Abstract
Background
Rural populations are older, have higher diabetes prevalence, and have less improvement in diabetes-related mortality rates compared to urban counterparts. Rural communities have limited access to diabetes education and social support services.
Objective
Determine if an innovative population health program that integrates medical and social care models improves clinical outcomes for patients with type 2 diabetes in a resource-constrained, frontier area.
Design/Participants
Quality improvement cohort study of 1764 patients with diabetes (September 2017–December 2021) at St. Mary’s Health and Clearwater Valley Health (SMHCVH), an integrated health care delivery system in frontier Idaho. The United States Department of Agriculture’s Office of Rural Health defines frontier as sparsely populated areas that are geographically isolated from population centers and services.
Intervention
SMHCVH integrated medical and social care through a population health team (PHT), where staff assess medical, behavioral, and social needs with annual health risk assessments and provide core interventions including diabetes self-management education, chronic care management, integrated behavioral health, medical nutritional therapy, and community health worker navigation. We categorized patients with diabetes into three groups: patients with two or more PHT encounters during the study (PHT intervention), one PHT encounter (minimal PHT), and no PHT encounters (no PHT).
Main Measures
HbA1c, blood pressure, and LDL over time for each study group.
Key Results
Of the 1764 patients with diabetes, mean age was 68.3 years, 57% were male, 98% were white, 33% had three or more chronic conditions, and 9% had at least one unmet social need. PHT intervention patients had more chronic conditions and higher medical complexity. Mean HbA1c of PHT intervention patients significantly decreased from baseline to 12 months (7.9 to 7.6%, p < 0.01) and sustained reductions at 18 months, 24 months, 30 months, and 36 months. Minimal PHT patients decreased HbA1c from baseline to 12 months (7.7 to 7.3%, p < 0.05).
Conclusion
The SMHCVH PHT model was associated with improved hemoglobin A1c among less well-controlled patients with diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07928-0.
KEY WORDS: rural, population health, diabetes mellitus
INTRODUCTION
Among frontier woodlands and rolling prairies, St. Mary’s Health and Clearwater Valley Health (SMHCVH) in north central Idaho strives toward a new frontier of diabetes care that integrates health care disciplines and mitigates the medical and social barriers that patients face. Frontier is the extreme limit of settled land beyond which lies wilderness, especially referring to the western USA before Pacific settlement. In a similar vein, frontier is the extreme limit of understanding or achievement in a particular area. Both definitions hold true for this critical access hospital and clinic network in rural Idaho developing and implementing innovative care models to care for patients with diabetes. Little research has explored what population health models are effective in frontier settings, what the United States Department of Agriculture’s Office of Rural Health defines as sparsely populated areas that are geographically isolated from population centers and services. Prior successful frontier interventions include a mobile health initiative to improve pregnancy outcomes among Wyoming Medicaid patients and a telecommunications intervention to improve diabetes self-management education access and outcomes in Idaho.1,2
Rural populations have a higher prevalence of diabetes, higher diabetes-related hospital mortality, and less improvement in overall diabetes-related mortality rates when compared to urban counterparts.3–5 Rural communities in the USA have limited access to certified diabetes educators (CDEs), diabetes self-management education (DSME) programs, and social services (e.g., food assistance, transportation, housing programs) that could support behavior change and improve health outcomes.6–8
As part of a 5-year grant initiative from the Merck Foundation, Bridging the Gap: Reducing Disparities in Diabetes Care, SMCHVH pilots primary care transformation activities for patients with diabetes and integrates medical and social care services. SMHCVH developed a dedicated population health team (PHT) to assess population health needs and support high-risk patients with clinic-based and community-based services. We describe SMHCVH’s PHT program, quantify the provision of services, and evaluate clinical outcomes among patients with diabetes.
METHODS
Overview
This quality improvement cohort study included the active adult population with diabetes at SMHCVH from September 2017 until December 2021. Electronic medical record (EMR) data were utilized to evaluate the effects of the PHT. This study was approved by the University of Chicago Institutional Review Board.
Setting
SMHCVH are critical access hospitals with level 4 trauma designations nestled among the high mountain prairies and rivers of North Central Idaho. SMHCVH formed an integrated health system between two hospitals and 8 clinics in 1998 and became affiliated with the Kootenai Health network in 2020. Residents of these counties are predominantly white (89.5%), Hispanic (3.9%), American Indian and Alaska Native (3.5%), and people who are two or more races (2.4%).9
SMHCVH’s predominant service area is a large (> 11,000 square miles including Clearwater, Idaho, and Lewis counties), geographically diverse area that requires resources and time to navigate. The Economic Research Service of the United States Department of Agriculture developed the Frontier and Remote (FAR) methodology to provide geographically detailed and adjustable delineations to describe conditions in sparsely settled and remote areas.10 Almost half of the 28,000+ people in North Central Idaho live in areas defined by the highest level of remoteness by the FAR methodology.
Intervention Goals and Components
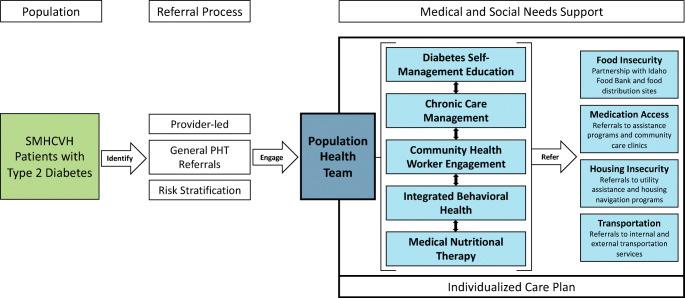
SMHCVH prioritized identifying and responding to the medical and social needs of underserved populations with type 2 diabetes and decreasing health disparities. It created a multi-disciplinary PHT that would develop and implement an individualized care plan that would address medical and social needs (Fig. 1). SMHCVH sought to improve access to diabetes self-management education (DSME), chronic care management (CCM), community health workers (CHWs), integrated behavioral health (IBH) services, and medical nutritional therapy (MNT). The referral process to the PHT included provider referrals, general referrals, and referrals based on risk stratification.
Fig. 1.
SMHCVH Comprehensive Diabetes Care Model. Abbreviations: SMHCVH—St. Mary’s Health and Clearwater Valley Health, PHT—population health team.
PHT Referral Process
During early implementation (2017–2019), patients were referred for services via provider referrals to specific PHT members. In 2020, SMHCVH implemented a new referral process where providers submit a general PHT referral, rather than an individual order for each PHT service. For each referral, the PHT collaborates with the provider to consider appropriate support services and develops an individualized care plan. In addition, PHT staff met regularly to discuss patient panels and new referrals, and to coordinate care plans among PHT members. Once the care plan was completed, the PHT and the primary care provider finalized exact orders for insurance coverage and authorization. In 2021, SMHCVH began piloting a proactive and data-driven patient engagement approach to expand PHT reach outside of provider referrals and community health worker engagement at screening events. SMHCVH identified patients with elevated risk for referral (i.e., HbA1c > 9%, blood pressure is ≥ 10 mm Hg above target, body mass index (BMI) > 30 kg/m2, current tobacco use).
Population Health Team
SMHCVH implemented the PHT in 2017, building upon an established patient-centered medical home team that included CDEs and nurse care managers. Between 2017 and 2021, the PHT expanded upon existing roles and includes 3 registered nurses involved in CCM and other clinical services, 2 clinical dietitians, 6 CHWs, 2 CDEs, 3 behavioral health therapists, and a population health director.
CCM at SMHCVH relies on the essential elements of the Chronic Care Model (e.g., community resources, health system, self-management support, delivery system design, decision support, clinical information systems) to provide care coordination and medical case management to patients, especially for those with multiple chronic conditions. In addition to direct support from CDEs and CCM nurses, SMHCVH CHWs offer community-based chronic disease self-management and chronic pain self-management sessions based on the Stanford self-management program curricula.11,12
The PHT sustained existing CDEs and offered individual DSME and group chronic disease education programs. CHWs conduct outreach through one-on-one visits or community-wide events and are conduits to other PHT services.13,14 The IBH model at SMHCVH integrates behavioral health providers directly into primary care clinics, and PHT team members provide warm hand-offs to ensure patient preferences and concerns are considered. Registered dietitians improve the integration of Medical Nutrition Therapy into existing diabetes education. The dietitians work directly with patients, providers, and community partners to support both patient-level and population-level nutrition goals.
Unmet Medical and Social Needs
SMHCVH implemented annual screenings to better understand and address medical, behavioral, and social needs of patients. SMHCVH began piloting a Health Risk Assessment (HRA) developed in partnership with an external consulting group in 2017 and made iterative, yet minor, changes to the assessment until 2020. After technical assistance from the National Program Office at the University of Chicago, SMHCVH critically evaluated the internal HRA and amended it to include questions and social needs domains from the Protocol for Responding to & Assessing Patients’ Assets, Risks & Experiences (PRAPARE).15 SMHCVH staff screened patients with this dynamic tool and utilized risk scoring to facilitate PHT referrals for patients who responded positively to medical or social need questions.
SMHCVH PHT staff developed high-functioning partnerships with community organizations to address challenges that patients may face (e.g., food access, transportation, medication access). For example, the SMHCVH population health director partnered with the Idaho Food Bank to co-locate CHWs at Idaho Food Bank distribution sites to reduce healthcare access barriers for community members.13 CHWs also built relationships with local Area Agencies on Aging to address specific needs for older patients (e.g., grab bars, wheelchair-accessible ramps, transportation). Each PHT member addresses medical and social needs, but CHWs, in particular, support critical screening and referral services.
Study Population
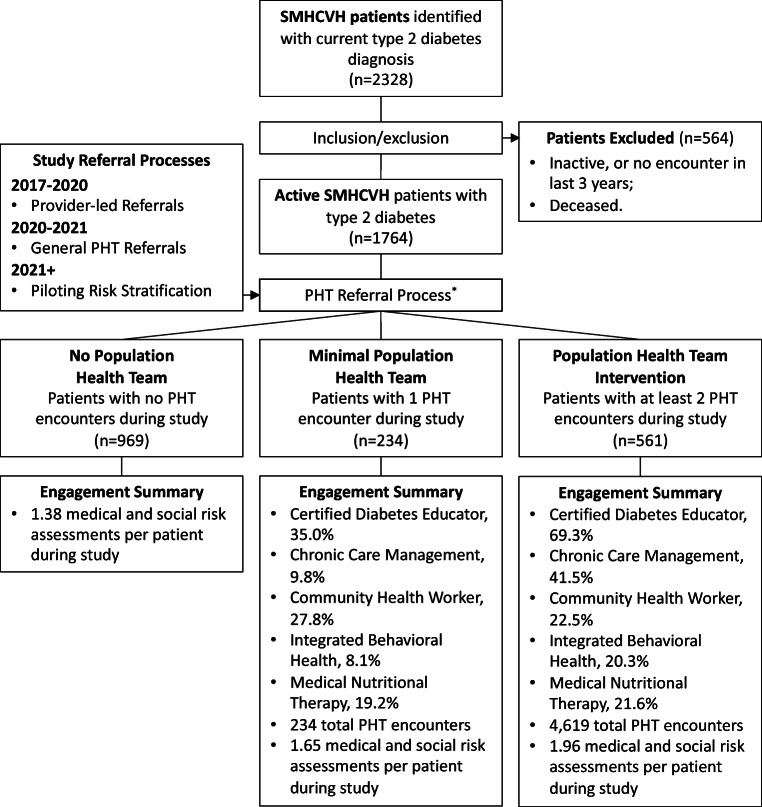
This study included patients with confirmed diagnosis of type 2 diabetes from September 1, 2017, to December 31, 2021. Inclusion criteria for analysis included active diabetes diagnosis (e.g., ICD-10 code verified in EMR) and active patient status. The EMR classifies inactive status when patients do not have a SMHCVH hospital or clinic encounter within the last 3 years. Patients with inactive and deceased statuses were excluded from analysis due to propensity for missing utilization, demographic, and clinical data (Fig. 2).
Fig. 2.
SMHCVH population flowchart for patients with diabetes. Abbreviations: SMCHVH—St. Mary’s Health and Clearwater Valley Health, PHT—Population health team. Definitions: no population health team: 0 visits with members of the population health team, minimal population health team: 1 visit with a member of the population health team, population health team intervention: 2 or more visits with a member of the population health team. *PHT referral process evolved during the initiative and included: (1) provider-led referrals—referrals to PHT were reliant on primary care physicians; (2) general PHT referral—providers submit general PHT referrals, instead of individual staff referrals. PHT staff develop care plans and coordinate referrals to individual PHT members; (3) piloting risk stratification—in addition to provider referrals, SMHCVH piloted an automated risk stratification process to identify and engage at-risk patients who may require additional support.
For descriptive purposes, groups were assigned to evaluate distributions of medical and social needs as well as trends in clinical outcomes. No PHT patients were defined as those with no encounters with the PHT (e.g., CCM, CHW, DSME, MNT, IBH) during the study. Minimal PHT was defined as those with only one confirmed encounter with a member of the PHT during the study. PHT intervention was defined as patients with at least two encounters with a member of the PHT during the study. These group definitions reflect referral processes and level of engagement.
SMHCVH’s diabetes care transformation efforts began in September 2017 and start dates were assigned to patients within the no PHT group based on their first recorded encounter with any SMHCVH service after September 1, 2017. Start dates for the minimal PHT and PHT intervention groups were assigned based on patients’ first encounter with a PHT member.
Data Measures
De-identified patient demographics, encounter data (e.g., CCM, DSME, MNT, IBH), clinical lab values (e.g., HbA1c, low-density lipoprotein (LDL), systolic blood pressure, diastolic blood pressure), problem lists, and HRA screening results were extracted from the EMR by SMHCVH and University of Chicago staff. CHWs entered their encounter details into a Research Electronic Data Capture (REDCap)16 database. CHW encounters were compiled with other intervention and non-intervention encounters.
Medical and social needs were evaluated from annual HRA data and categorized into at-risk and not at-risk responses based on SMHCVH’s internal risk scoring system that utilizes available clinical outcomes and responses from HRA assessments to enumerate a risk score (Appendix 1). Medical and social needs domains that overlapped across all survey versions (e.g., not taking medications as prescribed, taking 6 or more medications, recent hospital or emergency room visit, transportation, food insecurity, housing, difficulty paying medications) were evaluated at baseline across all groups.
Statistical Analyses
All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc.). Statistical analysis was mainly descriptive, due to the complexity of the multi-disciplinary interventions, and due to significant differences between groups in demographics, medical needs, social needs, and disease burden. However, we did compare baseline characteristics among groups and conducted self-comparison in clinical outcomes over time per group. Chi-square tests were conducted to compare binary and multinomial characteristics (e.g., sex, race, co-morbidity prevalence, at-risk medial or social needs) among groups. Kruskal Wallis test was used to compare age. We also conducted linear mixed models and generalized estimating equations within each group to evaluate any time trend effect and to compare each subsequent time point to baseline for continuous clinical outcomes and for dichotomized clinical performance metrics, i.e., HbA1C < 9%, blood pressure < 140/90 mm Hg, statin prescription.
Missing Data Analysis
Loss to follow-up or ineligibility led to missing data. We defined ineligibility as patients who had not yet reached the given time point. For example, a patient with a start date of December 1, 2020, would be eligible for the 6-month and 12-month time points on December 1, 2021, but would not be eligible for the 18-month, 24-month, 30-month, or 36-month time points yet. We examined whether the time trend effect on HbA1c was due to differential attrition. Within each group, we compared demographics and medical conditions of patients with available HbA1c values to those without values at 24 months. We included ineligible patients for a given time point in the group with HbA1c values to increase the power of rejecting the null hypothesis of no bias. We also assessed descriptive differences in population characteristics between groups using available HbA1c, blood pressure, and LDL data at each time point.
RESULTS
Population Description
We identified 2358 eligible patients with diabetes in the EMR, and patients were removed from the sample if their activity status indicated inactive or deceased (Fig. 1). A final sample of 1764 SMHCVH patients with diabetes was predominantly white (98.2%), older [median (IQR): 70 years (62.0, 77.2)], male (56.5%), and covered by Medicare (53.9%). There were significant differences between groups in sex, age, and insurance type (Table 1). PHT intervention and minimal PHT groups were younger (p = 0.012), more likely to be female (p < 0.001), and more often covered by Medicare and/or Medicaid when compared to the no PHT group (p = 0.004). PHT intervention had a higher prevalence of each medical factor (e.g., clinical conditions, medical complexity, utilization) except for diagnosis of heart failure and not taking your medications as prescribed. More people in the PHT intervention group reported transportation barriers (p = 0.021) and difficulty affording food (p = 0.059).
Table 1.
Demographic, Medical, and Social Characteristics of SMHCVH Patients with Diabetes
| Patients with diabetes (n = 1764) | No population health team (n = 969) | Minimal population health team (n = 234) | Population health team intervention (n = 561) | p value | |
|---|---|---|---|---|---|
| Age (mean, SD) | 68.7 (12.4) | 69.5 (12.5) | 67.5 (11.6) | 68.0 (12.6) | 0.012 |
| Race/ethnicity, n (%) | |||||
| White, non-Hispanic | 1718 (98.2) | 938 (96.8) | 230 (98.3) | 550 (99.0) | 0.341 |
| Non-White, non-Hispanic, and Hispanic | 31 (1.8) | 22 (3.2) | 4 (1.7) | 5 (1.0) | |
| Missing/unknown | 15 | 9 | 6 | ||
| Sex, n (%) | |||||
| Female | 767 (43.5) | 351 (36.2) | 127 (54.3) | 289 (51.5) | < 0.001 |
| Missing | 75 | 55 | 6 | 14 | |
| Insurance status, n (%) | |||||
| Medicare | 934 (53.9) | 525 (54.2) | 105 (49.0) | 327 (54.7) | 0.004 |
| Medicare + Medicaid | 172 (9.9) | 85 (8.8) | 25 (10.6) | 62 (11.6) | |
| Medicaid | 109 (6.3 | 45 (4.6) | 19 (9.1) | 45 (8.0) | |
| Commercial/third party | 473 (27.3) | 287 (29.6) | 56 (26.9) | 130 (23.2) | |
| Uninsured | 46 (2.7) | 27 (2.8) | 9 (4.3) | 10 (1.8) | |
| Missing | 30 | 0 | 26 | 4 | |
| Medical needs | |||||
| Chronic conditions, n (%) | |||||
| Anxiety and fear-related disease | 258 (14.6) | 103 (10.6) | 41 (17.5) | 114 (20.3) | < 0.001 |
| Heart failure | 155 (8.8) | 77 (7.9) | 22 (9.4) | 56 (10.0) | 0.374 |
| Chronic kidney disease | 269 (15.2) | 113 (11.7) | 44 (18.8) | 112 (20.0) | < 0.001 |
| Chronic obstructive pulmonary disorder | 210 (11.9) | 91 (9.4) | 27 (11.5) | 92 (16.4) | < 0.001 |
| Cardiovascular disease | 668 (37.9) | 326 (33.6) | 86 (36.8) | 256 (45.6) | < 0.001 |
| Depression | 322 (18.3) | 116 (12.0) | 42 (17.9) | 164 (29.2) | < 0.001 |
| Hypertension | 712 (40.4) | 371 (38.3) | 87 (37.2) | 254 (45.3) | 0.015 |
| Obesity | 461 (26.1) | 191 (19.7) | 54 (23.1) | 216 (38.5) | < 0.001 |
| Medical complexity, n (%) | |||||
| 3 or more chronic conditions | 580 (32.9) | 252 (26.0) | 79 (33.8) | 249 (44.4) | < 0.001 |
| Not taking medications as prescribed (n = 1121) | 65 (5.9) | 35 (6.2) | 6 (4.4) | 24 (5.9) | 0.701 |
| Taking 6 or more medications (n = 1189) | 626 (52.7) | 297 (49.3) | 71 (44.9) | 258 (60.3) | < 0.001 |
| Utilization, n (%) | |||||
| Self-reported, recent hospital admission (n = 1193) | 291 (24.4) | 138 (22.8) | 27 (16.9) | 126 (29.5) | 0.003 |
| Self-reported, recent emergency dept. use (n = 1193) | 319 (26.7) | 151 (25.0) | 35 (21.9) | 133 (31.1) | 0.030 |
| Social needs, n (%) | |||||
| Transportation barrier (n = 1194) | 66 (5.5) | 24 (4.0) | 8 (5.1) | 34 (7.9) | 0.021 |
| Difficulty affording food (n = 1117) | 62 (5.2) | 23 (4.1) | 8 (5.7) | 31 (7.6) | 0.059 |
| Current housing issue (n = 1203) | 15 (1.3) | 7 (1.2) | 2 (1.3) | 6 (1.4) | 0.941 |
| Difficulty paying meds (n = 1192) | 109 (9.1) | 51 (8.4) | 11 (7.0) | 47 (11.0) | 0.220 |
Definitions: No population health team: 0 visits with members of the population health team, minimal population health team: 1 visit with a member of the population health team, population health team intervention: 2 or more visits with a member of the population health team. Note: p values are two-sided and from chi-squared tests for categorical variables or Kruskal Wallis test for continuous variables, between study groups. p values < 0.05 indicate significant differences in at least 2 groups
SMHCVH St. Mary’s Health and Clearwater Valley Health
Intervention Engagement
Between September 1, 2017, and December 31, 2021, SMHCVH staff provided 4853 PHT intervention encounters to 895 patients with diabetes (Fig. 2). Within the PHT intervention group, engagement with the CDE was the most common intervention provided to patients, followed by CCM, CHW, MNT, and IBH, respectively. Within the minimal PHT group, engagement with the CDE was also the most common intervention provided to patients, followed by CHW, MNT, CCM, and IBH, respectively. PHT intervention had a higher number of HRA screening per patient during the study (mean: 1.96) compared to minimal PHT (1.63) and no PHT groups (1.38) (Fig. 2).
Primary Clinical Outcomes
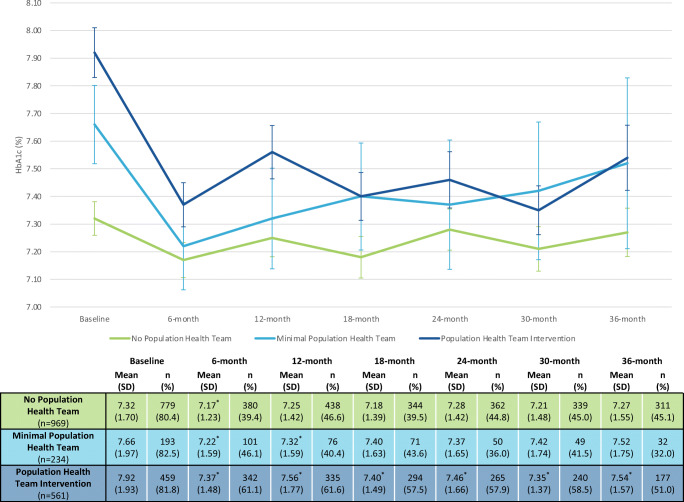
HbA1c, blood pressure, and LDL data for each group during the study are presented in Table 2. PHT intervention exhibited significantly reduced mean HbA1c at each subsequent time point when compared to baseline (e.g., baseline compared to 6 months, baseline compared to 12 months, etc.). The minimal PHT and no PHT groups also exhibited significantly reduced mean HbA1c at 6 months when compared to in-group baseline measures. These decreases were not sustained at any subsequent time point for the no PHT group. Minimal PHT sustained significant reductions at 12 months, but no significant decreases were observed after 12 months (Fig. 3). There were no significant changes in blood pressure (e.g., systolic, diastolic) in any of the groups (Table 2). PHT intervention had significant reductions in LDL, when compared to baseline measures, at 12 months, 24 months, and 36 months. Minimal PHT exhibited no significant reductions in LDL while no PHT had a significant reduction at 36 months (Table 2). Figure 3 describes the time trend of HbA1c and sample availability at each time point per group. Available clinical outcomes data across groups decreased initially from baseline to 6 months. However, data availability stabilized after 6 months for HbA1c and blood pressure and after 12 months for LDL (Appendix 2a–2c). There were few demographic, social, and clinical differences to note between patients with and without 24-month HbA1c except that PHT intervention patients with 24-month HbA1c were less white than those without HbA1c (Appendix 3). Additional missing data analyses found stable differences in medical and demographic characteristics between groups at each subsequent time point (Appendix 4a–4 g).
Table 2.
Clinical Outcomes for SMHCVH Patients with Diabetes by Intensity of Population Health Team Intervention
| No population health team (n = 969) | Minimal population health team (n = 234) | Population health team intervention (n = 561) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | SBP (mm Hg) | DBP (mm Hg) | LDL (mg/dl) | HbA1c (%) | SBP (mm Hg) | DBP (mm Hg) | LDL (mg/dl) | HbA1c (%) | SBP (mm Hg) | DBP (mm Hg) | LDL (mg/dl) | |
| Baseline | 7.32 (1.70) | 130.8 (15.9) | 77.7 (10.7) | 95.4 (38.3) | 7.66 (1.97) | 130.3 (15.8) | 76.8 (10.3) | 98.6 (36.6) | 7.92 (1.93) | 130.0 (14.7) |
76.1 (9.4) |
97.9 (39.5) |
| 6 months | 7.17* (1.23) | 130.6 (13.6) | 77.1 (9.0) | – | 7.22* (1.59) | 129.5 (14.4) | 77.0 (9.8) | – | 7.37* (1.48) | 128.6 (11.8) |
76.6 (7.5) |
– |
| 12 months | 7.25 (1.42) | 129.9 (13.6) | 76.8 (9.2) | 91.4 (33.8) | 7.32* (1.59) | 130.4 (11.9) | 77.8 (9.3) | 94.8 (39.7) | 7.56* (1.77) | 129.8 (13.7) |
77.2 (8.1) |
89.8* (35.4) |
| 18 months | 7.18 (1.39) | 130.3 (14.2) | 77.0 (9.6) | – | 7.40 (1.63) | 131.2 (14.0) | 77.5 (9.6) | – | 7.40* (1.49) | 129.1 (13.9) |
77.6 (22.6) |
– |
| 24 months | 7.28 (1.42) | 129.1 (15.6) | 76.7 (9.4) | 92.4 (37.6) | 7.37 (1.65) | 129.6 (11.7) | 75.7 (7.4) | 91.6 (36.7) | 7.46* (1.66) | 129.4 (12.6) |
76.5 (8.3) |
91.3* (35.6) |
| 30 months | 7.21 (1.48) | 131.4 (14.9) | 76.9 (9.4) | – | 7.42 (1.74) | 130.6 (14.5) | 77.4 (9.1) | – | 7.35* (1.37) | 129.6 (12.0) |
76.3 (8.6) |
– |
| 36 months | 7.27 (1.55) | 130.6 (13.9) | 75.8 (8.5) | 89.4* (33.8) | 7.52 (1.75) | 130.4 (13.4) | 77.3 (7.4) | 90.9 (33.8) | 7.54* (1.57) | 130.3 (13.4) |
77.3 (8.0) |
84.4* (31.7) |
Definitions: no population health team: 0 visits with members of the population health team, minimal population health team: 1 visit with a member of the population health team, population health team intervention: 2 or more visits with a member of the population health team. Note: Available clinical data at each time point and for each group is available in Appendix 2a–2c
SBP systolic blood pressure, DBP diastolic blood pressure, LDL low-density lipoprotein
*Denotes statistically significant change from self-compared baseline (p < 0.05)
Fig. 3.
HbA1c outcomes among SMHCHV patients with diabetes. Abbreviation: SMHCVH—St. Mary’s Health and Clearwater Valley Health. Definitions: no population health team: 0 visits with members of the population health team, minimal population health team: 1 visit with a member of the population health team, population health team intervention: 2 or more visits with a member of the population health team. *Denotes statistically significant change from self-compared baseline (p < 0.05).
Binary Clinical Performance Metrics
Table 3 presents the proportions of patients with diabetes who are meeting HbA1c, blood pressure, or statin prescription performance metrics. The PHT intervention group achieved statistically significant increases in HbA1c < 8% at each subsequent time point, when compared to baseline. The proportion of PHT intervention patients achieving an HbA1c < 8% increased from 59.9% at baseline to 72.5% at 24 months (p < 0.05). The no PHT group achieved significant increases in patients achieving blood pressure control (< 140/90 mm Hg) at each subsequent time point. All groups achieved significant increases in receipt of statin prescription at each subsequent time point.
Table 3.
Binary Clinical Performance Metrics for SMHCVH Patients with Diabetes by Intensity of Population Health Team Intervention
| No population health team (n = 969) | Minimal population health team (n = 234) | Population health team intervention (n = 561) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c < 8% | BP < 140/90 (mm Hg) | Statin Rx | HbA1c < 8% | BP < 140/90 (mm Hg) | Statin Rx | HbA1c < 8% | BP < 140/90 (mm Hg) | Statin Rx | |
| Baseline | 75.5% | 72.6% | 12.3% | 70.0% | 72.3% | 29.9% | 59.9% | 77.2% | 16.6% |
| 12 months | 74.7% | 81.2%* | 23.3%* | 71.1% | 80.8% | 27.6%* | 69.9%* | 80.5% | 25.6%* |
| 24 months | 75.1% | 77.8%* | 34.3%* | 78.0% | 83.5% | 33.3%* | 72.5%* | 81.2% | 34.3%* |
| 36 months | 74.3% | 78.5%* | 47.2%* | 75.0% | 82.0% | 43.8%* | 67.8%* | 78.3% | 44.1%* |
Definitions: No population health team: 0 visits with members of the population health team, minimal population health team: 1 visit with a member of the population health team, population health team intervention: 2 or more visits with a member of the population health team
Rx prescription, BP blood pressure
*Denotes statistically significant change from self-compared baseline (p < 0.05)
DISCUSSION
SMHCVH’s PHT model showed promising results for a quality improvement effort seeking to integrate medical and social care and improve clinical outcomes for people with diabetes in a frontier setting. SMHCVH PHT implementation illustrates how health systems may build a model to improve population health and assess and address social needs as part of standard medical care provided to all patients.17–19
First, medical complexity and prevalence of social needs in the three study groups reflect a PHT referral process that aimed to engage patients with medical and social needs barriers. SMHCVH successfully identified and connected the most medically complex patients, as well as those who reported more transportation barriers and difficulties affording food, to PHT services that extend beyond usual primary care (Table 1). Second, this frontier health system quality improvement effort provided an opportunity to assess clinical outcomes over an extended time frame of 36 months for changes in HbA1c, LDL, and blood pressure. Clinical outcomes improved, with significant reductions in HbA1c among PHT intervention patients baseline to 12 months and sustained reductions at 18 months, 24 months, 30 months, and 36 months. Significant reductions in HbA1c were also observed among minimal PHT intervention patients from baseline to 12 months. Third, proportions of patients with diabetes who are meeting HbA1c, blood pressure, or statin prescription performance metrics improved across all populations of interest, especially among the PHT intervention group, the patients with the greatest medical and social needs. The improvements validate implementation of the PHT model utilized to meet patients’ social needs (e.g., food access, transportation) and medical needs (e.g., behavioral health, nutrition education, primary care access). Future studies should continue to expand outcomes evaluations outside of clinical values and consider metrics that better align with patient preferences, including but not limited to thoughtful use of social needs screening data.
While this study documents associations between PHT intervention and improvements in diabetes outcomes, scaling integrated medical and social care and sustaining the structure of frontier population health is fraught with challenges for SMHCVH and other like-minded critical access hospitals.20 Many rural and frontier communities lack payment and reimbursement to facilitate the integration of medical and social care.21,22 Value-based payment methodologies (e.g., Delivery System Reform Incentive Payments, Advanced Payment Models) that incentivize and support integrated medical and social care are not readily available to SMHCVH, or other rural health systems. Although SMHCVH currently participates in value-based initiatives with payers, these programs have yielded little financial benefits (e.g., shared savings) due to suboptimal performance by other healthcare organizations and providers outside of SMHCVH that are still included in SMHCVH’s accountability metrics. Rural and frontier communities in the USA lack reimbursement and upfront payment for CHW engagement and care coordination, even though these programs and services could address uniquely rural medical and social needs (e.g., access to primary care, transportation, poverty).21,23,24 SMHCVH, and other rural healthcare institutions, must walk a critical line between offering comprehensive services to address both medical and social needs and remaining financially solvent in the current fee-for-service healthcare delivery and financing environment.
Study Limitations
Our study has several limitations. First, this study was an observational, quality improvement evaluation. Group definitions were retrospectively assigned and differences in clinical and demographic factors were expected partly due to risk-based referrals to PHT services. This limited between-group comparisons and generalizability of the findings. Second, more clinical lab data were missing at progressive 6-month follow-up intervals. In addition, study attrition across each interval could impact the overall findings. However, we found similar demographics, medical, and social characteristics between those with and without available HbA1c values across the entire study population for each time point, suggesting loss to follow-up did not impact the major findings of our analyses. Third, PHT referral data were not accessible and, thus, we could not assess whether the type of PHT referral process impacted outcomes. Fourth, we were unable to adjust for the effect that the COVID-19 pandemic had on outcomes, utilization, and HRA screening data. Lastly, our results may lack generalizability given the unique geographic environment and demographic makeup of Northern Idaho.
Conclusion
SMHCVH’s frontier PHT model demonstrates durable improvements in HbA1c outcomes for people with diabetes, especially among those who face significant medical and social challenges. These improvements validate the investment required to evolve care, but many rural healthcare organizations lack sufficient incentive and support for population health management that could mitigate medical and social needs for patients and expand the capacity of current healthcare teams. Although SMHCVH has creatively blended various funding sources to implement its PHT model, payment should instead be intentionally designed to support and incentivize care transformations that address medical and social needs and advance health equity.17,25,26
Supplementary Information
(DOCX 60 kb)
Acknowledgements
The authors would like to thank the members of St. Mary’s Health and Clearwater Valley Health’s Population Health Team for their pivotal role in providing diabetes care and support. The authors were supported in part by the Merck Foundation, Bridging the Gap: Reducing Disparities in Diabetes Care Program. Dr. Chin was supported in part by the Chicago Center for Diabetes Translation Research (NIDDK P30 DK092949). This paper has not previously been presented.
Declarations
Conflict of Interest
Dr. Chin co-chairs the Centers for Medicare and Medicaid Services Health Care Payment Learning and Action Network Health Equity Advisory Team. He is a consultant to the Patient-Centered Outcomes Research Institute and a lead subject matter expert for the Agency for Healthcare Research and Quality. He is a member of the Bristol-Myers Squibb Company Health Equity Advisory Board and Blue Cross Blue Shield Health Equity Advisory Panel.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jacob P. Tanumihardjo, Email: jtanumihardjo@uchicago.edu.
Cody Wilkinson, Email: cody.wilkinson@kh.org.
References
- 1.Bush J, Barlow DE, Echols J, Wilkerson J, Bellevin K. Impact of a mobile health application on user engagement and pregnancy outcomes among Wyoming Medicaid members. Telemed J E Health. 2017;23(11):891–898. doi: 10.1089/tmj.2016.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagnild G, MacCart JG, Mitchell S, Tyabah K, Leenknecht C, Meszaros JF. A telecommunications intervention for frontier patients with diabetes. Telemed J E Health. 2008;14(8):793–800. doi: 10.1089/tmj.2007.0133. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. CDC’s National Center for Chronic Disease Prevention and Health Promotion: Diabetes and Prediabetes Factsheet. https://www.cdc.gov/chronicdisease/pdf/factsheets/diabetes-H.pdf. Published 2018. Accessed April 11 2022.
- 4.Ferdinand AO AM, Callaghan TH, Towne SD Jr, Bolin JN. Diabetes-related hospital mortality in rural America: a significant cause for concern. In. Vol Policy Brief #3: Southwest Rural Health Research Center; 2017.
- 5.Callaghan T, Ferdinand AO, Akinlotan MA, Towne SD, Bolin J. The changing landscape of diabetes mortality in the United States across region and rurality, 1999-2016. J Rural Health. 2020;36(3):410–415. doi: 10.1111/jrh.12354. [DOI] [PubMed] [Google Scholar]
- 6.Feltner FJ, Thompson SP, Slone M. The need for certified diabetes educators and community health workers in community-based diabetes self-management education for high risk groups. In. Vol 5: Center of Excellence in Rural Health Workforce Reports; 2018.
- 7.Rutledge SA, Masalovich S, Blacher RJ, Saunders MM. Diabetes Self-Management Education Programs in Nonmetropolitan Counties - United States, 2016. MMWR Surveill Summ. 2017;66(10):1–6. doi: 10.15585/mmwr.ss6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belanger K, Stone W. The social service divide: service availability and accessibility in rural versus urban counties and impact on child welfare outcomes. Child Welfare. 2008;87(4):101–124. [PubMed] [Google Scholar]
- 9.US Census. QuickFacts: Lewis County, Idaho County, Clearwater County, Idaho. https://www.census.gov/quickfacts/fact/table/lewiscountyidaho,idahocountyidaho,clearwatercountyidaho/PST045221. Published 2021. Accessed April 11 2022.
- 10.USDA Economic Research Service. Frontier and remote area codes. https://www.ers.usda.gov/data-products/frontier-and-remote-area-codes/. Published 2019. Accessed July 26 2022.
- 11.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 12.LeFort SM, Gray-Donald K, Rowat KM, Jeans ME. Randomized controlled trial of a community-based psychoeducation program for the self-management of chronic pain. Pain. 1998;74(2-3):297–306. doi: 10.1016/S0304-3959(97)00190-5. [DOI] [PubMed] [Google Scholar]
- 13.Sommers I, Gunter KE, Chin MH, Peek ME, Wilkinson C. Trust dynamics of community health workers in frontier Idaho food banks and pantries. J Gen Intern Med. 2023. 10.1007/s11606‐022‐07921‐7. [DOI] [PMC free article] [PubMed]
- 14.McGrath K. Rural Healthcare disparities in the United States: can we get upstream?. J Gen Intern Med. 2023. 10.1007/s11606‐022‐07922‐6. [DOI] [PMC free article] [PubMed]
- 15.National Association of Community Health. The PRAPARE screening tool. https://prapare.org/the-prapare-screening-tool/. Published 2017. Accessed April 11 2022.
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed]
- 17.Gunter KE, Peek ME, Tanumihardjo JP, et al. Population health innovations and payment to address social needs among patients and communities with diabetes. Milbank Q. 2021;99(4):928–973. doi: 10.1111/1468-0009.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Academies of Sciences, Engineering, and Medicine. Integrating social care into the delivery of health care: moving upstream to improve the nation’s health. Washington, DC: The National Academies Press. 2019. 10.17226/25467. [PubMed]
- 19.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020. [DOI] [PMC free article] [PubMed]
- 20.Wright DB. Care in the country: a historical case study of long-term sustainability in 4 rural health centers. Am J Public Health. 2009;99(9):1612–1618. doi: 10.2105/AJPH.2008.146050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd J. Opportunities to advance complex care in rural and frontier areas. 2019; https://www.chcs.org/media/TCC-RURAL-BRIEF_050719.pdf. Accessed April 4 2022.
- 22.Maxwell J, Tobey R, Bourgoin A, et al. Rethinking value-based payment and care in the rural ambulatory safety net: recommendations for policymakers and funders. 2019; https://deltacenter.jsi.com/sites/default/files/uploads/documents/JSI_Rethinking-Value-Based-Payment-and-Care-in-the-Rural-Ambulatory-Safety-Net_072519.pdf. Accessed April 4 2022.
- 23.Association of State and Territorial Health Officials. State approaches for addressing rural social determinants of health. https://www.astho.org/globalassets/pdf/state-approaches-for-addressing-rural-social-determinants-of-health.pdf. Published 2018. Accessed April 4 2022.
- 24.Logan RI, Castañeda H. Addressing health disparities in the rural United States: advocacy as caregiving among community health workers and promotores de salud. Int J Environ Res Public Health. 2020;17(24). [DOI] [PMC free article] [PubMed]
- 25.Wang G, Gauthier R, Gunter KE, et al. Improving diabetes care through population health innovations and payments: lessons from Western Maryland. J Gen Intern Med. 2023; 10.1007/s11606-022-07918-2 [DOI] [PMC free article] [PubMed]
- 26.Tanumihardjo JP, Morganstern E, Altschuler S, et al. Community health collaborative facilitates health system and community change to address unmet medical and social needs in New Jersey. J Gen Intern Med. 2023; 10.1007/s11606-022-07927-1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 60 kb)