Abstract
Previously we demonstrated that the class II phosphoinositide 3-kinase C2β (PI3K-C2β) is rapidly recruited to a phosphotyrosine signaling complex containing the activated receptor for epidermal growth factor (EGF). Although this association was shown to be dependent upon specific phosphotyrosine residues present on the EGF receptor, the underlying mechanism remained unclear. In this study the interaction between PI3K-C2β and the EGF receptor is competitively attenuated by synthetic peptides derived from each of three proline-rich motifs present within the N-terminal region of the PI3K. Further, a series of N-terminal PI3K-C2β fragments, truncated prior to each proline-rich region, bound the receptor with decreased efficiency. A single proline-rich region was unable to mediate receptor association. Finally, an equivalent N-terminal fragment of PI3K-C2α that lacks similar proline-rich motifs was unable to affinity purify the activated EGF receptor from cell lysates. Since these findings revealed that the interaction between the EGF receptor and PI3K-C2β is indirect, we sought to identify an adaptor molecule that could mediate their association. In addition to the EGF receptor, PI3K-C2β(2-298) also isolated both Shc and Grb2 from A431 cell lysates. Recombinant Grb2 directly bound PI3K-C2β in vitro, and this effect was reproduced using either SH3 domain expressed as a glutathione S-transferase (GST) fusion. Interaction with Grb2 dramatically increased the catalytic activity of this PI3K. The relevance of this association was confirmed when PI3K-C2β was isolated by coimmunoprecipitation with anti-Grb2 antibody from numerous cell lines. Using immobilized, phosphorylated EGF receptor, recombinant PI3K-C2β was only purified in the presence of Grb2. We conclude that proline-rich motifs within the N terminus of PI3K-C2β mediate the association of this enzyme with activated EGF receptor and that this interaction involves the Grb2 adaptor.
The products of phosphoinositide 3-kinase (PI3K) activity play an important role in the regulation of many cellular functions, including proliferation, viability, motility, and vesicle transport (24). The PI3K molecules responsible for their production form a large enzyme family whose catalytic activity in vitro and sequence alignment form the basis of their division into three classes (14). Class I PI3K enzymes are recognized as mediators of receptor signaling, and their role in intracellular signal transduction has been examined extensively (15). The class III PI3Ks, such as Vps34p, regulate vesicle traffic, most likely in a ligand-independent manner (45). In contrast, little is known about the cellular role of the class II PI3K enzymes. These enzymes have a higher molecular mass than either the class I or class III PI3K enzymes, and they are distinguished by the presence of a PX and C2 domain at their C termini. Recent studies have revealed that like the class I enzymes, class II PI3Ks also act downstream of receptors for growth factors (5), for chemokines (44), and for integrins (49). In addition to the p85-p110α heterodimer, two class II PI3K enzymes, PI3K-C2α and PI3K-C2β, are recruited to a phosphotyrosine containing signaling complex following stimulation of the epidermal growth factor (EGF) receptor (EGFR) (2).
The EGFR (ErbB-1) is one member of the ErbB kinase family that includes ErbB-2 (p185erbB2/neu), ErbB-3 (p180erbB3), and ErbB-4 (p180erbB4) (30, 40). When EGF binds EGFR, this interaction promotes receptor subunit homo- and heterodimerization. In turn, receptor dimerization activates intrinsic tyrosine kinase activity to create a series of phosphotyrosine docking sites for signaling molecules that include Grb2, Shc, Src, and phospholipase Cγ (11). Although PI3K activity copurifies with the EGFR, this receptor does not directly bind the class I PI3K adaptor p85 (9, 37). Instead, the p85-p110α complex binds ErbB-3, which heterodimerizes with the activated EGFR (35). Furthermore, p85 may also be recruited to EGFR via the Grb2-associated binder Gab-1 (41, 48). Recruitment of the class II PI3K enzyme PI3K-C2β is dependent upon three phosphotyrosine residues on the activated EGFR; Y992, Y1068, and Y1173 (2). However, it is unclear if PI3K-C2β interacts directly with the EGFR or if its association is dependent upon an as yet unidentified intermediary signaling molecule.
Characterization of PI3K-C2β has established that its N-terminal residues 1 to 331 are sufficient for its interaction with the activated EGFR. This sequence lacks phosphotyrosine binding motifs; instead it has three proline-rich regions that have the potential to bind SH3 containing adaptor molecules (20, 39). The adaptor Grb2 consists of a single, phosphotyrosine binding SH2 domain flanked by two polyproline binding SH3 domains (25). Recruitment of Grb2 to the EGFR following ligand addition has been described extensively, and its interaction is dependent upon phosphotyrosine residues Y1068 and Y1173 (4, 42). In addition, Grb2 can be recruited to the EGFR indirectly through its association with the adaptor protein Shc. Grb2 is recognized for its association with the guanine nucleotide exchange factor SOS and the sequential activation of the ras GTPase. However, the Grb2 adaptor also interacts with and may serve to translocate other signaling molecules that contain proline-rich motifs, including Gab-1. In this study we examine the role of proline-rich regions in the recruitment of PI3K-C2β to the activated EGFR and the contribution made by Grb2.
MATERIALS AND METHODS
Cell culture.
Stock cultures of mammalian cells were passaged every 3 to 4 days in 90-mm-diameter dishes (Nunc) using Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin/ml, and 100 μg of streptomycin/ml (Life Technologies). Cultures were incubated in a humidified atmosphere of 10% CO2–90% air at 37°C. For experimental use, cells were switched either to DMEM containing ITS supplement (Sigma) or to 0.2% FBS. After 16 to 48 h, cultures were confluent and quiescent.
Transient expression in mammalian cells.
HEK293 cells were grown to 50 to 60% confluence and transfected with cDNA constructs encoding 5′ EE epitope-tagged PI3K-C2β in pcDNA 3.0 (Invitrogen) using calcium phosphate. Cells were harvested 48 h posttransfection, and protein was isolated following cell lysis at 4°C using 1 ml of lysis buffer (10 mM Tris-HCl [pH 7.6], 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 100 μM Na3VO4, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride).
Generation of recombinant protein.
cDNA representing the N terminus of PI3K-C2β (residues 2 to 130, 2 to 143, 2 to 157, 2 to 255, and 2 to 298) was produced by PCR using oligonucleotides containing 5′ SmaI and 3′ EcoRI restriction sites. This allowed the products to be cloned in frame into similarly digested pGex-2T bacterial expression vector (Pharmacia). The encoded proteins were expressed in Escherichia coli at the C terminus of glutathione S-transferase (GST) according to the manufacturer's instructions. Full-length human Grb2 and isolated N-terminal (residues 1 to 58) and C-terminal (residues 159 to 217) SH3 domains were also produced in this way using oligonucleotides containing a BamHI restriction site at the 5′ end and an EcoRI site at the 3′ end. The fidelity of all cDNA produced by PCR was verified by sequence analysis (MRC CSC core facility; Imperial College School of Medicine). Proteins expressed by this method were purified to homogeneity using glutathione-Sepharose affinity chromatography (Pharmacia). They were then eluted from beads upon incubation with buffer containing excess glutathione (10 mM) that was later removed by dialysis.
Immunoprecipitation and affinity purification.
Cultures treated in the absence or presence of EGF (100 nM) at 37°C were lysed at 4°C using the Triton X-100-based lysis buffer. Lysates were clarified by centrifugation (13,000 × g, 20 min), and the supernatants were transferred to a fresh tube. Immunoprecipitations were performed at 4°C over 4 h, and the immune complexes were collected on protein A-Sepharose (Pharmacia). Beads were briefly centrifuged and washed three times in lysis buffer prior to further analysis. Immobilized fusion proteins or GST alone were also assayed for their ability to affinity purify proteins from mammalian cell lysates. Recombinant protein was recovered on glutathione-Sepharose beads (4 h, 4°C).
Western blotting.
Immunoprecipitates were extracted in sample buffer (200 mM Tris-HCl, 6% sodium dodecyl sulfate [SDS], 2 mM EDTA, 4% 2-mercaptoethanol, 10% glycerol, pH 6.8) and fractionated by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes that were blocked with 5% nonfat dried milk in phosphate-buffered saline (PBS) containing 0.05% Tween 20, pH 7.2, and then were incubated for 2 to 4 h with antibody in PBS-Tween containing 3% nonfat milk. Immunoreactive proteins were detected using either anti-mouse or anti-rabbit coupled horseradish peroxidase (Amersham) and visualized by ECL (Amersham).
Cell-free protein phosphorylation.
Immunoprecipitates were washed twice with lysis buffer and again with in vitro kinase buffer (20 mM HEPES [pH 7.5], 100 mM NaCl, 10 mM MgCl2, and 15 mM MnCl2) at 4°C. Following the addition of 10 μM ATP, immunoprecipitates were incubated in a total volume of 50 μl for 20 min at 30°C. Reactions were terminated upon addition of excess kinase buffer at 4°C.
Assay of phosphoinositide kinase activity.
Lipid kinase assays were performed in a total volume of 30 μl containing 20 mM HEPES (pH 7.4), 100 mM NaCl, 0.1 mM EGTA, 0.1 mM EDTA, and 200 μg of phosphatidylinositol/ml. After preincubating sonicated lipid with sample for 10 min, reactions were initiated upon addition of divalent cation (6 mM) and 100 μM ATP (0.2 μCi of [γ-32P]ATP). Assays were incubated at 30°C for 20 min and then were terminated with acidified chloroform:methanol (1:1 [vol/vol]). The extracted lipid products were fractionated by thin-layer chromatography. Phosphoinositides were visualized by autoradiography and were quantified by scanning densitometry. All assays were linear with respect to time and enzyme addition.
Inhibition of SH3 domain-mediated binding to proline-rich regions.
Peptides corresponding to each of three PI3K-C2β proline-rich motifs at residues 127 to 140 (KKLSPPPLPPRASI), 140 to 153 (IWDTPPLPPRKGSP), and 252 to 265 (SKTMPPQVPPRTYA) or a control peptide representing residues 69 to 82 (NSLSPLEGPPNHST) were synthesized, high-performance liquid chromatography purified, and lyophilized (Alta Bioscience, Birmingham, United Kingdom). After reconstitution in PBS and neutralization with NaOH (0.1 N), the concentration was determined spectrophotometrically. Each peptide (25 μM) was added to an EGFR–PI3K-C2β association assay.
RESULTS
The N terminus of PI3K-C2β but not PI3K-C2α affinity purifies the activated EGFR.
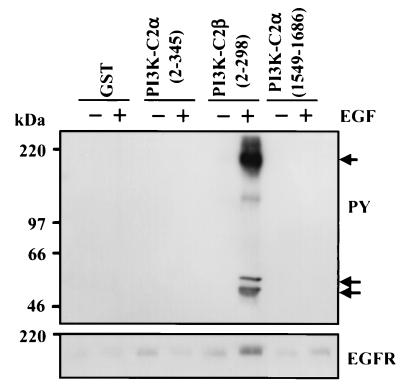
Lysates prepared from A431 cells were treated for 10 min in the presence or absence of EGF (100 nM). To these, recombinant protein representing GST, GST–PI3K-C2α(2-345), GST–PI3K-C2β(2-298), or GST–PI3K-C2α(1549-1686) (C2 domain) was added (10 μg) together with glutathione-Sepharose beads. After 4 h at 4°C, the beads were isolated by centrifugation and washed three times with lysis buffer. Associated proteins were extracted with sample buffer. Once fractionated by SDS-PAGE and transferred onto PVDF, proteins were Western blotted with anti-phosphotyrosine or anti-EGFR antibody. A major band at approximately 170 kDa representing phosphorylated EGFR (Fig. 1, upper panel) was isolated only with PI3K-C2β(2-298) from EGF-stimulated lysates. Importantly, the equivalent region of PI3K-C2α(2-345) was unable to bind EGFR in the same manner. Two minor phosphotyrosine-containing proteins at approximately 47 and 52 kDa were also isolated by the N-terminal PI3K-C2β fusion protein. Western blotting with anti-EGFR antibody confirmed that the major tyrosine phosphoprotein band represented EGFR (Fig. 1, lower panel).
FIG. 1.
The N terminus of PI3K-C2β but not PI3K-C2α interacts with the EGFR. A431 cells were incubated in the absence (−) or presence (+) of EGF (100 nM) for 10 min. Cell lysates were prepared and incubated with either GST, GST–PI3K-C2α(2-345), GST–PI3K-C2β(2-298), or GST–PI3K-C2α(1549-1686) for 4 h at 4°C. Fusion protein was isolated using glutathione-Sepharose beads, and associated proteins were fractionated by SDS-PAGE. Membranes were Western blotted with antiphosphotyrosine antibody (upper panel) or anti-EGFR antibody (lower panel). Arrows indicate the EGFR (approximately 180 kDa) and associated phosphoproteins. PY, phosphotyrosine.
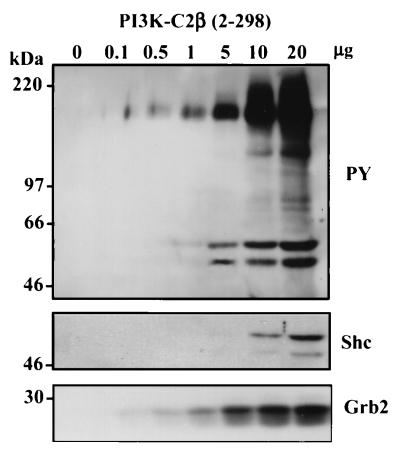
Both Shc and Grb2 are present with activated EGFR following its isolation by PI3K-C2β(2-298).
Lysates prepared from EGF-stimulated A431 cells were incubated for 4 h with various concentrations of recombinant PI3K-C2β(2-298) fusion protein as previously described. The fusion and associated proteins were recovered using glutathione-Sepharose beads and were Western blotted following fractionation by SDS-PAGE. Figure 2, upper panel, shows that the N-terminal fragment of PI3K-C2β affinity purifies the activated EGFR in a dose-dependent manner. Again, phosphoproteins at 47 and 52 kDa were clearly evident in these samples, their appearance correlating with increased EGFR binding. The association of the adaptor protein Shc with phosphorylated EGFR has been well documented, and it exists as isoforms with a molecular mass similar to that of the lower-molecular-mass phosphoproteins detected with the antiphosphotyrosine antibody. The presence of p47 and p54 Shc was confirmed using anti-Shc antibody (Fig. 2, middle panel). Similarly, Western blotting also demonstrated the presence of Grb2 in these samples (Fig. 2, lower panel).
FIG. 2.
PI3K-C2β(2-298) affinity purifies EGFR, Shc, and Grb2 in a dose-dependent manner. Lysates from EGF-stimulated A431 cells were incubated with various additions of recombinant GST–PI3K-C2β(2-298) protein as shown. After 4 h at 4°C the fusion and associated proteins were isolated by centrifugation and were washed. They were then extracted, fractionated by SDS-PAGE, and Western blotted with antiphosphotyrosine (upper panel), anti-Shc (middle panel), or anti-Grb2 (lower panel). PY, phosphotyrosine.
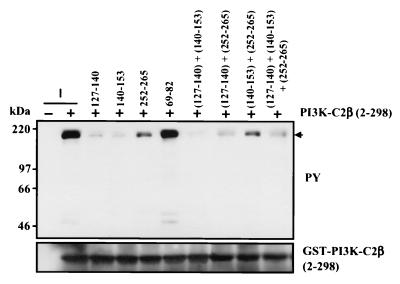
Association of PI3K-C2β(2–298) with activated EGFR is competitively inhibited by proline-rich peptides.
Since PI3K-C2β(2-298) but not the equivalent region of PI3K-C2α was able to affinity purify the activated EGFR, we examined the contribution of the proline-rich motifs that characterize this N-terminal fragment. Proline-rich regions with the consensus PXXP are established binding sites for SH3 domain-containing molecules (20). Since several signaling molecules that directly bind activated EGFR contain SH3 domains, they could potentially serve as docking sites for molecules with such proline-rich motifs. Lysates of A431 cells stimulated with EGF were preincubated with synthetic peptides derived from each of the three proline-rich regions in PI3K-C2β for 30 min at 4°C. Recombinant PI3K-C2β(2-298) was then added to these samples for 4 h. Fusion protein was then isolated using glutathione-Sepharose beads, washed, and fractionated by SDS-PAGE. Samples were Western blotted to reveal the association of phosphorylated EGFR. Figure 3 shows that in the presence of peptide derived from each PI3K-C2β proline-rich region, the interaction between PI3K-C2β(2-298) and the EGFR was markedly attenuated (residues 127 to 140, 91%; 140 to 153, 92%; and 252 to 265, 81%). In contrast, a control sequence (residues 69 to 82) exerted no inhibitory effect. When combinations of these peptides were used, the isolation of the EGFR could be completely abolished.
FIG. 3.
Polyproline-rich peptides competitively attenuate the association of PI3K-C2β with the EGFR. Peptides (25 μM) corresponding to each of the three PI3K-C2β polyproline-rich regions at residues 127 to 140 (KKLSPPPLPPRASI), 140 to 153 (IWDTPPLPPRKGSP) and 252 to 265 (SKTMPPQVPPRTYA) or control residues 69 to 82 (NSLSPLEGPPNHST) were added to lysates of EGF-stimulated A431 cells. Samples were incubated at 4°C, and recombinant PI3K-C2β(2-298) (5 μg) was added 30 min later together with glutathione-Sepharose beads. After 4 h at 4°C, fusion protein was isolated by centrifugation, fractionated by SDS-PAGE, and Western blotted with antiphosphotyrosine (upper panel) or anti-PI3KC2β antibody (lower panel). PY, phosphotyrosine.
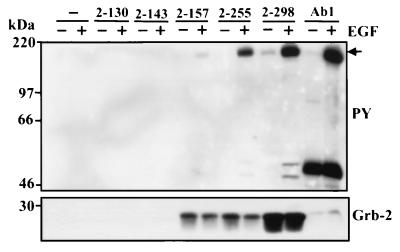
Affinity purification of the EGFR by N-terminal fragments of PI3K-C2β systematically lacking proline-rich motifs.
To further demonstrate the role of the proline-rich motifs in the association of PI3K-C2β with the EGFR, we used PCR to produce cDNA encoding a series of N-terminal fragments (residues 2 to 130, 2 to 143, 2 to 157, and 2 to 255) and compared the degree of receptor binding to the 2-298 construct. Fragments were truncated around each proline-rich region such that 2-130 has no proline-rich motifs, 2-143 has one, 2-157 and 2-255 have two, and 2-298 has three. Proteins were expressed as GST fusions and incubated in lysates of EGF-stimulated cells. Figure 4 (upper panel) shows that their ability to isolate the phosphorylated EGFR was dependent upon the number of proline-rich motifs each fragment contained. A minimum of two proline-rich motifs were required for receptor association, since the protein 2-143, containing the first proline-rich region alone, was unable to purify the EGFR. Interestingly, proteins representing residues 2 to 157 and 2 to 252 of PI3K-C2β both contained two proline-rich regions yet the latter bound EGFR with higher avidity. The reason for this discrepancy is unclear, since both proteins were able to bind endogenous Grb2 similarly (Fig. 4, lower panel).
FIG. 4.
N-terminal fragments of PI3K-C2β establish that two proline-rich motifs are required for association with EGFR. A series of GST fusion proteins representing residues 2 to 130, 2 to 143, 2 to 157, 2 to 255, and 2 to 298 were incubated for 4 h at 4°C with lysates of A431 cells that had been treated in the absence (−) or presence (+) of EGF (100 nM) for 10 min. For control, EGFR was also immunoprecipitated from these lysates (Ab1; Oncogene Science). Each fusion and associated protein was isolated using glutathione-Sepharose beads, washed, fractionated by SDS-PAGE, and Western blotted with antiphosphotyrosine antibody to visualize the activated EGFR (upper panel) and anti-Grb2 antibody (lower panel). PY, phosphotyrosine.
PI3K-C2β directly associates with Grb2.
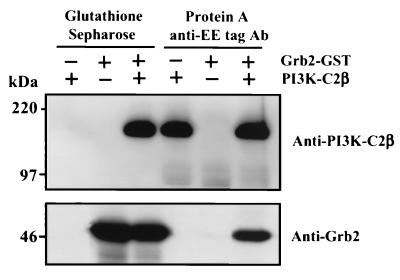
Although the results presented above demonstrated that the interaction between the N terminus of PI3K-C2β and EGFR is dependent upon proline-rich regions, they did not identify the intermediary protein responsible. A consistent finding in our Western blots is the presence of the SH2/SH3 domain-containing adaptor Grb2 in these PI3K-C2β complexes (Fig. 2 and 4). Although this might have been a consequence of isolating the activated EGFR to which Grb2 is recruited, we examined whether EE epitope-tagged PI3K-C2β directly bound recombinant Grb2 in vitro. PI3K-C2β expressed in HEK293 cells was isolated with anti-EE tag antibody on protein A-Sepharose and was eluted from the beads using an excess of a peptide derived from the epitope tag. Aliquots of the PI3K enzyme (1 μg) were added to lysis buffer containing recombinant Grb2 GST (1 μg). Following a 4-h incubation at 4°C, either PI3K-C2β was isolated using anti-EE tag antibody and protein A-Sepharose or GST-Grb2 was isolated with glutathione-Sepharose beads. Figure 5 demonstrates that isolation of both Grb2-GST and epitope-tagged PI3K-C2β reciprocally copurified the other protein.
FIG. 5.
Recombinant Grb2 and PI3K-C2β associate in vitro. Grb2-GST fusion protein (1 μg) was added to Triton lysis buffer (500 μl) in the absence or presence of EE epitope-tagged PI3K-C2β (1 μg) and either glutathione-Sepharose beads or anti-EE tag antibody (Ab) and protein A-Sepharose. After incubation at 4°C for 4 h, beads were isolated by centrifugation and washed. Associated proteins were extracted, fractionated by SDS-PAGE, and Western blotted with either anti-PI3K-C2β antibody (upper panel) or anti-Grb2 antibody (lower panel) and visualized with ECL.
Both the N-terminal and C-terminal Grb2 SH3 domains bind PI3K-C2β proline-rich regions.
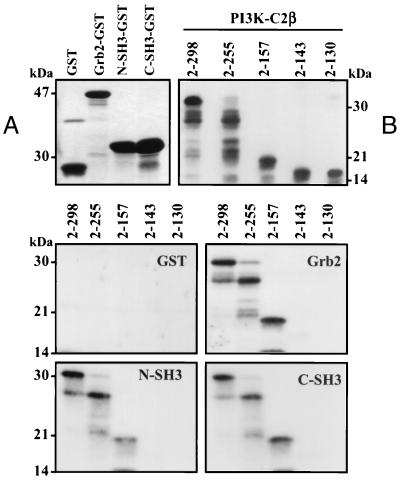
cDNA encoding both the N-terminal (residues 1 to 58) and C-terminal (residues 159 to 217) SH3 domains of Grb2 were amplified by PCR, subcloned into pGex2T vector, and expressed as GST fusion proteins. Figure 6A shows aliquots of each soluble fusion protein extracted, fractionated by SDS-PAGE, and visualized by Coomassie blue staining. Soluble PI3K-C2β N-terminal fragments were produced upon their cleavage from GST with thrombin. Aliquots were fractionated by SDS-PAGE and Western blotted with anti-PI3K-C2β antisera to demonstrate antibody cross-reaction (Fig. 6B). Each SH3 domain fusion, full-length Grb2, or GST alone was incubated (2 h, 4°C) with the N-terminal PI3K-C2β fragments. Following recovery with glutathione-Sepharose, the GST fusions together with their associated proteins were fractionated by SDS-PAGE and Western blotted with anti-PI3K-C2β antibody. The middle and lower panels of Fig. 6 show that no PI3K-C2β N-terminal fragments bound GST. Fragments containing either two proline-rich regions (residues 2 to 157 and 2 to 255) or three proline-rich regions (residues 2 to 298) were affinity purified with full-length Grb2 and each SH3 domain. The fragment lacking a proline-rich region (residues 2 to 130) or containing only one (residues 2 to 143) did not bind Grb2 or either SH3 domain.
FIG. 6.
Both N-terminal and C-terminal Grb2 SH3 domains (N-SH3 and C-SH3, respectively) bind PI3K-C2β. The N-terminal and C-terminal SH3 domains of Grb2 together with full-length Grb2 adaptor were expressed as GST fusion proteins and visualized by Coomassie blue staining following SDS-PAGE (panel A). The N-terminal PI3K-C2β fragments 2-130, 2-143, 2-157, 2-255, and 2-298 were expressed as GST fusion proteins, purified using glutathione-Sepharose beads, and cleaved with 5 μg of thrombin/ml (2 h, 21°C). Each protein was fractionated by SDS-PAGE and Western blotted with anti-PI3K-C2β antisera to confirm cross-reaction (panel B). Aliquots of each PI3K-C2β N-terminal fragment were incubated (2 h, 4°C) with either GST, full-length Grb2, or N-terminal or C-terminal Grb2 SH3 domain. Each GST fusion was isolated with glutathione-Sepharose beads, washed, and fractionated by SDS-PAGE. The N-terminal fragments of PI3K-C2β were visualized by Western blotting with anti-PI3K-C2β antibody.
Grb2 association with PI3K-C2β in intact cells.
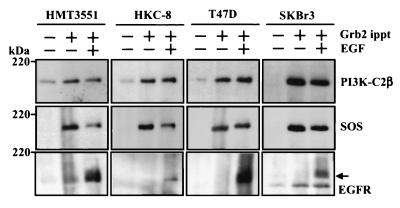
Confluent and quiescent cultures of human breast cancer cell lines (HMT3551, T47D, and SKBr3) and a human renal tubular epithelial cell line (HKC-8) were stimulated with EGF (100 nM) for 10 min. Lysates were prepared and incubated with anti-Grb2 antibody (Santa Cruz) and protein A-Sepharose beads for 4 h at 4°C. After isolation and washing, the resultant immune complexes were extracted, fractionated by SDS-PAGE, and Western blotted with antisera to either PI3K-C2β (Fig. 7, upper panels), SOS1/2 (Santa Cruz) (middle panels), or anti-EGFR (Santa Cruz) (lower panels). In each cell type, both PI3K-C2β and SOS were constitutively associated with Grb2. Following EGF stimulation, binding of Grb2 to EGFR was observed and PI3K-C2β exhibited a slight but consistent retardation of its electrophoretic mobility. This effect was more evident with SOS.
FIG. 7.
Isolation of PI3K-C2β, SOS, and EGFR in anti-Grb2 immunoprecipitates (ippt). Confluent and quiescent cultures of human renal epithelial cells (HKC-8) and breast cancer cells (HTM3551, T47D, and SKBr3) were incubated with (+) or without (−) EGF (100 nM) for 10 min. Lysates were prepared to which anti-Grb2 antibody (Santa Cruz C-23) was added for 4 h at 4°C. Immune complexes were isolated following addition of protein A-Sepharose and centrifugation. Proteins were extracted, fractionated by SDS-PAGE, and Western blotted with antisera to PI3K-C2β (upper panels), SOS 1/2 (D21; Santa Cruz) (middle panels), and EGFR (1005; Santa Cruz) (lower panels).
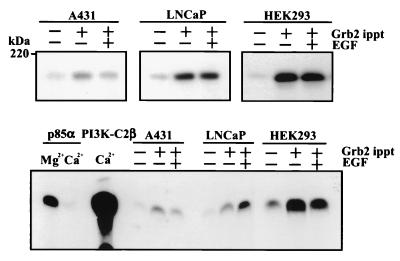
Cultures of a squamous cell carcinoma cell line (A431), a prostate cancer cell line (LNCaP), and a human embryonic kidney cell line (HEK293) transfected with PI3K-C2β were also examined. Following incubation with EGF, lysates were prepared and similarly immunoprecipitated with anti-Grb2 antibody. Immune complexes were then isolated and aliquots were either fractionated by SDS-PAGE and Western blotted with anti-PI3K-C2β antisera (Fig. 8, upper panel) or used for a phosphoinositide kinase assay using PtdIns as the substrate and Ca2+ as the divalent cation (lower panel). In agreement with data presented in Fig. 7, PI3K-C2β was constitutively associated with Grb2. The lower panel of Fig. 8 shows that the lipid kinase activity associated with anti-p85α immunoprecipitates from A431 cell lysates was abolished when Ca2+ instead of Mg2+ was used as the divalent cation in the kinase reaction. The catalytic activity of recombinant PI3K-C2β and anti-Grb2 immunoprecipitates was evaluated in the presence of Ca2+. Results obtained with each cell line support the constitutive association of PI3K-C2β with Grb2 identified by Western blotting. Intriguingly, the effect of EGF stimulation differed depending on the cell type examined. In A431 and HEK293 cells, EGF stimulation slightly decreased the activity while in LNCaP cells catalytic activity in the Grb2 immunoprecipitates was elevated.
FIG. 8.
Grb2 associates with PI3K-C2β in vivo. Cultures of confluent and quiescent A431, LNCaP, and HEK293 cells that overexpress epitope-tagged PI3K-C2β were incubated in the absence (−) or presence (+) of EGF (100 nM) for 10 min. Lysates were prepared and immunoprecipitated (ippt) with anti-Grb2 antisera for 4 h at 4°C. Immune complexes were isolated with protein A-Sepharose. These were either extracted, fractionated by SDS-PAGE, and Western blotted with anti-PI3K-C2β antisera (upper panels) or used for lipid kinase assay with PtdIns as the substrate and Ca2+ as the divalent cation (lower panels). The activity of recombinant PI3K-C2β is shown as a control, as is the class IA PI3K activity immunoprecipitated from A431 cell lysates using anti-p85α antibody.
Association with Grb2 increases the specific activity of the PI3K-C2β enzyme in vitro.
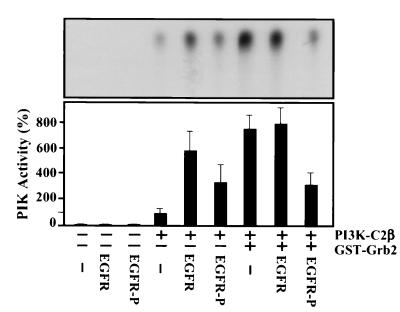
To assess the functional significance of the observed interaction between Grb2 and PI3K-C2β, we examined its effect upon the catalytic activity of the PI3K enzyme. Aliquots of recombinant EE-tagged PI3K-C2β (100 ng) were incubated in PI3K assay buffer in the presence or absence of GST-Grb2 (100 ng) for 2 h at 4°C. This mixture was added to EGFR immunoprecipitated from cultures of quiescent A431 cells and subsequently autophosphorylated in vitro upon addition of ATP. Controls included GST and mock EGFR immunoprecipitates. Incubation was continued for a further 1 h. At the end of this time, PtdIns was added and a lipid kinase assay was performed in the presence of [γ-32P]ATP. Figure 9 shows that association of PI3K-C2β with Grb2 produced a greater than sevenfold increase in the catalytic activity of this PI3K enzyme. This stimulation did not further increase in the presence of nonphosphorylated receptor, although phosphorylated EGFR attenuated this effect (3.3-fold potentiation). Unexpectedly, the presence of EGFR immunoprecipitated from quiescent cells alone also markedly stimulated PI3K-C2β enzyme activity (5.8-fold). Using phosphorylated receptor, this effect was also less marked (3.5-fold).
FIG. 9.
Grb2 increases the catalytic activity of PI3K-C2β. Aliquots of recombinant EE-tagged PI3K-C2β (100 ng) were incubated in PI3K assay buffer for 2 h at 4°C with either Grb2-GST fusion protein (100 ng) or GST. After this time, immobilized EGFR previously immunoprecipitated (Ab-1; Oncogene Science) from lysates of quiescent A431 cells and autophosphorylated in vitro or mock control was added. Incubation was continued for a further 1 h. Following addition of kinase buffer, phosphatidylinositol (200 μg/ml) and [γ-32P]ATP, samples (50 μl) were incubated at room temperature for 20 min. Radiolabeled phosphoinositides were extracted and aliquots were fractionated by thin-layer chromatography and visualized by autoradiography. Representative data are shown in the upper panel. Quantification of PtdIns3P was undertaken by scanning densitometry. In the lower panel, data are displayed as means ± standard errors of the means (n = 6). Under the conditions used, all reactions displayed linear kinetics.
Formation of a EGFR-Grb2–PI3K-C2β complex in vitro.
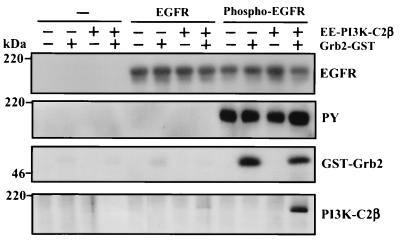
Finally, we examined whether a complex containing EGFR, Grb2, and PI3K-C2β could be formed in vitro. As described above, recombinant EE-tagged PI3K-C2β was incubated with GST-Grb2 for 2 h at 4°C. EGFR was immunoprecipitated from quiescent A431 cultures and autophosphorylated in vitro. Aliquots of phosphorylated and nonphosphorylated receptors were then added and the incubation was continued for a further 1 h. The EGFR and associated proteins were isolated by centrifugation, washed with lysis buffer, and extracted. After fractionation by SDS-PAGE, proteins were Western blotted to determine the presence of EGFR, phosphorylated EGFR, Grb2, and PI3K-C2β. Figure 10 shows that recombinant GST Grb2 associated only with immunoprecipitated EGFR that was autophosphorylated in vitro (Phospho-EGFR). Critically, PI3K-C2β only associated with phosphorylated EGFR in the presence of GST-Grb2. No significant association of this PI3K to nonphosphorylated EGFR was evident under these conditions.
FIG. 10.
Formation of the EGFR-Grb2–PI3K-C2β complex in vitro. Recombinant EE-tagged PI3K-C2β (100 ng) was incubated in lysis buffer for 2 h at 4°C in the absence or presence of Grb2-GST fusion protein (100 ng) or GST. Immobilized EGFR, isolated by immunoprecipitation (Ab1; Oncogene Science) from lysates of confluent and quiescent cultures of A431 cells, was phosphorylated for 30 min at 30°C in protein kinase buffer upon addition of ATP. Either phosphorylated EGFR, nonphosphorylated EGFR, or mock immunoprecipitate was added to the Grb2–PI3K-C2β sample, and the incubation was continued for a further 1 h. Beads containing immobilized receptor and associated proteins were isolated by centrifugation, washed, fractionated by SDS-PAGE, and Western blotted with either anti-EGFR antibody, antiphosphotyrosine, anti-Grb2, or anti-PI3K-C2β antibody. PY, phosphotyrosine.
DISCUSSION
In this study we sought to delineate the binding site on PI3K-C2β involved in recruiting this enzyme to the activated EGFR (2). Although the N terminus of this PI3K enzyme mediates this association, it lacks any domain that can directly bind the receptor. We have produced three independent pieces of data to demonstrate the involvement of its proline-rich motifs in this effect. Figure 1 shows that the N-terminal fragment of PI3K-C2β but not PI3K-C2α affinity purified activated EGFR from cell lysates. The N terminus of PI3K-C2α (13) and PI3K-C2β both contain motifs that form the P-X-X-P consensus that favors interaction with SH3 domains (20, 39). Secondly, peptides based on each proline-rich region were able to competitively attenuate the interaction between PI3K-C2β and EGFR (Fig. 3). In contrast, a control peptide based on an adjacent sequence had no effect. This approach was successfully used in many previous studies to define the sites of protein-protein interaction (3, 4, 28). Finally, fragments of the PI3K-C2β N terminus expressed as GST fusions demonstrated that either two proline-rich motifs are required for EGFR binding or the proline-rich motif at residues 144 to 149 is critical for this interaction (Fig. 4). However, data presented in Fig. 3 show that peptides derived from all three proline-rich motifs are equally able to displace EGFR binding, making this latter possibility less likely.
Given the importance of the proline-rich motifs in PI3K-C2β–EGFR association we sought to identify a molecule that could mediate this interaction. From our earliest experiments we recognized that both the Shc and Grb2 adaptor proteins were isolated by the PI3K-C2β N terminus together with EGFR from cell lysates (Fig. 2 and 4). Of these two molecules, only Grb2 contains an SH3 domain capable of binding proline-rich motifs. The Grb2 adaptor (also termed Ash) is the human homologue of the Caenorhabditis elegans protein Sem-5 and consists of a single SH2 domain flanked by two SH3 domains. Recruitment of Grb2 to activated EGFR is mediated by the SH2 domain and can occur either directly or via phosphorylated Shc (4, 7). The degree to which Grb2 associates directly with the EGFR or indirectly via Shc appears to be dependent upon the cell type examined, although direct interaction with the receptor is often favored (36). Each SH3 domain preferentially binds proteins containing proline-rich motifs that adopt a left-handed polyproline type II helix with the minimal consensus sequence P-X-X-P (33). However, structural and binding studies have since refined this consensus to P-X-X-P-X-R (6). Each of the three proline-rich motifs present within the N terminus of PI3K-C2β obeys this consensus. The best-characterized binding partner of the Grb2 SH3 domain is the nucleotide exchange factor SOS that in turn acts as a positive regulator of p21ras (6, 7). Many studies have demonstrated that the Grb2 SH3 domains bind effectors in addition to SOS. These include adaptor proteins (Gab 1 and 2, Cbl, and Slp-76) (21, 26, 41, 48), phosphotyrosine phosphatases (SHP-2, PEST) (10, 47), serine/threonine kinases (MEKK1) (34), several proteins implicated in cytoskeletal reorganization (dynamin, N-Wasp) (20, 27), receptors (CD28) (29), and other guanine nucleotide exchange factors for ras-related proteins (Vav, C3G) (38, 43).
We show that recombinant Grb2 directly binds full-length PI3K-C2β in vitro (Fig. 5). Data presented in Fig. 6 demonstrate that both the N-terminal and C-terminal Grb2 SH3 domain can bind PI3K-C2β although, in agreement with Fig. 4, two proline-rich regions were required. It remains unclear why Grb2 binds PI3K-C2β(2-157) and PI3K-C2β(2-255) with equal affinity yet EGFR binding was significantly greater using fragment 2-255. It suggests that the association of Grb2 and PI3K-C2β occurs independently of the EGFR and that this complex forms first within the cell before translocating to the activated receptor. Although it is possible that residues 158 to 255 of PI3K-C2β contain a motif that stabilizes the PI3K-C2β–EGFR interaction, we have expressed this region as a GST fusion protein and found that it does not bind phosphorylated EGFR independently (data not shown). Constitutive interaction between endogenous Grb2 and PI3K-C2β was also demonstrated when the PI3K enzyme was identified in immunoprecipitates of Grb2 from numerous cell lysates (Fig. 7 and 8), including one cell line (HEK293) that was transfected with recombinant PI3K-C2β. Figure 7 shows that while EGF stimulation translocates Grb2 to the activated EGFR, it does not alter the stoichiometry of PI3K-C2β or SOS binding, nor does it display a dramatic effect on the catalytic activity of the PI3K enzyme in situ (Fig. 8). In contrast, data presented in Fig. 9 show that the interaction of Grb2 and immunoprecipitated EGFR dramatically increased (sevenfold) PI3K-C2β lipid kinase activity. The effect of EGFR was unexpected, given that the receptor does not directly bind PI3K-C2β. However, since the EGFR was isolated by immunoprecipitation from cell lysates, these preparations might contain associated molecules that exert this effect. The increase in specific activity conferred by EGFR and Grb2 was neither synergistic nor additive, suggesting that they share the same mechanism of activation. It is also unclear why addition of phosphorylated EGFR produced a lesser activation than nonphosphorylated receptor. However, this finding was consistent with data presented in Fig. 8 for A431 and HEK293 cells.
Further studies are required to determine if Grb2 is the only adaptor able to mediate association of PI3K-C2β to EGFR. Other SH3 domain-containing proteins recruited to the activated EGFR may also play a role. Potential candidates include Nck (17), phospholipase Cγ, ras GTPase-activating protein GAP1(m), and vav-2 (31). Since our data suggest the need for at least two proline-rich motifs (Fig. 4 and 6), it seems unlikely that an adaptor containing a single SH3 domain could mediate the interaction between EGFR and PI3K-C2β without the formation of a higher-order complex. Indeed, despite the presence of an SH3 domain, p85 adaptor protein cannot bind PI3K-C2β (1). Our previous failure to identify a protein that coimmunoprecipitates with either PI3K-C2α or PI3K-C2β in high stoichiometry contrasts with the association of class IA catalytic subunits and the p85 adaptor (2). However, those experiments were performed using antisera directed against the N-terminal sequence of the class II PI3K enzymes. Consequently, it is possible that proteins associated with PI3K-C2β could block its antigenic epitopes or that an excess of antibody competes with binding partners for similar or overlapping epitopes, resulting in their dissociation.
Important questions remain regarding the significance of class II PI3K recruitment for biological responses downstream of EGFR. One possibility is their involvement in the regulation of receptor internalization. Although the mechanism of EGFR internalization has attracted particular attention, little is understood about which signals trigger their recruitment into clathrin-coated vesicles. Pharmacological inhibition using wortmannin clearly demonstrates a role for PI3K activity (22, 23), and we have recently confirmed the presence of PI3K-C2α in clathrin-coated vesicle preparations and its ability to regulate clathrin-mediated endocytosis and sorting in the trans Golgi network (12, 16). Ligand binding triggers recruitment of EGFR to clathrin-coated pits and its subsequent delivery to lysosomes for degradation. Experiments using fluorescent GFP-tagged p85α and a chimeric EGFR–ErbB-3 receptor show that despite recruitment of the class I PI3K adaptor and a clustering of staining into patches, there was no evidence of receptor internalization or its association with clathrin-containing endosomes following EGF stimulation (19). Interestingly, Grb2 is also required for efficient receptor endocytosis (46). Characterization of the FYVE domain (8, 18, 32) has illustrated how PI3K-dependent vesicle trafficking is regulated by generation of PtdIns3P rather than by PtdIns(3,4)P2 or PtdIns(3,4,5)P3, which are considered to be the principle products of class IA PI3K enzyme activity. Although production of PtdIns3P has been largely attributed to the class III PI3K vps34p homologue, the class II PI3K enzymes may also contribute to its generation in vivo and therefore play a regulatory role in these events.
Since both PI3K-C2α and PI3K-C2β share an extensive and overlapping tissue distribution and play a role downstream of several receptors, it is important to establish if they are regulated in a similar or disparate manner. Although both class II PI3K isozymes lie downstream of the activated EGFR, the results of this study demonstrate that PI3K-C2β and not PI3K-C2α is recruited to the receptor by a mechanism involving proline-rich regions and the Grb2 adaptor. Consequently, this observation offers scope for the differential regulation of PI3K-C2α and PI3K-C2β catalytic activity and thus the development of compounds to selectively antagonize the action of each class II PI3K isozyme.
ACKNOWLEDGMENTS
We thank Tony Pawson for his generous provision of human Grb2 pcDNA3.0 cDNA. We are grateful to Charles Coombes, Imperial College School of Medicine, for provision of the T47D, SKBr3, and HMT3522 breast cancer cell lines and to L. Racusen, The John Hopkins University School of Medicine, Baltimore, Md., for the human renal tubular epithelial cell line HKC-8.
This work was supported by grants from The Wellcome Trust and BBSRC.
REFERENCES
- 1.Arcaro A, Volinia S, Zvelebil M J, Stein R, Watton S J, Layton M J, Gout I, Ahmadi K, Downward J, Waterfield M D. Human PI3-kinase C2β—the role of calcium and the C2 domain in enzyme activity. J Biol Chem. 1998;273:33082–33091. doi: 10.1074/jbc.273.49.33082. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro A, Zvelebil M J, Wallasch C, Ullrich A, Waterfield M D, Domin J. Class II phosphoinositide 3-kinase are downstream targets of activated polypeptide growth factor receptors. Mol Cell Biol. 2000;20:3817–3830. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batzer A G, Blaikie P, Nelson K, Schlessinger J, Margolis B. The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol Cell Biol. 1995;15:4403–4409. doi: 10.1128/mcb.15.8.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batzer A G, Rotin D, Urena J M, Skolnik E Y, Schlesinger J. Hierachy of binding sites for grb-2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown R A, Domin J, Arcaro A, Waterfield M D, Shepherd P R. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. J Biol Chem. 1999;274:14529–14532. doi: 10.1074/jbc.274.21.14529. [DOI] [PubMed] [Google Scholar]
- 6.Bunday L. Membrane-targeting of signalling molecules by SH2/SH3 domain-containing adaptor proteins. Biochim Biophys Acta. 1999;1422:187–204. doi: 10.1016/s0304-4157(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 7.Bunday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 8.Burd C G, Emr S D. Phosphatidylinositol (3)-phosphate signalling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 9.Carter A N, Downes C P. Phosphatidylinositol 3-kinase is activated by nerve growth factor and epidermal growth factor in PC12 cells. J Biol Chem. 1992;267:14563–14567. [PubMed] [Google Scholar]
- 10.Charest A, Wagner J, Kwan M, Tremblay M. Coupling of the murine protein tyrosine phosphatase PEST to the epidermal growth factor (EGF) receptor through a Src homology 3 (SH3) domain-mediated association with Grb2. Oncogene. 1997;14:1643–1651. doi: 10.1038/sj.onc.1201008. [DOI] [PubMed] [Google Scholar]
- 11.Cohen G B, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 12.Domin J, Gaidarov I, Smith M, Keen J, Waterfield M. The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J Biol Chem. 2000;275:11943–11950. doi: 10.1074/jbc.275.16.11943. [DOI] [PubMed] [Google Scholar]
- 13.Domin J, Pages F, Volinia S, Rittenhouse S E, Zvelebil M J, Stein R C, Waterfield M D. Cloning of a human phosphatidylinositol 3-kinase with a C2 domain which displays reduced sensitivity to the inhibitor wortmannin. Biochem J. 1997;326:139–147. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domin J, Waterfield M D. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997;410:91–95. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- 15.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 16.Gaidarov I, Smith M E, Domin J, Keen J H. The class II phosphoinositide 3-kinase C2 alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7:443–449. doi: 10.1016/s1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- 17.Galisteo M, Chernoff J, Su Y, Skolnik E, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 18.Gaullier J M, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 19.Gillham H, Golding M C H M, Pepperkok R, Gullick W J. Intracellular movement of green fluorescent protein-tagged phosphatidylinositol 3-kinase in response to growth factor receptor signaling. J Cell Biol. 1999;146:869–880. doi: 10.1083/jcb.146.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gout I, Dhand R, Hiles I D, Fry M J, Panayotou G, Das P, Truong O, Totty N F, Hsuan J, Booker G W, Campbell I D, Waterfield M D. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- 21.Jackman J, Motto D, Sun Q, Tanemoto M, Turck C, Peltz G, Koretzky G, Findell P. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 22.Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- 23.Joly M, Kazlauskas A, Fay F S, Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994;263:684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- 24.Leevers S, Vanhaesebroeck B, Waterfield M. Signalling through phosphoinositide 3-kinases: the lipids take center stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 25.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar-Sagi D, Schlesinger J. The SH2 and SH3 domain containing protein Grb2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 26.Meisner H, Conway B, Hartley D, Czech M. Interactions of Cbl with Grb2 and phosphatidylinositol 3′ kinase in activated Jurkat cells. Mol Cell Biol. 1995;15:3571–3578. doi: 10.1128/mcb.15.7.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 28.Okabayashi Y, Kido Y, Okutani T, Sugimoto Y, Sakaguchi K, Kasuga M. Tyrosines 1148 and 1173 of activated human epidermal growth factor receptors are binding sites of Shc in intact cells. J Biol Chem. 1994;269:18674–18678. [PubMed] [Google Scholar]
- 29.Okkenhaug K, Rottapel R. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J Biol Chem. 1998;273:21194–21202. doi: 10.1074/jbc.273.33.21194. [DOI] [PubMed] [Google Scholar]
- 30.Olayioye M, Neve R, Lane H, Hynes N E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey A, Podtelejnikov A, Blagoev B, Bustelo X, Mann M, Lodish H. Analysis of receptor signaling pathways by mass spectrometry: identification of vav-2 as a substrate of the epidermal and platelet-derived growth factor receptors. Proc Natl Acad Sci USA. 2000;97:179–184. doi: 10.1073/pnas.97.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patki V, Lawe D C, Corvera S, Virbasius J V, Chawla A. A functional PtdIns(3)P-binding motif. Nature. 1998;394:433–434. doi: 10.1038/28771. [DOI] [PubMed] [Google Scholar]
- 33.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 34.Pomerance M, Multon M, Parker F, Venot C, Blondeau J, Tocque B, Schweighoffer F. Grb2 interaction with MEK-kinase 1 is involved in regulation of Jun-kinase activities in response to epidermal growth factor. J Biol Chem. 1998;273:24301–24304. doi: 10.1074/jbc.273.38.24301. [DOI] [PubMed] [Google Scholar]
- 35.Prigent S A, Gullick W J. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prigent S, Nagane M, Lin H, Huvar I, Boss G, Feramisco J, Cavenee W, Huang H. Enhanced tumorigenic behavior of glioblastoma cells expressing a truncated epidermal growth factor receptor is mediated through the Ras-Shc-Grb2 pathway. J Biol Chem. 1996;271:25639–25645. doi: 10.1074/jbc.271.41.25639. [DOI] [PubMed] [Google Scholar]
- 37.Raffioni S, Bradshaw R A. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor and nerve growth factor in PC12 phaeochromocytoma cells. Proc Natl Acad Sci USA. 1992;89:9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos-Morales F, Romero F, Schweighoffer F, Bismuth G, Camonis J, Tortolero M, Fischer S. The proline-rich region of Vav binds to Grb2 and Grb3-3. Oncogene. 1995;11:1665–1669. [PubMed] [Google Scholar]
- 39.Ren R, Mayer B, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 40.Riese D J, II, Stern D F. Specificity within the EGF family/ErbB receptor family signalling network. BioEssays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues G A, Falasca M, Zhang Z, Ong S H, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, Matuoka K, Takenawa T, Kurata T, Nagashima K, et al. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci USA. 1994;91:3443–3447. doi: 10.1073/pnas.91.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner S J, Domin J, Waterfield M D, Ward S G, Westwick J. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2α. J Biol Chem. 1998;273:25987–25995. doi: 10.1074/jbc.273.40.25987. [DOI] [PubMed] [Google Scholar]
- 45.Volinia S, Dhand R, Vanhaesebroeck B, MacDougall L K, Stein R, Zvelebil M J, Domin J, Panaretou C, Waterfield M D. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J. 1995;14:3339–3348. doi: 10.1002/j.1460-2075.1995.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Moran M. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- 47.Wong L, Johnson G R. Epidermal growth factor induces coupling of protein-tyrosine phosphatase 1D to GRB2 via the COOH-terminal SH3 domain of GRB2. J Biol Chem. 1996;271:20981–20984. doi: 10.1074/jbc.271.35.20981. [DOI] [PubMed] [Google Scholar]
- 48.Yart A, Laffargue M, Mayeux P, Chretien S, Peres C, Tonks N, Roche S, Payrastre B, Chap H, Raynal P. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J Biol Chem. 2001;276:8856–8864. doi: 10.1074/jbc.M006966200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Banfic H, Straforini F, Tosi L, Volinia S, Rittenhouse S. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source of phosphatidylinositol 3-phosphate. J Biol Chem. 1998;273:14081–14084. doi: 10.1074/jbc.273.23.14081. [DOI] [PubMed] [Google Scholar]