Abstract
Background
Global budgets might incentivize healthcare systems to develop population health programs to prevent costly hospitalizations. In response to Maryland’s all-payer global budget financing system, University of Pittsburgh Medical Center (UPMC) Western Maryland developed an outpatient care management center called the Center for Clinical Resources (CCR) to support high-risk patients with chronic disease.
Objective
Evaluate the impact of the CCR on patient-reported, clinical, and resource utilization outcomes for high-risk rural patients with diabetes.
Design
Observational cohort study.
Participants
One hundred forty-one adult patients with uncontrolled diabetes (HbA1c > 7%) and one or more social needs who were enrolled between 2018 and 2021.
Interventions
Team-based interventions that provided interdisciplinary care coordination (e.g., diabetes care coordinators), social needs support (e.g., food delivery, benefits assistance), and patient education (e.g., nutritional counseling, peer support).
Main Measures
Patient-reported (e.g., quality of life, self-efficacy), clinical (e.g., HbA1c), and utilization outcomes (e.g., emergency department visits, hospitalizations).
Key Results
Patient-reported outcomes improved significantly at 12 months, including confidence in self-management, quality of life, and patient experience (56% response rate). No significant demographic differences were detected between patients with or without the 12-month survey response. Baseline mean HbA1c was 10.0% and decreased on average by 1.2 percentage points at 6 months, 1.4 points at 12 months, 1.5 points at 18 months, and 0.9 points at 24 and 30 months (P<0.001 at all timepoints). No significant changes were observed in blood pressure, low-density lipoprotein cholesterol, or weight. The annual all-cause hospitalization rate decreased by 11 percentage points (34 to 23%, P=0.01) and diabetes-related emergency department visits also decreased by 11 percentage points (14 to 3%, P=0.002) at 12 months.
Conclusions
CCR participation was associated with improved patient-reported outcomes, glycemic control, and hospital utilization for high-risk patients with diabetes. Payment arrangements like global budgets can support the development and sustainability of innovative diabetes care models.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07918-2.
KEY WORDS: diabetes, population health, global budgets, care coordination, social drivers of health
Introduction
As health care costs and chronic disease prevalence rise in the USA, policymakers are increasingly implementing value-based payment reforms that aim to reduce health care expenditures while improving health outcomes.1,2 Diabetes is an important target given its significant impact on morbidity and mortality. Despite advances in treatment and prevention, over 34 million people in the USA have diabetes and one in four health care dollars is attributable to patients with diabetes.3,4 Additionally, to reduce persistent disparities in health outcomes, effective diabetes care programs must involve multidisciplinary teams and address social drivers of health such as food insecurity.5–8 However, these programs remain difficult to fund and sustain without changes in health care policy and payment systems.
Individual states can accelerate value-based care through policy and payment reforms. Maryland provides a unique example of state-driven care transformation efforts. Maryland established the country’s only all-payer rate-setting system for hospital services in the late 1970s, introduced hospital global budgets in the early 2010s, and in 2019 launched its Total Cost of Care model with the Center for Medicare and Medicaid Innovation.9–11 Under Maryland’s global budget model, all payers in aggregate pay hospitals a fixed annual amount of revenue for inpatient and outpatient services, adjusted for various factors such as population size and quality performance and irrespective of utilization.12,13 This financial model flips the traditional fee-for-service incentive structure by allowing hospitals to focus on outcomes rather than volume and rewards them for investing in population health strategies that can reduce costly hospitalizations. Previous studies analyze the overall experience of global budgets in Maryland and have shown significant cost savings, improved quality, and reduced readmissions.14,15 However, little research has focused on the impact of global budgets on patient-level outcomes for chronic conditions like diabetes, nor studied how these state-level changes have enabled Maryland hospitals to address social drivers of health and improve population health.
We aimed to evaluate the impact of state-level policy changes at University of Pittsburgh Medical Center (UPMC) Western Maryland (previously Western Maryland Health System) on health system innovations to improve outcomes for high-risk patients with type 2 diabetes. UPMC Western Maryland is an integrated healthcare network in Cumberland, Maryland, and grantee in Bridging the Gap: Reducing Disparities in Diabetes Care, a 5-year initiative supported by the Merck Foundation that aims to improve diabetes inequities by transforming primary care and addressing medical and social needs.16,17 In 2013, supported by the shift to global budgets, the hospital opened the Center for Clinical Resources (CCR), an outpatient facility focused on co-managing high-risk patients with chronic diseases such as diabetes through intensive, team-based interventions that provide enhanced medical support, social needs assistance, and patient education. We conducted an observational cohort study to assess how CCR interventions impacted patient-reported, clinical, and utilization outcomes for high-risk patients with type 2 diabetes. These findings are relevant to other states and stakeholders interested in creating delivery and payment environments that enable diabetes care transformation.
Methods
Study Setting and Design
We performed an observational cohort study of 141 patients with diabetes at UPMC Western Maryland. The hospital cares for a rural, aging population of approximately 123,000 in Allegany County and surrounding counties in West Virginia and Pennsylvania.18 Allegany County is ranked among the least healthy counties in Maryland, with rates of smoking, obesity, and food insecurity that exceed both state and national averages.19 Additionally, the hospital service area has a diabetes prevalence of 15%, making it an important region for intervention.
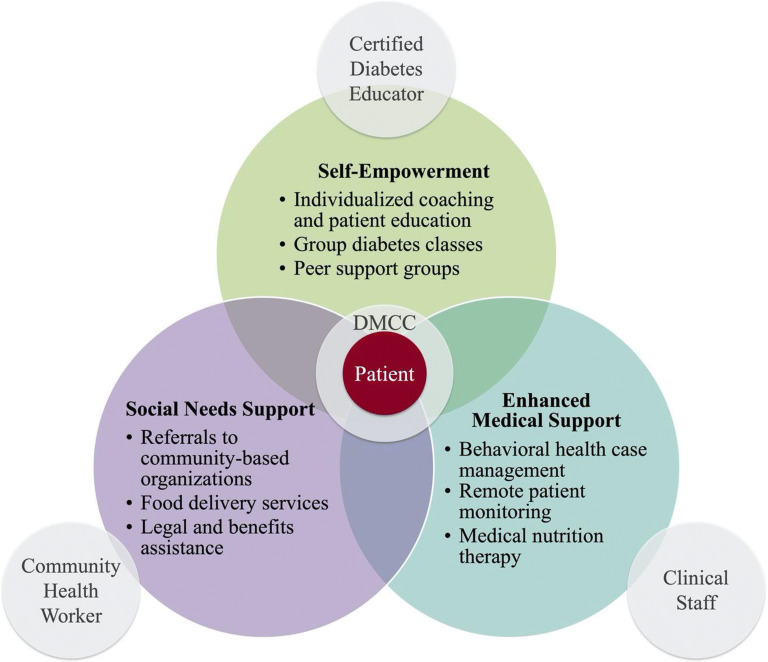
Unique to UPMC Western Maryland, the CCR is an outpatient chronic disease management facility that offers a wide array of supports such as interdisciplinary care coordination, nutritional counseling, food delivery services, behavioral health case management, and other services aimed at addressing unmet social and medical needs for patients with diabetes (Fig. 1). We collected and analyzed patient-reported, clinical, and utilization outcomes for patients engaged at the UPMC Western Maryland CCR. This study was approved by the University of Chicago Institutional Review Board.
Figure 1.
UPMC Western Maryland Center for Clinical Resources Care Model. DMCC, Diabetes Management Care Coordinator.
Eligibility Criteria
Patients were enrolled in the CCR by Diabetes Management Care Coordinators (DMCCs), registered nurses with expertise in working with patients with diabetes. DMCCs received patient referrals from primary care, endocrinologists, and inpatient providers and then recommended patients for inclusion if they had uncontrolled diabetes (HbA1c > 7.0%), more than one unmet social need (e.g., issues accessing food, housing, transportation), and a score of “moderate” or “high” on a diabetes risk stratification screening. The screening tool was created internally using validated sources including the Arizona Self-Sufficiency Matrix and Accountable Health Communities Health-Related Social Needs Screening Tool and considered factors such as medication regimen, comorbidities, and self-management knowledge (see Appendices A and B for additional information on patient eligibility and screening criteria).20,21 Patients were included in the present study if they enrolled in CCR services and were seen more than once by the DMCC. Enrollment occurred on a rolling basis between February 2018 and June 2021.
Intervention
Each patient was assigned to a dedicated DMCC who served as the patient’s main point of contact and advocate throughout the healthcare system. Initially, the DMCC met with each patient in the CCR or the primary care provider’s (PCP) office for an intake process, identification of unmet social needs via the WellRx questionnaire, and development of an individualized diabetes management plan.22 After intake, DMCCs initiated referrals for any urgent social needs and collaborated with PCPs and allied team members (e.g., Community Health Workers (CHWs), Diabetes Care and Education Specialists, registered dietitians) to coordinate care goals. For example, if a DMCC identified food insecurity as a barrier, then they would refer to a CHW who would assess program eligibility (e.g., SNAP), frequency of needs, and options for local resources. DMCCs formally followed patients at 3-month intervals for a minimum of 6 months through telephone and in-person encounters. They then continued existing management or de-escalated to lower intensity support or self-management as indicated by reassessment of the risk stratification screening tool. CHWs and other allied providers typically worked with patients at weekly or monthly frequencies as indicated by patients’ specific needs and until goals were met. The length of patient enrollment in CCR services ranged from 4 to 41 months, with a mean of 28 months. Further characterization of enrollment data is available in Appendix 3.
Data Collection
Patient clinical and utilization data were collected via electronic health record (EHR) extraction every 6 months through June 2021. Patient-reported data were received on a rolling basis as patients were interviewed at baseline (i.e., 0 months) and 12 months. Each patient was assigned a unique identifier and all patient data were de-identified and stored in a secure, web-based file storage platform compliant with University of Chicago IRB HIPAA security standards.
Patient-reported outcomes (PROs) were collected via a 22-item survey (Appendix 4) with questions pertaining to diabetes self-care, quality of life, and patient experience from several validated measures (Summary of Diabetes Self-Care Activities, Behavioral Risk Factor Surveillance System, CDC Health-Related Quality of Life).23–29 Patients were administered the same survey at baseline and 12 months.
Clinical outcomes were collected from outpatient visit and laboratory data extracted from the hospital’s EHR, including Hemoglobin A1c (HbA1c), systolic and diastolic blood pressure (BP), lipids (including LDL, HDL, triglycerides), weight, and body mass index (BMI). These outcomes were collected at multiple timepoints between baseline and 36 months to enable longitudinal evaluation.
Utilization outcomes were collected from internal claims data including emergency department (ED) visits, hospitalizations, and charge data associated with each stay. These data were also collected for 12 months pre- and post-enrollment to enable comparison of patient outcomes before and after intervention.
Data Analysis
Survey and EHR data were imported into RStudio (version 3.6.1; RStudio, PBC) for statistical analysis.30 Patients’ demographic, PRO, clinical, and utilization data were summarized by descriptive statistics. To conduct self-comparisons in patient outcomes before and after intervention, we used McNemar’s tests for binary outcomes, paired t-tests for continuous outcomes, and Wilcoxon signed-rank tests for ordinal outcomes. Given varying sample sizes at each follow-up timepoint (e.g., decreasing sample sizes over time either due to loss to follow-up or patients not yet reaching that timepoint), we also used a linear mixed model to model clinical outcomes over time and to test time trend effects, adjusting for age, sex, and insurance type. In addition, we conducted analyses to detect any patterns in missing data, using chi-square tests, Fishers’ exact tests, and two-sample t-tests to compare groups with and without certain follow-up data (e.g., 12-month PRO data, 12-month HbA1c). We used the Clinical Classifications Software Refined software from the Agency for Healthcare Research and Quality to categorize comorbidities from problem lists and primary diagnoses from ED visits and hospitalizations.31 Significance was defined as a two-sided P-value less than 0.05.
Results
Demographics
In total, 141 patients were included in the study sample. Patient demographics are displayed in Table 1. Most patients were over the age of 50 (76%), were female (57%), spoke English as their preferred language (100%), and were publicly insured (Medicare, Medicaid, or both; 83%). Social needs at baseline included difficulty accessing food (71%), transportation (42%), and medications (33%). Patients had an average of 4.9 chronic conditions, with the most common comorbidities being hyperlipidemia (77%), hypertension (75%), and obesity (64%).
Table 1.
Demographics of study participants at baseline
| Category | N (%) |
|---|---|
| Age | |
| 18–39 | 9 (6.4) |
| 40–49 | 25 (17.7) |
| 50–59 | 50 (35.5) |
| 60–64 | 17 (12.1) |
| 65+ | 40 (28.4) |
| Sex | |
| Female | 81 (57.4) |
| Race | |
| Black or African Descent | 5 (3.6) |
| White or Caucasian | 134 (95.7) |
| Hispanic or Latino | 1 (0.7) |
| Unknown | 1 (0.7) |
| Preferred language | |
| English | 141 (100) |
| Insurance type | |
| Medicaid | 48 (34.1) |
| Medicare | 44 (31.2) |
| Dual eligible | 25 (17.7) |
| Commercial | 20 (14.2) |
| Uninsured | 4 (2.8) |
| Social needs | |
| Food | 100 (70.9) |
| Transportation | 59 (41.8) |
| Medications | 47 (33.3) |
| Utilities | 42 (29.8) |
| Housing | 28 (19.9) |
| Number of chronic conditions | |
| 1–3 | 34 (24.1) |
| 4–5 | 59 (41.8) |
| 6+ | 46 (32.6) |
| Comorbidities | |
| Hyperlipidemia | 109 (77.3) |
| Essential hypertension | 106 (75.2) |
| Obesity | 90 (63.8) |
| Depressive disorders | 38 (27.0) |
| Coronary artery disease | 34 (24.1) |
| Chronic kidney disease | 33 (23.4) |
| Chronic obstructive pulmonary disease | 24 (17.0) |
Patient-Reported Outcomes
Patient-reported outcomes improved across all 22 items in the survey delivered at baseline and 12 months (Table 2). The response rate for 12-month surveys was 56% (79/141). All survey items were optional, leading to some variability in response rates across questions. No statistically significant differences were detected between the groups with and without the 12-month response when accounting for sex, race, age, insurance type, and number of comorbidities. The greatest improvements occurred in healthful eating, self-efficacy, health ratings, and experiences with PCPs. Days per week eating five or more servings of fruits and vegetables increased from 2.46 to 4.14 days per week (P<0.001). Confidence in managing blood sugar levels increased from 4.38 to 7.00 on a 10-point scale (P<0.001). Additionally, patients reporting that their overall health was better than 6 months prior increased from 3 to 47% (P<0.001). Furthermore, patients reporting that their PCP always knew important information about their life and always asked for their own ideas about managing their health increased from 15 to 46% (P<0.001) and 14 to 46% respectively (P<0.001).
Table 2.
Patient-reported outcomes
| Question* | Scale notes | N | Baseline mean (SD) | 12-month mean (SD) | P-value† |
|---|---|---|---|---|---|
| 1 Healthful Eating Plan | Days per week | 79 | 2.46 (2.06) | 4.14 (1.87) | <0.001 |
| 2 Fruits and Vegetables | Days per week | 78 | 1.86 (2.16) | 3.73 (2.09) | <0.001 |
| 3 High Fat Foods | Days per week | 78 | 3.33 (1.88) | 2.91 (1.62) | 0.08 |
| 4 Exercise | Days per week | 77 | 1.62 (2.29) | 2.48 (2.40) | 0.01 |
| 5 Blood Sugar Test | Days per week | 76 | 5.51 (2.56) | 6.10 (2.07) | 0.06 |
| 6 Glucose Test Adherence | Days per week | 74 | 4.47 (3.06) | 5.69 (2.26) | 0.004 |
| 7 Foot Care | Days per week | 76 | 3.03 (2.58) | 3.91 (2.40) | 0.01 |
| 8 Diabetes Treatment Adherence | Days per week | 79 | 5.80 (2.35) | 6.24 (1.96) | 0.21 |
| 9 Sugared Beverages | Times per day | 75 | 0.89 (1.09) | 0.41 (0.66) | <0.001 |
| Self-efficacy composite | 3.84 | 6.15 | |||
| 10 Food Choices | 10-point confidence‡ | 79 | 3.66 (2.19) | 6.35 (2.22) | <0.001 |
| 11 Exercise | 10-point confidence‡ | 78 | 3.08 (2.64) | 4.63 (3.40) | <0.001 |
| 12 Managing Blood Sugar | 10-point confidence‡ | 79 | 4.38 (2.47) | 7.00 (2.14) | <0.001 |
| 13 Diabetes Control | 10-point confidence‡ | 76 | 4.22 (2.22) | 6.63 (2.29) | <0.001 |
| Current health rating | |||||
| 14 Health=Excellent, Very Good, Good, Fair, Poor | 79 | 0.01 | |||
| 14 Excellent | 3% | 0% | |||
| 14 Very Good | 3% | 8% | |||
| 14 Good | 22% | 35% | |||
| 14 Fair | 56% | 51% | |||
| 14 Poor | 18% | 6% | |||
| Health Change Rating | |||||
| 15. Health=Better, Same, Worse vs 6 months ago | 79 | <0.001 | |||
| 15 Better | % vs 6 months ago | 3% | 47% | ||
| 15 Same | % vs 6 months ago | 47% | 39% | ||
| 15 Worse | % vs 6 months ago | 51% | 14% | ||
| 16 Physical Health Not Good | Days per month | 62 | 15.30 (9.94) | 10.10 (9.53) | <0.001 |
| 17 Mental Health Not Good | Days per month | 66 | 11.20 (9.69) | 8.03 (8.73) | 0.01 |
| 18 Physical or Mental Health Barrier Days | Days per month if Q16 or Q17 >0 | 62 | 13.69 (10.54) | 9.13 (9.41) | 0.002 |
| 19 Any Health Limitation | % Yes | 69 | 72% | 52% | 0.06 |
| 20 Pain Barriers | Days per month | 64 | 12.20 (11.20) | 8.65 (10.50) | 0.03 |
| 21 Primary Care Provider Always Knew Personal Information | % in past 6 months | 79 | 15% | 46% | <0.001 |
| 22 Primary Care Provider Always Asked for Your Ideas | % in past 6 months | 79 | 14% | 46% | <0.001 |
*See Appendix 3 for survey questions. †Paired t-test used for continuous variables, Wilcoxon signed-rank test for ordinal variables, and McNemar’s test for binary variables. ‡1 = not at all confident, 10 = totally confident
Clinical Outcomes
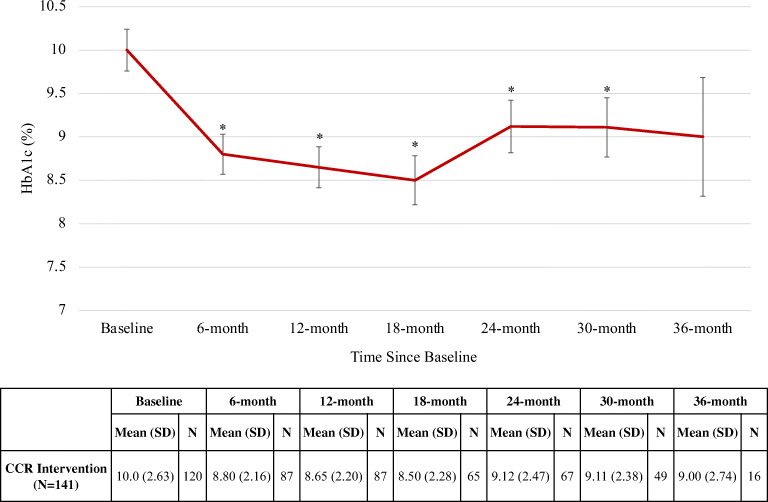
Baseline mean HbA1c was 10.0% and decreased on average by 1.2 percentage points at 6 months, 1.4 points at 12 months, 1.5 points at 18 months, 0.9 points at 24 and 30 months, and 1.0 points at 36 months (Fig. 2). In linear mixed modeling adjusting for age, sex, and insurance type, these reductions were statistically significant at all time points between baseline and 30 months (P<0.001). There was no significant difference in baseline HbA1c between those with and without a 12-month HbA1c value (P=0.12). No significant changes were observed in BP, LDL, weight, or BMI between baseline and 36 months (Table 3).
Figure 2.
Mean HbA1c over time. HbA1c, Hemoglobin A1c; CCR, Center for Clinical Resources. Error bars represent standard error. *Denotes statistically significant change from baseline (P<0.001).
Table 3.
Timepoint means for clinical outcomes other than HbA1c
| Clinical outcomes | Baseline | 6 months | 12 months | 18 months | 24 months | 30 months | 36 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood pressure (mmHg) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Systolic blood pressure | 126 | 130 (13) | 122 | 131 (17) | 103 | 129 (16) | 82 | 132 (14) | 67 | 130 (16) | 56 | 132 (14) | 17 | 137 (25) |
| Diastolic blood pressure | 126 | 76 (8) | 122 | 75 (8) | 103 | 74 (8) | 82 | 74 (8) | 67 | 75 (9) | 56 | 75 (9) | 17 | 71 (16) |
| Low-density lipoprotein cholesterol (mg/dL) | 100 | 95 (37) | 90 | 92 (37) | 68 | 86 (42) | 29 | 93 (43) | ||||||
| Weight (lbs) | 135 | 233 (68) | 130 | 233 (71) | 106 | 233 (68) | 85 | 233 (70) | 78 | 236 (70) | 54 | 235 (73) | 18 | 251 (88) |
| Body mass index (kg/m2) | 135 | 37.6 (10.5) | 130 | 37.7 (10.6) | 106 | 37.7 (10.4) | 85 | 37.1 (10.8) | 78 | 37.3 (10.2) | 54 | 38.1 (11.4) | 18 | 41.6 (16.4) |
Resource Utilization
The all-cause hospitalization rate decreased by 11 percentage points (34 to 23%, P=0.01) between the pre- and post-intervention periods (12 months before and after baseline) (Table 4). When limited to the 81 patients with baseline and 12-month HbA1c, hospitalizations decreased from 38 to 27% between time periods (P=0.11). Mean total days length of stay across all hospitalizations in each period and mean length of stay per hospitalization also decreased from 2.45 to 1.90 days (P=0.04) and 1.70 to 1.16 days (P=0.02) respectively. Additionally, the proportion of ED visits due to diabetes-related complications decreased by 11 percentage points (14 to 3%, P=0.002) between periods. No significant changes were observed for mean number of ED visits or hospitalizations, percentage of patients with an ED visit, or mean total charges for ED visits or hospitalizations among program participants.
Table 4.
Emergency department and hospital inpatient utilization outcomes
| Emergency department (ED) utilization (N=141) | |||
|---|---|---|---|
| −12–0 months | 0–12 months | P-value* | |
| Percentage of patients with ED usage | 40% | 41% | 1.00 |
| Mean number of ED visits (SD) | 1.01 (2.00) | 1.01 (2.20) | 1.00 |
| Mean $ total charges (SD) | 833 (1764) | 891 (2032) | 0.88 |
| Percentage of visits due to diabetes-related complications | 14% | 3% | 0.002 |
| Inpatient utilization (N=141) | |||
| −12–0 months | 0–12 months | P-value* | |
| Percentage of patients with hospitalizations | 34% | 23% | 0.01 |
| Mean number of hospitalizations (SD) | 0.52 (0.91) | 0.37 (0.78) | 0.08 |
| Mean $ total charges (SD) | 7262 (15683) | 6868 (18422) | 0.25 |
| Mean total days length of stay (SD) | 2.45 (4.89) | 1.90 (4.98) | 0.04 |
| Mean days length of stay per hospitalization | 1.70 (3.18) | 1.16 (2.99) | 0.02 |
*Wilcoxon signed-rank tests and McNemar’s tests used to determine P-values
Discussion
Global budget models might incentivize health care systems to develop innovative population health programs to improve patient outcomes and reduce avoidable utilization. In a case study for diabetes care innovation under Maryland’s global budget system, we found that participation in UPMC Western Maryland’s Center for Clinical Resources was associated with improved quality of life and self-efficacy, durable reductions in HbA1c, and reductions in diabetes-related ED visits and inpatient utilization.
Integrated models that address unmet medical, social, and psychological needs are effective for improving diabetes outcomes. Our findings reinforce principles of the Chronic Care Model, which identifies the importance of community resources, self-management support, and delivery system redesign in providing high-quality chronic disease care.32,33 In particular, the CCR leverages several strengths to drive patient engagement and improve outcomes. First, it is a centralized outpatient unit that uses a team-based model of care with nurses, dieticians, CHWs, and other allied health professionals to address patient self-management and social needs while also augmenting physician capacity. Second, DMCCs longitudinally build trust with patients, provide direct coaching, and adapt care plans to patients’ changing needs. High-touch, personalized relationships with care coordinators and shared decision-making with medical providers have been found to meaningfully impact patient engagement and health outcomes.34–36 Third, as a program designed to address the holistic needs of high-risk patient populations, the CCR engages in cross-sector partnerships to extend care into the community and address unmet social needs. For example, they partner with a large food service provider to provide home meals and grocery delivery services upon discharge to patients facing food insecurity. These model elements provide individualized services to diabetic patients with complex medical and social needs to improve outcomes.
While core concepts around chronic care management and interdisciplinary care coordination are not new, these programs remain difficult to develop and fund. Thus, Maryland provides a promising case study of the unique opportunities afforded by global budgets. Alternative payment models (APMs) like global budgets can help align hospital financial realities with population health goals by reorienting the incentives from increasing volume of services rendered in a fee-for-service environment to developing strategies to keep patients healthy and out of the hospital.37,38 Maryland’s global budget model differs from other APMs like Accountable Care Organizations (ACOs) by prospectively setting annual funding to cover inpatient and outpatient services for an attributed patient population across payers and putting hospitals at greater financial risk than in ACO shared savings models in which providers share in a smaller percentage of savings or losses.39 By providing fixed, predictable revenue that is de-linked from volume, hospitals have increased flexibility to allocate resources efficiently under the budget constraint to improve population health. At UPMC Western Maryland, this spending flexibility, in combination with strong vision and leadership, enabled the development of the CCR rather than primarily relying on grants and other fragmented sources of funding.40 Additionally, the Maryland all-payer rate setting system enforces reimbursement parity across all payers, mitigating issues of lower reimbursements from Medicaid seen in other states.41 While payment reform is not a panacea to all difficulties in diabetes care management, the promising results seen in this study suggest that payment reform can be an important catalyst for new care delivery models that emphasize prevention and strategies to address social drivers of health.
Additionally, this study highlights how innovative diabetes care models can be achieved in rural settings with the right policy context and leadership. Significant geographic disparities exist in quality and access to diabetes care, with rural populations suffering from 16% higher prevalence of type 2 diabetes and 20% higher diabetes-related hospital mortality.42,43 Despite operating in a lower-resource environment, UPMC Western Maryland’s success in building and maintaining the CCR suggest that other rural hospitals may be able to replicate features of the CCR model and adapt them to their local circumstances. However, it also emphasizes the imperative of driving state investments such that rural hospitals have the capacity to build, hire, and acquire necessary technology and personnel to make such a model viable. Telehealth represents another strategy to improve rural diabetes care and became a critical modality of CCR care during the COVID-19 pandemic.44–46
Our study has several limitations. First, this was a real-world observational study and did not include randomization or a control group. While we collected retrospective data for utilization measures to evaluate changes across the pre- and post-intervention time periods, we cannot determine causality of the interventions on outcomes of interest and can only state associations. Outcome data may also be subject to regression to the mean, which we attempt to mitigate through retrospective data and long-term follow-up (e.g., 36 months for clinical outcomes) but cannot fully eliminate without a randomized design. Second, there was loss to follow-up for surveys and laboratory values. Reasons for loss to follow-up included resolution of social needs and patient discontinuation (e.g., disengagement, relocation, or death). While no statistically significant demographic differences were detected in sensitivity analyses between the groups included in the analytic sample versus those lost to follow-up, there may be some level of bias in the reported data and longer-term data (e.g., 36-month HbA1c) may be subject to greater variability. Third, the COVID-19 pandemic may be a confounder in our results and may have impacted patients’ ability to engage with the healthcare system. Significant physical and social stressors on individuals may have also affected various outcomes. While most patients were enrolled and reached their 12-month timepoint before the pandemic, we are unable to definitively isolate the intervention effect from the pandemic at later timepoints. Finally, this was a single-site study within a unique payment environment. While we believe that there are important lessons to be learned from UPMC Western Maryland’s holistic approach to diabetes care, our findings may not be generalizable and may need to be adapted for the unique care and payment circumstances of different healthcare settings.
Conclusions
New payment models, such as hospital global budgets, can provide the flexibility and incentives needed to develop and sustain innovative care models. UPMC Western Maryland provides a first case study in Maryland that suggests how these payment changes can lead to care programs that are associated with durable improvements in patient-reported outcomes, glycemic control, and hospitalizations for high-risk diabetes patients with substantial medical and social needs. Further research is needed to test whether these models can be effectively translated to other hospital settings.
Supplementary Information
(DOCX 27 kb)
Funding
This study was supported by the Merck Foundation Bridging the Gap: Reducing Disparities in Diabetes Care Program. Dr. Chin was supported in part by the Chicago Center for Diabetes Translation Research (NIDDK P30 DK092949). Mr. Wang was supported in part by the University of Chicago Bucksbaum Institute for Clinical Excellence.
Declarations
Conflict of interest
Dr. Chin co-chairs the Centers for Medicare and Medicaid Services Health Care Payment Learning and Action Network Health Equity Advisory Team and co-directs the Robert Wood Johnson Foundation Advancing Health Equity: Leading Care, Payment, and Systems Transformation National Program Office. He is a consultant to the Patient-Centered Outcomes Research Institute and a lead subject matter expert for the Agency for Healthcare Research and Quality. He is a member of the Bristol-Myers Squibb Company Health Equity Advisory Board and Blue Cross Blue Shield Health Equity Advisory Panel.
Footnotes
Prior Presentations:
Society of General Internal Medicine Meeting, April 2022, Orlando, FL; AcademyHealth Annual Research Meeting, June 2022, Washington, DC.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dieleman JL, Cao J, Chapin A, et al. US Health Care Spending by Payer and Health Condition, 1996-2016. JAMA. 2020;323(9):863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burwell SM. Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 3.National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States. Published online 2020:32.
- 4.Association AD. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. Published online March 21, 2018. 10.2337/dci18-0007
- 5.Chin MH. New Horizons-Addressing Healthcare Disparities in Endocrine Disease: Bias, Science, and Patient Care. J Clin Endocrinol Metab. 2021;106(12):e4887–e4902. doi: 10.1210/clinem/dgab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association AD 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S7–S14. doi: 10.2337/dc21-S001. [DOI] [PubMed] [Google Scholar]
- 7.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care. 2021;44(1):258–279. doi: 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–1639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray R. Setting Hospital Rates To Control Costs And Boost Quality: The Maryland Experience. Health Aff (Millwood). 2009;28(5):1395–1405. doi: 10.1377/hlthaff.28.5.1395. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar R, Patel A, Murphy K, et al. Maryland’s all-payer approach to delivery-system reform. N Engl J Med. 2014;370(6):493–495. doi: 10.1056/NEJMp1314868. [DOI] [PubMed] [Google Scholar]
- 11.Sapra KJ, Wunderlich K, Haft H. Maryland Total Cost of Care Model: Transforming Health and Health Care. JAMA. 2019;321(10):939–940. doi: 10.1001/jama.2019.0895. [DOI] [PubMed] [Google Scholar]
- 12.Patel A, Rajkumar R, Colmers JM, Kinzer D, Conway PH, Sharfstein JM. Maryland’s Global Hospital Budgets--Preliminary Results from an All-Payer Model. N Engl J Med. 2015;373(20):1899–1901. doi: 10.1056/NEJMp1508037. [DOI] [PubMed] [Google Scholar]
- 13.Global Budgets. The Maryland Health Services Cost Review Commission. . https://hscrc.maryland.gov/Pages/default.aspx
- 14.Maryland’s All-Payer Model—Achievements, Challenges, And Next Steps | Health Affairs Blog. Accessed June 13, 2021. https://www.healthaffairs.org/do/10.1377/hblog20170131.058550/full/
- 15.Pines JM, Vats S, Zocchi MS, Black B. Maryland’s Experiment With Capitated Payments For Rural Hospitals: Large Reductions In Hospital-Based Care. Health Aff Proj Hope. 2019;38(4):594–603. doi: 10.1377/hlthaff.2018.05366. [DOI] [PubMed] [Google Scholar]
- 16.Gunter KE, Peek ME, Tanumihardjo JP, et al. Population Health Innovations and Payment to Address Social Needs Among Patients and Communities With Diabetes. Milbank Q. 2021;99(4):928–973. doi: 10.1111/1468-0009.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanumihardjo JP, Gunter KE, Chin MH, et al. Integrating Technology and Human Capital to Address Social Needs: Lessons to Promote Health Equity in Diabetes Care. J Health Care Poor Underserved. 2021;32(2):241–261. doi: 10.1353/hpu.2021.0061. [DOI] [Google Scholar]
- 18.Allegany County Health Department UWM. Allegany County Community Health Needs Assessment. Accessed August 20, 2021. https://health.alleganymedia.com/wp-content/uploads/2020/07/CHNA-Narrative-FINAL-62920.pdf
- 19.Allegany County, Maryland. County Health Rankings & Roadmaps. . https://www.countyhealthrankings.org/app/maryland/2021/overview
- 20.Culhane D, Gross K, Parker W, Poppe B, Sykes E. Accountability, Cost-Effectiveness, and Program Performance: Progress Since 1998. Dep Pap SPP. Published online February 11, 2008. https://repository.upenn.edu/spp_papers/114
- 21.Centers for Medicare and Medicaid Services, Billioux A, Verlander K, et al. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspect. 2017;7(5). 10.31478/201705b
- 22.Page-Reeves J, Kaufman W, Bleecker M, et al. Addressing Social Determinants of Health in a Clinic Setting: The WellRx Pilot in Albuquerque, New Mexico. J Am Board Fam Med. 2016;29(3):414–418. doi: 10.3122/jabfm.2016.03.150272. [DOI] [PubMed] [Google Scholar]
- 23.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 24.Behavioral Risk Factor Surveillance System (BRFSS). Accessed March 29, 2022. https://www.ark.org/adh_brfss_questions/Detail.aspx?id=31980
- 25.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 2009;35(4):641–651. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 26.BRFSS 2017 Questionnaire. Published online 2017:93.
- 27.Healthy Days Core Module: HRQOL-14 Measure | HRQOL | CDC. Published November 5, 2018. Accessed March 29, 2022. https://www.cdc.gov/hrqol/hrqol14_measure.htm
- 28.Moriarty DG, Zack MM, Kobau R. The Centers for Disease Control and Prevention’s Healthy Days Measures – Population tracking of perceived physical and mental health over time. Health Qual Life Outcomes. 2003;1:37. doi: 10.1186/1477-7525-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fryer AK, Friedberg MW, Thompson RW, Singer SJ. patient perceptions of integrated care and their relationship to utilization of emergency, inpatient and outpatient services. Healthcare. 2017;5(4):183–193. doi: 10.1016/j.hjdsi.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 30.R: The R Project for Statistical Computing. Accessed August 20, 2021. https://www.r-project.org/
- 31.Clinical Classifications Software Refined (CCSR). Accessed March 28, 2022. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
- 32.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 33.Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: a systematic review. Prev Chronic Dis. 2013;10:E26. doi: 10.5888/pcd10.120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenigsberg MR, Corliss J. Diabetes Self-Management: Facilitating Lifestyle Change. Am Fam Physician. 2017;96(6):362–370. [PubMed] [Google Scholar]
- 35.Glenn LE, Nichols M, Enriquez M, Jenkins C. Impact of a community-based approach to patient engagement in rural, low-income adults with type 2 diabetes. Public Health Nurs Boston Mass. 2020;37(2):178–187. doi: 10.1111/phn.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saheb Kashaf M, McGill ET, Berger ZD. Shared decision-making and outcomes in type 2 diabetes: A systematic review and meta-analysis. Patient Educ Couns. 2017;100(12):2159–2171. doi: 10.1016/j.pec.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Saulsberry L, Peek M. Financing Diabetes Care in the U.S. Health System: Payment Innovations for Addressing the Medical and Social Determinants of Health. Curr Diab Rep. 2019;19(11):136. doi: 10.1007/s11892-019-1275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarron D. Alternative Payment Model (APM) Framework. 2017;(17):47.
- 39.Japinga M, McClellan M. Uniquely Similar: New Results from Maryland’s All-Payer Model and Paths Forward for Value-Based Care. :12.
- 40.Ronan BP. Remaining Financially Viable in a Time of Healthcare Transition. Front Health Serv Manage. 2017;34(2):14–24. doi: 10.1097/HAP.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 41.Meaningful Value-Based Payment Reform, Part 2: Expanding The Maryland Model To Other States | Health Affairs Forefront. . https://www.healthaffairs.org/do/10.1377/forefront.20220207.85767/full/
- 42.Krishna S, Gillespie KN, McBride TM. Diabetes Burden and Access to Preventive Care in the Rural United States. J Rural Health. 2010;26(1):3–11. doi: 10.1111/j.1748-0361.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- 43.Dugani SB, Mielke MM, Vella A. Burden and management of type 2 diabetes in rural United States. Diabetes Metab Res Rev. 2021;37(5):e3410. doi: 10.1002/dmrr.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis TC, Hoover KW, Keller S, Replogle WH. Mississippi Diabetes Telehealth Network: A Collaborative Approach to Chronic Care Management. Telemed J E-Health Off J Am Telemed Assoc. 2020;26(2):184–189. doi: 10.1089/tmj.2018.0334. [DOI] [PubMed] [Google Scholar]
- 45.Vadheim LM, Patch K, Brokaw SM, et al. Telehealth delivery of the diabetes prevention program to rural communities. Transl Behav Med. 2017;7(2):286–291. doi: 10.1007/s13142-017-0496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu T, Pujara S, Sutton S, Rhee M. Telemedicine in the Management of Type 1 Diabetes. Prev Chronic Dis. 2018;15:E13. doi: 10.5888/pcd15.170168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 27 kb)