] [PubMed] [Google Scholar]
- 112.Naughton JA, Bell A. Studies on cell-cycle synchronization in the asexual erythrocytic stages of Plasmodium falciparum. Parasitology. 2007;134:331–337. doi: 10.1017/S0031182006001466 [DOI] [PubMed] [Google Scholar]
- 113.Inselburg J, Banyal HS. Plasmodium falciparum: Synchronization of asexual development with aphidicolin, a DNA synthesis inhibitor. Exp Parasitol. 1984;57:48–54. doi: 10.1016/0014-4894(84)90061-4 [DOI] [PubMed] [Google Scholar]
- 114.Prior KF, Rijo-Ferreira F, Assis PA, Hirako IC, Weaver DR, Gazzinelli RT, et al. Periodic Parasites and Daily Host Rhythms. Cell Host Microbe. 2020;27:176–187. doi: 10.1016/j.chom.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stanway RR, Witt T, Zobiak B, Aepfelbacher M, Heussler VT. GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol Cell. 2009;101:415–435. doi: 10.1042/BC20080202 [DOI] [PubMed] [Google Scholar]
- 116.Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger T-W, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011;2:165. doi: 10.1038/ncomms1169 [DOI] [PubMed] [Google Scholar]
- 117.Zeeshan M, Brady D, Stanway RR, Moores CA, Holder AA, Tewari R. Plasmodium berghei Kinesin-5 Associates With the Spindle Apparatus During Cell Division and Is Important for Efficient Production of Infectious Sporozoites. Front Cell Infect Mi. 2020;10:583812. doi: 10.3389/fcimb.2020.583812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Broichhagen J, Kilian N. Chemical Biology Tools To Investigate Malaria Parasites. Chembiochem. 2021;22:2219–2236. doi: 10.1002/cbic.202000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lukinavičius G, Reymond L, D’Este E, Masharina A, Göttfert F, Ta H, et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods. 2014;11:731–733. doi: 10.1038/nmeth.2972 [DOI] [PubMed] [Google Scholar]
- 120.Wang L, Tran M, D’Este E, Roberti J, Koch B, Xue L, et al. A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy. Nat Chem. 2020;12:165–172. doi: 10.1038/s41557-019-0371-1 [DOI] [PubMed] [Google Scholar]
- 121.Chen B-C, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science. 2014;346:1257998. doi: 10.1126/science.1257998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geoghegan ND, Evelyn C, Whitehead LW, Pasternak M, McDonald P, Triglia T, et al. 4D analysis of malaria parasite invasion offers insights into erythrocyte membrane remodeling and parasitophorous vacuole formation. Nat Commun. 2021;12:3620. doi: 10.1038/s41467-021-23626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eshar S, Dahan-Pasternak N, Weiner A, Dzikowski R. High resolution 3D perspective of Plasmodium biology: advancing into a new era. Trends Parasitol. 2011;27:548–554. doi: 10.1016/j.pt.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 124.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, et al. Super-Resolution Dissection of Coordinated Events during Malaria Parasite Invasion of the Human Erythrocyte. Cell Host Microbe. 2011;9:9–20. doi: 10.1016/j.chom.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 125.Rosario MD, Periz J, Pavlou G, Lyth O, Latorre-Barragan F, Das S, et al. Apicomplexan F-actin is required for efficient nuclear entry during host cell invasion. EMBO Rep. 2019;20:e48896. doi: 10.15252/embr.201948896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hell SW. Far-Field Optical Nanoscopy. Science. 2007;316:1153–1158. doi: 10.1126/science.1137395 [DOI] [PubMed] [Google Scholar]
- 127.Burda P-C, Schaffner M, Kaiser G, Roques M, Zuber B, Heussler VT. A Plasmodium plasma membrane reporter reveals membrane dynamics by live-cell microscopy. Sci Rep. 2017;7:9740. doi: 10.1038/s41598-017-09569-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mehnert A-K, Simon CS, Guizetti J. Immunofluorescence staining protocol for STED nanoscopy of Plasmodium-infected red blood cells. Mol Biochem Parasitol. 2019;229:47–52. doi: 10.1016/j.molbiopara.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 129.Schloetel J-G, Heine J, Cowman AF, Pasternak M. Guided STED nanoscopy enables super-resolution imaging of blood stage malaria parasites. Sci Rep. 2019;9:4674. doi: 10.1038/s41598-019-40718-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Looker O, Blanch AJ, Liu B, Nunez-Iglesias J, McMillan PJ, Tilley L, et al. The knob protein KAHRP assembles into a ring-shaped structure that underpins virulence complex assembly. PLoS Pathog. 2019;15:e1007761. doi: 10.1371/journal.ppat.1007761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sanchez CP, Karathanasis C, Sanchez R, Cyrklaff M, Jäger J, Buchholz B, et al. Single-molecule imaging and quantification of the immune-variant adhesin VAR2CSA on knobs of Plasmodium falciparum-infected erythrocytes. Commun Biol. 2019;2:172. doi: 10.1038/s42003-019-0429-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tillberg PW, Chen F. Expansion Microscopy: Scalable and Convenient Super-Resolution Microscopy. Annu Rev Cell Dev Bi. 2019;35:1–19. doi: 10.1146/annurev-cellbio-100818-125320 [DOI] [PubMed] [Google Scholar]
- 133.Gambarotto D, Zwettler FU, Guennec ML, Schmidt-Cernohorska M, Fortun D, Borgers S, et al. Imaging cellular ultrastructures using expansion microscopy (U-ExM). Nat Methods. 2019;16:71–74. doi: 10.1038/s41592-018-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aikawa M. The Fine Structure of the Erythrocytic Stages of Three Avian Malarial Parasites, Plasmodium Fallax, P. Lophurae, and P. Cathemerium. Am J Trop Med Hyg. 1966;15:449–471. doi: 10.4269/ajtmh.1966.15.449 [DOI] [PubMed] [Google Scholar]
- 135.Canning EU, Sinden RE. The organization of the ookinete and observations on nuclear division in oocysts of Plasmodium berghei. Parasitology. 1973;67:29–40. doi: 10.1017/s0031182000046266 [DOI] [PubMed] [Google Scholar]
- 136.Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. A Brief Illustrated Guide to the Ultrastructure of Plasmodium falciparum Asexual Blood Stages. Parasitol Today. 2000;16:427–433. doi: 10.1016/s0169-4758(00)01755-5 [DOI] [PubMed] [Google Scholar]
- 137.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, et al. Hemoglobins S and C Interfere with Actin Remodeling in Plasmodium falciparum–Infected Erythrocytes. Science. 2011;334:1283–1286. doi: 10.1126/science.1213775 [DOI] [PubMed] [Google Scholar]
- 138.Weiner A, Dahan-Pasternak N, Shimoni E, Shinder V, von Huth P, Elbaum M, et al. 3D nuclear architecture reveals coupled cell cycle dynamics of chromatin and nuclear pores in the malaria parasite Plasmodium falciparum. Cell Microbiol. 2011;13: 967–977. doi: 10.1111/j.1462-5822.2011.01592.x [DOI] [PubMed] [Google Scholar]
- 139.Birnbaum J, Scharf S, Schmidt S, Jonscher E, Hoeijmakers WAM, Flemming S, et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science. 2020;367:51–59. doi: 10.1126/science.aax4735 [DOI] [PubMed] [Google Scholar]
- 140.Birnbaum J, Flemming S, Reichard N, Soares AB, Mesén-Ramírez P, Jonscher E, et al. A genetic system to study Plasmodium falciparum protein function. Nat Methods. 2017;14:450–456. doi: 10.1038/nmeth.4223 [DOI] [PubMed] [Google Scholar]
- 141.Flueck C, Bartfai R, Volz J, Niederwieser I, Salcedo-Amaya AM, Alako BTF, et al. Plasmodium falciparum Heterochromatin Protein 1 Marks Genomic Loci Linked to Phenotypic Variation of Exported Virulence Factors. PLoS Pathog. 2009;5:e1000569. doi: 10.1371/journal.ppat.1000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kehrer J, Kuss C, Andres-Pons A, Reustle A, Dahan N, Devos D, et al. Nuclear Pore Complex Components in the Malaria Parasite Plasmodium berghei. Sci Rep. 2018;8:11249. doi: 10.1038/s41598-018-29590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeeshan M, Shilliday F, Liu T, Abel S, Mourier T, Ferguson DJP, et al. Plasmodium kinesin-8X associates with mitotic spindles and is essential for oocyst development during parasite proliferation and transmission. PLoS Pathog. 2019;15:e1008048. doi: 10.1371/journal.ppat.1008048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wichers JS, Wunderlich J, Heincke D, Pazicky S, Strauss J, Schmitt M, et al. Identification of novel inner membrane complex and apical annuli proteins of the malaria parasite Plasmodium falciparum. Cell Microbiol. 2021;23:e13341. doi: 10.1111/cmi.13341 [DOI] [PubMed] [Google Scholar]
- 145.Engelberg K, Ivey FD, Lin A, Kono M, Lorestani A, Faugno-Fusci D, et al. A MORN1-associated HAD phosphatase in the basal complex is essential for Toxoplasma gondii daughter budding. Cell Microbiol. 2016;18:1153–1171. doi: 10.1111/cmi.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]