Abstract
In light of the adverse prognosis related to severe mitral regurgitation, heart failure, or sudden cardiac death in a subset of patients with mitral valve prolapse (MVP), identifying those at higher risk is key. For the first time in decades, researchers have the means to rapidly advance discovery in the field of MVP thanks to state-of-the-art imaging techniques, novel omics methodologies, and the potential for large-scale collaborations using web-based platforms. The National Heart, Lung, and Blood Institute recently initiated a webinar-based workshop to identify contemporary research opportunities in the treatment of MVP. This report summarizes 3 specific areas in the treatment of MVP that were the focus of the workshop: 1) improving management of degenerative mitral regurgitation and associated left ventricular systolic dysfunction; 2) preventing sudden cardiac death in MVP; and 3) understanding the mechanisms and progression of MVP through genetic studies and small and large animal models, with the potential of developing medical therapies.
Keywords: cardiac magnetic resonance imaging, echocardiography, mitral regurgitation, mitral valve prolapse, sudden cardiac death
Mitral valve prolapse (MVP) is a common heritable valvulopathy affecting over 170 million individuals worldwide.1,2 It is the direct cause of degenerative mitral regurgitation (DMR), which represents the most frequent form of mitral regurgitation (MR) requiring surgery.3,4 MVP is characterized by fibromyxomatous changes, defined structurally by expansion of the middle spongiosa layer of leaflets caused by proteoglycan accumulation, structural alterations of collagen in all components of the leaflet, and by abnormal chordae.5,6 Macroscopically, MVP is characterized by redundant mitral valve (MV) tissue, which clinically translates by echocardiography in a displacement ≥2 mm of 1 or both leaflets beyond the annular high points at end-systole toward the left atrium (Central Illustration).7-9 Leaflet displacement may yield malcoaptation and consequent DMR. Although most individuals with MVP in the general population have mild or no MR,10 severe DMR affects 10% of subjects in MVP cohorts1 and up to 25% in longitudinal samples in association with aging.4,11 MV repair is generally associated with low risk, superior late survival to valve replacement, and when performed before symptoms and before development of left ventricular (LV) dysfunction,12 to restoration of life expectancy. However, early surgery remains a Class II indication for low-risk patients based on current valvular guidelines.13 Hence, risk stratification and management of older, higher-risk patients remains challenging.
CENTRAL ILLUSTRATION. Research Opportunities: Degenerative Mitral Regurgitation and Arrhythmic Mitral Valve Prolapse.
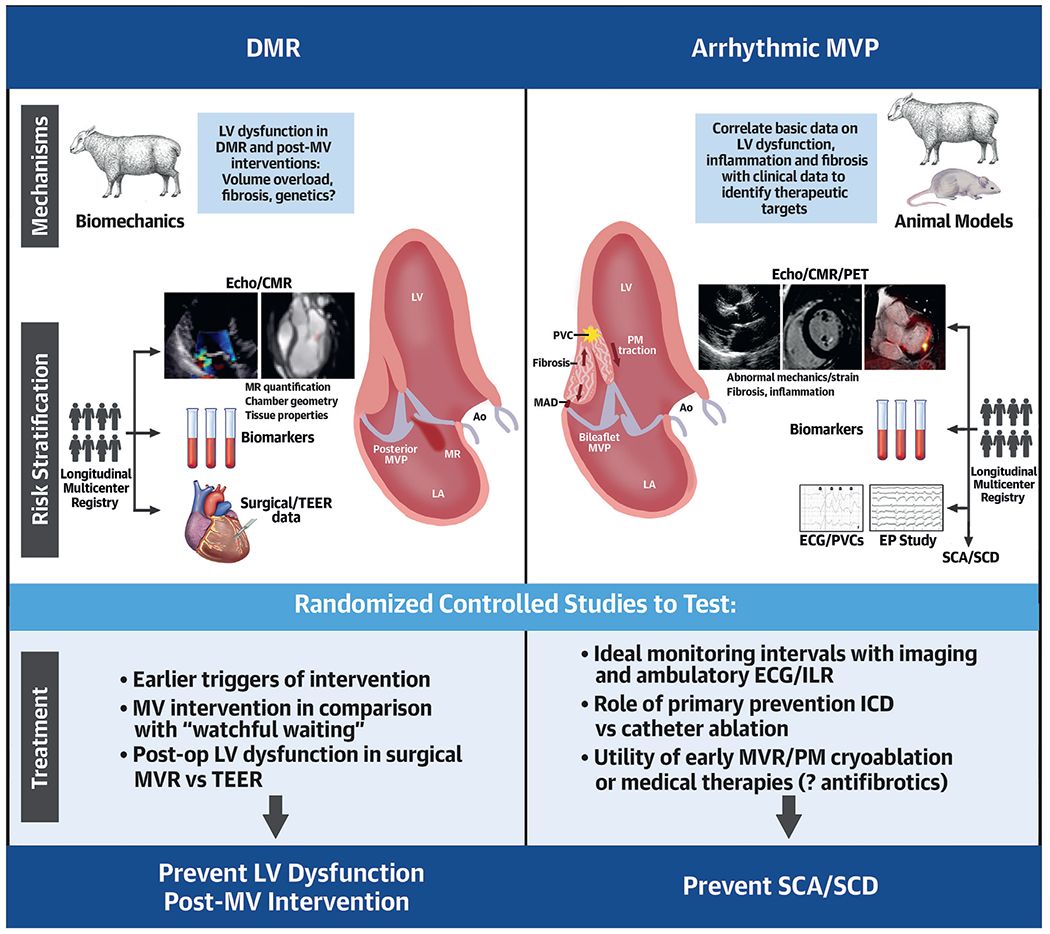
Valvular-ventricular interactions and potential fibrotic stimuli and arrhythmogenic triggers in mitral valve prolapse (MVP) include (right): papillary muscle (PM) traction, associated curling motion (black arrows in opposite directions), and increased systolic annular expansion augmented by mitral annular disjunction (MAD). MV schematics adapted with permission from Nagata et al.87 AO = aorta; CMR = cardiac magnetic resonance; DMR = degenerative mitral regurgitation; ECG = electrocardiography; EP = electrophysiology; ICD = implantable cardioverter-defibrillator; ILR = implantable loop recorder; LA = left atrium; LV = left ventricular; PET = positron emission tomography; PVC = premature ventricular contraction; SCA = sudden cardiac arrest; SCD = sudden cardiac death; TEER = transcatheter edge-to-edge repair.
Recent studies have emphasized the risk of ventricular arrhythmias associated with MVP. Overall, 0.4% to 1.8% of individuals with MVP will develop sudden cardiac arrest (SCA) or sudden cardiac death (SCD) every year (32,000-152,000/y in the United States alone).14,15 Far more patients (up to 30%) have frequent ventricular ectopy and/or syncope,16 hence the uncertainty regarding whether they are at risk for SCA. Severe DMR explains only 20% of SCD cases in MVP.17 SCD/SCA risk has also been linked to a malignant bileaflet phenotype with mitral annular disjunction (MAD), abnormal valvular-myocardial interactions, and LV fibrosis, even in the absence of severe DMR (Central Illustration).18-22 Complex ventricular ectopy is a common feature of malignant MVP (with or without severe DMR),18 and is associated with higher mortality.23 However, routine monitoring for ventricular arrhythmias is currently not recommended in valvular guidelines. Indications for implantation of a primary prevention implantable cardioverter-defibrillator (ICD) in MVP or valve interventions to reduce arrhythmias are lacking.
Given the adverse prognosis in a subset of MVP patients, imaging becomes essential to better understand mechanisms and identify those at higher risk. Moreover, recent genetic discoveries may provide additional clues to MVP mechanisms, with the potential for developing medical therapies. State-of-the-art imaging techniques, novel omics methodologies, and the potential for large-scale international collaborations using web-based platforms could rapidly advance discovery in the field of MVP.
To engage the scientific community in identifying contemporary research opportunities in the treatment of MVP, the National Heart, Lung, and Blood Institute (NHLBI) recently initiated a webinar-based workshop. The following research opportunities were discussed during the workshop: 1) improve management of DMR and associated LV systolic dysfunction; 2) prevent SCD in MVP; and 3) understand MVP mechanisms and progression through genetic studies and development of small and large animal models.
DMR, CARDIAC REMODELING, AND HEART FAILURE
HEART FAILURE AND INDICATIONS FOR INTERVENTION.
MVP is the most frequent cause of clinically significant DMR.24 The burden of moderate to severe DMR is large, affecting 1.4 to 1.6 million persons in the United States.25,26 Early cohorts of DMR emphasized excess-mortality, frequent heart failure and atrial fibrillation during follow-up,27,28 mostly in proportion to DMR severity.29 Despite the high burden and serious outcomes of DMR, whether MVP and DMR represent a public health problem may not appear obvious, as an effective treatment is available in the form of MV repair, which is superior to valve replacement30 at all ages and long after surgery.31 MV repair restores life expectancy and markedly reduces heart failure risk when indicated early in the course of the disease.32 However, despite this therapeutic progress, there remains an unmet need for treatment, as attested by the profound undertreatment of affected patients in the community, which is in turn associated with persistent excess mortality.26,33
Current consensus (American College of Cardiology/American Heart Association and European Society of Cardiology) guidelines for treatment of MVP are predicated on confirmation of severe MR.13,34 Current triggers for intervention are based upon symptoms, LV dysfunction, left atrial remodeling, progressive LV remodeling, and high likelihood of surgical repair.13,34 These guidelines do not fully take into account clinical modifiers, and largely rely on linear chamber indexes rather than volumetric cutoffs, which are quantifiable by 3-dimensional (3D) echocardiography and cardiac magnetic resonance (CMR) technologies.
Knowledge gaps
MVP is heterogeneous–anatomically, physiologically, and in its consequences11,35–and algorithms for combined and individualized risk assessment are lacking (Figure 1).
DMR is often a disease of aging,25,26 and as such, its symptoms are affected by limited activity or comorbidities, ventricular alterations may be extraneous to DMR, and outcomes under medical management are markedly worse.36 Conversely, with aging, risks of interventions increase.37
Although DMR severity is a cardinal determinant of outcome,11,29,35 interactions with sex/body size are uncertain, yielding poor outcomes.38
“Moderate” DMR, currently not part of guideline-based surgical indications,13 shows association with excess-mortality,29,35 warranting renewed attention.
Progression of MVP lesions and DMR severity are poorly defined.39,40
The rhythmic,41,42 left atrial,43,44 and hormonal (“omics”) responses45 to MVP with DMR are highly variable and have differential impact on survival after diagnosis and postsurgery. Such variability is not fully integrated into management algorithms. Consequently, MVP with DMR is an unmet need for treatment often linked to inadequate risk assessment in an aging population, warranting comprehensive reassessment of clinical algorithms.
FIGURE 1. Research Opportunities in DMR.
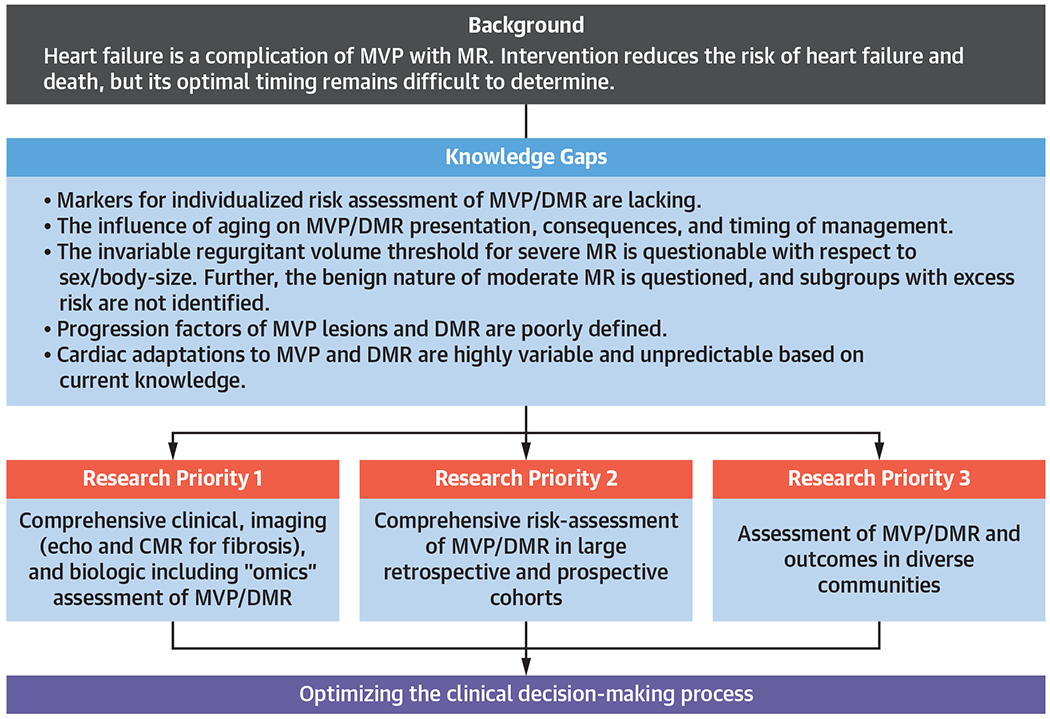
Preventing heart failure through identification of earlier triggers for intervention in degenerative mitral regurgitation (DMR). CMR = cardiac magnetic resonance; MR = magnetic resonance; MVP = mitral valve prolapse.
Imaging advances in the acquisition and postprocessing of both echocardiography and CMR hold substantial potential to enhance clinical decisionmaking regarding optimal timing of therapeutic interventions for MVP. The following knowledge gaps were identified with regards to imaging triggers of intervention for DMR:
Utility of cardiac chamber volumes (vs echocardiographic linear indexes) as modifiers of timing and strategy for MVP interventions.
Utility of different cutoffs for severe MR for echocardiography and CMR.
Significance of myocardial tissue substrate remodeling (as assessed by strain echocardiography, diffuse interstitial or regional replacement fibrosis by CMR T1 mapping and late gadolinium enhancement [LGE], and edema on T2 mapping) on timing and therapeutic strategy for MVP.
Incremental utility of new technologies (such as 4-dimensional flow CMR, exercise CMR, and 3D echocardiography) for MR quantification.
Research opportunities
Comprehensive assessment of patients with MVP/DMR using clinical, imaging (echocardiography/CMR for reliable MR quantification, chamber geometry, myocardial tissue properties), and biological (metabolomics, proteomics, and genomics) tools to evaluate the association to heterogeneous anatomic/physiological/cardiac-response presentation and progression of MVP/DMR (Figure 1, Central Illustration).
Comprehensive risk assessment of MVP/DMR in large retrospective and prospective cohorts within a large multicenter network. Extensive use of electronic medical record data, facilitated by artificial intelligence/machine learning, will enable data sharing and efficient implementation of large-scale randomized clinical trials for refinement of triggers of intervention and improved treatment of DMR.
Assessment of MVP/DMR, progression, and outcomes and their determinants in diverse geographically defined communities to minimize bias and evaluate coherence of outcome markers across populations.
PERSISTENT CARDIAC DYSFUNCTION/REMODELING FOLLOWING VALVE INTERVENTIONS
Severe DMR leads to progressive LV systolic dysfunction, heart failure, and death if left untreated. MV repair is indicated in MVP patients with symptoms, LV dilation, or dysfunction13 and is associated with a normalization of long-term survival in patients presenting with no or mild symptoms.46 In addition, MV repair surgery may be associated with improved outcomes compared with conservative management (“watchful waiting”) in asymptomatic patients with severe MR.47 Persistent LV dysfunction occurs in approximately 10% to 20% of patients post-MV surgery, even in patients with normal preoperative LV ejection fraction, and is associated with incomplete LV reverse remodeling and poor long-term survival.48 Less is known about cardiac remodeling following transcatheter edge-to-edge repair (TEER), but preliminary data suggest that it may be associated with worse long-term survival.49
Besides the conventional LV remodeling indexes, such as LV volumes, ejection fraction, and measures of global longitudinal strain that may be linked to persistent LV dysfunction post-MR correction,50,51 other forms of cardiac remodeling have been suggested as profoundly affecting outcome. The most studied are left atrial enlargement/dysfunction,43,44 and right ventricular characteristics,52 but these remain incompletely analyzed in sizeable cohorts.
Mechanistic aspects of cardiac remodeling in MVP and DMR are poorly defined. Whether these responses are directly elicited by the severity of volume overload, by genetic characteristics independent of MVP, or associated with the MVP-linked defect remains unknown (Central Illustration). A contributor to cardiac remodeling is replacement fibrosis. Myocardial fibrosis is detected by CMR imaging in approximately one-third of MVP patients, may be more common than with other MR formsx, and is associated with ventricular arrhythmias.53-57 However, its role and more generally the causes of cardiac remodeling/dysfunction remain undefined.
Knowledge gaps
MV repair is applied in a small proportion of eligible patients.33 It is now being performed more frequently in patients with asymptomatic MR to prevent long-term sequelae of LV volume over-load,58 but this practice is based largely on retrospective data.46,47
TEER offers a less-invasive therapeutic option and may be more attractive to patients, resulting in a larger proportion of eligible individuals being treated. However, TEER is primarily performed in patients at high surgical risk. In addition, longterm efficacy data on TEER is lacking, which is an important consideration if this treatment modality is applied to younger, lower-risk patients. Moreover, the prevalence of post-TEER LV dysfunction in MVP patients is unknown, and a comparison to LV dysfunction post-MV repair surgery is lacking.
Biomechanical studies assessing the effects of TEER and MV repair on LV function and strain are required.
Mechanistic insights into post-MV intervention LV dysfunction are sparse. Multiple possible contributors, particularly myocardial fibrosis, must be assessed as potential causative factors. Based on the results of such studies, methods to delay or prevent the onset of LV dysfunction–including pharmacotherapy and optimal timing of MV intervention–can be investigated.33,58
Research opportunities
Mechanistic studies to gain more insight into etiology and consequences of persistent LV dysfunction post-MV intervention (Central Illustration).
Clinical studies comparing persistent LV dysfunction post-MV surgery vs TEER.
Clinical studies comparing MV intervention to “watchful waiting” in asymptomatic MVP patients with severe DMR, with a particular focus on the identification of LV inflammation, fibrosis, and postoperative LV dysfunction (Central Illustration). Shared decision making and patient engagement in trial design are essential for the successful completion of such studies.
RECURRENT MR FOLLOWING SURGICAL OR PERCUTANEOUS MV INTERVENTION.
The central tenet of understanding failure of MV interventions is ascertaining the difference between residual and recurrent MR, which reflects the bimodal prevalence of early vs late echocardiographic evidence of MR in relation to the time of corrective MV intervention. The most common cause of significant MR in the early postoperative period (days to months) is inadequate surgical repair at the time of operation.59 This is often caused by untreated pathology (ie, excess posterior leaflet height causing systolic anterior motion) or incomplete repair strategy (ie, unaddressed clefts or adjacent segment prolapse following TEER) at the time of the index procedure. On the contrary, delayed failure with recurrent MR is mostly associated with progression of the native valve disease (ie, prolapse from new chordal elongation/rupture) or new pathology (ie, endocarditis, calcification) in a previously competent surgical repair.59 Progression of native valve disease, leaflet tear at the device site, and single leaflet detachment can result in recurrent MR after TEER.60
Knowledge gaps
Mid-and long-term risks of recurrent MR are not well defined based on current literature.
Evidence on long-term durability of surgical mitral repair is limited to published reports from a small number of tertiary referral centers with sufficiently high operative volume and resources to conduct long-term follow-up (Figure 2).61
Multicenter, randomized clinical TEER trials with standardized follow-up intervals and echocardio-graphic core laboratory-adjudicated outcomes improved our understanding of the timing and mechanisms of recurrent MR associated with this procedure,62,63 notwithstanding limitations of almost universal short-term follow-up periods,60,63 as well as the constant evolution of the transcatheter device arena. National TEER registries only include site-reported, short-term outcomes.64 It is thus challenging to apply such evidence to inform clinical practice and adjudicate patient and procedural risk of repair failure in the context of a constant flux of new device therapies, increased early failure rates, and suboptimal mid-term outcomes with a poorly understood mechanism of failure.
The most important issue affecting outcomes following MV interventions is low operative volume and not the need for new techniques or devices. This pattern was observed in both surgical and structural interventions,65 with incremental procedural mortality and residual MR linked with low procedural volume.66
The American College of Cardiology/American Heart Association guidelines recommend referral of patients with DMR to centers of excellence,13 but these are poorly defined in many aspects, because recognition of an academic institution as an overall center of excellence does not automatically translate to excellence in mitral repair specifically.67 There are thus no reliable sources for patients, physicians, or insurers regarding volume, outcomes, and quality related to valve surgeons, structural interventionalists, or centers to facilitate patient access to mitral repair centers of excellence (Figure 2).
Little is known regarding the impact of socioeconomic status on access to high quality MV disease care or subsequent outcomes following a valve intervention.68,69
FIGURE 2. Research Opportunities in DMR.
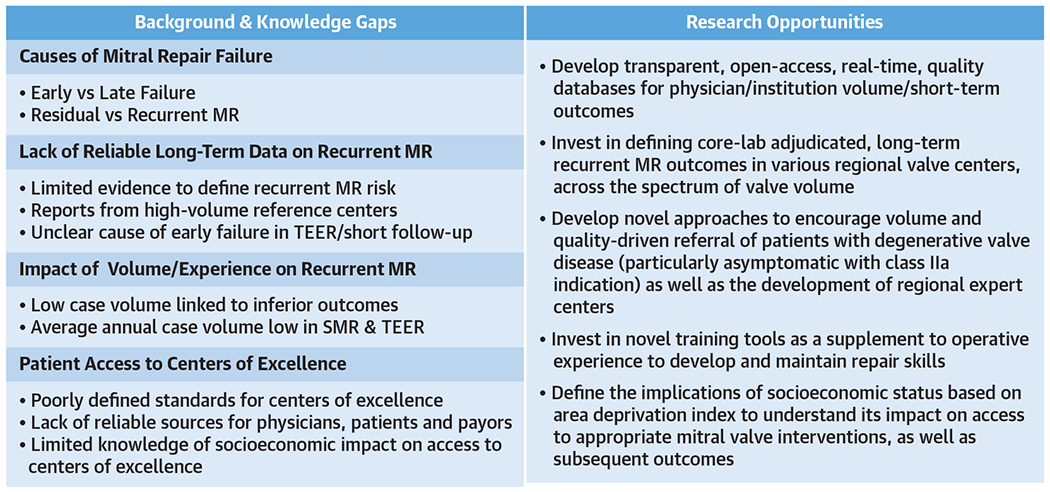
Preventing recurrent MR postintervention through development of large databases and centers of excellence. SMR = surgical mitral valve repair; TEER = transcatheter edge-to-edge repair; other abbreviations as in Figure 1.
Research opportunities
Development of transparent, open-access, realtime, quality databases for physician/institution volume/short-term outcomes (Figure 2).
Definition of core laboratory-adjudicated, longterm recurrent MR outcomes in various regional valve centers across the spectrum of valve volume.
Development of novel approaches to encourage volume and quality-driven referral of patients with DMR (particularly asymptomatic patients with a Class IIa indication), as well as the development of regional expert centers with strong patient involvement and shared decision making.
Assessment of the implications of socioeconomic status based on area deprivation index to understand its impact on access to appropriate MV interventions and subsequent outcomes.
SCD IN MVP
IDENTIFYING MVP AT RISK FOR VENTRICULAR ARRHYTHMIA AND SCD.
For years, the risk of SCD, overt in MVP patients with severe DMR,14 has remained uncertain for those without severe DMR,1 and was mostly implied by case reports with MVP confirmed by pathology.70,71 A consensus on SCD risk in MVP was reached when recent work elucidated the “malignant MVP” phenotype, ie, bileaflet MVP with multisegmental myxomatous disease, often mild MR, complex ventricular ectopy, and SCA/SCD not explained by ischemia, cardiomyopathy, or channelopathy.18 This phenotype, initially identified by John Barlow in the 1960s,72 was later confirmed in larger postmortem studies.19,22,73 Although the subset of MVP patients who experience SCA/SCD is small, it is not trivial.14,15,74 Yearly incidence of SCA/SCD can be as high as 1.8% in patients with flail leaflet and severe DMR, 0.4% to 0.8% among all comers in a tertiary care MVP population, and 0.14% in the community.75 MVP as cause as SCD may even be underestimated on autopsy.22 Importantly, up to 30% of MVP patients have frequent ventricular ectopy and/or syncope.16 Hence, it is crucial to identify, among many benign MVP cases, those at higher risk for SCD, a devastating outcome that often affects younger, asymptomatic individuals with MVP.19 Potential screening methods discussed during the workshop were as follows: 1) standard and novel imaging tools (echocardiography, CMR, and positron emission tomography [PET]) to detect arrhythmic substrates in the LV myocardium; and 2) ambulatory electrocardiography (ECG) monitoring to detect “intermediate” arrhythmic MVP phenotypes with complex ventricular ectopy.
Echocardiography.
Echocardiography provides nonin-vasive diagnosis of MVP relative to the 3D annulus7 with MR quantification, and reveals the cardiac consequences of MVP. Early studies associated MR severity with SCD risk, but primarily in the context of LV failure.14,76 More recently, it was demonstrated that MVP patients with SCD may or may not have MR, but are commonly characterized by so called “Barlow’s disease” with elongated leaflets,77 severe myxomatous degeneration, bileaflet involvement, and MAD18,78 (Central Illustration). MAD is defined as the separation between the left atrial wall at the level of MV junction and the LV free wall.21,79 MAD, which is known to be interspersed with regions of normal mitral annulus, is typically diagnosed in the parasternal long-axis view, but can also be identified in apical views.80 Recent studies have focused on valvular-ventricular interactions and abnormal mechanics capable of altering ventricular biology and rhythm (Central Illustration): papillary muscle (PM) traction,81 associated curling motion (exaggerated apical-inward displacement of the inferolateral LV as demonstrated by high tissue Doppler velocities), and increased systolic annular expansion and flattening can increase the force exerted on the LV.82-87 Such valvular-ventricular interactions, augmented by MAD and dispersion of segmental contraction (ie, increased mechanical dispersion by speckle tracking strain echocardiography),88 can lead to ventricular arrhythmias and fibrosis.21,80,84,88 Fibrosis localization to the PMs and basal inferolateral LV suggests possible mechanical linkage to MVP,21,53,89 augmented by MR severity.53 MAD has been linked to increased risk of ventricular arrhythmias at a population level. However, the risk of SCD in the presence of MAD may not be immediate,90 but is rather mediated by complex ventricular ectopy.23
CMR imaging.
The echocardiographic phenotype of bileaflet MVP with MAD is supported by a postmortem and CMR phenotype characterized by replacement fibrosis and LGE at the level of the basal inferolateral LV wall and PMs.19,55,91,92 Thus, a relatively uniform phenotype of MVP with severe myxomatous degeneration and redundancy, MAD, and replacement fibrosis appears at the center of defining the subset at risk for SCD.55 In those without evidence of replacement fibrosis by LGE or histology in postmortem samples,22 interstitial or diffuse fibrosis (identified by T1 mapping CMR methods in living individuals),20 may represent an alternative substrate for SCD. Other new CMR strain-based techniques suggest a tissue abnormality associated with reduced segmental circumferential and radial strain in the basal and mid LV inferolateral walls.93
Due to its high spatial resolution, robust delineation of endocardial borders, and 3D assessment of the mitral annulus, CMR also provides improved visualization of MAD compared with echocardiography.80
Positron emission tomography.
Although a majority of patients who experienced MVP-related SCD have evidence of myocardial fibrosis, ~25% of such patients do not based on advanced imaging or at autopsy.22,53 Subclinical myocardial inflammation, which is known to be proarrhythmic in a variety of other substrates, may be part of the disease process of MVP.94 The mechanical forces of the prolapsing leaflets transmitted to the chordae and surrounding myocardium may be activating myofibroblasts and inflammatory pathways. The presence of subclinical myocardial inflammation might explain progressive development of fibrosis, ventricular ectopy out of proportion to either the burden of fibrosis or degree of DMR, and how patients with no fibrosis, or minimal fibrosis, can experience ventricular tachycardia (VT) or ventricular fibrillation (VF). In a recent study assessing the burden and distribution of myocardial inflammation (using 18F-fluorodeoxyglucose PET) and fibrosis (using LGE) in patients with bileaflet MVP, significant DMR, and ventricular ectopy, focal, or focal-on-diffuse uptake of 18F-fluorodeoxyglucose (PET+) was detected in 85% of patients, with FDG uptake colocalizing with areas of LGE (PET+/CMR+) in 70%.95 These findings suggest a relationship between extensive myxomatous degeneration typical of bileaflet MVP, ventricular ectopy, and occult myocardial inflammation. This relationship was recently reaffirmed by a study demonstrating histopathological evidence of regionalized LV inflammation and activated myofibroblasts in MVP.89
Electrocardiography and complex ventricular ectopy.
Biphasic or inverted T waves in the inferior leads on 12-lead ECG have been described in MVP patients with SCA or SCD. However, inferior T-wave abnormalities are present in 40% of MVPs even in the absence of prior ventricular arrhythmias.96 Complex ventricular ectopy in MVP is defined in most publications as >5% burden of mitral apparatus premature ventricular contractions (PVCs), pleomorphic PVCs, or couplets/triplets/nonsustained VT of any morphology.18,97 Typically, pleomorphic ventricular ectopy in MVP originates from the outflow tract alternating with PM or fascicular origin and is thought to be a trigger for VF in the presence of a fibrotic substrate.18,19,98 Complex ventricular ectopy, particularly when VT is ≥180 beats/min, was associated with excess subsequent mortality and higher rates of ICD implantation and VT ablation in 1 study.23
Knowledge gaps.
The association of abnormal valve-related motion to myocardial inflammation, fibrosis, and ventricular arrhythmias requires further mechanistic understanding.
Is PM traction alone capable of triggering arrhythmias, and can it initiate ventricular arrhythmias of fibrotic myocardium?99,100
Are myocardial changes independent or closely linked to MVP, with implications for preventive repair?
Do systolic annular expansion and MAD indicate primary annular pathologies, and are they linked?
Data on fibrosis in DMR and LV remodeling are needed as well as clinic-pathological correlation with ventricular arrhythmias.
As pathology studies report a variable prevalence of MVP as a cause of SCD, including the histological myocardial substrates, standardization of postmortem examination is an essential step toward improved mechanistic understanding.
The interaction of mechanical alterations with the specific genetic type of MVP and possible links to myocardial dysfunction and alterations of proteomics and metabolomic changes remain undefined.
CMR imaging
Despite the comprehensive assessment of myocardial involvement provided by CMR, the lack of a standardization protocol (both for acquisition and postprocessing) limits comparison of results among different studies and sites.
Multiple different methods of delineating LGE extent and defining the presence and extent of MAD further increase data heterogeneity.
Is diffuse fibrosis by T1 mapping a precursor of LGE or is it independently responsible for increased arrhythmic risk?
Does the burden of LGE matter for arrhythmic risk stratification?
Positron emission tomography
The relationship between FDG uptake pattern/intensity on PET/MR and arrhythmic burden.
Whether FDG uptake precedes the development of myocardial fibrosis.
FDG uptake and LGE in patients with less than severe MR, and correlation with markers of mechanical traction (including biomarkers) and arrhythmic burden.
Whether FDG uptake is a prognostic marker for LV remodeling and whether MV repair impacts FDG uptake.
Ambulatory ECG
There is a lack of standardized nomenclature, definition, and documentation of complex ventricular ectopy.
Little is known about the ideal timing of ambulatory ECG monitoring and clinical follow-up, especially in those MVP patients who are asymptomatic for palpitations. Indications for an implantable loop recorder are unclear.
Finally, clinical, imaging, and ECG parameters of arrhythmic risk described so far have been studied mostly in retrospective or single-center investigations, and need to be assessed prospectively in the context of a multivariable risk prediction model similar to what has been developed for hypertrophic cardiomyopathy.101,102 This represents an essential step toward development of guidelines for a primary prevention ICD.
PREVENTIVE MEASURES FOR VENTRICULAR ARRHYTHMIA AND SCD.
Various research efforts have been made to understand whether the arrhythmic risk in MVP could be reduced by targeting either the myocardial substrate–through catheter ablation of the scar area, or the trigger–by removing the mechanical stretch on the myocardium through prophylactic valve repair (Central Illustration).
Electrophysiology study, radiofrequency catheter ablation, and primary prevention ICD.
In symptomatic patients with MVP and a high burden of pleomorphic ventricular ectopy, medical therapy alone has not been shown to reduce the risk of SCD.19 As such, there is likely a role for electrophysiology study (EPS)/ablation and ICD implantation in selected patients with MVP and high-risk features.
Several groups have reported single-center experiences with EPS and catheter ablation in a variety of patient phenotypes. Although these studies are small, a few key insights have been gleaned. First, although arrhythmic triggers most often arise from the mitral apparatus, a minority are localized to sites remote from the MV or PMs, including the RVOT, TV annulus, or LV apex.103 The majority of MVP patients with complex ventricular ectopy do not have evidence of myocardial scar by CMR,103,104 and endocardial voltage maps obtained during EPS are often normal.105 Successful sites of ablation are often distinguished by local Purkinje potentials, particularly when ectopy arises from the PMs,105 or in cases of PVC-triggered VF.106 Although acute procedural success rates appear to be acceptable (>70%), the recurrence rates are high, and up to 42% at 1.3 years. Thus, ablation itself does not mitigate the risk of SCD.104-106 Inducible VT during EPS appears to be a marker of risk.104
MV repair.
When patients develop current guideline triggers for MV repair, evidence on the effect of surgery in reducing the PVC burden and postoperative SCD risk is inconsistent.107,108 Because treating the VT/VF trigger (ie, PVCs) may reduce SCD risk, the effect of direct-access, adjunctive PM cryoablation in selected patients with PM-PVCs at the time of their index MV repair was recently investigated and shown to reduce postoperative PVC burden by >90%.109 Early experience from this pivotal series may serve as the foundation for a future randomized trial of adjunctive PM cryoablation at the time of MV repair in patients with the malignant MVP phenotype.
Knowledge gaps
Our current understanding of the spectrum of disease evident during EPS is derived from fewer than 100 patients evaluated at 4 high-volume, quaternary referral centers.
There is no unified approach to patient selection for EPS, and the procedural techniques, targets for ablation, and endpoints are not well-defined.
There are no defined approaches to substrate modification, and identification of triggers/targets for ablation remains speculative.
Although some procedural success has been reported, the rate of recurrence after ablation remains unacceptably high, and it is uncertain if ablation effectively mitigates the risk of subsequent arrhythmias and SCA/SCD.
We lack knowledge about selection of patients with MVP and high-risk features who could be candidates for primary prevention ICD implantation. A systematic, quantitative clinical/imaging-based risk assessment tool, possibly enriched by EPS data in those with higher PVC burden, non-sustained VT, or history of syncope, would be valuable in informing decision-making for these patients.
Research opportunities
There is a need for clinically linked basic investigations of MVP-induced ventricular dysfunction, inflammation, and fibrosis, correlating small-and large-animal models with clinical data to determine the fundamental mechanisms of serious ventricular arrhythmias in MVP and identify therapeutic targets (Central Illustration).
To improve risk prediction of SCD in MVP there is a need for a large, longitudinal multicenter/international registry with serial evaluations, imaging (echo, CMR, PET if available), ambulatory ECG monitoring or implantable loop recorders, biological samples (proteomics, genomics, and metab-olomics), EPS, and assessment of clinical outcomes (sustained VT, SCA/SCD, and appropriate ICD shocks). Retrospective studies would be encouraged, but a prospective investigation is needed to include more recent advances in imaging that identify the abnormal ventricular mechanics associated with serious ventricular arrhythmias, and SCA/SCD (Central Illustration).
Once risk prediction is improved, there is a need for randomized controlled trials to establish ideal monitoring intervals with imaging and ambulatory ECG or implantable loop recorders, utility of primary prevention ICD vs radiofrequency catheter ablation, and utility of early MV repair and surgical PM cryoablation. Patient engagement in the design of such trials is key (Central Illustration).
GENETIC STUDIES AND ANIMAL MODELS TO UNDERSTAND MVP MECHANISMS AND PROGRESSION
GENETIC STUDIES.
Genes involved in MVP development play key roles in extracellular matrix deposition and organization, which are influenced by TGF-beta and/or ciliogenic signaling nodes. Among such genes, DCHS1, a member of the cadherin super family, is essential for cell alignment during valve development.110 As highlighted by defects in the DZIP1 gene, the loss of primary cilia during development also leads to progressive myxomatous degeneration of the MV in mice and humans.111 At the population level, MVP mostly occurs as a result of mild dysfunction of the many complex biological mechanisms required during development and/or valve function. Genome-wide association studies (GWAS) have identified predisposition loci,112-114 particularly those near TNS1, a focal adhesion protein, further supporting the importance of cytoskeleton organization revealed by the study of the polyvalvulopathy syndrome caused by FLNA sequence variants.115 Globally, genes located in MVP loci are involved in valve and heart development and potentially aging.116
Knowledge gaps
MVP presents significant clinical heterogeneity and substantial heritability.117 The existing genetic investigations conducted on small pedigrees and medium-sized case control studies described so far present limited power to comprehensively investigate the full phenotypic spectrum of MVP, which can manifest with SCD, severe DMR, or both, and with differences in sex/ethnicity.
Known genetic loci involved in MVP susceptibility only explain a small fraction of the interindividual genetic variability, and given the polygenic feature of MVP, most genetic factors are yet to be discovered.112,3,116 Target genes and their underlying biological mechanisms have been discovered only for a minority of GWAS loci.118 Studies reporting expression quantitative trait loci specific to the MV are lacking,118 making genomic annotation and the search for target genes at MVP GWAS loci even more challenging.
Specific to arrhythmic MVP, the study of the genetic underpinnings of ventricular arrhythmia or SCD in MVP is limited to case reports,119,120 and would greatly benefit from a larger sample of arrhythmic cases.
Research opportunities
Perform large-scale genetic studies covering clinical heterogeneity (arrhythmic MVP vs DMR), and diversity of human populations as it pertains to gender differences and social/ethnic factors.
Create large collections of myxomatous and non-myxomatous valves to explore the specificities of genomic organization, and establish expression quantitative trait loci data for MVs (Figure 3).
Apply multiplex and high throughput methods (eg, clustered regularly interspaced short palindromic repeats interference) to dissect specific regulatory mechanisms of genetic variants involved in MVP (Figure 3).
FIGUER 3. Research Opportunities in the Genetics of MVP.
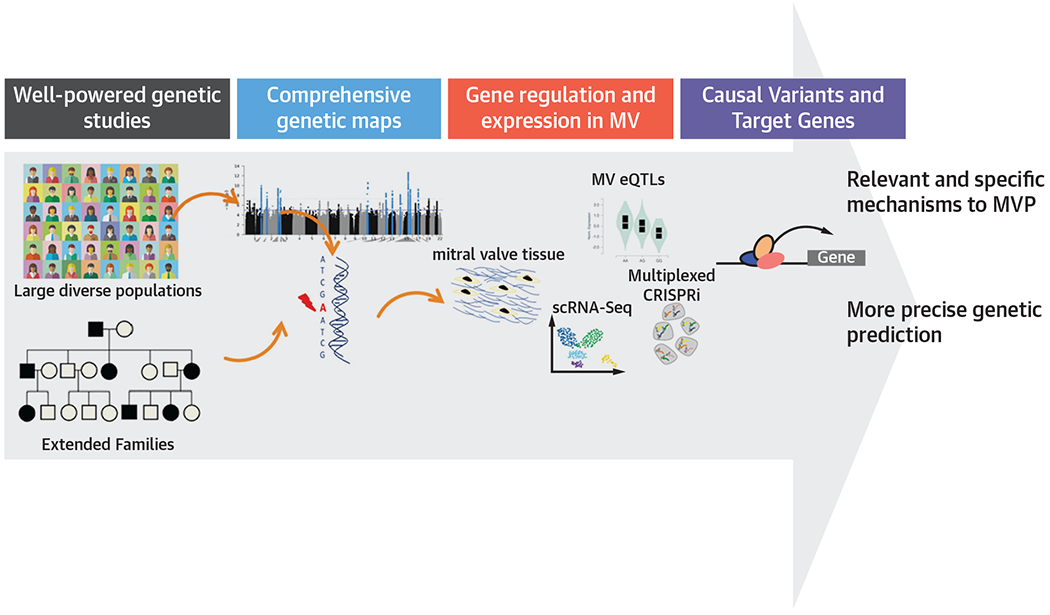
The importance of comprehensive genetic maps and understanding of gene regulation and expression in the mitral valve. CRISPRi = clustered regularly interspaced short palindromic repeats interference; eQTLs = expression quantitative trait loci; MVP = mitral valve prolapse; scRNA-Seq = single-cell RNA sequencing.
DEVELOPMENTAL BASIS OF MVP AND MOUSE MODELS.
Over the past 10 years, MVP variants have been validated in cell culture or in vivo settings through creation of animal models.110-112,114,121-123 Such models have revealed a developmental basis for disease.110,111,121 Specifically, subtle changes in valve geometry during embryogenesis set in motion a process that evolves, over time, into disease pathology. Yet, how these changes are exacerbated over time to give rise to a clinically relevant disease is unknown. Genome-wide and familial studies of large patient cohorts with nonsyndromic MVP have revealed sequence variants in various cytoskeletal and ciliary genes. In turn, such variants may lead to altered interactions of valve endothelial cells (VECs), valve interstitial cells (VICs), and inflammatory cells (Figure 4). Uncovering how these genes orchestrate cell biology and tissue anatomy will provide significant inroads to disease causation with the added potential of informing new therapeutic discoveries.
FIGURE 4. Understanding the Developmental Basis of MVP.
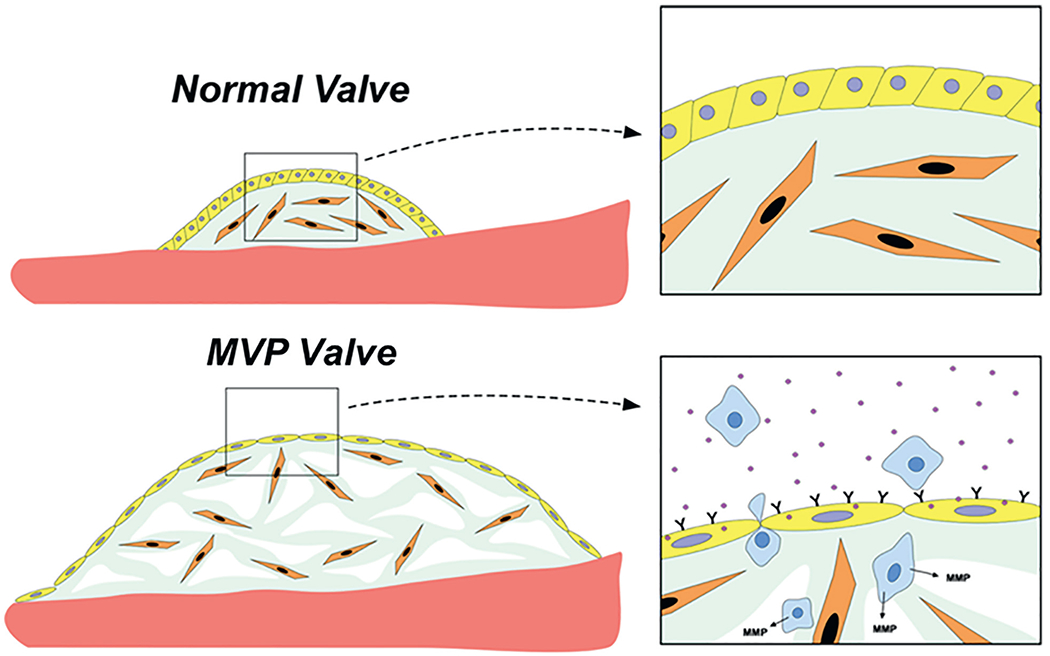
Demonstration of the altered interactions between valve endothelial (yellow)/interstitial (orange)/inflammatory cells (blue) using mouse models. MVP = mitral valve prolapse.
Knowledge gaps
What is exact role of VICs, VECs, and the cytoskeleton in the development of MVP and how do these cells communicate with each other?
How do extracardiac cells infiltrate into the valve and how/why does this occur at a greater rate in the disease94,124 context?
The anatomy and geometry of the valve are critical for normal function, and defects in establishing proper form can result in tears of the endothelium. Is this physical and mechanical change a driving force for disease?
MECHANISMS OF MYOCARDIAL FIBROSIS AND VENTRICULAR ARRHYTHMIA IN MOUSE MODELS.
Studies have shown that increased tension by a prolapsing valve can induce a reactive response in the suspensory apparatus (chordae tendineae, annulus, and PMs) as well as regions within the LV wall.19,20,48,53,55,89,98,125-127 Induction of fibrosis and inflammation likely commences at regions of highest mechanical strain such as the PM-chordal junction.55,89 As time proceeds, pathogenic signals sustain and facilitate propagation and spread of fibrosis throughout the PM and inferobasal myocardium. What likely starts as interstitial and/or perivascular fibrosis evolves into replacement fibrosis with effects on cardiomyocyte viability. Once established, fibrosis provides an arrhythmogenic substrate that may lead to SCD. Recently, an animal model for MVP was tested for regional fibrosis in areas that are most affected in patients.89 This mouse (Dzip1S14R/+) recapitulated the molecular and cellular changes observed in MVP patients and confirmed the progressive nature of LV changes.
Knowledge gaps
Is fibrosis a true consequence of a prolapsing valve and altered mechanics, more related to genetic predisposition, or both?
Can replacement fibrosis be treated? What are the cell types and cellular sensors that respond to the change in mechanical tension?
Does inflammation precede fibrosis or is fibrosis initiated independent of circulating cells? Can the mouse model be used as a testbed to understand disease or treatments and how similar to the human is the murine phenotype? Is the fibrosis in MVP patients or mice arrhythmogenic?
Research opportunities
Understand mechanisms of fibrosis and ventricular arrhythmia in MVP through analysis of the molecular and mechanosensing crosstalk between VIC/VEC/inflammatory cells driving development and/or disease processes using existing and new mouse models, electrophysiology, and optical mapping.
Harness mechanistic insight from developmental discoveries to test therapeutic remediation in mouse models through unbiased fibrotic drug screens.
SURGICAL AND TRANSCATHETER CORRECTION OF ABNORMAL VALVULAR-MYOCARDIAL MECHANICS IN MVP: BIOMECHANICAL SIMULATORS AND LARGE ANIMAL MODELS.
From a therapeutic standpoint, there is some evidence that surgical correction of MVP may reduce the susceptibility to arrhythmias in patients.107,109,128,129 It is possible that reducing the abnormal traction forces that the valve imposes on annular and ventricular structures may decrease overall arrhythmic burden. Ex vivo MV biosimulators and large animal models may correlate the extent of prolapse reduction to changes in traction forces or the remodeling of the myocardial substrate. MV biosimulators have been used extensively130-132 by isolating the MV apparatus from cadaver hearts, instrumenting them with transducers and sensors, and mounting them into systems in which pulsatile hemodynamics can be generated.133,134 The effects of different repair strategies on the valve biomechanics can also be investigated.130,132,135
Knowledge gaps
We need a quantitative understanding of the magnitude of tugging forces on the annulus and sub-PMs from MVP, and the effect of MV repair (surgical and transcatheter) or replacement strategies on these biomechanical perturbations.
Abnormal valvular-myocardial biomechanics need to be mimicked in large animal models,136 such as swine and sheep, which, compared with ex vivo biosimulators allow the use of echocardiography, strain imaging, FDG-PET scanning, and electroanatomic mapping.
The relationship among abnormal biomechanical stimuli, tissue ultrastructure, and the onset of ventricular arrhythmias remains to be investigated in large animal models of MVP combining noninvasive imaging techniques and serial tissue biopsies. Such models may also investigate whether removing the abnormal biomechanical stimuli with MV repair can halt or reverse myocardial changes.
Research opportunities
Develop realistic biomechanical heart valve simulators that can mimic MVP and in which the annular, leaflet, PM and sub-PM biomechanics may be quantified, before and after various MV interventions including surgical and transcatheter techniques.
Invest in large animal models in which MVP can be mimicked, and in novel instrumentation techniques to quantify valvular and ventricular biomechanics. Combine these models with noninvasive imaging modalities to quantify tissue deformation, inflammation, and fibrosis.
Develop patient imaging-derived computational models to understand the heterogeneity in arrhythmogenesis in relation to biomechanical stimuli, and their relief after MV intervention.
CONCLUSIONS
MVP is associated with adverse prognosis in a subset of patients who develop severe DMR, heart failure, SCD, or persistent LV dysfunction despite MV intervention. Research efforts should focus on better understanding mechanisms underlying hemodynamic and arrhythmic complications in MVP through development of large-scale genetic studies, biomechanical simulators, and small and large-animal models. Linking basic to clinical data is of paramount importance to develop novel therapies. To identify those patients at highest risk for complications, standard and novel imaging tools for improved myocardial tissue characterization, such as strain echocardiography, CMR, and PET, should be evaluated in association with “omics,” ECG/EPS, and surgical data for SCD and DMR/LV dysfunction risk, respectively. Prospective evaluation of such parameters in large, international multicenter registries leveraging sex and race differences across populations is key for improved risk stratification and consequent design of randomized controlled trials able to improve treatment of DMR and develop preventative measures for SCD in MVP (Central Illustration).
HIGHLIGHTS.
Severe DMR, SCD, and postoperative LV dysfunction develop in a subset of patients with MVP.
Better risk stratification is essential to improve management and prevent adverse events in patients with MVP.
Retrospective and observational cardiac imaging, genetic, and molecular studies have suggested mechanisms that may underlie adverse events, but prospective multicenter collaborations are needed to identify patients at highest risk.
ACKNOWLEDGMENTS
The authors thank Donna Lloyd-Jones for the assistance in manuscript formatting and preparation; National Heart, Lung, and Blood Institute staff members (Kathleen Fenton, MD, Marissa Miller, DVM, and Vandana Sachdev, MD) for their contribution to planning the virtual workshop; and Yasufumi Nagata, Philippe Bertrand, and Dimosthenis Pandis for their help in figure drafting or formatting.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Delling is supported by the UCSF Resource Allocation Program 7501268 and by National Institutes of Health (NIH) research grant R01HL153447. Dr Noseworthy has received research funding from the NIH, including the National Heart, Lung, and Blood Institute (R21AG 62580-1, R01HL 131535-4, R01HL 143070-2), the National Institute on Aging (R01AG 062436-1), Agency for Healthcare Research and Quality (R01HS 25402-3), Food and Drug Administration (FD 06292), and the American Heart Association (18SFRN34230146; has filed patents related to the application of artificial intelligence to the ECG for diagnosis and risk stratification and has licensed several A-ECG algorithms to Anumana; is involved in potential equity/royalty relationship with AliveCor; is a study investigator in an ablation trial sponsored by Medtronic; and has served on an expert advisory panel for OptumLabs. Dr Adams’s institution, the Icahn School of Medicine at Mount Sinai, receives royalties for mitral and tricuspid valve repair prostheses related to his intellectual property from Edwards Life-sciences and Medtronic. Dr Borger’s institution receives speakers’ honoraria and/or consulting fees on his behalf from Edwards Life-sciences, Medtronic, Abbott, and CryoLife. Dr Elmariah has received research funding from the NIH (R01HL 151838) and the American Heart Association (19TPA34910170); has received research grants from Edwards Lifesciences, Medtronic, and Abbott Vascular; and is a consultant for Edwards Lifesciences. Dr Gerstenfeld has received lecture honoraria from Medtronic, Boston Scientific, and Abbott; has received research funding from, is on the scientific advisory board, and has received compensation from Biosense Webster; is on the scientific advisory board for and is PI of a clinical ablation trial sponsored by Farapulse; and is on the Data and Safety Monitoring Board for trials sponsored by Thermedical Inc and Abbott. Dr Norris has received research funding from the NIH (HL131546, GM103444, HL149696, HL122906), and the American Heart Association (19TPA34850095, 20SRG35540029,19TPA34900016,17CSA33590067). Dr Padala is supported by the National Heart, Lung, and Blood Institute (R01HL135145, R01HL140325, R01HL133667, R01HL144714, R01HL135505) and by the National Institute of Biomedical Imaging and Bioengineering (R01EB031101); has significant stock ownership and a role as a director in Nyra Medical; and has received consulting fees from Heart Repair Technologies Inc in the last 12 months. Dr Weinsaft is supported by NIH research grants R01HL128278 and R01HL151686. Dr Enriquez-Sarano has served as a consultant for Edwards, Cryolife, ChemImage, and HighLife. Dr Levine is supported by NIH research grants R01HL128099 and R01HL141917, American Heart Association 963793/Levine/2022, and funding of the Ellison Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CMR
cardiac magnetic resonance
- DMR
degenerative mitral regurgitation
- EPS
electrophysiology study
- LGE
late gadolinium enhancement
- ICD
implantable cardioverter-defibrillator
- LV
left ventricular
- MAD
mitral annular disjunction
- MVP
mitral valve prolapse
- PET
positron emission tomography
- PM
papillary muscle
- PVC
premature ventricular contraction
- SCA
sudden cardiac arrest
- SCD
sudden cardiac death
- TEER
transcatheter edge-to-edge repair
- VEC
valve endothelial cells
- VF
ventricular fibrillation
- VIC
valve interstitial cell
- VT
ventricular tachycardia
Footnotes
The views expressed in this paper are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2.Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129(21):2158–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol. 1995;75(15):1028–1032. [DOI] [PubMed] [Google Scholar]
- 4.Delling FN, Rong J, Larson MG, et al. Evolution of mitral valve prolapse: insights from the Framingham Heart Study. Circulation. 2016;133(17): 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux RB, Kramer-Fox R, Shear MK, Kligfield P, Pini R, Savage DD. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987;113(5):1265–1280. [DOI] [PubMed] [Google Scholar]
- 6.Luxereau P, Dorent R, De Gevigney G, Bruneval P, Chomette G, Delahaye G. Aetiology of surgically treated mitral regurgitation. Eur Heart J. 1991;12(Suppl B):2–4. [DOI] [PubMed] [Google Scholar]
- 7.Levine RA, Handschumacher MD, Sanfilippo AJ, et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80(3):589–598. [DOI] [PubMed] [Google Scholar]
- 8.Levine RA, Stathogiannis E, Newell JB, Harrigan P, Weyman AE. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1988;11(5):1010–1019. [DOI] [PubMed] [Google Scholar]
- 9.Levine RA, Triulzi MO, Harrigan P, Weyman AE. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75(4):756–767. [DOI] [PubMed] [Google Scholar]
- 10.Freed LA, Benjamin EJ, Levy D, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40(7):1298–1304. [DOI] [PubMed] [Google Scholar]
- 11.Avierinos JF, Gersh BJ, Melton LJ 3rd, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106(11):1355–1361. [DOI] [PubMed] [Google Scholar]
- 12.Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation. 1994;90(2):830–837. [DOI] [PubMed] [Google Scholar]
- 13.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. [DOI] [PubMed] [Google Scholar]
- 14.Grigioni F, Enriquez-Sarano M, Ling LH, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34(7):2078–2085. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura RA, McGoon MD, Shub C, Miller FA Jr, Ilstrup DM, Tajik AJ. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med. 1985;313(21):1305–1309. [DOI] [PubMed] [Google Scholar]
- 16.Kelley BP, Chaudry AM, Syed FF. Developing a mechanistic approach to sudden death prevention in mitral valve prolapse. J Clin Med. 2022;11(5): 1285. 10.3390/jcm11051285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han HC, Ha FJ, Teh AW, et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 2018;7(23):e010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriram CS, Syed FF, Ferguson ME, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62(3):222–230. [DOI] [PubMed] [Google Scholar]
- 19.Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132(7):556–566. [DOI] [PubMed] [Google Scholar]
- 20.Bui AH, Roujol S, Foppa M, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. 2017;103(3):204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9(8):e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLLing FN, Aung S, Vittinghoff E, et al. Antemortem and post-mortem characteristics of Lethal mitral valve prolapse among all countywide sudden deaths. JAm Coll Cardiol EP. 2021;7(8):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essayagh B, Sabbag A, Antoine C, et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76(6):637–649. [DOI] [PubMed] [Google Scholar]
- 24.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373(9672): 1382–1394. [DOI] [PubMed] [Google Scholar]
- 25.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. [DOI] [PubMed] [Google Scholar]
- 26.Dziadzko V, Dziadzko M, Medina-Inojosa JR, et al. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Ear Heart J. 2019;40(27):2194–2202. [DOI] [PubMed] [Google Scholar]
- 27.Ling H, Enriquez-Sarano M, Seward J, et al. Clinical outcome of mitral regurgitation due to flail leaflets. N Engl J Med. 1996;335:1417–1423. [DOI] [PubMed] [Google Scholar]
- 28.Grigioni F, Tribouilloy C, Avierinos JF, et al. Outcomes in mitral regurgitation due to flail leaflets. J Am Coll Cardiol Img. 2008;1:133–141. [DOI] [PubMed] [Google Scholar]
- 29.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352(9):875–883. [DOI] [PubMed] [Google Scholar]
- 30.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. Circulation. 1995;91:1022–1028. [DOI] [PubMed] [Google Scholar]
- 31.Lazam S, Vanoverschelde JL, Tribouilloy C, et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017;135(5):410–422. [DOI] [PubMed] [Google Scholar]
- 32.Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310(6):609–616. [DOI] [PubMed] [Google Scholar]
- 33.Dziadzko V, Clavel MA, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391(10124):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;43(7):561–632. [Google Scholar]
- 35.Antoine C, Benfari G, Michelena HI, et al. Clinical outcome of degenerative mitral regurgitation: critical importance of echocardiographic quantitative assessment in routine practice. Circulation. 2018;138(13):1317–1326. [DOI] [PubMed] [Google Scholar]
- 36.Avierinos JF, Tribouilloy C, Grigioni F, et al. Impact of ageing on presentation and outcome of mitral regurgitation due to flail Leaflet: a multicentre international study. Eur Heart J. 2013;34(33):2600–2609. [DOI] [PubMed] [Google Scholar]
- 37.Detaint D, Sundt TM, Nkomo VT, et al. Surgical correction of mitral regurgitation in the elderly: outcomes and recent improvements. Circulation. 2006;114(4):265–272. [DOI] [PubMed] [Google Scholar]
- 38.Avierinos JF, Inamo J, Grigioni F, Gersh B, Shub C, Enriquez-Sarano M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann Intern Med. 2008;149(11):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delling FN, Gona P, Larson MG, et al. Mild expression of mitral valve prolapse in the Framingham offspring: expanding the phenotypic spectrum. J Am Soc Echocardiogr. 2014;27(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enriquez-Sarano M, Basmadjian AJ, Rossi A, Bailey KR, Seward JB, Tajik AJ. Progression of mitral regurgitation: a prospective doppler echocardiographic study. J Am Coll Cardiol. 1999;34: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 41.Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and longterm outcome. J Am Coll Cardiol. 2002;40(1):84–92. [DOI] [PubMed] [Google Scholar]
- 42.Grigioni F, Benfari G, Vanoverschelde JL, et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. JAm Coll Cardiol. 2019;73(3):264–274. [DOI] [PubMed] [Google Scholar]
- 43.Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010;56(7):570–578. [DOI] [PubMed] [Google Scholar]
- 44.Essayagh B, Antoine C, Benfari G, et al. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J Am Coll Cardiol. 2019;74(7):858–870. [DOI] [PubMed] [Google Scholar]
- 45.Clavel MA, Tribouilloy C, Vanoverschelde JL, et al. Association of B-type natriuretic peptide with survival of patients with degenerative mitral regurgitation. J Am Coll Cardiol. 2016;68(12):1297–1307. [DOI] [PubMed] [Google Scholar]
- 46.David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127(14):1485–1492. [DOI] [PubMed] [Google Scholar]
- 47.Kang DH, Park SJ, Sun BJ, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014;63(22):2398–2407. [DOI] [PubMed] [Google Scholar]
- 48.Quintana E, Suri RM, Thalji NM, et al. Left ventricular dysfunction after mitral valve repair–the fallacy of “normal” preoperative myocardial function. J Thorac Cardiovasc Surg. 2014;148(6):2752–2760. [DOI] [PubMed] [Google Scholar]
- 49.Hagnas MJ, Grasso C, Di Salvo ME, et al. Impact of post-procedural change in left ventricle systolic function on survival after percutaneous edge-to-edge mitral valve repair. J Clin Med. 2021;10(20):4748. 10.3390/jcm10204748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suri RM, Schaff HV, Dearani JA, et al. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovasc Surg. 2009;137(5):1071–1076. [DOI] [PubMed] [Google Scholar]
- 51.Witkowski TG, Thomas JD, Debonnaire PJ, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. 2013;14(1):69–76. [DOI] [PubMed] [Google Scholar]
- 52.Le Tourneau T, Deswarte G, Lamblin N, et al. Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment. Circulation. 2013;127(15):1597–1608. [DOI] [PubMed] [Google Scholar]
- 53.Kitkungvan D, Nabi F, Kim RJ, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72(8):823–834. [DOI] [PubMed] [Google Scholar]
- 54.Lim SJ, Koo HJ, Cho MS, Nam GB, Kang JW, Yang DH. Late gadolinium enhancement of left ventricular papillary muscles in patients with mitral regurgitation. Korean J Radiol. 2021;22(10): 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. 2021;143(18):1763–1774. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Friera L, Salguero R, Vannini L, Arguelles AF, Arribas F, Solis J. Mechanistic insights of the left ventricle structure and fibrosis in the arrhythmogenic mitral valve prolapse. Glob Cardiol Sci Pract. 2018;2018(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero Daza A, Chokshi A, Pardo P, et al. Mitral valve prolapse morphofunctional features by cardiovascular magnetic resonance: more than just a valvular disease. J Cardiovasc Magn Reson. 2021;23(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou T, Li J, Lai H, et al. Benefits of early surgery on clinical outcomes after degenerative mitral valve repair. Ann Thorac Surg. 2018;106(4):1063–1070. [DOI] [PubMed] [Google Scholar]
- 59.Anyanwu AC, Adams DH. Why do mitral valve repairs fail? J Am Soc Echocardiogr. 2009;22(11):1265–1268. [DOI] [PubMed] [Google Scholar]
- 60.Ikenaga H, Makar M, Rader F, et al. Mechanisms of mitral regurgitation after percutaneous mitral valve repair with the MitraClip. Eur Heart J Cardiovasc Imaging. 2020;21(10):1131–1143. [DOI] [PubMed] [Google Scholar]
- 61.Castillo JGAD. Mitral valve repair and replacement. In: Otto C, Bonow R, eds. Valvular Heart Disease: A Companion to Braunwald’s Heart Disease. 4th ed. Saunders Elsevier; 2018:326–340. [Google Scholar]
- 62.Feldman T, Kar S, Elmariah S, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66(25):2844–2854. [DOI] [PubMed] [Google Scholar]
- 63.Buck T, Eiswirth N, Farah A, et al. Recurrence of functional versus organic mitral regurgitation after transcatheter mitral valve repair: implications from three-dimensional echocardiographic analysis of mitral valve geometry and left ventricular dilation for a point of no return. J Am Soc Echocardiogr. 2021;34(7):744–756. [DOI] [PubMed] [Google Scholar]
- 64.Sorajja P, Vemulapalli S, Feldman T, et al. Outcomes With Transcatheter Mitral Valve Repair in the United States: An STS/ACC TVT Registry Report. J Am Coll Cardiol. 2017;70(19):2315–2327. [DOI] [PubMed] [Google Scholar]
- 65.Chikwe J, Toyoda N, Anyanwu AC, et al. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol. 2017;69(19):2397–2406. 10.1016/j.jacc.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 66.Chhatriwalla AK, Vemulapalli S, SzerLip M, et al. Operator experience and outcomes of transcatheter mitral valve repair in the United States. J Am Coll Cardiol. 2019;74(24):2955–2965. [DOI] [PubMed] [Google Scholar]
- 67.Bonow RO, O’Gara PT, Adams DH, et al. 2019 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutionaL recommendations and requirements for transcatheter mitraL vaLve intervention: a joint report of the American Association for Thoracic Surgery, the American CoLLege of CardioLogy, the Society for CardiovascuLar Angiography and Interventions, and The Society of Thoracic Surgeons. J Am Coll Cardiol. 2020;76(1):96–117. [DOI] [PubMed] [Google Scholar]
- 68.VemuLapaLLi S, Grau-SepuLveda M, Habib R, Thourani V, Bavaria J, Badhwar V. Patient and hospitaL characteristics of mitraL vaLve surgery in the United States. JAMA Cardiol. 2019;4(11):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ALkhouLi M, ALqahtani F, Holmes DR, Berzingi C. RaciaL disparities in the utiLization and outcomes of structuraL heart disease interventions in the United States. J Am Heart Assoc. 2019;8(15):e012125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shappell SD, Marshall CE, Brown RE, Bruce TA. Sudden death and the familial occurrence of mid-systolic click, Late systolic murmur syndrome. Circulation. 1973;48(5):1128–1134. [DOI] [PubMed] [Google Scholar]
- 71.Bharati S, Granston AS, Liebson PR, Loeb HS, Rosen KM, Lev M. The conduction system in mitral valve prolapse syndrome with sudden death. Am Heart J. 1981;101(5):667–670. [DOI] [PubMed] [Google Scholar]
- 72.Barlow JB, Bosman CK, Pocock WA, Marchand P. Late systoLic murmurs and nonejection (“mid-Late”) systoLic clicks. An analysis of 90 patients. Br Heart J. 1968;30(2):203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han HC, Parsons SA, Teh AW, et al. Characteristic histopathological findings and cardiac arrest rhythm in isoLated mitral valve prolapse and sudden cardiac death. J Am Heart Assoc. 2020;9(7):e015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sabbag A, Essayagh B, Barrera JDR, et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace. Published online August 11, 2022. 10.1093/europace/euac125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nalliah CJ, Mahajan R, Elliott AD, et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 2019;105(2):144–151. [DOI] [PubMed] [Google Scholar]
- 76.Kligfield P, Levy D, Devereux RB, Savage DD. Arrhythmias and sudden death in mitral valve prolapse. Am Heart J. 1987;113(5):1298–1307. [DOI] [PubMed] [Google Scholar]
- 77.Farb A, Tang AL, Atkinson JB, McCarthy WF, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenLy to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am J Cardiol. 1992;70(2):234–239. [DOI] [PubMed] [Google Scholar]
- 78.Hourdain J, Clavel MA, Deharo JC, et al. Common phenotype in patients with mitraL valve prolapse who experienced sudden cardiac death. Circulation. 2018;138(10):1067–1069. [DOI] [PubMed] [Google Scholar]
- 79.Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med. 1986;314(9):535–540. [DOI] [PubMed] [Google Scholar]
- 80.Dejgaard LA, Skjolsvik ET, Lie OH, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72(14):1600–1609. [DOI] [PubMed] [Google Scholar]
- 81.Vohra J, Sathe S, Warren R, Tatoulis J, Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16(3 Pt 1): 387–393. [DOI] [PubMed] [Google Scholar]
- 82.Clavel MA, Mantovani F, Malouf J, et al. Dynamic phenotypes of degenerative myxomatous mitral valve disease: quantitative 3-dimensional echocardiographic study. Circ Cardiovasc Imaging. 2015;8(5):e002989. [DOI] [PubMed] [Google Scholar]
- 83.Hei S, Iwataki M, Jang JY, et al. Possible mechanism of late systolic mitral valve prolapse: systolic superior shift of leaflets secondary to annular dilatation that causes papillary muscle traction. Am J Physiol Heart Circ Physiol. 2019;316(3):H629–H638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muthukumar L, Rahman F, Jan MF, et al. The pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. J Am Coll Cardiol Img. 2017;10(9):1078–1080. [DOI] [PubMed] [Google Scholar]
- 85.Lee AP, Hsiung MC, Salgo IS, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation. 2013;127(7):832–841. [DOI] [PubMed] [Google Scholar]
- 86.Jensen MO, Jensen H, Smerup M, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008;118(14 Suppl):S250–S255. [DOI] [PubMed] [Google Scholar]
- 87.Nagata Y, Bertrand PB, Levine RA. Malignant mitral valve prolapse: risk and prevention of sudden cardiac death. Curr Treat Options Cardiovasc Med. 2022;24(5):61–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ermakov S, Gulhar R, Lim L, et al. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart. 2019;105(14):1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morningstar JE, Gensemer C, Moore R, et al. Mitral valve prolapse induces regionalized myocardial fibrosis. J Am Heart Assoc. 2021;10(24):e022332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Essayagh B, Sabbag A, Antoine C, et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. J Am Coll Cardiol Img. 2021;14(11):2073–2087. [DOI] [PubMed] [Google Scholar]
- 91.Han Y, Peters DC, Salton CJ, et al. CardiovascuLar magnetic resonance characterization of mitral valve prolapse. J Am Coll Cardiol Img. 2008;1(3):294–303. [DOI] [PubMed] [Google Scholar]
- 92.Han HC, Teh AW, Hare DL, Farouque O, Lim HS. The clinical demographics of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76(22):2689–2690. [DOI] [PubMed] [Google Scholar]
- 93.Guglielmo M, Fusini L, Muscogiuri G, et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur Radiol. 2021;31(2):1100–1109. [DOI] [PubMed] [Google Scholar]
- 94.Kim AJ, Xu N, Umeyama K, et al. Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation. 2020;141(2):132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller MA, Adams DH, Pandis D, et al. Hybrid positron emission tomography/magnetic resonance imaging in arrhythmic mitral valve prolapse. JAMA Cardiol. 2020;5(9):1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhutto ZR, Barron JT, Liebson PR, Uretz EF, Parrillo JE. Electrocardiographic abnormalities in mitral valve prolapse. Am J Cardiol. 1992;70(2): 265–266. [DOI] [PubMed] [Google Scholar]
- 97.Guenancia C, Pace N, Hossu G, et al. Prevalence and determinants of PVCs originating from the mitral apparatus in patients with MVP. J Am Coll Cardiol EP. 2022;8(4):526–528. [DOI] [PubMed] [Google Scholar]
- 98.Miller MA, Dukkipati SR, Turagam M, Liao SL, Adams DH, Reddy VY. Arrhythmic mitral valve prolapse: JACC review topic of the week. J Am Coll Cardiol. 2018;72(23 Pt A):2904–2914. [DOI] [PubMed] [Google Scholar]
- 99.Gornick CC, Tobler HG, Pritzker MC, Tuna IC, Almquist A, Benditt DG. Electrophysiologic effects of papillary muscle traction in the intact heart. Circulation. 1986;73(5):1013–1021. [DOI] [PubMed] [Google Scholar]
- 100.Fulton BL, Liang JJ, Enriquez A, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 2018;29(1):146–153. [DOI] [PubMed] [Google Scholar]
- 101.O’Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014;35(30):2010–2020. [DOI] [PubMed] [Google Scholar]
- 102.Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779. [DOI] [PubMed] [Google Scholar]
- 103.Bumgarner JM, Patel D, Kumar A, et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin Electrophysiol. 2019;42(4):447–452. [DOI] [PubMed] [Google Scholar]
- 104.Marano PJ, Lim LJ, Sanchez JM, et al. Longterm outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J Interv Card Electrophysiol. 2021;61(1):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enriquez A, Shirai Y, Huang J, et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: electrophysio-logic substrate and catheter ablation outcomes. J Cardiovasc Electrophysiol. 2019;30(6):827–835. [DOI] [PubMed] [Google Scholar]
- 106.Syed FF, Ackerman MJ, McLeod CJ, et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9(5):e004005. [DOI] [PubMed] [Google Scholar]
- 107.Naksuk N, Syed FF, Krittanawong C, et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J. 2016;16(6):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaidya VR, DeSimone CV, Damle N, et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol. 2016;46(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Eshmawi A, Pandis D, Miller MA, et al. Surgical cryoablation of papillary muscle PVCs during mitral valve surgery: therapeutic consideration for malignant MVP. J Am Coll Cardiol. 2020;76(25):3061–3062. [DOI] [PubMed] [Google Scholar]
- 110.Durst R, Sauls K, Peal DS, et al. Mutations in DCHS1 cause mitral valve prolapse. Nature. 2015;525(7567):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toomer KA, Yu M, Fulmer D, et al. Primary cilia defects causing mitral valve prolapse. Sci Transl Med. 2019;11(493):eaax0290. 10.1126/scitranslmed.aax0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu M, Georges A, Tucker NR, et al. Genomewide association study-driven gene-set analyses, genetic, and functional follow-up suggest GLIS1 as a susceptibility gene for mitral valve prolapse. Circ Genom Precis Med. 2019;12(5):e002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dina C, Bouatia-Naji Ni, Tucker N, et al. Genetic association analyses highlight biological pathways underlying mitral valve prolapse. Nat Genet. 2015;47(10):1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roselli C, Yu M, Nauffal V, et al. Genome-wide association study reveals novel genetic loci: a new polygenic risk score for mitral valve prolapse. Eur Heart J. 2022;43(17):1668–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le Tourneau T, Le Scouarnec S, Cueff C, et al. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J. 2018;39(15):1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu M, Kyryachenko S, Debette S, et al. Genome-wide association meta-analysis supports genes involved in valve and cardiac development to associate with mitral valve prolapse. Circ Genom Precis Med. 2021;14(5):e003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delling FN, Li X, Li S, et al. Heritability of mitral regurgitation: observations from the Framingham heart study and Swedish population. Circ Cardiovasc Genet. 2017;10(5):e001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kyryachenko S, Georges A, Yu M, et al. Chromatin accessibility of human mitral valves and functional assessment of MVP risk loci. Circ Res. 2021;128(5):e84–e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bains S, Tester DJ, Asirvatham SJ, Noseworthy PA, Ackerman MJ, Giudicessi JR. A novel truncating variant in FLNC-encoded filamin C may serve as a proarrhythmic genetic substrate for arrhythmogenic bileaflet mitral valve prolapse syndrome. Mayo Clin Proc. 2019;94(5):906–913. [DOI] [PubMed] [Google Scholar]
- 120.Mahajan AM, Itan Y, Cerrone M, et al. Sudden cardiac arrest in a patient with mitral valve prolapse and LMNA and SCN5A mutations. J Am Coll Cardiol Case Rep. 2021;3(2):242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sauls K, de Vlaming A, Harris BS, et al. Developmental basis for filamin-A-associated myxomatous mitral valve disease. Cardiovasc Res. 2012;96(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duval D, Labbe P, Bureau L, et al. MVP-associated filamin A mutations affect FlnA-PTPN12 (PTP-PEST) interactions. J Cardiovasc Dev Dis. 2015;2(3):233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levine RA, Hagege AA, Judge DP, et al. Mitral valve disease-morphology and mechanisms. Nat Rev Cardiol. 2015;12(12):689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sauls K, Toomer K, Williams K, et al. Increased infiltration of extra-cardiac cells in myxomatous valve disease. J Cardiovasc Dev Dis. 2015;2(3):200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morningstar JE, Nieman A, Wang C, Beck T, Harvey A, Norris RA. Mitral valve prolapse and its motley crew-syndromic prevalence, pathophysiology, and progression of a common heart condition. J Am Heart Assoc. 2021;10(13):e020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Basso C, Iliceto S, Thiene G, Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140(11):952–964. [DOI] [PubMed] [Google Scholar]
- 127.Han HC, Parsons SA, Curl CL, et al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm. 2021;18(4):570–576. [DOI] [PubMed] [Google Scholar]
- 128.Ross A, DeWeese JA, Yu PN. Refractory ventricular arrhythmias in a patient with mitral valve prolapse. Successful control with mitral valve replacement. J Electrocardiol. 1978;11(3):289–295. [DOI] [PubMed] [Google Scholar]
- 129.Pocock WA, Barlow JB, Marcus RH, Barlow CW. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral valve prolapse. Am Heart J. 1991;121(1 Pt 1):199–202. [DOI] [PubMed] [Google Scholar]
- 130.Padala M, Powell SN, Croft LR, Thourani VH, Yoganathan AP, Adams DH. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J Thorac Cardiovasc Surg. 2009;138(2):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Padala M, Cardinau B, Gyoneva LI, Thourani VH, Yoganathan AP. Comparison of artificial neochordae and native chordal transfer in the repair of a flail posterior mitral leaflet: an experimental study. Ann Thorac Surg. 2013;95(2):629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Erek E, Padala M, Pekkan K, et al. Mitral web–a new concept for mitral valve repair: improved engineering design and in-vitro studies. J Heart Valve Dis. 2009;18(3):300–306. [PubMed] [Google Scholar]
- 133.Imbrie-Moore AM, Paulsen MJ, Thakore AD, et al. Ex vivo biomechanical study of apical versus papillary neochord anchoring for mitral regurgitation. Ann Thorac Surg. 2019;108(1):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhan-Moodie S, Xu D, Suresh KS, et al. Papillary muscle approximation reduces systolic tethering forces and improves mitral valve closure in the repair of functional mitral regurgitation. JTCVS Open. 2021;7:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Imbrie-Moore AM, Paulsen MJ, Zhu Y, et al. A novel cross-species model of Barlow’s disease to biomechanically analyze repair techniques in an ex vivo left heart simulator. J Thorac Cardiovasc Surg. 2021;161(5):1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chawla SK, Frater RWM, Cunningham M, Padala M. Performance and healing of an expanded polytetrafluoroethylene multichordal device at 6 months after repair of mitral leaflet flail in swine. J Thorac Cardiovasc Surg. 2019;157(3):932–940.e933. [DOI] [PMC free article] [PubMed] [Google Scholar]


