Abstract
Axonal transport is an essential function in neurons, as mutations in either motor proteins or their adaptors cause neurodegeneration. While some mutations cause a complete block in axonal transport, other mutations affect transport more subtly. This is especially true of mutations identified in human patients, which impair but do not block motor function in the cell. Dissecting the pathogenic mechanisms of these more subtle mutations requires assays that can tease apart the distinct phases of axonal transport, including transport initiation, sustained/ regulated motility, and cargo-specific sorting or delivery. Here, we describe a live-cell photobleaching assay to assess retrograde flux from the distal axon tip, a measure for distal transport initiation. We have previously used this method to show that the CAP-Gly domain of DCTN1 is required for efficient retrograde transport initiation in the distal axon, but it is not required to maintain retrograde flux along the mid-axon. This approach has allowed us to examine the effects of disease-causing mutations in the axonal transport machinery, and in combination with other assays, will be useful in determining the mechanisms and regulation of axonal transport in normal and diseased conditions.
Keywords: axonal transport, DCTN1, dynactin, dynein, LAMP-1, live-cell imaging, microtubule-based transport, neurodegeneration, photobleaching
Introduction
The development of video-enhanced microscopy (Allen, Travis, Allen, & Yilmaz, 1981; Inoue, 1981) allowed the first direct visualization of organelle motility in squid axons (Allen, Metuzals, Tasaki, Brady, & Gilbert, 1982) and extruded squid axoplasm (Brady, Lasek, & Allen, 1982). This was followed by the discovery of the microtubule motors kinesin-1 (Vale, Reese, & Sheetz, 1985) and cytoplasmic dynein (Paschal & Vallee, 1987), which were later identified as the motors driving anterograde (Nobutaka Hirokawa et al., 1991) and retrograde (N. Hirokawa, Sato-Yoshitake, Yoshida, & Kawashima, 1990) transport, respectively. Since then, live-cell imaging and neuron culture have continued to play a critical role in our understanding of axonal transport. Here, we will focus on cytoplasmic dynein and the dynactin complex, which form the primary motor complex responsible for long-distance retrograde axonal transport.
Cellular studies examining the mechanisms of dynein-mediated retrograde transport have been complemented by genetic approaches, which have identified mutations in dynein/ dynactin and other adaptors or cytoskeleton associated proteins in a number of neurodevelopmental and neurodegenerative diseases, reviewed in (Maday, Twelvetrees, Moughamian, & Holzbaur, 2014; Perlson, Maday, Fu, Moughamian, & Holzbaur, 2010). Approaches to study the effects of these mutations at the cellular level have involved overexpressing wild type or mutant protein and visualizing the axonal transport of fluorescently labeled proteins/ vesicles. These approaches have been successful in identifying mutations that cause gross disruption of axonal transport. However, since dynein and many other cytoskeletal proteins have essential functions in all cells (e.g. the essential role of dynein/ dynactin in mitosis), mutations that cause complete loss of function are embryonic lethal. Thus, mutations that persist into adulthood and cause late-onset neurodegenerative diseases likely cause subtle defects or target specific phases of axonal transport, such as transport initiation, sustained/ regulated motility, or cargo-specific sorting or delivery.
Here, we describe an assay to measure retrograde flux from the distal axon. Using this assay, we confirm that the N-terminal Cytoskeletal-associated Protein Glycine-rich (CAP-Gly) domain of dynactin is required for retrograde transport initiation, but is not required for mid-axonal transport maintenance (Lloyd et al., 2012; Moughamian & Holzbaur, 2012). This assay, and the development of additional novel assays that are sensitive to distinct phases of axonal transport will be useful in determining the pathogenic mechanisms of subtle, novel mutations in motor proteins, adaptors, or their associated cytoskeletal components.
Methods
Primary neuron culture
Imaging organelle and vesicle dynamics in primary neuron cultures is a well-established method to probe the molecular mechanisms of axonal transport. We use mouse dorsal root ganglion sensory neurons as a robust system to examine the effects of mutations in motor proteins or their adaptors on axonal transport. Results from DRG neurons have been corroborated in other neuronal subtypes using the photobleaching approach. Here we focus on the photobleaching assay and analysis of transport initiation. For more information on neuron isolation, enrichment, and culture please refer to any of the recent protocols on the topic (Kaech & Banker, 2006; Malin, Davis, & Molliver, 2007; Owen & Egerton, 2012).
Live-cell imaging
Materials
Neurons transfected with fluorescently-tagged proteins of interest, cultured in glass-bottomed dishes.
Glass bottomed dish for live-cell imaging. We use 35mm Fluorodish (FD35–100) or Mat-Tek (P35G-1.5–20-C) glass bottomed dishes.
Inverted microscope.
Spinning disk confocal equipped with a photobleaching module.
Controller software: Volocity (PerkinElmer), MetaMorph (Molecular Devices), or μManager.
Hibernate A low fluorescence medium (BrainBits)
B27 Supplement (Gibco 17504–44)
GlutaMAX supplement (Invitrogen 35050061)
General considerations for photobleaching assays
It is important to calibrate the photobleaching module prior to each imaging session, especially if multiple users are using the same microscope or if the photobleaching module has not been used recently. To calibrate the system, take a uniformly fluorescent test slide or sample and select a region of interest (ROI) using your integrated microscope manager software. Bleach the test slide/sample and compare the area of photobleaching with your ROI. If the system is calibrated, the ROI and bleached region should completely overlap with minimal bleaching outside your ROI. If not, re-calibrate the photobleaching module according to your system’s User Manual.
- Prior to starting the experiment, perform test experiments to determine:
- the minimal laser power and photobleaching cycles required to bleach a region of axon.
- the frame rate required to capture the dynamics of your labeled protein.
- the image acquisition settings (exposure, gain, sensitivity, laser power) and duration of time-lapse imaging.
Setup of photobleaching and image acquisition
In your microscope manager software, set up a new image acquisition protocol in the photobleaching module. Set the desired number of photobleaching cycles, laser power, and frame rate (frames per second or fps). When using LAMP-1-RFP, a marker of late endosomes/ lysosomes, we use 25 photobleaching cycles at 100% laser power and a frame rate of 3fps post-bleaching.
Set the camera to acquire time-lapse series pre-bleach and another series post-bleach. For LAMP-1-RFP vesicles, which exhibit rapid, bidirectional transport, we use at least 5– 10 seconds pre-bleach and 120 seconds post-bleach. These settings will depend on the fluorescently labeled protein.
Next, prepare your transfected neurons for imaging. Gently remove the neuron culture media and replace with 37°C, low fluorescence imaging media pre-equilibrated in CO2 (Hibernate A + 2% B27 supplement + 2mM Glutamax).
Transfer the live-cell dish onto the stage and get the coverslip into focus.
Next, find the cell body or axon of a healthy, low expressing neuron. Manually set the contrast to a window where the relative level of fluorescent protein expression is easily visualized. Avoid cells that appear saturated due to very high expression of fluorescent protein.
Trace the axon to the distal axon terminal, adjusting the Z focus to follow the axon as it moves over/ under non-transfected neurons. After identifying the distal axon, capture a static image. Next, use the “measure” tool to trace 10 μm proximal to the distal axon tip and draw an ROI to photobleach. When we image at 100X, we draw an ROI beginning 10 μm proximal to the distal axon and extending retrograde 40 μm toward the cell body. We find that photobleaching is much faster with small, thin, rectangular ROIs than with freehand ROIs.
Acquire images using the protocol described above.
In the same neuron, move at least 100 −150 μm proximal from the distal axon tip and acquire a time-lapse movie from the mid-axon. We recommend imaging paired distal and mid-axons to compare distal transport initiation with mid-axonal retrograde flux within the same neuron. Record the neuron and the orientation of the axons (anterograde/ retrograde).
Data Analysis
We use manual kymograph analysis to measure retrograde flux in this assay. A kymograph is a 1 dimensional projection of the fluorescent intensity along the long axis of the axon over time. The X-axis is the distance along the axon and the Y-axis is the time elapsed. The primary output of this analysis is the vesicles per minute that have moved retrograde into the bleached region a specified distance (retrograde flux). In the distal axon, just before the axon tip or growth cone, this provides a measure of the number of vesicles starting transport and moving retrograde.
Materials
ImageJ (NIH) or other image processing software (e.g. Volocity, MetaMorph)
- Open source kymograph analysis tools:
- KymographDirect (https://www.nat.vu.nl/~erwinp/downloads.html)
Kymograph analysis of retrograde flux
Draw a 1 pixel wide segmented line along the axon beginning with the distal region of the bleached ROI and extending proximally toward the soma. Save the ROI line selection or take an RGB reference image indicating where the line was drawn.
Generate a kymograph with time displayed on the Y-axis. In ImageJ, use the ‘MultipleKymograph’ plugin.
Determine the point at which you will measure retrograde flux. In our analysis, we make a line ~50 pixels (3–4 microns with a 100x objective) retrograde from the distal end of the ROI.
Trace the cargo trajectories using automated kymograph tracing tools such as Icy (de Chaumont et al., 2012), KYMOMAKER (Chiba, Shimada, Kinjo, Suzuki, & Uchida, 2014), or KymographDirect (Peterman lab) or manual tracing. Kymographs with low signal to noise ratios or high cargo density may require manual tracing. Then, count the total number of vesicles crossing this distance threshold vertical line. This represents the Retrograde Flux (vesicles·min−1) in an axonal region. In the distal axon, this measurement represents the number of vesicles initiating from the distal axon.
Repeat the process for the mid-axon and compare whether there is selective disruption of distal retrograde flux vs. mid axonal flux.
Conclusions
Here we describe a photobleaching assay to analyze retrograde flux in the distal axon, as a measure of retrograde transport initiation. We have previously used this approach to show that the CAP-Gly domain of dynactin is required for efficient retrograde transport initiation from the distal axon (Moughamian & Holzbaur, 2012; Moughamian, Osborn, Lazarus, Maday, & Holzbaur, 2013). This assay may be applied to study the role of dynein, dynactin, or other microtubule plus-end binding in retrograde transport initiation and to investigate how mutations in these proteins disrupt axonal transport and contribute to human neurodegenerative diseases.
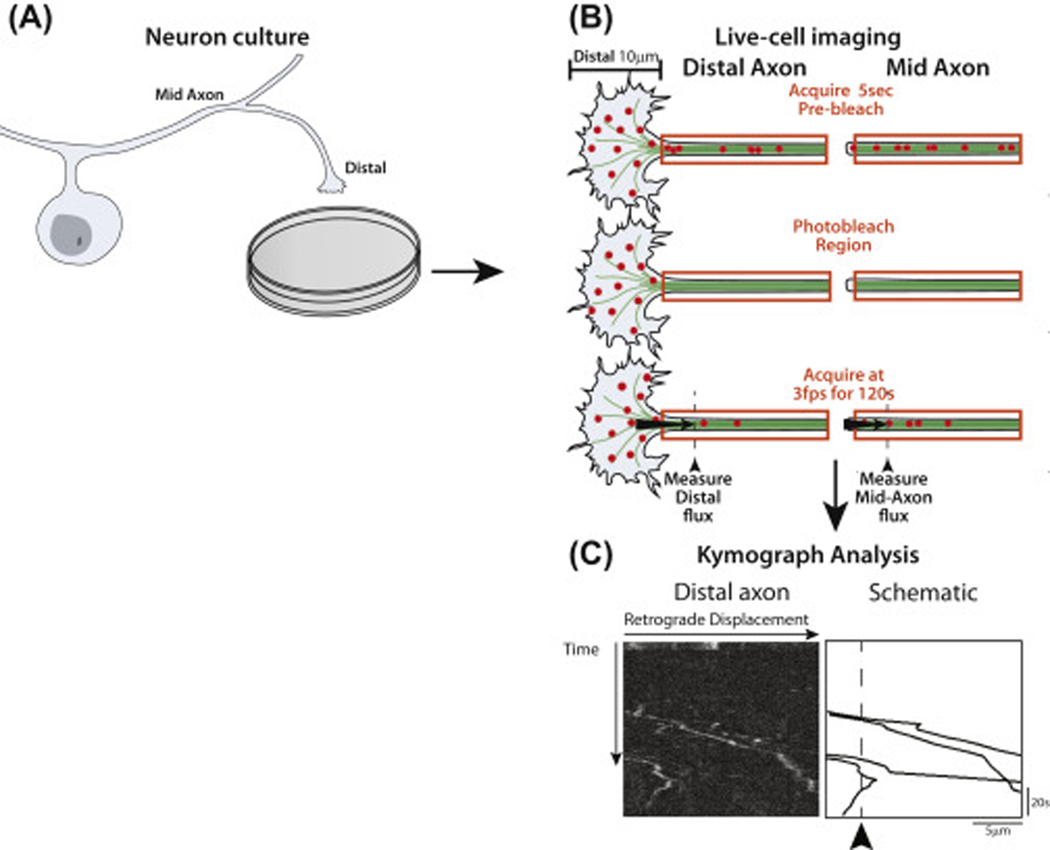
Figure 1.
Schematic overview of the approach used in the photobleaching assay. (A) Primary neuron culture. (B) Live cell photobleaching assay in paired distal and mid-axon segments. Red circles represent fluorescently labeled LAMP-1-RFP vesicles. (C) Kymograph analysis: representative kymographs from the distal axon of wild type DRG neurons shows the tracks of vesicles that recently initiated retrograde transport from the axon tip. The arrowhead and dotted line in the schematic indicate the pre-defined distance threshold for analyzing retrograde flux. Only retrograde-moving vesicles were traced.
Acknowledgements
We would like to thank Armen Moughamian, Mariko Tokito, and Karen Wallace for their help in developing this protocol, cloning the constructs, and mouse handling. J.J.N. was supported by training grant T32GM7170 and the Neuroscience Graduate Group Hearst Fellowship; ELFH was supported by NIH GM48661.
References
- Allen RD, Metuzals J, Tasaki I, Brady ST, & Gilbert SP (1982). Fast axonal transport in squid giant axon. Science (New York, N.Y.), 218(4577), 1127–1129. doi: 10.1126/science.6183744 [DOI] [PubMed] [Google Scholar]
- Allen RD, Travis JL, Allen NS, & Yilmaz H. (1981). Video-enhanced contrast polarization (AVEC-POL) microscopy: a new method applied to the detection of birefringence in the motile reticulopodial network of Allogromia laticollaris. Cell Motility, 1, 275–289. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, & Allen RD (1982). Fast axonal transport in extruded axoplasm from squid giant axon. Science (New York, N.Y.), 218(4577), 1129–1131. doi: 10.1126/science.6183745 [DOI] [PubMed] [Google Scholar]
- Chiba K, Shimada Y, Kinjo M, Suzuki T, & Uchida S. (2014). Simple and direct assembly of kymographs from movies using KYMOMAKER. Traffic (Copenhagen, Denmark), 15(1), 1–11. doi: 10.1111/tra.12127 [DOI] [PubMed] [Google Scholar]
- De Chaumont F, Dallongeville S, Chenouard N, Hervé N, Pop S, Provoost T, … Olivo-Marin J-C (2012). Icy: an open bioimage informatics platform for extended reproducible research. Nature Methods, 9(7), 690–6. doi: 10.1038/nmeth.2075 [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, & Brady ST (1991). Kinesin associates with anterogradely transported membranous organelles in vivo. Journal of Cell Biology, 114(2), 295–302. doi: 10.1083/jcb.114.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, & Kawashima T. (1990). Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. Journal of Cell Biology, 111(September), 1027–1037. doi: 10.1083/jcb.111.3.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S. (1981). Video image processing greatly enhances contrast, quality, and speed in polarization-based microscopy. Journal of Cell Biology, 89(2), 346–356. doi: 10.1083/jcb.89.2.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, & Banker GA (2006). Culturing hippocampal neurons. Nature Protocols, 1(5), 2406–15. doi: 10.1038/nprot.2006.356 [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Machamer J, O’Hara K, Kim JH, Collins SE, Wong MY, … Kolodkin AL (2012). The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron, 74(2), 344–60. doi: 10.1016/j.neuron.2012.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, & Holzbaur ELF (2014). Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron, 84(2), 292–309. doi: 10.1016/j.neuron.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S.a, Davis BM, & Molliver DC (2007). Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nature Protocols, 2(1), 152–160. doi: 10.1038/nprot.2006.461 [DOI] [PubMed] [Google Scholar]
- Moughamian AJ, & Holzbaur ELF (2012). Dynactin Is Required for Transport Initiation from the Distal Axon. Neuron, 74(2), 331–343. doi: 10.1016/j.neuron.2012.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughamian AJ, Osborn GEG, Lazarus JE, Maday S, & Holzbaur ELF (2013). Ordered Recruitment of Dynactin to the Microtubule Plus-End is Required for Efficient Initiation of Retrograde Axonal Transport. The Journal of Neuroscience, 33(32), 13190–203. doi: 10.1523/JNEUROSCI.0935-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, & Egerton J. (2012). Neurotrophic Factors. In Skaper SD (Ed.), Methods in Molecular Biology (Vol. 846, pp. 213–222). doi: 10.1007/978-1-61779-536-7 [DOI] [PubMed] [Google Scholar]
- Paschal B, & Vallee R. (1987). Retrograde transport by the microtubule-associated protein MAP 1C. Nature, 300, 181–183. [DOI] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu M-M, Moughamian AJ, & Holzbaur EL (2010). Retrograde axonal transport: pathways to cell death? Trends in Neurosciences, 33(7), 335–44. doi: 10.1016/j.tins.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Reese TS, & Sheetz MP (1985). Identification of a Novel Force-Generating Protein, Kinesin, Involved in Microtubule-Based Motility, 42(August), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]