Abstract
Antigen receptor signaling is known to activate NF-κB in lymphocytes. While T-cell-receptor-induced NF-κB activation critically depends on novel protein kinase C θ (PKCθ), the role of novel PKCs in B-cell stimulation has not been elucidated. In primary murine splenic B cells, we found high expression of the novel PKCs δ and ɛ but only weak expression of the θ isoform. Rottlerin blocks phorbol ester (phorbol myristate acetate [PMA])- or B-cell receptor (BCR)-mediated NF-κB and c-Jun N-terminal kinase (JNK) activation in primary B and T cells to a similar extent, suggesting that novel PKCs are positive regulators of signaling in hematopoietic cells. Mouse 70Z/3 pre-B cells have been widely used as a model for NF-κB activation in B cells. Similar to the situation in splenic B cells, rottlerin inhibits BCR and PMA stimulation of NF-κB in 70Z/3 cells. A derivative of 70Z/3 cells, 1.3E2 cells, are defective in NF-κB activation due to the lack of the IκB kinase (IKKγ) protein. Ectopic expression of IKKγ can rescue NF-κB activation in response to lipopolysaccharides (LPS) and interleukin-1β (IL-1β), but not to PMA. In addition, PMA-induced activation of the mitogen-activated protein kinase JNK is blocked in 1.3E2 cells, suggesting that an upstream component common to both pathways is either missing or mutated. Analysis of various PKC isoforms revealed that exclusively PKCθ was absent in 1.3E2 cells while it was expressed in 70Z/3 cells. Stable expression of either novel PKCθ or -δ but not classical PKCβII in 1.3E2 IKKγ-expressing cells rescues PMA activation of NF-κB and JNK signaling, demonstrating a critical role of novel PKCs for B-cell activation.
Transcription factor NF-κB is critically involved in many cellular processes such as inflammation, immune response, proliferation, and apoptosis. In most cells, NF-κB resides in the cytoplasm and activation is triggered in response to multiple stimuli, e.g., proinflammatory cytokines (tumor necrosis factor alpha [TNF-α] or interleukin-1β [IL-1β]), bacterial lipopolysaccharides (LPS), mitogenic signals (i.e., phorbol myristate acetate [PMA]), or antigen receptor signaling. The signal transduction pathways which are initiated by all these signals converge at the IκB kinase (IKK) complex, which upon activation phosphorylates NF-κB-inhibitory molecules (IκBs). Phosphorylated IκBs are prone to rapid ubiquitination and proteasomal degradation, liberating NF-κB, which subsequently translocates to the nucleus and activates gene transcription (23). In addition, most NF-κB-inducing agents also stimulate mitogen-activated protein kinase (MAPK) signaling cascades, leading to the activation of the transcription factor AP-1 (8). NF-κB and AP-1 cooperate at the level of transcription, and the induction of certain promoters (e.g., IL-2 promoter) depends on the activation of both transcription factors (1, 22).
Intensive work has focused on the signaling cascades upstream of the IKK complex. Especially the events leading to NF-κB activation in response to pro-inflammatory cytokines and LPS have been analyzed in great detail. Ligand binding induces the association of effector molecules (e.g., TRAFs, RIP) into the respective receptor complexes. Nevertheless, the exact mechanism by which activation of the IKK complex is accomplished remains to be determined, but there is good evidence that MAPK kinase kinases play a role (16, 23).
More recently it was shown that signaling events downstream of the T-cell receptor (TCR), TCR/CD3, are distinct from cytokine-mediated signaling in that they require Vav and the novel protein kinase C θ (PKCθ) for the activation of the IKK complex (9, 11, 29). In mature T cells from PKCθ-deficient mice, NF-κB activation was blocked in response to T-cell stimulation, proving the essential and selective role of PKCθ (40). TCR/CD28 costimulation can be mimicked by PMA plus Ca2+ ionophore (39). Yet, it is not clear how PKCθ activates the IKK complex in response to TCR/CD28 or PMA stimulation. Endogenous PKCθ associates with the activated IKK complex, and TCR/CD28 signaling induces recruitment of PKCθ and IKK into membrane lipid rafts (24). Furthermore, Bcl10 is required for NF-κB activation in response to TCR signaling and PMA treatment, but not LPS, IL-1, or TNF-α, and Bcl10 probably acts either at the level of or downstream of PKCθ (38). However, due to the absence of well-defined substrates for PKCs, the exact mechanism by which PKCθ triggers NF-κB activation remains to be identified. Recently, the IKK-related NF-κB-activating kinase has been proposed to act as a direct target of PKCs and to function as an intermediate for IKK activation in response to PMA (41). It was also reported that different PKC isoenzymes can activate IKKβ directly (27). Still, the relevance of both findings for T-cell activation is not clear.
Using Jurkat T cells, several studies have demonstrated that PKCθ, but not other PKC isoforms, also mediate c-Jun N-terminal kinase (JNK) activation in a T-cell specific manner (2, 14, 39, 43). However, peripheral T cells from PKCθ-deficient mice display no defect in TCR/CD28-induced activation of JNK, even though AP-1 activation was reduced, questioning the physiological relevance of MAPK signaling for T-cell activation (1, 40).
In primary B cells, immunoglobulin M (IgM) cross-linking of the B-cell receptor (BCR) as well as CD40 ligation have been shown to activate NF-κB (12, 13, 30, 35). CD40 receptor is a member of the TNF receptor superfamily, and signal transduction through the receptor involves association of TRAFs and subsequent IKK activation (19–21). Considerably less is known about BCR-triggered NF-κB activation. Recently, it was demonstrated that Bruton's tyrosine kinase activates phospholipase C-γ2, which couples the BCR to the IKK complex and subsequently activates NF-κB (3, 33, 34). Furthermore, antigen receptor-induced NF-κB activation in B cells and T cells is abrogated in Bcl10-deficient mice, suggesting that Bcl10 is a common constituent of both pathways (38). Still, nothing is known about other signaling intermediates, especially about the role of PKCs for BCR-induced NF-κB activation.
In this study we provide evidence that novel PKCs are necessary for BCR- and PMA-induced NF-κB and JNK activation in B cells. Pharmacological inhibitors indicate that novel PKCs transmit PMA and BCR signaling in primary splenic B cells as well as in 70Z/3 pre-B cells. 1.3E2 cells, a derivative of 70Z/3 pre-B cells, carry a defect in PMA signaling that is independent of a functional IKK complex. Novel PKCθ was found to be weakly expressed in 70Z/3 cells but was absent in 1.2E3 cells. Stable expression of either novel PKCθ or -δ but not classical PKCβΙΙ could rescue PMA-mediated NF-κB and JNK activation in 1.3E2 cells, suggesting that novel PKCs are critically involved in B-cell activation.
MATERIALS AND METHODS
Cell culture and treatment.
70Z/3 and 1.3E2 cells were grown in RPMI medium supplemented with 7.5% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin and streptomycin per ml, and β-mercaptoethanol. Jurkat T cells were grown in RPMI supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 100 U of penicillin and streptomycin per ml. The stable 1.3E2 IKKγ clone was cultured using 1 μg of G418/ml, and stable 1.3E2 IKKγ/PKCθ clones were grown using 1 μg of G418/ml and 1 μg of hygromycin/ml. Cells were treated with the following agents and concentrations: 200 ng of PMA (Calbiochem) per ml, 10 μg of LPS (Sigma) per ml or 10 ng of IL-1β (Promega) per ml, 20 ng of TNF-α (Biomol) per ml, 12 μg of anti-mouse IgM F(ab′)2 fragment (Jackson Laboratories) per ml, and 10 ng of gamma interferon (IFNγ; Endogene) per ml. PKC inhibitors Gö6976 and rottlerin were purchased from Calbiochem. For UV irradiation, cells were exposed to 150 J/m2 at 254 nm (UV-C) in a Stratagene UV cross-linker.
Stable and transient transfection.
1.3E2 cells were transfected by electroporation. Generation of 1.3E2 IKKγ-expressing clones was described previously (17). For stable transfections of PKC isoenzymes, cells were electroporated using 28 μg of either PEF-PKCθ, PEF-PKCδ, or MTH-PKCβΙΙ together with 2 μg of pTK-hygromycin (Clontech) in a Bio-Rad gene pulser at 950 μF and 220 V. After 2 days, selection was started using 2.5 μg of hygromycin/ml. For transient transfections, cells were electroporated using 1 μg of pTK-luciferase (Clontech), 2 μg of 6xNF-κB-luciferase (6), and 27 μg of pcDNA3. Thirty-six hours posttransfection, the cells were stimulated for 6 h as indicated and luciferase activity was determined using a dual luciferase assay kit (Promega).
Antibodies.
The following antibodies were used: IκBα (C-21), IκBβ (N-20), IKKα (H744), IKKγ (FL419), PKCβΙ (C-16), PKCβΙΙ (C-18), PKCδ (C-17), and PKCɛ (C-15) were obtained from Santa Cruz; monoclonal PKCα, PKCγ, and PKCθ antibodies were purchased from Transduction Laboratories. Further antibodies used were monoclonal JNK1 (Pharmingen), phospho-SAPK/JNK (Cell Signaling), ERK1/2 (Calbiochem), and phospho-ERK1/2 (Biomol).
Extracts, EMSA, Western blotting, and kinase assay.
Whole cell extracts were prepared and analyzed by electrophoretic mobility shift assay (EMSA) and Western blotting essentially as described previously (26). Preparation of nuclear and cytoplasmic extracts was performed using low-salt lysis with 0.1% NP-40 and subsequent high-salt lysis essentially as described previously (25).
For the IKK and JNK1 kinase assays, lysis of cells, immunoprecipitation using specific antibodies (see above), and kinase reactions were performed as described previously (18). For IKK activity, GSTIκBα1–53 was used as substrate, and for JNK1 activity GSTJun1–79 was used as substrate. PKC kinase assays were performed with purified rat brain PKC (Promega) and 1 μg of myelin basic protein as a substrate in 30 mM Tris-HCl (pH 7.5), 100 mM KCl, 6 mM MgCl2, 0.5 mM CaCl2, 10 μg of phospatidylserine/ml, 1 μg of diolein/ml, 10 μM ATP, and 5 μCi of [γ-32P]ATP for 15 min at 30°C.
Purification of splenic B and T cells and flow cytometry.
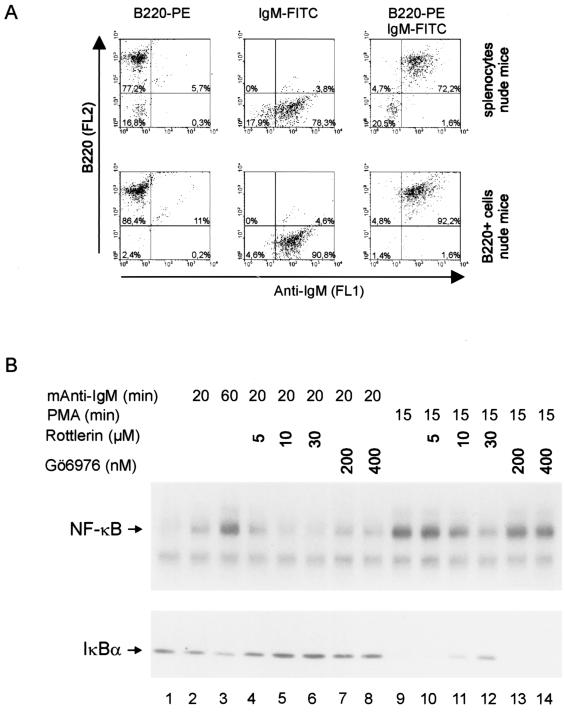
Either BALB/c wild-type or BALB/c nude mice were used for the preparation of splenic B and T cells. Spleens were homogenized using a 70-μm cell strainer (Falcon), and erythrocytes were removed by ACK lysis buffer (150 mM NH4Cl [pH 7.3], 1 mM KHCO3, 0.1 mM EDTA). Magnetic-activated cell sorting (MACS) was performed according to the manufacturer's protocol using B220 or Thy1.2 microbeads and VS+ columns (Miltenyi Biotech.). In order to get maximal purification of B220-positive B cells, splenocytes from BALB/c wild-type mice were first sorted for Thy1.2-positive cells and then the Thy1.2-negative cells were subsequently sorted using B220 microbeads. B220-positive cells from BALB/c nude mice were obtained in a single round of purification. Purification efficiency was determined by fluorescence-activated cell sorter flow cytometry using fluorescein isothiocyanate (FITC)-labeled Thy1.2 (Caltag Laboratories) and phycoerythrin-labeled B220 (Southern Biotech) antibodies. IgM surface expression was analyzed with FITC-labeled anti-mouse IgM antibody (R6–60.2; Pharmingen). Analysis and quantification were done using WinMDI.
Indirect immunofluorescence.
70Z/3 and 1.3E2 cells were grown on chamber slides in the absence of serum. After 16 h the cells completely attached to the surface of the chamber slide. Cells were fixed for 15 min in 4% paraformaldehyde and permeabilized for 5 min with 0.1% Triton X-100. For the detection of monoclonal PKCθ antibody, a donkey anti-mouse tetramethyl rhodamine isocyanate-conjugated antibody for the detection of the polyclonal IKKγ antibody and a donkey anti-rabbit FITC-conjugated antibody (both Jackson Laboratories) were used. Chamber slides were analyzed and photographed by indirect immunofluorescence microscopy using an Axioplan 2 microscope (Zeiss).
RESULTS
Novel PKCs are involved in PMA- and BCR-induced NF-κB and JNK activation in splenic B cells.
In lymphocytes, stimulation with PMA, a pleiotropic PKC activator, induces activation of NF-κB and MAPK signaling pathways. To assess the expression and the function of novel PKCs in primary lymphocytes, splenic B and T cells were obtained from wild-type and nude mice (Fig. 1). Splenic B and T cells were purified by MACS using Thy1.2 (CD90) and B220 (CD45R) microbeads. The purification led to almost homogenous B-cell and T-cell populations with minimal cross-contamination (Fig. 1A). Expression of PKC isoforms was analyzed by Western blotting (Fig. 1B). In agreement with previously published results (32), we found high expression of PKCδ and PKCɛ in primary B220-positive B cells compared to Thy1.2-positive T cells. In contrast, there was only a weak signal for PKCθ in the B220-positive B cells and strong expression of PKCθ in Thy1.2-positive T cells. After 24 h in culture, the B and T cells were stimulated with PMA in the absence or presence of the PKC inhibitors rottlerin and Gö6976 and analyzed for NF-κB and JNK kinase activation (Fig. 1C and D). Rottlerin, an inhibitor with high specificity towards Ca2+-independent (novel) PKCs (15, 42), interfered with NF-κB and JNK activation in B and T cells at a concentration of 30 μM, whereas the PKC inhibitor Gö6976, which inhibits Ca2+-dependent (classical) PKCs, had no effect on either signaling pathway. The specificity was confirmed in a kinase reaction on purified rat brain PKC, which consists primarily of the α, β, and γ isoforms, with lesser amounts of δ and ζ isoforms (Fig. 1E). Whereas 200 nM Gö6976 strongly inhibited the activity of rat brain PKCs, rottlerin exerted no effect at concentrations up to 30 μM. Loss of NF-κB signaling was most likely due to impaired upstream signaling events because IκBα was significantly stabilized in the presence of rottlerin (Fig. 1D, upper panel). These results indicate that similar signaling pathways which involve novel PKCs contribute to NF-κB and JNK1 activation in primary B and T cells.
FIG. 1.
Novel PKCs are involved in NF-κB and JNK activation in response to PMA in primary murine splenic B and T cells. (A) Splenocytes, MACS-separated Thy1.2-positive cells (Thy1.2+), or MACS-separated B220-positive cells (B220+) from either wild-type (BALB/c) or nude mice were analyzed for Thy1.2 and B220 expression by flow cytometry. (B) Whole cell extracts of B220- or Thy1.2-positive cells were analyzed for the expression of different PKC isoenzymes, as indicated. A nonspecific band that migrates slightly faster than PKCθ is indicated by a circle. (C and D) T cells from BALB/c mice and B cells from nude mice were pretreated for 30 min with the indicated concentrations of PKC inhibitors Gö6976 or rottlerin and subsequently stimulated with PMA. NF-κB activity was determined by EMSA (C) and IκBα degradation and JNK phosphorylation were determined by Western blotting (D). (E) Specificities of Gö6976 and rottlerin were confirmed in an in vitro kinase reaction using purified rat brain PKCs and myelin basic protein as a substrate.
It has been shown that NF-κB is activated in response to stimulation of the BCR (3, 34). To elucidate the role of novel PKCs for BCR-mediated NF-κB activation we treated primary B cells from nude mice with anti-IgM antibody. We checked expression of surface IgM (sIgM) in splenocytes and B220-positive cells from nude mice (Fig. 2A). Nearly all B220-positive cells were also sIgM positive. NF-κB was activated in B220-positive B cells in response to IgM cross-linking (Fig. 2B). Activation of NF-κB correlated with reduced IκBα protein levels, and the process was inhibited by rottlerin but not by Gö6976. We did not detect MAPK activation in response to IgM ligation (data not shown). These data demonstrate that novel PKCs are involved in BCR-induced activation of NF-κB and provide evidence that a conserved pathway links antigen receptors from B and T cells to their downstream targets.
FIG. 2.
BCR-directed activation of NF-κB requires novel PKCs. (A) Splenocytes from nude mice and B220-positive cells obtained after MACS (Fig. 1) were analyzed for B220 and IgM surface expression by flow cytometry. Left and middle panels represent single stains; right panels represent double stains. (B) NF-κB activation and ΙκBα degradation of B220 cells in response to anti-IgM or PMA stimulation was analyzed by EMSA or Western blotting, respectively. Rottlerin or Gö6976 was added at the indicated concentrations 30 min prior to stimulation.
Novel PKCs are involved in NF-κB and JNK activation in 70Z/3 pre-B cells.
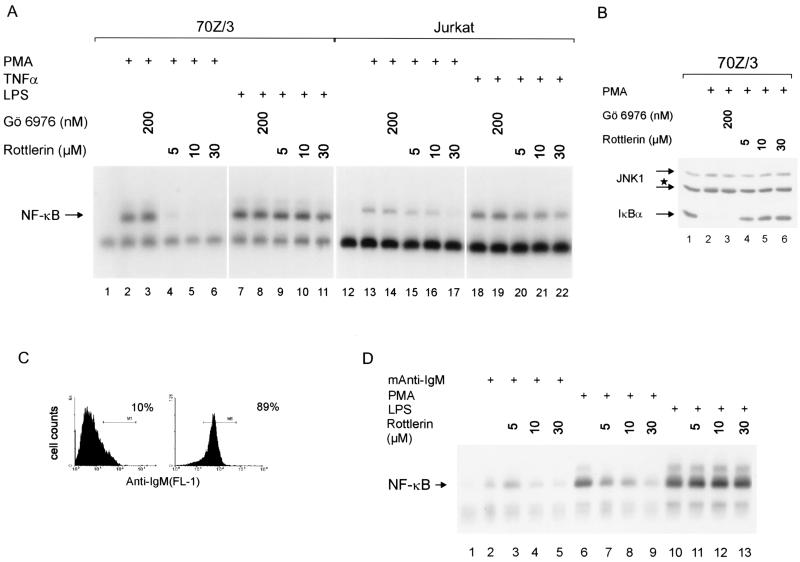
We analyzed the requirement of individual PKCs for the activation of NF-κB in mouse 70Z/3 pre-B cells as a model system. This well-characterized cell line can be activated to express surface IgM in response to LPS or IFN-γ (36). We first investigated by pharmacological inhibition which PKC subfamilies are responsible for NF-κB and JNK activation in 70Z/3 cells (Fig. 3). Rottlerin was a potent inhibitor of PMA-induced NF-κB activation with an effective concentration of 5 to 10 μM (Fig. 3A). In Jurkat T cells, where PKCθ has been implicated in PMA-mediated NF-κB activation (42), complete inhibition was exerted with 30 μM rottlerin. These concentrations had no effect on NF-κB activation in response to either LPS (70Z/3; Fig. 3A, lanes 9 to 11) or TNF-α (Jurkat; Fig. 3A, lanes 20 to 22), showing that rottlerin specifically inhibited PMA-induced activation of NF-κB. Furthermore, administration of rottlerin inhibited PMA-induced degradation of IκBα (Fig. 3B), demonstrating that PKC inhibition affected upstream signaling events. Gö6976 did not elicit an effect on NF-κB activation at a concentration of 200 nM in 70Z/3 and Jurkat T cells. Similar to NF-κB activation, JNK1 phosphorylation in 70Z/3 cells was inhibited at a rottlerin concentration of 5 μM and Gö6976 had no effect (Fig. 3B).
FIG. 3.
Inhibition of novel PKCs blocks NF-κB and JNK kinase activation in 70Z/3 cells. (A) 70Z/3 pre-B or Jurkat T cells were pretreated for 30 min with the indicated amounts of Gö6976 or rottlerin and subsequently stimulated with PMA, TNF-α, or LPS for 15 min. Whole cell extracts were analyzed for NF-κB activation by EMSA. (B) 70Z/3 cells were treated as for panel A, and JNK1 as well as ΙκBα were analyzed by Western blotting. The star indicates the position of the hyperphosphorylated 46-kDa JNK1 isoform. (C) 70Z/3 cells were stimulated for 24 h with 10 ng of IFN-γ/ml, and IgM surface expression was determined by flow cytometry (untreated and stimulated cells, left and right panels, respectively). (D) IFN-γ-stimulated cells were pretreated with the indicated concentrations of rottlerin for 30 min and stimulated with mouse anti-IgM antibody (30 min), PMA (10 min), or LPS (30 min). NF-κB DNA binding activity was determined by EMSA.
We treated 70Z/3 pre-B cells for 24 h with IFN-γ to induce expression of sIgM (Fig. 3C). IFN-γ pretreatment induced sIgM expression without any effect on NF-κB (Fig. 3 and data not shown). Incubation of the IgM-positive cells with an anti-IgM antibody led to a weak NF-κB activation in 70Z/3 cells (Fig. 3D). NF-κB activation was strictly dependent on sIgM expression, because in 70Z/3 cells that had not been pretreated with IFN-γ, IgM ligation did not activate NF-κB (data not shown). NF-κB activation in response to IgM cross-linking or PMA was inhibited by rottlerin to a similar extent. Again, LPS induction of NF-κB was not affected by rottlerin. As in primary B cells, we did not observe activation of JNK in response to IgM ligation (data not shown). We conclude that NF-κB activation in 70Z/3 pre-B cells and in primary B cells by PMA or IgM cross-linking involves the same signaling pathways that depend on novel PKCs. Therefore, 70Z/3 pre-B cells are a suitable model system to investigate the contribution of novel PKCs in response to B-cell activation.
Defective activation of NF-κB and JNK by PMA in the mutant pre-B-cell line 1.3E2.
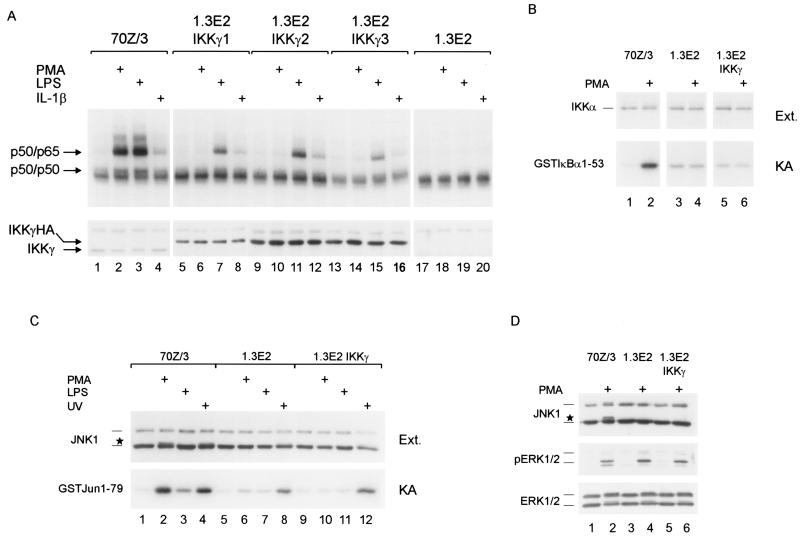
1.3E2 cells, derivatives of 70Z/3 cells, are unable to activate NF-κB in response to either LPS, IL-1, PMA, Taxol, or double-stranded RNA (10). This deficiency is due to the lack of IKKγ (44). In order to analyze the pathway for NF-κB activation by PMA, we reintroduced IKKγ into the 1.3E2 cells. Stable expression of IKKγHA rescued NF-κB activation after stimulation with either LPS or IL-1β but, surprisingly, IKKγ failed to rescue NF-κB activation in response to PMA in three different IKKγ-expressing clones (Fig. 4A). Also, in transient transfections N-terminally Flag-tagged IKKγ could rescue LPS- but not PMA-induced activation of an NF-κB reporter, ruling out that the position or sequence of the epitope tag has an effect on NF-κB activation (S. Tegethoff, unpublished results). NF-κB activation in 1.3E2 IKKγ clones by LPS or IL-1β was slightly reduced in comparison to 70Z/3 cells, which was most likely due to the increased amounts of IKKγ (Fig. 4A; lower panel). Overexpression of IKKγ alone has been shown to inhibit IKK and NF-κB activation, probably through competition for upstream activators of the IKK complex (25, 28). Next, we tested whether PMA led to the activation of IKKs by performing in vitro kinase reactions after immunoprecipitation of the IKK complex (Fig. 4B). PMA efficiently activated the IKK complex in 70Z/3 cells but failed to do so in either 1.3E2 or 1.3E2 IKKγ cells. Therefore, the loss of NF-κB activation must be due to an upstream defect that is independent of IKKγ deficiency.
FIG. 4.
(A) IKKγ expression rescues LPS- and IL-1β- but not PMA-induced NF-κB activation of 1.3E2 cells. 70Z/3 cells, three 1.3E2 IKKγ-expressing clones, and 1.3E2 cells were treated with PMA, LPS, or IL-1β for 30 min and NF-κB DNA-binding activity was determined by EMSA. IKKγ protein was determined by Western blotting (lower panel). (B and C) Activation of IKK and JNK1 in response to PMA is defective in 1.3E2 and 1.3E2 IKKγ cells. (B) 70Z/3, 1.3E2, and 1.3E2 IKKγ2 cells were stimulated with PMA (10 min), and extracts were analyzed for IKKα protein expression. IKK activity was determined in an in vitro kinase reaction after IKK immunoprecipitation with an anti-IKKα antibody. GstIκBα1–53 was used as a substrate. (C) 70Z/3, 1.3E2, and 1.3E2 IKKγ2 cells were stimulated with PMA (10 min), LPS (20 min), or UV light (20 min) and cellular extracts were analyzed for JNK1 expression (upper panel). The hyperphosphorylated 46-kDa JNK1 isoform is indicated by a star. JNK1 kinase activity was determined in an in vitro kinase reaction after immunoprecipitation of JNK1. Kinase activity was determined with recombinant GstJun1–79 as substrate. (D) Activation of ERK1/2 is not defective in 1.3E2 cells. Cells were stimulated with PMA for 10 min and cellular extracts were analyzed by Western blotting using JNK1, phospho-ERK1/2, or ERK1/2 antibodies (as indicated). Migration of the hyperphosphorylated 46-kDa JNK1 is indicated by a star.
Since PMA is a potent inducer of MAPK signaling pathways, we tested its ability to activate JNK1 in the different cell lines (Fig. 4C). JNK1 kinase assays using GstJun1–79 as a substrate were performed after immunoprecipitation with JNK1 antibody. PMA or UV light led to an activation of JNK1 in 70Z/3 cells, whereas LPS only weakly activated JNK1. In contrast, in neither 1.3E2 nor 1.3E2 IKKγ cells could JNK1 kinase activity be stimulated by PMA. Since JNK1 could still be activated by UV light, JNK signaling is not generally defective in 1.3E2 cells. To further test MAPK signaling in 1.3E2 cells, we analyzed activation of ERK1/2 using a phospho-specific antibody (Fig. 4D). Phosphorylation of ERK1/2 in response to PMA did not differ in 70Z/3, 1.3E2, and 1.3E2 IKKγ cells. We did not observe activation of p38 in either 70Z/3 or 1.3E2 cells when a phospho-specific antibody was used (data not shown). These results suggest that the loss of PMA-mediated NF-κB and JNK activation in 1.3E2 cells is caused by a common defect upstream of the IKK complex that does not affect activation of ERKs.
Novel PKCθ is absent in 1.3E2 cells.
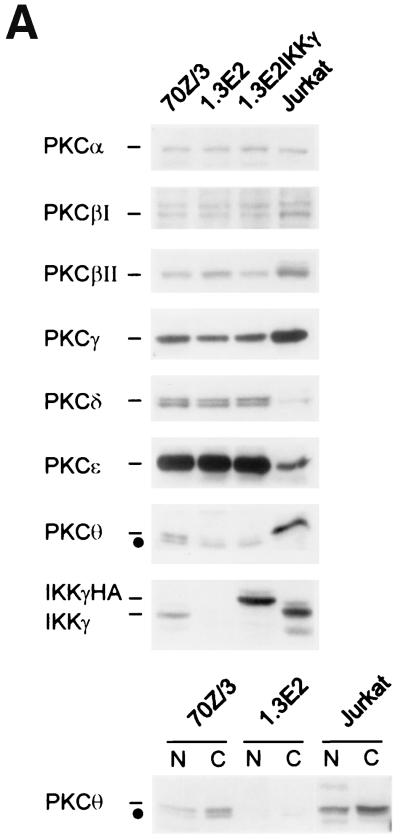
Classical and novel PKC isoforms are directly activated by phorbol ester. We compared the protein amounts of various PKC family members in 70Z/3, 1.3E2, 1.3E2 IKKγ, and Jurkat cells by Western blotting (Fig. 5A). As expected, compared to Jurkat T cells the pre-B cell line 70Z/3 as well as its 1.3E2 derivative contained high amounts of the Ca2+-independent PKCδ and PKCɛ, both of which have been shown to be expressed in the B-cell lineage (32). Nevertheless, novel PKCs δ and ɛ and the classical Ca2+-dependent PKCs α, βI, βII, and γ (Fig. 5A) as well as the atypical phorbol ester-independent PKC isoforms ι and λ (data not shown) were all equally expressed in 70Z/3 cells compared to 1.3E2 cells. In contrast, PKCθ was only weakly expressed in 70Z/3 cells versus Jurkat T cells, and it was absent in 1.3E2 and 1.3E2 IKKγ cells. We confirmed this result by nuclear-cytoplasmic fractionation of 70Z/3, 1.3E2, and Jurkat cells (Fig. 5; lower panel). As expected, PKCθ was predominantly localized in the cytoplasmic compartment and only weakly in the nucleus. Again, PKCθ expression was observed in Jurkat and, albeit more weakly, in 70Z/3 cells, but it was lacking in 1.3E2 cells. Due to the weak expression of PKCθ in 70Z/3 cells, a nonspecific band appears that migrates slightly faster than the specific signal.
FIG. 5.
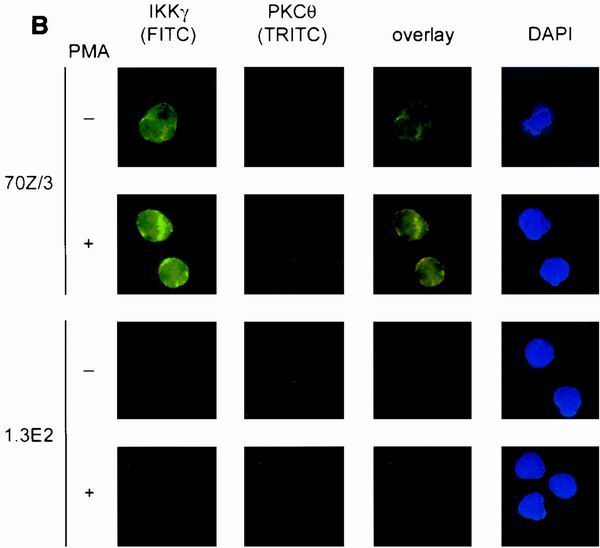
PKCθ is absent in 1.3E2 and 1.3E2 IKKγ cells. (A) Whole cell extracts were prepared from 70Z/3, 1.3E2, 1.3E2 IKKγ2, and Jurkat T cells and the expression of various PKC family members was determined by Western blotting. Lower panel, nuclear (N) and cytoplasmic (C) extracts of 70Z/3, 1.3E2, and Jurkat cells were analyzed for PKCθ expression. A nonspecific band migrating faster than PKCθ is indicated by a dot. (B) In response to PMA, PKCθ translocates to the membrane but does not quantitatively associate with IKKγ. Cells were attached to the surface of the chamber slides by culturing in the absence of serum for 16 h. 70Z/3, 1.3E2, 1.3E2 IKKγ/PKCθ, or 1.3E2 IKKγ cells were left untreated or stimulated with PMA for 20 min. After fixation and permeabilization, cells were costained with polyclonal IKKγ and monoclonal PKCθ primary antibodies. For the detection, FITC-conjugated donkey anti-rabbit and tetramethyl rhodamine isocyanate-conjugated donkey anti-mouse secondary antibodies were used.
Previous studies have suggested that the function of PKCθ in the hematopoietic lineage is restricted to T cells (32, 40). Therefore, by performing indirect immunofluorescence in 70Z/3 pre-B cells, we tested if PKCθ translocates to the membrane in response to PMA (Fig. 5B). In unstimulated 70Z/3 cells, PKCθ resided predominantly in the cytoplasm, but upon activation the majority was localized to the cytosolic membrane. In parallel, we costained IKKγ in the same cells and found that IKKγ resided in the cytoplasm and to a small degree also in the nuclear compartment, with no change upon stimulation. Congruent with the Western blotting data, no staining of either PKCθ or IKKγ was observed in 1.3E2 cells, which confirms the specificity of the staining in 70Z/3 cells. PKCθ deficiency in 1.3E2 cells together with defective PMA signaling suggested that this isoform could be engaged in NF-κB and JNK activation in 70Z/3 pre-B cells and encouraged us to rescue PMA signaling by ectopic expression of PKCθ.
Stably expressed novel PKCθ or PKCδ, but not classical PKCβΙΙ, rescues NF-κB and JNK activation in 1.3E2 IKKγ-expressing cells.
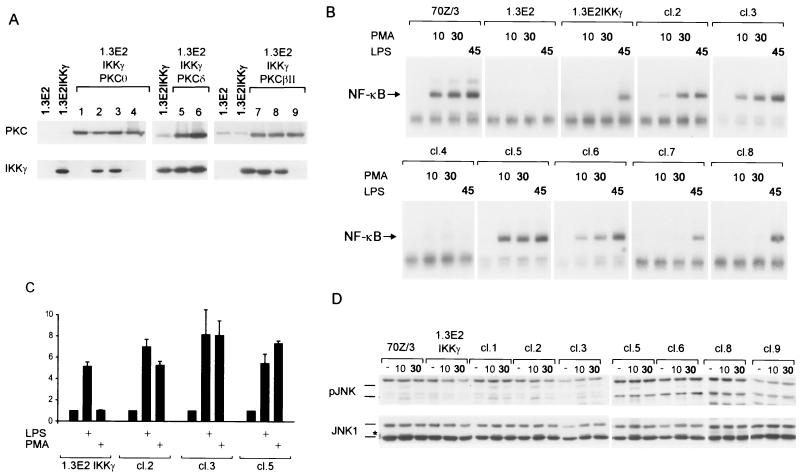
We decided to introduce different PKC isoenzymes into 1.3E2 IKKγ-expressing cells to see if defective PMA signaling can be rescued. Novel PKCθ and PKCδ as well as classical PKCβΙΙ were stably transfected in 1.3E2 IKKγ cells and PKC-positive clones were selected (Fig. 6A). In some cases IKKγ protein expression was lost during the process of selection (clones 1, 4 and 9), making it possible to determine the role of PKCs in the absence of a functional IKK complex.
FIG. 6.
Stable expression of PKCθ or PKCδ, but not PKCβΙΙ, rescues PMA activation of NF-κB and JNK in 1.3E2 IKKγ cells. (A) Expression of the different PKC isoforms and IKKγ in the stable transfectants was analyzed by Western blotting. Note that some clones (1, 4, and 9) lost IKKγ expression during the process of selection. (B) Activation of NF-κB in 70Z/3, 1.3E2, and 1.3E2 IKKγ cells and various PKC-expressing clones in response to PMA (10 and 30 min) or LPS (45 min), as determined by EMSA. (C) NF-κB activation in response to LPS and PMA was determined by NF-κB reporter assays in 1.3E2 IKKγ cells and either PKCθ- or PKCδ-expressing clones. Each bar represents the average of three independent experiments, with standard deviations indicated. (D) PMA-induced JNK activation in 70Z/3 cells and in various 1.3E2 clones was determined in a Western blot using phospho-JNK and anti-JNK1 antibodies. Migration of transiently phosphorylated JNK is marked by a star.
NF-κB activation by PMA (10 and 30 min) or LPS (45 min) was determined by EMSA (Fig. 6B). Stable expression of either PKCθ or PKCδ in 1.3E2 IKKγ cells (clones 2, 3, 5, and 6) rescued NF-κB activation in response to PMA. In contrast, ectopic PKCβΙΙ failed to reinstate PMA responsiveness in 1.3E2 IKKγ cells. NF-κB activation was abrogated in response to LPS only in 1.3E2 cells and in the PKCθ-expressing clone 4, which had lost any detectable IKKγ protein. Thus, LPS responsiveness was unaffected as long as a functional IKK complex was present. NF-κB activation was functional, as observed in a luciferase reporter assay (Fig. 6C). Whereas LPS activated the NF-κB reporter independent of ectopic PKCθ or PKCδ, PMA could only activate the reporter in the presence of either kinase. We also tested NF-κB activation by IgM ligation in 1.3E2 cells or in the IKKγ- or IKKγ/PKC-expressing clones. No activation was detectable; however, only about 50% of the IFNγ-treated 1.3E2 cells were sIgM positive. In addition, the overall level of sIgM in positive cells was decreased compared to IFNγ-induced 70Z/3 cells (data not shown). Therefore, given the relatively weak activation of NF-κB in 70Z/3 cells (see Fig. 3D), the lack of activation in 1.3E2 cells is probably due to the limiting amount of the BCR on the cell surface.
Besides NF-κB activation, stable expression of novel PKCθ and PKCδ also rescued JNK activation in 1.3E2 cells (Fig. 6D). Western analysis using anti-phospho-JNK and anti-JNK1 antibodies showed that JNK was transiently phosphorylated in response to PMA in 70Z/3 cells as well as in all 1.3E2 cell clones, which were expressing either PKCθ or PKCδ. JNK kinase activation was independent of a functional IKK complex, since JNK phosphorylation was also obtained in the clones which had lost IKKγ expression. Since several clones (two PKCθ- and one PKCδ-expressing clone; Fig. 6D and data not shown) displayed JNK activation in the absence of IKKγ, clonal differences cannot account for the maintenance of JNK signaling. Again, ectopic expression of PKCβII did not rescue JNK signaling in 1.3E2 cells.
We conclude that a defect in NF-κB and JNK activation in response to PMA in the 70Z/3-derived 1.3E2 cell line can be overcome by the ectopic expression of novel PKCθ or -δ. Expression of classical PKCβII cannot promote NF-κB and JNK activation. Taken together, the results present evidence for a specific role of novel PKCs for PMA- or BCR-specific activation of NF-κB in B cells.
DISCUSSION
During the last years much attention has been focused on the role of novel PKCs and especially of PKCθ for TCR/CD3, and CD28 costimulation with PMA-ionomycin is thought to mimic this stimulation in T cells (2, 9, 11, 14, 24, 29, 40, 42). Using the PKC inhibitor rottlerin, we showed that novel PKCs are also involved in PMA-induced NF-κB activation in primary B220-positive B cells derived from spleens of wild-type and nude mice. It was demonstrated that stimulation of the BCR leads to activation of NF-κB (5, 35), a process which involves Bruton's tyrosine kinase, phospholipase C-γ2, and the IKK complex (3, 33, 34). We now demonstrate that BCR activation of NF-κB requires novel PKCs in 70Z/3 pre-B cells as well as in primary B cells. In contrast to peripheral T cells, which are characterized by high expression of novel PKCθ, B cells express high amounts of PKCδ and -ɛ. Congruent with this observation, BCR signaling was shown to activate PKCδ (4). We did not observe MAPK activation after BCR cross-linking in 70Z/3 cells or primary B cells (data not shown). Interestingly, Ruland et al. (38) have now shown that Bcl10−/− mice are defective in NF-κB activation in response to both antigen receptors (TCR and BCR). Our data suggest that the upstream signaling events leading to IKK activation in response to antigen receptor signaling in B and T cells are distinct from those of pathways initiated by TNF-α, IL-1, or LPS. Signal transduction in both cases involves novel PKCs, Bcl10, the IKK complex, and subsequently NF-κB, which shows that signaling initiated by TCR and BCR is highly conserved.
We looked for a cellular model to study NF-κB activation in a well-characterized cell line of the B-lymphocytic lineage. 70Z/3 pre-B cells were chosen because expression of sIgM can be induced and it was previously shown that cross-linking of sIgM leads to NF-κB activation (35). We found that, just like in primary B cells, IgM ligation activated NF-κB in 70Z/3 cells, demonstrating that these cells express a functional BCR and contain all components necessary for downstream signaling. Importantly, both BCR- and PMA-induced signaling required novel PKCs. The 1.3E2 cell line has been isolated by immunoselection as a variant of 70Z/3 cells that is unable to express surface IgM in response to LPS (31). Subsequently, it was shown that the cells are defective in NF-κB and Oct-2 activation (7, 10, 36). 1.3E2 cells do not functionally express the NF-κB essential modulator (NEMO) or IKKγ, which is essential for activation of the IKK complex (37, 44). NF-κB activation in response to multiple stimuli can be recovered by NEMO/IKKγ expression. In contrast to previous results by Yamaoka et al. (44), we found that defective NF-κB activation by PMA was not complemented by the stable expression of IKKγ, even though LPS and IL-1 responses could be recovered. Possibly, clonal differences depending on the lots of the 1.3E2 cells used may explain this discrepancy. Nevertheless, all other molecular and cellular parameters which we determined for 1.3E2 cells (i.e., lack of NEMO/IKKγ protein, reduced Oct-2 DNA binding, active ERK1/2 kinases, sIgM expression in response to IFNγ but not to LPS) are congruent with previously published observations. We have used the human NEMO/IKKγ cDNA for complementation, but the fact that LPS and IL-1 responses as well as PMA stimulation in the presence of novel PKCs could be rescued in 1.3E2 cells strongly argues against species-specific functional differences of IKKγ. The reproducible finding of defective PMA responses of NF-κB and JNK (see also below) in 1.3E2 cells as well as in 1.3E2 IKKγ clones made it possible to specifically investigate the requirements for PKC-mediated antigen receptor signaling in pre-B cells.
The observation that 1.3E2 cells are not only defective in NF-κB activation but also fail to activate the MAPK JNK provides further evidence that loss of PMA responsiveness is caused by a defect in a component common to both pathways. Defective JNK activation was shown with antibodies recognizing the hyperphosphorylated form of JNK as well as in vitro kinase reactions using GSTJun1–79 as a substrate. Importantly, UV light-induced JNK signaling was still intact, providing evidence that the defect resides in PMA-induced signal cascades and is not a consequence of a mutation in JNK itself. NF-κB and JNK signaling pathways diverge somewhere upstream of the IKK complex, making it very likely that either the loss of or a mutation at the level of PKCs is responsible for the signaling defect. Interestingly, MAPK signaling is not generally affected because ERK1 and -2 are still active in 1.3E2 cells (Fig. 4D) (10), showing that distinct pathways activate individual MAPKs (also reviewed in reference 8). Also, in Jurkat T cells it has been demonstrated that PKCθ contributes to JNK but not to ERK activation (14; see also below).
Since PMA directly activates PKCs, we determined the expression of a panel of PKC family members. Just like in primary B cells, strong expression of PKCδ and -ɛ could be observed in the pre-B cell lines. However, of all PKC isoenzymes tested in Western blotting, only PKCθ was differentially expressed in 70Z/3 versus 1.3E2 cells. This result was confirmed by indirect immunofluorescence, showing that PKCθ is not only expressed in 70Z/3 cells but also translocates to the membrane in response to PMA. In T cells it has been shown that upon stimulation with PMA-ionomycin or CD3/CD28, PKCθ associates with IKKα or -β and colocalizes at the membrane (24). Therefore, we investigated if endogenous IKKγ is at least partially shifted to the membrane in response to stimulation, but we found that IKKγ resides in the cytoplasm and maybe partially in the nucleus (see also reference 28). Since IKKγ quantitatively associates with IKKβ and IKKα in 70Z/3 cells (25), we find no evidence that membrane translocation of the IKK complex precedes its activation by PMA. Nevertheless, a transient translocation of the IKK complex to the membrane might precede its activation.
The finding that PKCθ was differentially expressed in the pre-B cell lines was quite surprising, because an analysis of PKCs in hematopoietic cells reveals that PKCθ is predominantly expressed in T cells (Fig. 1B) (32). However, the differential expression of PKCθ in 70Z/3 and 1.3E2 cells clearly suggests that this isoenzyme should have a key function for NF-κB and JNK activation. Due to the extremely poor transfection efficiency in 70Z/3 cells, we failed to block NF-κB activation using kinase-inactive mutants of either PKCθ, PKCδ, or PKCɛ (data not shown), and we therefore attempted to rescue PMA signaling by stable expression of PKCs in 1.3E2 IKKγ cells. In line with the hypothesis that PKCθ is essential for NF-κB and MAPK activation by PMA in the pre-B-cell lines, we could rescue both signaling pathways by ectopic expression of PKCθ (Fig. 6). Surprisingly, PKCδ, another novel PKC isoform, could also rescue NF-κB and JNK activation. In contrast, PMA activation was not restored by expression of the Ca2+-dependent classical PKC isoform βΙΙ. Therefore, in agreement with the data obtained using the pharmacological inhibitor rottlerin, a rescue was observed only when novel PKCs were transfected. The question remains why PKC function could be rescued with either PKCθ or -δ, even though PKCδ, in contrast to PKCθ, was expressed in considerable amounts in 70Z/3 as well as in 1.3E2 cells. As a likely explanation, strong overexpression of one novel PKC isoform might suffice to compensate for the loss of the other. In fact, among the novel PKCs, PKCδ is the closest relative of PKCθ, with an overall extended identity of 59% suggesting that both isoforms could be functionally redundant. The similarity between PKCθ and other novel PKC isoforms is considerably lower, with 38% identity for PKCɛ as the next relative. Significant homologies to classical PKCs are restricted to the kinase and PMA binding domains. Furthermore, NF-κB and JNK activation in 1.3E2 cells was strictly dependent on PKC activation by PMA, and PKC overexpression alone did not suffice to activate signaling nonspecifically. Moreover, in the mutant 1.3E2 cells, ectopically expressed PKCθ translocates to the membrane in response to PMA, indicating that all other components necessary for PMA signaling are intact. It was shown recently that Bcl10 is necessary for NF-κB activation in response to TCR stimulation and acts downstream or at the level of PKCθ (38). We found no change in Bcl10 protein expression between 70Z/3 and 1.3E2 cells (data not shown). Furthermore, Bcl10 deficiency does not affect MAPK activation (38), indicating that the point where NF-κB and MAPK pathways diverge is upstream of Bcl10 at the level of PKC activation. Therefore, it is most likely that PKCθ deficiency in 1.3E2 cells is responsible for the PMA signaling defect, but this defect can be overcome either by reintroduction of PKCθ or by increasing the levels of the related δ isoform.
The data thus suggest that besides the specific function of PKCθ in T-cell lines, mature primary T cells, and the 70Z/3 and 1.3E2 pre-B cell lines, PKCθ plays a key role in NF-κB signaling in primary B cells. The analysis of PKC isoenzyme expression in primary B and T cells revealed that PKCθ levels are highest in T cells. Nevertheless, we did observe expression of PKCθ, albeit weaker, also in primary B cells. A detailed analysis of B cells from PKCθ- and/or PKCδ-deficient mice needs to be performed to answer the question of which PKC isoform plays a crucial role for pre-B cells or at any other stage of B-cell differentiation. In this respect, it is interesting that PKCθ is essential for TCR-mediated NF-κB activation in mature but not immature T lymphocytes (40). The PKC isoform responsible for NF-κB activation in immature T lymphocytes remains to be identified. In addition, in mice, MAPK signaling in T cells was not influenced by PKCθ deficiency, even though PKCθ is an upstream regulator for JNK in Jurkat T cells (2, 14). The data indicate that each individual PKC isoform carries out specific functions which are not only dependent on the cell type but which also differ in the downstream signaling pathway that they activate.
In this study we established a role for novel PKCs in BCR signaling to NF-κB, and thereby we underscored a striking conservation for antigen receptor signaling in T and B cells. Future studies will need to address the mechanism by which novel PKCs are linking the antigen receptor activation to the IKK complex.
ACKNOWLEDGMENTS
We thank Gottfried Baier for providing us with PKCθ and PKCδ, Harald Mischak for the gift of PKCβΙΙ, and Robert Newton for the 6xNF-κBluc constructs. We thank Carol Sibley for the gift of the 70Z/3 and 1.3E2 cells. Furthermore, we thank Erika Scharschmidt for excellent technical assistance, Benjamin Mordmüller for purification of GstJun1–79, and Susanne Preiss for the help with the lymphocyte purification.
REFERENCES
- 1.Altman A, Isakov N, Baier G. Protein kinase Cθ: a new essential superstar on the T-cell stage. Immunol Today. 2000;21:567–573. doi: 10.1016/s0167-5699(00)01749-7. [DOI] [PubMed] [Google Scholar]
- 2.Avraham A, Jung S, Samuels Y, Seger R, Ben-Neriah Y. Co-stimulation-dependent activation of a JNK-kinase in T lymphocytes. Eur J Immunol. 1998;28:2320–2330. doi: 10.1002/(SICI)1521-4141(199808)28:08<2320::AID-IMMU2320>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Bajpai U D, Zhang K, Teutsch M, Sen R, Wortis H H. Bruton's tyrosine kinase links the B cell receptor to nuclear factor kappaB activation. J Exp Med. 2000;191:1735–1744. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbazuk S M, Gold M R. Protein kinase C-delta is a target of B-cell antigen receptor signaling. Immunol Lett. 1999;69:259–267. doi: 10.1016/s0165-2478(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 5.Bendall H H, Sikes M L, Ballard D W, Oltz E M. An intact NF-kappa B signaling pathway is required for maintenance of mature B cell subsets. Mol Immunol. 1999;36:187–195. doi: 10.1016/s0161-5890(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann M, Hart L, Lindsay M, Barnes P J, Newton R. IκBα degradation and nuclear factor-κB DNA binding are insufficient for interleukin-1β and tumor necrosis factor-alpha-induced κB-dependent transcription. Requirement for an additional activation pathway. J Biol Chem. 1998;273:6607–6610. doi: 10.1074/jbc.273.12.6607. [DOI] [PubMed] [Google Scholar]
- 7.Briskin M, Damore M, Law R, Lee G, Kincade P W, Sibley C H, Kuehl M, Wall R. Lipopolysaccharide-unresponsive mutant pre-B-cell lines blocked in NF-kappa B activation. Mol Cell Biol. 1990;10:422–425. doi: 10.1128/mcb.10.1.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 9.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci USA. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtois G, Whiteside S T, Sibley C H, Israel A. Characterization of a mutant cell line that does not activate NF-kappaB in response to multiple stimuli. Mol Cell Biol. 1997;17:1441–1449. doi: 10.1128/mcb.17.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dienz O, Hehner S P, Droge W, Schmitz M L. Synergistic activation of NF-kappa B by functional cooperation between vav and PKCtheta in T lymphocytes. J Biol Chem. 2000;275:24547–24551. doi: 10.1074/jbc.C000177200. [DOI] [PubMed] [Google Scholar]
- 12.Francis D A, Karras J G, Ke X Y, Sen R, Rothstein T L. Induction of the transcription factors NF-kappa B, AP-1, and NF-AT during B cell stimulation through the CD40 receptor. Int Immunol. 1995;7:151–161. doi: 10.1093/intimm/7.2.151. [DOI] [PubMed] [Google Scholar]
- 13.Francis D A, Sen R, Rice N, Rothstein T L. Receptor-specific induction of NF-kappaB components in primary B cells. Int Immunol. 1998;10:285–293. doi: 10.1093/intimm/10.3.285. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari-Tabrizi N, Bauer B, Villunger A, Baier-Bitterlich G, Altman A, Utermann G, Uberall F, Baier G. Protein kinase Ctheta, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur J Immunol. 1999;29:132–142. doi: 10.1002/(SICI)1521-4141(199901)29:01<132::AID-IMMU132>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Gschwendt M, Muller H J, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 16.Hatada E N, Krappmann D, Scheidereit C. NF-kappaB and the innate immune response. Curr Opin Immunol. 2000;12:52–58. doi: 10.1016/s0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 17.Heissmeyer V, Krappmann D, Hatada E N, Scheidereit C. Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol Cell Biol. 2001;21:1024–1035. doi: 10.1128/MCB.21.4.1024-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heissmeyer V, Krappmann D, Wulczyn F G, Scheidereit C. NF-kappaB p105 is a target of IkappaB kinases and controls signal induction of Bcl-3-p50 complexes. EMBO J. 1999;18:4766–4778. doi: 10.1093/emboj/18.17.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H M, O'Rourke K, Boguski M S, Dixit V M. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J Biol Chem. 1994;269:30069–30072. [PubMed] [Google Scholar]
- 20.Ishida T, Kobayashi N, Tojo T, Ishida S, Yamamoto T, Inoue J. CD40 signaling-mediated induction of Bcl-XL, Cdk4, and Cdk6. Implication of their cooperation in selective B cell growth. J Immunol. 1995;155:5527–5535. [PubMed] [Google Scholar]
- 21.Ishida T K, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 23.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 24.Khoshnan A, Bae D, Tindell C A, Nel A E. The physical association of protein kinase C theta with a lipid raft-associated inhibitor of kappa B factor kinase (IKK) complex plays a role in the activation of the NF-kappa B cascade by TCR and CD28. J Immunol. 2000;165:6933–6940. doi: 10.4049/jimmunol.165.12.6933. [DOI] [PubMed] [Google Scholar]
- 25.Krappmann D, Hatada E N, Tegethoff S, Li J, Klippel A, Giese K, Baeuerle P A, Scheidereit C. The I kappa B kinase (IKK) complex is tripartite and contains IKK gamma but not IKAP as a regular component. J Biol Chem. 2000;275:29779–29787. doi: 10.1074/jbc.M003902200. [DOI] [PubMed] [Google Scholar]
- 26.Krappmann D, Wulczyn F G, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-kappaB inhibitor IkappaB alpha in vivo. EMBO J. 1996;15:6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 27.Lallena M J, Diaz-Meco M T, Bren G, Paya C V, Moscat J. Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Kang J, Friedman J, Tarassishin L, Ye J, Kovalenko A, Wallach D, Horwitz M S. Identification of a cell protein (FIP-3) as a modulator of NF-kappaB activity and as a target of an adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis. Proc Natl Acad Sci USA. 1999;96:1042–1047. doi: 10.1073/pnas.96.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin X, O'Mahony A, Mu Y, Geleziunas R, Greene W C. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J L, Chiles T C, Sen R J, Rothstein T L. Inducible nuclear expression of NF-kappa B in primary B cells stimulated through the surface Ig receptor. J Immunol. 1991;146:1685–1691. [PubMed] [Google Scholar]
- 31.Mains P E, Sibley C H. LPS-nonresponsive variants of mouse B cell lymphoma, 70Z/3: isolation and characterization. Somatic Cell Genet. 1983;9:699–720. doi: 10.1007/BF01539475. [DOI] [PubMed] [Google Scholar]
- 32.Meller N, Elitzur Y, Isakov N. Protein kinase C-theta (PKCtheta) distribution analysis in hematopoietic cells: proliferating T cells exhibit high proportions of PKCtheta in the particulate fraction. Cell Immunol. 1999;193:185–193. doi: 10.1006/cimm.1999.1478. [DOI] [PubMed] [Google Scholar]
- 33.Petro J B, Khan W N. Phospholipase C-gamma 2 couples Bruton's tyrosine kinase to the NF-kappaB signaling pathway in B lymphocytes. J Biol Chem. 2001;276:1715–1719. doi: 10.1074/jbc.M009137200. [DOI] [PubMed] [Google Scholar]
- 34.Petro J B, Rahman S M, Ballard D W, Khan W N. Bruton's tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–1754. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rooney J W, Dubois P M, Sibley C H. Cross-linking of surface IgM activates NF-kappa B in B lymphocyte. Eur J Immunol. 1991;21:2993–2998. doi: 10.1002/eji.1830211214. [DOI] [PubMed] [Google Scholar]
- 36.Rooney J W, Emery D W, Sibley C H. 1.3E2, a variant of the B lymphoma 70Z/3, defective in activation of NF-kappa B and OTF-2. Immunogenetics. 1990;31:73–78. doi: 10.1007/BF00661216. [DOI] [PubMed] [Google Scholar]
- 37.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 38.Ruland J, Duncan G S, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, Millar D G, Bouchard D, Wakeham A, Ohashi P S, Mak T W. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 39.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 40.Sun Z, Arendt C W, Ellmeier W, Schaeffer E M, Sunshine M J, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg P L, Littman D R. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 41.Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, Karin M, Nakanishi M. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 42.Villalba M, Kasibhatla S, Genestier L, Mahboubi A, Green D R, Altman A. Protein kinase Ctheta cooperates with calcineurin to induce Fas ligand expression during activation-induced T cell death. J Immunol. 1999;163:5813–5819. [PubMed] [Google Scholar]
- 43.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]