Abstract
Parkinson's disease (PD) is a neurodegenerative disorder that results when the dopaminergic neurons (DNs) present in the substantia nigra necessary for voluntary motor control are depleted, making patients with this disorder ideal candidates for cell replacement therapy. Human induced pluripotent stem cells (hiPSCs), obtained by reprogramming adult cells, possess the properties of pluripotency and immortality while enabling the possibility of patient-specific therapies. An effective cell therapy for PD requires an efficient, defined method of DN generation, as well as protection from the neuroinflammatory environment upon engraftment. Although similar in pluripotency to human embryonic stem cells (hESCs), hiPSCs differentiate less efficiently into neuronal subtypes. Previous work has shown that treatment with guggulsterone can efficiently differentiate hESCs into DNs. Our work shows that guggulsterone is able to derive DNs from hiPSCs with comparable efficiency, and furthermore, this differentiation can be achieved inside three-dimensional fibrin scaffolds that could enhance cell survival upon engraftment.
Keywords: tissue engineering, biomaterials, pluripotent stem cells, differentiation, neuroscience
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the progressive loss of the dopaminergic neurons (DNs) located in the substantia nigra. 1 These neurons are responsible for regulating voluntary movement through the neurochemical signaling of dopamine. Symptoms of this disease, including tremors, muscle rigidity, and impaired balance, vary with individuals. There is no long-term cure for PD, and the most effective treatment to date is the pharmaceutical agent levodopa, 2 which is a metabolic precursor of dopamine that works by replenishing the dopamine levels in the substantia nigra to restore function. 3 However, it becomes less effective over time, eventually causing the development of motor fluctuations (dyskinesias).
Cell replacement could serve as an ideal therapy for the treatment of PD as effective treatment requires replacing only the DNs of the substantia nigra. Both human pluripotent stem cells and neural stem cells possess the ability to renew themselves indefinitely and to differentiate into neurons, making them attractive options for cell therapies. Accordingly, human fetal and embryonic stem cell (hESC)-sourced grafts of DNs lead to improvement in symptoms of PD, but they require the controversial use of embryonic tissue along with additional immunosuppressive therapy to avoid rejection.4–7 Isolated from the inner cell mass of a blastocyst, hESCs have the potential to replace specific cells affected by injury or disease.8,9 However, in addition to posing the risk of immune rejection upon transplantation, their use raises ethical concerns as the blastocyst does not survive during cell extraction. Similar concerns arise when using fetus-derived cells.
Human induced pluripotent stem cells (hiPSCs) can serve as a promising alternative to these cell types. These iPSC lines were originally established by Takahashi et al. 10 and Okita et al. 11 , who demonstrated that pluripotency can be induced in adult mouse fibroblasts by introducing the transcription factors octamer-binding transcription factor 3/4 (OCT3/4), (SRY) sex determining region y-box 2 (SOX2), v-myc avian myelocytomatosis viral oncogene homolog (C-MYC), and kruppel-like factor 4 (KLF4), along with Nanog, to restore germ line competence. 11 hiPSCs offer the opportunity both to replace cells lost in PD with patient-specific cells and to study the individual pathophysiology of the diseaseto shed light on its causes and possible treatments.12–15 Additionally, recent advancements in gene-editing techniques make it possible to correct genetic mutations found in cell lines, enhancing the potential of hiPSCs for use in cell replacement therapies for the treatment of PD.13,14
Grafts of hiPSC-derived DNs demonstrate the ability to survive, innervate the degenerated striatum, and restore dopamine levels in animal models of PD.15–19 In these models, the number of DNs that survive upon engraftment and the extent to which they innervate the striatum are directly correlated with motor recovery,16,18,20 highlighting the importance of efficient derivation of DNs within these grafts. Moreover, undifferentiated hiPSCs within the graft could potentially contribute to tumor formation.15,16,19 Thus, to be clinically relevant, an efficient differentiation protocol for the derivation of a pure population of DNs from hiPSCs is required.
In PD, the substantia nigra is trapped in a self-perpetuating cycle of inflammation wherein activated microglia, consisting of the macrophages present in the central nervous system, engulf damaged neurons while releasing harmful reactive oxygen species and inflammatory cytokines. 21 Accordingly, engrafting DNs into rat models of PD results in poor survival (3%-20%) within 24 hours. 21 A second inhibitory aspect of the inflammatory environment is the promotion of astroglial cell differentiation by microglia-secreted cytokines, resulting in the development of astroglial tissue from engrafted neural progenitors (NPs). 21 Fibrin, a natural bioscaffold, can serve as a permissive environment for neural adhesion, proliferation, and differentiation while providing protection from the destructive aspects of the inflammatory site in vivo. 22 Formed at sites of injury by the enzymatic polymerization of the protein fibrinogen by thrombin and the cross-linking action of factor XIII, 23 fibrin possesses biochemical and mechanical cues similar to those of soft tissue. Fibrin-based scaffolds are compatible with stem cell cultures, and they are naturally degraded by cell-secreted proteases.23–27 Fibrin scaffolds can be further functionalized by the addition of drug delivery systems, allowing for the controlled release of morphogens such as neurotrophins. Such engineered fibrin scaffolds populated with ESC-derived and hiPSC-derived NPs allow for robust differentiation into neurons under inflammatory conditions present in the damaged central nervous system, with extensive neurite growth into host tissue and establishment of synaptic connections with host neurons.28–31
Recently, Gonzalez et al. 32 screened a small molecule library consisting of 1,120 biologically active compounds and found that the naturally occurring steroid guggulsterone was the most efficient inducer of DNs to date. In their study, hESC-derived NPs treated with fibroblast growth factor (FGF8) and sonic hedgehog (SHH) for 7 days, followed by treatment with the small molecule guggulsterone for 14 days, produced DNs with >97% efficiency, a significant increase over previous efficiencies obtained using xeno-free methods. Furthermore, they confirmed these neurons to be developing toward a DN fate by reverse transcription polymerase chain reaction (RT-PCR) and genetic profile analysis compared to substantia nigra tissue. These neurons increased dopamine production in culture by five times, and after 80 days of maturation in guggulsterone, these cells displayed electrophysical properties unique to DNs. 32
This study sought, primarily, to assess firstly the ability of guggulsterone to derive DNs from hiPSCs based on the protocol described by Gonzalez et al. 32 , and secondly, the ability of guggulsterone to derive DNs from hiPSCs seeded within fibrin scaffolds. hiPSCs were cultured in xeno-free, defined, and feeder-free conditions. Before treatment with gugglesterone, NPs were produced using two different protocols: 1) neuralization by an EB-like structure-formation step and 2) the TeSR-E6 method for neural rosette formation described by Lippmann et al. 33 After the neural rosettes were isolated, the cells were treated with FGF8 and purmorphamine (PUR, an activator of the SHH signaling pathway), followed by treatment with guggulsterone in the final stage of differentiation, as was done by Gonzalez et al. 32 (Fig. 1). These two-dimensional (2D) cultures were characterized for their dopaminergic properties. Neural aggregates (NAs) and rosettes produced using these protocols were also seeded into three-dimensional fibrin scaffolds and treated with FGF8, PUR, and guggulsterone for 14 days and then characterized accordingly.
Figure 1.

Overview of the two protocols used for differentiating human induced pluripotent stem cells (hiPSCs) into dopaminergic neurons (DNs) using guggulsterone. (A) Schematic representation for differentiating hiPSCs into DNs from neural aggregates (NAs), and (B) Schematic diagram for differentiating hiPSCs into DNs from neural rosettes derived using the TeSR-E6 method described by Lippmann et al. 33 FGF8 refers to fibroblast growth factor and PUR refers to the small molecule purmorphamine.
Materials
Vitronectin XF substrate, TeSR-E8 medium, TeSR-E6 medium, ReLeSR reagent, Aggrewell800 plates, neural induction medium (NIM), neural rosette selection agent, and antityrosine hydroxylase (anti-TH) antibody were all purchased from STEMCELL Technologies. Poly-L-ornithine, laminin, guggulsterone isotopes E and Z, normal goat serum (NGS), and Triton X-100 were purchased from Sigma Aldrich. FGF8, phosphate-buffered saline (PBS), secondary antibody AlexaFluor488 goat anti-mouse IgG, and thrombin were purchased from Life Technologies. Moreover, 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid stain was purchased from Invitrogen. PUR was purchased from Stemgent. Fibrinogen (plasminogen-depleted, human plasma) was purchased from Calbiochem. Formaldehyde and calcium chloride were purchased from Anachemia. Potassium chloride and Tris base were purchased from Caledon. Tris-HCl was purchased from BioBasic Canada Incorporated. The Human/Mouse Embryonic Stem Cell Multi-Color Flow Cytometry Kit was purchased from R&D Systems. The Dopamine Fast-Track ELISA kit was purchased from Labor Diagnostics Nord GmbH & Co. KG.
Methods
Culture of undifferentiated hiPSCs in defined conditions
hiPSCs (1-DL-01 line 34 obtained from WiCell Research Institute) were cultured under standard conditions consisting of 5% CO2 and 37 °C on Vitronectin XF substrate in the presence of TeSR-E8 medium as previously described. 22 Cells were passaged every 5 days using the ReLeSR™ reagent to dissociate the colonies and to obtain a pure population of undifferentiated hiPSCs for passage and for further differentiation studies.
Neural differentiation of hiPSCs using an aggregate-formation step
To induce differentiation into NPs, undifferentiated hiPSCs were seeded into the wells of Aggrewell 800 plates in the presence of NIM. After 5 days of culture, this process produces aggregates containing only NPs, which will be referred to as NAs. On Day 5, the NAs were collected and seeded at high density onto laminin (10 μg/mL)-coated plates, wherein they formed neural rosettes after 7 days or were seeded into fibrin scaffolds. The laminin-coated plates were produced by treating tissue culture plates with poly-L-ornithine (15 μg/mL) followed by treatment with laminin. After 7 days of culture, rosettes were collected using Neural Rosette Selection Reagent and seeded onto laminin-coated plates in the presence of NIM (Fig. 1A).
Neural differentiation of hiPSCs using the TeSR-E6 method
Neural rosettes were generated by the method of Lippmann et al. 33 , whereby neuralization was accomplished by switching to TeSR-E6 medium once confluency was reached in cultures seeded on Vitronectin XF surfaces. These cultures formed neural rosettes after 6 days, as previously described. 33 On Day 7, rosettes were collected using Neural Rosette Selection Reagent and plated onto poly-L-ornithine/laminin substrates until they reached confluency or were seeded into fibrin scaffolds (Fig. 1B).
Dopaminergic differentiation of NPs
To derive DNs, PUR (200 ng/mL) – an activator of the SHH pathway – and FGF8 (100 ng/mL) were added to cultures following the plating of NAs or rosettes in NIM for 14 days or 7 days, respectively. For the final stage of differentiation, NPs were cultured in NIM with guggulsterone isotopes E and Z (2.5 μM) for 38 days or 14 days (38 days for NA-derived NPs or 14 days for rosette-derived NPs; Fig. 1).
Characterization of hiPSCs using flow cytometry
Undifferentiated hiPSCs were collected and stained for SSEA4, SOX2 (pluripotency markers), and SSEA1 (differentiation marker). The cells were stained for SSEA4-carboxyfluorscein (CFS) with immunoglobulin G3 (IgG3)-CFS isotype control, Human/Mouse SSEA1 PerCP Mouse IgM (Clone MC-480) with Mouse IgM PerCP Control, and an SOX2-PE Mouse IgG2A (Clone 245610) with Mouse IgG2A-PE Isotype Control as per the manufacturer's instructions using the R&D Human/Mouse Embryonic Stem Cell Multi-Color Flow Cytometry Kit. Cells were incubated in the fixation/permeabilization buffer for 30 minutes, washed, and resuspended in permeabalization/wash buffer and 10 μL of each antibody for at least 45 minutes. Cells were then washed in permeabilization/wash buffer and resuspended in PBS. Data were collected using the Millipore Guava EasyCyte HT flow cytometer. Cells were gated to exclude small debris and clustered cells, and a maximum of 1,000 gated events were collected for each sample. All gains were set so that isotype controls were fluorescing below 10. 1 All analysis was completed using Guava-Soft EasyCyte software.
Immunostaining of cultures
Cells were fixed with 3.7% formaldehyde/PBS solution for 1 hour, followed by permeabilization in 0.1% Triton X-100 in PBS for 45 minutes at 4 °C, and blocked with 5% NGS in PBS for 2 hours at 4 °C. The cell cultures were incubated with the primary antibody anti-TH diluted 1:500 in 5% NGS overnight at 4 °C, followed by washes with PBS three times for 15 minutes each, and incubation with secondary antibody (AlexaFluor488 goat anti-mouse IgG diluted 1:200 in 5% NGS) for 4 hours at 4 °C. Cells were washed three times as previously described. For cells counter-stained with DAPI nucleic acid stain, a 300-nM DAPI solution in PBS was added to the cultures after the final wash and incubated for 3 minutes, followed by rinsing with PBS.
Phase contrast and fluorescence imaging
Cells were visualized with a Leica DMI 3000B microscope equipped with an XCite Series 120Q fluorescent light source and QImaging RETIGA 2000R camera at 100 × magnification. Images were captured using QCapture Software 2.9.12. Fluorescently labeled cells were visualized with a green filter for green fluorescent protein.
Time-lapse microscopy and quantitative analysis
Time-lapse microscopy images were taken with an IncuCyte ZOOM™ kinetic imaging system from Essen BioScience Videos and quantitative analysis of images for neurite length and branch points were done using Kinetic NeuroTrack™ Assay software. This software automatically processes IncuCyte ZOOM images to allow analysis of neurite dynamics, including average neurite length and the numbers of branch points present per axon in cultures that exhibit neuronal differentiation. This analysis was performed at Day 38 for neurons produced using an NA-based differentiation protocol and at Day 14 for neurons produced using the TeSR-6 differentiation protocol. Additionally, this software was used to image neurons produced using an NA-based differentiation protocol over Days 19–26 of the differentiation process (Supplementary Video 1).
Seeding in fibrin scaffolds
Fibrin scaffolds were prepared as previously described,23,35 with modified volumes of a 400-μL base and a 150-μL top, due to an increased rate of fibrin degradation seen with aggregates derived from hiPSC protocols, as opposed to murine iPSC-derived EB protocols. A single NA or rosette was placed between the base and top layers with 1mL of NIM being added to each well. The resulting cultures were analyzed as previously described.
Quantification of dopamine release
Supernatant was collected from hiPSCs cultured for 41 days and analyzed for concentration of dopamine using a dopamine-specific enzyme-linked immunosorption assay (ELISA) kit as per manufacturer's instructions, and the output read using TECAN infinite M200 Pro microtiter plate reader.
Statistical analysis
Statistical analysis was performed using the two-tailed Student's t-test with a 95% confidence level (α = 0.05) and P<0.05, and in the case of the dopaminespecific ELISA using a right-sided Student's t-test with a 95% confidence level (α = 0.05) and P<0.05. Results are presented as the mean ± standard deviation.
Results
Morphology of hiPSC-derived cultures
Characterization of the undifferentiated hiPSC cultures
The purity of the hiPSCs used for experiments plays an important role in the resulting differentiation. Accordingly, our undifferentiated hiPSC cultures were characterized both using microscopy and flow cytometry. Our hiPSCs were cultured in xeno-free, chemically defined conditions consisting of vitronectin-coated substrates in the presence of TeSR-E8 medium. Figure 2A shows that our hiPSCs formed colonies under these culture conditions as anticipated. Flow cytometry was also used to characterize these hiPSCs based on their expression of SSEA4 (glycolipid associated with pluripotency), Sox2 (transcription factor associated with pluripotency), and SSEA1 (glycolipid associated with pluripotency in mouse iPSCs and with differentiation in hiPSCs).
Figure 2.

Xeno-free culture of human induced pluripotent stem cells (hiPSCs) on recombinant vitronectin surfaces in the presence of E8 medium results in maintenance of pluripotency. (A) Phase-contrast image showing hiPSC colonies. Scale bar is 100 μm. Representative histograms showing flow cytometry analysis of our undifferentiated hiPSCs for the following markers: (B) SSEA4, a pluripotency marker based on carbohydrate epitopes expressed during oogenesis; (C) SOX2, a transcription factor that is essential for maintaining pluripotency; (D) SSEA1, a marker expressed by differentiating hiPSCs; (E) Time line of SSEA4, SOX2, and SSEA1 expression during neural differentiation.
Figure 2B–D shows representative histograms that result after flow cytometry for these markers. Figure 2E shows the time line of expression for these markers. Our hiPSC cultures were found to be pure, expressing high levels of the pluripotency markers SSEA4 (82.8% ± 2.1%) and SOX2 (88.8% ± 1.8%), with very little expression of differentiation marker SSEA1 (1.6% ± 0.7%) (n = 4 for all markers). The high level of purity obtained can be attributed to the use of ReLeSR reagent, a selective dissociation reagent used to target pluripotent stem cells for collection, leaving differentiating cells adhered to the substrate.
Characterization of neural rosettes produced using an NA-based method and the TeSR-E6 method
Neural rosettes were generated using one method that required NA formation and another method that avoids the use of an aggregate-formation step. Figure 3A shows successful formation of an NA after 24 hours of culture in an AggreWell800 plate. After 5 days of incubation in an Aggrewell plate, the resulting NPs were seeded at high density on to laminin-coated substrates and cultured in the presence of FGF8 and PUR. Figure 3B shows the morphology of the early-stage neural rosettes that formed 3 days after seeding, on Day 7. Figure 3C shows the morphology of these neural rosettes after 7 days of culture, on Day 13. Figure 3D shows the formation of early neural rosettes on Day 3 in the presence of TeSR-E6 medium on a 2D vitronectin-coated surface using a protocol that avoids an aggregate-formation step. Figure 3E shows the morphology of the early-stage neural rosettes that formed by Day 6. Figure 3F shows the morphology of these neural rosettes replated on laminin after 1 day of culture, on Day 7.
Figure 3.

The formation of neural rosettes from human induced pluripotent stem cells (hiPSCs) using two different methods. (A) Successful formation of neural aggregates (NAs) in an AggreWell 800 microwell. (B) Early-stage neural rosettes at Day 7 plated and cultured on a 2D laminin substrate. (C) Selected neural rosette, obtained using Neural Rosette Selection reagent, plated on a 2D laminin substrate at Day 13. (D) Early neural rosettes on Day 3 cultured on Vitronectin XF substrate in TeSR-E6 produced without an aggregate-formation step. (E) Neural rosettes at Day 6 cultured on Vitronectin XF substrate in TeSR-E6 medium. (F) Selected neural rosette, obtained using Neural Rosette Selection reagent, plated onto a 2D laminin substrate. All scale bars are 100 μm.
Long-term characterization of neural cultures produced using an NA-based method
After selection and plating of neural rosettes onto 2D laminin substrates, differentiation into DNs was performed as detailed in Figure 1A, including treatment with the small molecule guggulsterone from Day 19 onward. The morphology of the resulting cultures was tracked daily from Day 18 until Day 60 ((Fig. 4A-I). Figure 4J shows the extension of neurites at Day 60 as masked when using Neurotracker software analysis. Supplementary Video 1 shows this differentiation process occurring over time.
Figure 4.

Human induced pluripotent stem cell-derived neural progenitors (NPs) after the selection of neural aggregate (NA)-derived neural rosettes and seeding as single cells onto 2D laminin substrate in the presence of guggulsterone. (A) Neural progenitors (NPs) seeded at low density in Neural Induction Medium (NIM) supplemented with purmorphamine (PUR) and fibroblast growth factor 8 (FGF8) at Day 18; (B) NPs at high density in NIM with PUR and FGF8 at Day 20; (C) NPs in NIM treated with guggulsterone at Day 24; (D) NPs in NIM treated with guggulsterone for 14 days at Day 36; (E) NPs in NIM treated with guggulsterone for 17 days at Day 39; (F) NPs in NIM treated with guggulsterone at Day 43; (G) NPs in NIM treated with guggulsterone at Day 46; (H) NPs in NIM treated with guggulsterone at Day 53; (I) NPs in NIM treated with guggulsterone at Day 60; and (J) NPs in NIM treated with guggulsterone at Day 60 using Neurotracker software to generate neurite masking in purple and cell body masking in brown. All scale bars are 100 μm.
Long-term morphological characterization of neural cultures produced using the TeSR-E6 method
After selection and plating of neural rosettes onto 2D laminin substrates, differentiation into DNs was performed as detailed in Figure 1B, including treatment with the small molecule guggulsterone from Day 13 onward. The morphology of the resulting cultures was tracked daily from Day 10 until Day 28 (Fig. 5A–F). Figure 5G shows the extension of neurites at Day 28 as masked when using Neurotracker software analysis.
Figure 5.
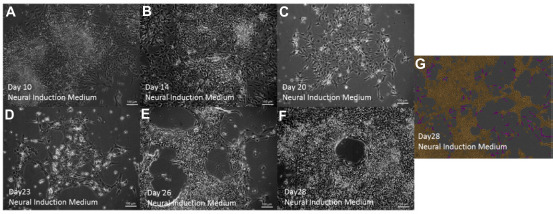
Human induced pluripotent stem cell-derived neural progenitors (NPs) after the selection of TeSR-E6 medium-derived neural rosettes and seeding as single cells onto 2D laminin substrates in the presence of guggulsterone. (A) Neural progenitors (NPs) in Neural Induction Medium (NIM) with purmorphamine (PUR) and fibroblast growth factor 8 (FGF8) at Day 10; (B) NPs in NIM treated with guggulsterone at Day 14; (C) NPs in NIM treated with guggulsterone for 6 days at Day 20; (D) NPs in NIM treated with guggulsterone at Day 23; (E) NPs in NIM treated with guggulsterone for 12 days at Day 26; (F) NPs in NIM treated with guggulsterone at Day 28; and (G) NPs in NIM treated with guggulsterone at Day 28 using Neurotracker software to generate neurite masking in purple and cell body masking in brown. All scale bars are 100 μm.
Quantitative analysis of neurite extension and branch points between cultures differentiated in DNs produced using the two differentiation methods
As detailed in the Methods section, Neurotrack software was used to quantitatively analyze the average length of neurites present and the average number of branch points found in the extended axons. Neurite length and branching were quantified after the final differentiation stage in both cultures (38 days in NIM with guggulsterone for NA-derived cultures, or 14 days in NIM with guggulsterone for E6 medium-derived cultures). Table 1 shows the results of this analysis. Average neurite length and the average number of neurite branching points were significantly higher in the culture produced using the differentiation protocol using an NA-formation step compared to the TeSR-E6 method. However, neurite extension and branching were observed in both cultures.
Table 1.
Quantification of neurite length and branching of human induced pluripotent stem cell-derived dopaminergic neurons after the final differentiation stage.
| PROTOCOL | AVERAGE NEURITE LENGTH (MM/MM) 2 | AVERAGE NUMBER OF NEURITE BRANCHING POINTS (1/MM) 2 |
|---|---|---|
| NA formation protocol (n = 16) | 7.21 ± 4.02 | 71.47 ± 38.79 |
| TeSR-E6 protocol (n = 81) | 1.77 ± 0.11 | 19.28 ± 1.07 |
Notes: Neurite length and branch point analysis of human induced pluripotent stem cell-derived dopaminergic neurons was conducted after 38 days in NIM with guggulsterone (NA formation-based protocol), or 14 days in NIM with guggulsterone (E6 medium-based protocol). Results are automatically normalized by Neurotracker software and presented with the standard deviation. Significance was determined using a two-tailed t-test with P = 0.05.
Immunostaining of hiPSC-derived neuronal cultures for dopaminergic markers produced using both protocols
At the end of the time points given in Figure 1, the resulting hiPSC-derived neuronal cultures were fixed and stained to determine whether these cells were expressing TH, the marker expressed by DNs. The NA-derived neurons matured for 38 days using guggulsterone showed nearly complete expressions of TH (Fig. 6A). Neuronal cultures produced by neuralizing hiPSCs in TeSR-E6 Medium were also positive for TH expression after 14 days of guggulsterone treatment (Day 28 of differentiation; Fig. 6B). Supplementary Figure 1 shows a negative control for TH staining as cells derived using an NA-formation step do not express TH after 17 days of culture.
Figure 6.

Human induced pluripotent stem cell-derived dopaminergic neurons (DNs) immunostained for dopaminergic marker tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine production. TH staining is indicated in green, cells were counterstained with DAPI (nuclear marker), which is shown in blue. (A) DNs derived using a protocol requiring a neural aggregate (NA) step on Day 60, and (B) DNs derived using the protocol requiring TeSR-E6 medium at Day 28. All scale bars are 100 μm.
Quantification of dopamine release from hiPSC-derived neuronal cultures
DNs regulate voluntary movement by dopamine secretion; thus their ability to release dopamine is an important feature of their physiology. Accordingly, supernatants collected from the hiPSC-derived neuronal cultures produced using an NA step were analyzed for their dopamine content relative to controls (undifferentiated hiPSCs), as these cultures had higher levels of neurite length and branching points as was determined by Neurotracker software analysis. The concentration of dopamine released from cells was determined using a dopamine-specific ELISA. The guggulsterone-treated cultures secreted 2.23 ± 0.29 ng/mL of dopamine (n = 4), which was a significant increase compared to supernatants collected from undifferentiated hiPSCs (1.29 ng/mL).
Seeding hiPSC-derived NPs into three-dimensional fibrin scaffolds
All of the aforementioned work was conducted using 2D substrates. If used as a cell therapy, these cells would be injected into the three-dimensional (3D) microenvironment present in the brain. As mentioned in the Introduction, the microenvironment present during PD presents a hostile environment for cell survival and integration. The use of a biomaterial scaffold can address these issues posed by the microenvironment and enhance cell survival and differentiation. This work determined whether hiPSC-derived NPs could be differentiated in neurons inside of 3D fibrin scaffolds. NAs generated using Aggrewells and neural rosettes produced using the TeSR-E6 method were seeded within 3D fibrin scaffolds, wherein they were exposed to FGF8, PUR, and guggulsterone for 14 days, followed by immunostaining for TH to confirm their dopaminergic identity (Fig. 7). Both NAs and rosettes got attached, then spread, and differentiated within the fibrin to express TH after 14 days. NAs seeded into scaffolds exhibited both robust spreading into the fibrin and TH expression (Fig. 7A–B). TH was also expressed in the NAs that did not spread, but to a lesser extent (n = 20). Of the rosettes seeded, 40% got attached, spread, and expressed TH, although not as robustly as the NAs (Fig. 7C-D). Those that did not spread when seeded as rosettes showed no TH expression (n = 22).
Figure 7.

Human induced pluripotent stem cells (hiPSCs) differentiated in fibrin scaffolds and immunostained for dopaminergic marker tyrosine hydroxylase (TH). (A) Neural aggregates (NAs) immunostained for TH after 14 days inside of 3D fibrin scaffolds and exposure to morphogenic factors fibroblast growth factor 8 (FGF8), purmorphamine (PUR), and guggulsterone; (B) Phase-contrast image of NAs after 14 days in fibrin and exposure to FGF8, PUR, and guggulsterone; (C) Neural rosettes derived by TeSR-E6 medium, immunostained for TH after 14 days in 3D fibrin scaffolds and exposure to FGF8, PUR, and guggulsterone; and (D) Phase-contrast image of neural rosettes derived by TeSR-E6 medium, after 14 days in fibrin and exposure to FGF8, PUR, and guggulsterone. All scale bars are 100 μm.
Discussion
Characterization of undifferentiated hiPSC cultures
The most commonly used protocols maintaining human pluripotent stem cells in an undifferentiated state require the use of mouse-derived Matrigel surfaces or feeder layers of mouse embryonic fibroblasts in combination with media formulations that contain bovine serum albumin. 35 These methods are undesirable because they contain products derived from animals (specifically mice and cows), which can be taken up by the human cells, increasing the possibility of immune rejection upon transplantation. 36 However, recent work has shown that chemically defined, xeno-free culture systems can be used to maintain human pluripotent stem cells in an undifferentiated state. 35 A pure population of undifferentiating, pluripotent stem cells at the onset of differentiation is important for efficient neuralization, because spontaneously differentiating hiPSCs have the potential to generate nonneural cell lineages, which can lead to tumor formation when engrafted. As shown in the Results section, the use of vitronectin surfaces in combination with TeSR-E8 medium maintains a high level of pluripotency. Such methods also lend themselves readily for applications in clinical translation due to their defined nature, which results in a high level of reproducibility. In addition, the use of ReLeSR dissociation reagent enables selection of a highly pure population of pluripotent stem cells for NA or rosette formation, ensuring a highly pure starting population in our cultures, as was determined by flow cytometry. The use of the ReLeSR reagent ensures that mainly undifferentiated cells were selected even though some differentiated cells are present in Figure 2A.
Morphology of hiPSC-derived neuronal cultures
As a further purification step, the use of a Neural Rosette Selection dissociation reagent selects only neural epithelial cells for engraftment and downstream differentiation (leaving behind neural crest cells). While both methods produced neural rosettes, the TeSR-E6 protocol requires significantly less time, which is an important consideration when developing a cell therapy. The amount of time required for differentiation should be minimized, which is why both protocols were evaluated. After a period of maturation, neurons produced using both protocols displayed a fusiform cell body and long dendritic processes typical of the hiPSC-derived DN phenotype, recently characterized in a comprehensive study by Hartfield et al. 37 As differentiation progressed, flat underlying cells gave rise to overlying clusters of cells with fusiform bodies and long processes (Supplementary Video 1). The observed morphologies were consistent with observations made by Yang et al. 38 in their study on the effects of in vitro conditions on transplanted embryonic DNs. Our quantitative analysis showed that the protocol involving an NA-formation step resulted in greater neurite length and increased branching compared to the results with the TeSR-E6 protocol. This difference could be due to the extended time period required for differentiation and it is possible that similar levels of branching could be observed with the TeSR-E6 protocol with additional culture time.
Immunostaining of hiPSC-derived neuronal cultures
The dopaminergic identity of the hiPSC-derived neurons was confirmed by immunostaining. After 38 days of maturation (60 days of differentiation), NA-derived neurons expressed TH at high levels comparable to the efficiency achieved by Gonzales et al. 32 using hESC-derived neurons. 33 Neurons derived using the TeSR-E6 medium protocol were also seen to express high levels of TH after only 14 days of maturation (28 days of differentiation) despite the shorter neurite extension and lesser degree of branching observed. The mechanism by which guggulsterone directs neural progenitors to become DNs has not been described to date, but it is interesting to note that previous studies have determined guggulsterone to be a potent inhibitor of the signal transducer and activator of transcription 3 (STAT3) pathway, an intracellular pathway responsible for directing neurons toward an astroglial fate, which is inhibited during neural differentiation by proneural pathway neurogeninl (Ngn1) and Mashl.39,40 A possible mechanism contributing to the efficiency of dopaminergic differentiation by guggulsterone may be inhibition of the STAT3 pathway, leading to greater yields of neurons.
Quantification of dopamine release from hiPSC-derived neuronal cultures
DNs communicate through dopamine secretion and uptake; thus their ability to release dopamine is an important feature of their physiology. hiPSC-derived neurons derived by NA formation secreted dopamine at Day 41 of differentiation. The amount of dopamine secreted, compared to controls (1.72 times more), was less than the amount reported with hESC-derived DNs by Gonzalez et al. 32 , who reported a five-fold increase in dopamine production. These results suggest that hiPSC-derived DNs may be slower to develop than their hESC-derived counterparts. These findings are consistent with the studies detailed in the Introduction regarding previous observed differences in differentiation efficiency observed between hESCs and hiPSCs. 26
Seeding hiPSC-derived NPs into 3D fibrin scaffolds
Both NAs and neural rosettes showed the ability to differentiate into DNs when seeded into fibrin scaffolds and exposure to guggulsterone, regardless of their stage of neural induction, illustrating that there is no restriction on the timing of guggulsterone exposure during differentiation. It is also important to note that these protocols could be successfully implemented in a 3D microenvironment that more closely mimics the environment that the cells would experience in vivo. However, NAs appeared to differentiate with greater efficiency, indicating a potential correlation between aggregate bodies and an enhanced ability to differentiate to a dopaminergic fate, which is consistent with previous findings of neurosphere-derived DNs in culture41,42 It was observed that the hiPSC-derived neural cultures also degraded the 3D fibrin scaffolds at a much quicker rate than that observed when mouse iPSC-derived NPs where seeded within 3D fibrin scaffolds as reported by Montgomery et al. 24 Future work will investigate the use of aprotinin, a protease inhibitor, to slow the rate of scaffold degradation. Overall, these findings suggest that fibrin scaffolds populated with hiPSC-derived NAs offer a promising way to deliver DNs as a cell therapy for PD.
Conclusion
This work demonstrated that protocols using guggulsterone to achieve DN differentiation can be successfully adapted for use with hiPSCs. Our protocol based on using an NA-formation step followed by treatment with FGF8, PUR, and guggulsterone can be used to achieve effective differentiation of hiPSCs into DNs, as suggested by the high levels of TH expression and the ability of hiPSC-derived DNs to secrete dopamine. Additionally, when seeded inside of fibrin-based scaffolds, these hiPSC-derived neuronal cells can adopt a dopaminergic fate as indicated by immunostaining. This combination of hiPSC-derived DNs and fibrin scaffolds could potentially serve as a way for restoring DNs lost in PD.
Author Contributions
Conceived and designed the experiments: LS, MR, SW, BRC. Analyzed the data: LS, MR. Wrote the first draft of the manuscript: MR. Contributed to the writing of the manuscript: MR, SW, SY, NG, EB. All authors agree with manuscript results and conclusions. Jointly developed the structure and arguments for the paper: MR, SW, SY. Made critical revisions and approved final version: SY, BRC, SW. All authors reviewed and approved of the final manuscript.
Supplementary Material
Supplementary Video 1
Morphology of human induced pluripotent stem cell-derived neural progenitors exposed to guggulsterone derived using the neural aggregate method during days 19–26 of differentiation. A cluster of flat underlying cells can be seen to give way over time to fusiform cells with neurite extensions. This video portrays a very low confluence culture to better image the neurite extensions and branching over time. We could not maintain the cells for long at this confluence as they exhibited very poor survival; therefore, this video is for the purpose of clarifying the morphology of the neurites during guggulsterone treatment and was not used as a supplement to any of the other results presented.
Supplementary Figure 1
Neurons differentiated from neural aggregate-derived neural rosettes at Day 17 of culture stain negative for tyrosine hydroxylase. These cultures have not yet been exposed to guggulsterone.
References
- 1.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003; 39(6): 889–909. [DOI] [PubMed] [Google Scholar]
- 2.Grimes D., Gordon J., Snelgrove B. et al. Canadian Nourological Sciences Federation. Canadian guidelines on Parkinson's disease. Can J Neurol Sci. 2012; 39(4 Suppl 4): S1–30. [DOI] [PubMed] [Google Scholar]
- 3.Nutt J. Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord. 2008 2008; 23: S580–4. [DOI] [PubMed] [Google Scholar]
- 4.Swijnenburg R.J., Schrepfer S., Govaert J.A. et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA. 2008; 105(35): 12991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freed C.R., Zhou W., Breeze R.E. Dopamine cell transplantation for Parkinson's disease: the importance of controlled clinical trials. Neurotherapeutics. 2011; 8(4): 549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindvall O., Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol Sci. 2009; 30(5): 260–7. [DOI] [PubMed] [Google Scholar]
- 7.Freed C.R., Greene P.E., Breeze R.E. et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001; 344(10): 710–9. [DOI] [PubMed] [Google Scholar]
- 8.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292(5819): 154–6. [DOI] [PubMed] [Google Scholar]
- 9.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S. et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282(5391): 1145–7. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K., Tanabe K., Ohnuki M. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131(5): 861–72. [DOI] [PubMed] [Google Scholar]
- 11.Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007; 448(7151): 313–7. [DOI] [PubMed] [Google Scholar]
- 12.Park I.H., Arora N., Huo H. et al. Disease-specific induced pluripotent stem cells. Cell. 2008; 134(5): 877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldner F., Laganière J., Cheng A.W. et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011; 146(2): 318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross C.A., Akimov S.S. Human-induced pluripotent stem cells: potential for neurodegenerative diseases. Hum Mol Genet. 2014; 23(R1): R17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee Y.H., Ko J.Y., Chang M.Y. et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011; 121(6): 2326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernig M., Zhao J.P., Pruszak J. et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA. 2008; 105(15): 5856–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swistowski A., Peng J., Liu Q. et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010; 28(10): 1893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hargus G., Cooper O., Deleidi M. et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA. 2010; 107(36): 15921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai J., Yang M., Poremsky E., Kidd S., Schneider J.S., Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010; 19(7): 1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björklund L.M., Isacson O. Regulation of dopamine cell type and transmitter function in fetal and stem cell transplantation for Parkinson's disease. Prog Brain Res. 2002; 138: 411–20. [DOI] [PubMed] [Google Scholar]
- 21.Li F., Zhu S., Wu C., Yan C., Liu Y., Shugan L. Neuroinflammation and cell therapy for Parkinson's disease. Front Biosci (Schol Ed). 2011; 3: 1407–20. [DOI] [PubMed] [Google Scholar]
- 22.Mohtaram N.K., Ko J., King C. et al. Electrospun biomaterial scaffolds with varied topographies for neuronal differentiation of human-induced pluripotent stem cells. J Biomed Mater Res A. 2014. [DOI] [PubMed] [Google Scholar]
- 23.Kolehmainen K., Willerth S.M. Preparation of 3D fibrin scaffolds for stem cell culture applications. J Vis Exp. 2012(61): e3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery A., Wong A., Gabers N., Willerth S.M. Engineering personalized neural tissue by combining induced pluripotent stem cells with fibrin scaffolds. Biomater Sci. 2015. 3, 401–13. [DOI] [PubMed] [Google Scholar]
- 25.Willerth S.M., Arendas K.J., Gottlieb D.I., Sakiyama-Elbert S.E. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006; 27(36): 5990–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willerth S.M., Faxel T.E., Gottlieb D.I., Sakiyama-Elbert S.E. The effects of soluble growth factors on embryonic stem cell differentiation inside of fibrin scaffolds. Stem Cells. 2007; 25(9): 2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willerth S.M., Rader A., Sakiyama-Elbert S.E. The effect of controlled growth factor delivery on embryonic stem cell differentiation inside fibrin scaffolds. Stem Cell Res. 2008; 1(3): 205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu P., Woodruff G., Wang Y. et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014; 83(4): 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P., Graham L., Wang Y., Wu D., Tuszynski M. Promotion of survival and differentiation of neural stem cells with fibrin and growth factor cocktails after severe spinal cord injury. J Vis Exp. 2014; (89): e50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson P.J., Tatara A., McCreedy D.A., Shiu A., Sakiyama-Elbert S.E. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010; 6(20): 5127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson P.J., Tatara A., Shiu A., Sakiyama-Elbert S.E. Controlled release of neu-rotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant. 2010; 19(1): 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez R., Garitaonandia I., Abramihina T. et al. Deriving dopaminergic neurons for clinical use. A practical approach. Sci Rep. 2013; 3: 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippmann E.S., Estevez-Silva M.C., Ashton R.S. Defined human pluripotent stem cell culture enables highly efficient neuroepithelium derivation without small molecule inhibitors. Stem Cells. 2014; 32(4): 1032–42. [DOI] [PubMed] [Google Scholar]
- 34.Yu J., Vodyanik M.A., Smuga-Otto K. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318(5858): 1917–20. [DOI] [PubMed] [Google Scholar]
- 35.Ko J., Mohtaram N.K., Ahmed F. et al. Fabrication of poly (-caprolactone) micro-fiber scaffolds with varying topography and mechanical properties for stem cell-based tissue engineering applications. J Biomater Sci Polym Ed. 2014; 25(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 36.Mohtaram N.K., Montgomery A., Willerth S.M. Biomaterial-based drug delivery systems for the controlled release of neurotrophic factors. Biomed Mater. 2013; 8(2): 022001. [DOI] [PubMed] [Google Scholar]
- 37.Hartfield E.M., Yamasaki-Mann M., Ribeiro Fernandes H.J. et al. Physiological characterisation of human iPS-derived dopaminergic neurons. PLoS One. 2014; 9(2): e87388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M., Donaldson A.E., Jiang Y., Iacovitti L. Factors influencing the differentiation of dopaminergic traits in transplanted neural stem cells. Cell Mol Neurobiol. 2003; 23(4-5): 851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn K.S., Sethi G., Sung B., Goel A., Ralhan R., Aggarwal B.B. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP–1. Cancer Res. 2008; 68(11): 4406–15. [DOI] [PubMed] [Google Scholar]
- 40.Wen S., Li H., Liu J. Dynamic signaling for neural stem cell fate determination. Cell Adh Migr. 2009; 3(1): 107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho M.S., Hwang D.Y., Kim D.W. Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc. 2008; 3(12): 1888–94. [DOI] [PubMed] [Google Scholar]
- 42.Schulz T.C., Noggle S.A., Palmarini G.M. et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004; 22(7): 1218–38. [DOI] [PubMed] [Google Scholar]


