Abstract
BACKGROUND:
Despite the biopsychosocial underpinnings of chronic noncancer pain, relatively little is known about the contribution of psychosocial factors to chronic cancer pain. The authors aimed to characterize associations between biopsychosocial factors and pain and opioid use among individuals with chronic pain and cancer.
METHODS:
The authors conducted a retrospective, cross-sectional study of 700 patients with chronic pain and cancer seeking treatment at an academic tertiary pain clinic. Patients completed demographic questionnaires and validated psychosocial and pain measures. Multivariable, hierarchical linear and logistic regressions assessed the relative contributions of biopsychosocial factors to the primary dependent variables of pain severity, pain interference, and opioid use.
RESULTS:
Participants were 62% female and 66% White with a mean age of 59 ± 15 years, and 55% held a college degree or higher. Older age, African American or “other” race, sleep disturbance, and pain catastrophizing were significantly associated with higher pain severity (F(5,657) = 22.45; P ≤ .001; R2 = 0.22). Depression, sleep disturbance, pain catastrophizing, lower emotional support, and higher pain severity were significantly associated with pain interference (F(5,653) = 9.47; P ≤ .001; R2 = 0.44). Lastly, a poor cancer prognosis (Exp(B) = 1.62) and sleep disturbance (Exp(B) = 1.02) were associated with taking opioids, whereas identifying as Asian (Exp(B) = 0.48) or Hispanic (Exp(B) = 0.47) was associated with lower odds of using opioids.
CONCLUSIONS:
Modifiable psychological factors—specifically sleep disturbance, depression, and pain catastrophizing—were uniquely associated with pain and opioid use in patients with chronic pain and diverse cancer diagnoses. Future behavioral pain interventions that concurrently target sleep may improve pain among patients with cancer.
Keywords: cancer, cancer pain, catastrophizing, Collaborative Health Outcomes Information Registry (CHOIR), disparities, opioids, sleep
LAY SUMMARY:
• Feeling depressed, worrying about pain, and bad sleep are related to higher pain symptoms in individuals with chronic pain and cancer.
• Specifically, those who struggle to sleep have worse pain and use more opioids.
• Also, individuals who have a bad prognosis for their cancer are more likely to be using opioid pain medications.
• Although race and cancer are related to chronic pain in patients, psychological well-being is also strongly related to this same pain.
INTRODUCTION
Approximately 40% to 90% of patients with cancer suffer from pain.1 Pain significantly affects functional, psychological, and social well-being for patients with cancer.2 Cancer pain frequently results from malignant growth or treatment3; however, similarly to noncancer pain, data suggest that psychosocial factors may also influence pain associated with cancer.4,5 Individuals with cancer experience elevated rates of psychosocial distress after their diagnosis and during cancer treatment, and this possibly exacerbates their pain.6 Cancer pain management, however, infrequently engages psychosocial resources in pain treatment and often exclusively emphasizes pharmacologic analgesia.7 A large proportion of patients continue to report undertreated pain.8
The World Health Organization analgesic ladder designates the use of opioid analgesics as a treatment for cancer pain with a focus on relieving acute pain from active disease/treatment and for palliative treatment of end-stage disease.9,10 As advances in cancer treatment extend longevity and increase survivorship, pain management approaches require personalized adjustments to treat pain both effectively and safely.11 For instance, although long-term opioid therapy persists as an appropriate treatment for some patients, most could benefit from less risky approaches that emphasize self-management.5,9 This may be especially true for patients with early-stage disease or a high likelihood of a cure, for whom adverse effects associated with long-term opioid use are more likely.9,12,13 Although the physical causes of cancer pain are indisputable, understanding the relative influence of biopsychosocial factors may inform treatment personalization to achieve patient-centered cancer pain management.14,15
Investigating psychosocial variables may elucidate modifiable factors that explain undertreated cancer pain. Studies evaluating the role of biopsychosocial influences in cancer pain have primarily focused on acute postsurgical pain16–20 or have been limited to smaller cohorts of patients with a single cancer type (primarily breast cancer).21–25 These studies show that patients who have higher symptoms of depression and anxiety or are younger, of non-White race, and less educated report higher levels of pain severity and interference4,16,18–23,26–31 Yet to date, few studies have simultaneously evaluated the influence of demographic, disease, and psychosocial risk factors on pain symptoms and prescription opioid use, and even fewer have validated these relationships among diverse samples with chronic pain and cancer. Identifying the relative contributions of modifiable psychosocial factors to cancer pain symptoms and opioid use could help to inform the development of effective and patient-centered cancer pain interventions.
The Collaborative Health Outcomes Information Registry (CHOIR) learning health system is an informatics platform that systematically collects pain-relevant biopsychosocial patient-reported outcome measures throughout the course of patient care.32,33 This study used CHOIR data from patients with chronic pain and cancer receiving an initial medical evaluation at the Stanford Pain Management Center, an academic tertiary pain clinic. Our goal was to assess and compare the relative associations of sociodemographic, medical, psychological, and social factors with pain symptoms and active opioid use in a diverse sample of patients with chronic pain and cancer.
MATERIALS AND METHODS
The study was approved by the Stanford University School of Medicine and Dana-Farber Cancer Institute institutional review boards.
Procedures
All patients being seen at the Stanford Pain Management Center in Redwood City, California, complete initial and follow-up surveys within their routine care to assess multiple dimensions of physical, psychological, and social functioning. Patients with chronic pain are referred to this tertiary pain management center from primary and specialty clinics throughout California, including Stanford’s Comprehensive Cancer Center, nationally, and internationally.
Patients complete the CHOIR questionnaires at home, through a secure email link the night before, or on a tablet provided during the check-in for their initial evaluation. Patients complete questionnaires evaluating their demographics, pain symptoms, psychosocial measures, and self-reported medication use. CHOIR is an open-source learning health care system platform (http://choir.stanford.edu)32,33 and integrates computerized adaptive testing; this allows for fewer items to be tested and yields greater precision in domain assessment. This system affords the ability to comprehensively evaluate biopsychosocial patient-reported outcomes for pain disorders in real-world clinical samples.
From the CHOIR data set, we identified 841 patients with a cancer diagnosis identified through International Classification of Diseases, Ninth Revision (ICD-9) claims. One hundred forty-one patients with early-stage nonmelanoma skin cancer, thyroid cancer, or carcinoma in situ were excluded because these conditions are infrequent causes of chronic pain. Our final sample included 700 patients with oncologic diagnoses presenting for an evaluation for chronic pain.
Measures
Sociodemographic and clinical characteristics
Demographic data were extracted from electronic medical records. Patients self-reported their level of education, race, and ethnicity. Cancer types were extracted from electronic medical records with the ICD-9 codes present in medical claims (2016–2019). We further classified ICD-9 codes for cancer diagnoses by referencing previously published categories.34,35 We characterized a patient’s cancer as “poor prognosis” if the ICD-9 code corresponded to a cancer frequently diagnosed at an advanced stage and/or with a high mortality rate (eg, pancreatic cancer, lung cancer, esophagogastric cancer, or acute myeloid leukemia) or if a nonlymphatic metastatic code was present (1960–1991; 20970–20979).36–38 All other patients were considered to have a “good prognosis” cancer.
Physical, psychological, and social factors
The Patient-Reported Outcomes Measurement Information System (PROMIS) is a National Institutes of Health consensus-based framework that includes validated self-reported health measures.39 CHOIR uses PROMIS measures, including Pain Intensity, Pain Interference, and Sleep Disturbance for physical health; Depression, Anxiety, and Anger for psychological factors; and Emotional Support and Social Isolation for social health. PROMIS measures are standardized with a t score of 50, which represents the population mean (standard deviation [SD] = 10; range = 0–100). Pain catastrophizing, the magnification of pain-related negative thoughts, was measured with the validated 13-item Pain Catastrophizing Scale.40
Pain symptoms and opioid use
Average pain intensity, pain interference, and current opioid use were used as our primary outcomes. Patients self-reported whether they were currently taking opioid and non-opioid pain medications that were prescribed before their current appointment. The use of opioids, antidepressants, anxiolytics, and gabapentinoids was included because these medications are commonly prescribed analgesics for chronic pain. Patients self-reported opioid use by responding to “Are you currently taking any opioid medications?” (Vicodin, Oxycontin, oxycodone, methadone, morphine, MS-Contin, codeine, Actiq, methadone, Duragesic, Dilaudid, Percocet, Opana, Nucynta, Stadol, Ultram, and Norco). A binomial response (0 or 1) was used to represent the use of each opioid separately. Patients were asked to rate their average, current, and worst pain intensity in the previous 7 days on an 11-point numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain).41 Pain interference, a clinically meaningful measure identifying the extent to which pain affects an individual’s function, was evaluated with the PROMIS Pain Interference scale.39
Statistical Analysis
Associations between biopsychosocial variables and average pain intensity and pain interference were evaluated with 2 separate hierarchical linear regressions (model building: 1) sociodemographics, 2) clinical characteristics, 3) social factors, and 4) psychological factors). Hierarchical linear regressions were used because this methodology allows for the differentiation of the unique variance explained by a set of independent variables on the outcome, while controlling for the impact of variables previously applied. Models were built by including theoretically modifiable factors later in the progression of analyses. Sociodemographic and disease variables were added in the first 2 steps to isolate their relative effects on pain outcomes. Social and psychological factors were added thereafter to isolate the impact of each factor on pain symptoms while controlling for sociodemographic/disease associations. The relationship between biopsychosocial factors and opioid use was evaluated with a multiple logistic regression model.
All analyses were conducted with SPSS version 26. Data were screened for outliers and missing data with no remarkable findings. All variables were normally distributed. The data met necessary assumptions for hierarchical linear regression and logistic regression analyses.
RESULTS
Participants were 62% female (n = 436) and 66% White (n = 454), 55% held a college degree (n = 388), and the mean age was 59 years (SD = 15 years; range = 19–93 years; see Table 1). Patients had a variety of cancer diagnoses. In total, 56.9% (n = 398) reported currently using opioids, with a higher rate of patients with a poor prognosis using opioids (62%; n = 236) in comparison with those with a good prognosis (49%; n = 102) or hematologic/lymph malignancies (55%; n = 59; Fig. 1). Patients reported a mean score of 5.4/10 (SD = 2.33) for pain intensity and a mean t score of 62.82/100 (t-score, SD = 7.72) for pain interference (Fig. 2).
TABLE 1.
Patient Sociodemographic, Disease, Psychosocial, and Pain Factors (n = 700)
| Parameter | No. (%) | |
|
| ||
| Age, mean (SD), years | 59 (15.3) | |
| Sex | ||
| Female | 436 (62.3) | |
| Male | 264 (37.7) | |
| Education | ||
| ≤High school | 95 (13.6) | |
| Some college | 207 (29.6) | |
| Bachelor’s degree | 182 (26.0) | |
| Master’s degree | 126 (18.0) | |
| Doctorate | 80 (11.4) | |
| Race/ethnicity | ||
| White | 454 (65.6) | |
| African American | 23 (3.3) | |
| American Indian/Alaska Native | 6 (0.90) | |
| Asian | 90 (13.0) | |
| Other race | 35 (5.1) | |
| Hispanic | 84 (12.1) | |
| Disease type | ||
| Breast | 141 (20.1) | |
| Hematologic/lymph | 109 (15.6) | |
| Bone/connective tissue | 72 (10.3) | |
| Colorectal | 66 (9.4) | |
| Brain/CNS | 53 (7.6) | |
| Genital (male) | 43 (6.1) | |
| Thoracic | 43 (6.1) | |
| Urologic | 38 (5.4) | |
| Genital (female) | 34 (4.9) | |
| Head/neck | 31 (4.4) | |
| Unknown | 27 (3.9) | |
| Liver | 22 (3.1) | |
| Melanoma | 21 (3.0) | |
| Prognosis | ||
| Good prognosis | 209 (29.9) | |
| Poor prognosis | 382 (54.6) | |
| Hematologic/lymph | 108 (15.4) | |
| Medications | ||
| Gabapentinoid use | 179 (25.6) | |
| Antidepressant use | 185 (26.4) | |
| Anxiolytic use | 121 (17.3) | |
| Opioid use | 398 (56.9) | |
| Aberrant opioid use behaviors | ||
| “Taking more than prescribed” | 23 (3.3) | |
| ‘Changing the dosing frequency” | 25 (3.6) | |
| “Stockpiling medications for a rainy day” | 9 (1.3) | |
| “Getting additional pills from friends and family” | 4 (0.6) | |
| Currently smoking | 33 (4.4) | |
|
| ||
| Mean | SD | |
|
| ||
| Pain variables | ||
| Average pain intensity | 5.42 | 2.329 |
| Pain interference | 62.82 | 7.721 |
| Psychosocial variables | ||
| Emotional support | 50.52 | 9.420 |
| Social isolation | 45.69 | 9.006 |
| Satisfactional roles | 43.41 | 9.139 |
| Depression | 52.67 | 9.779 |
| Anxiety | 53.90 | 9.704 |
| Sleep impairment | 54.94 | 9.389 |
| Sleep disturbance | 55.19 | 9.146 |
| Anger | 48.26 | 9.966 |
| Fatigue | 57.52 | 9.399 |
| Pain catastrophizing | 20.37 | 12.335 |
Abbreviations: CNS, central nervous system; PROMIS, Patient-Reported Outcomes Measurement Information System; SD, standard deviation.
Psychosocial variables were measured with the PROMIS scales and the Pain Catastrophizing Scale. The pain intensity was measured with an 11-point numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). The aberrant drug-taking behavior scale was adapted from the Potential Aberrant Drug-Related Behavior section of the Pain Assessment and Documentation Tool42: “Are you taking your opioid pain medications any differently than prescribed by your doctor?” (0 = no; 1 = yes).
Figure 1.
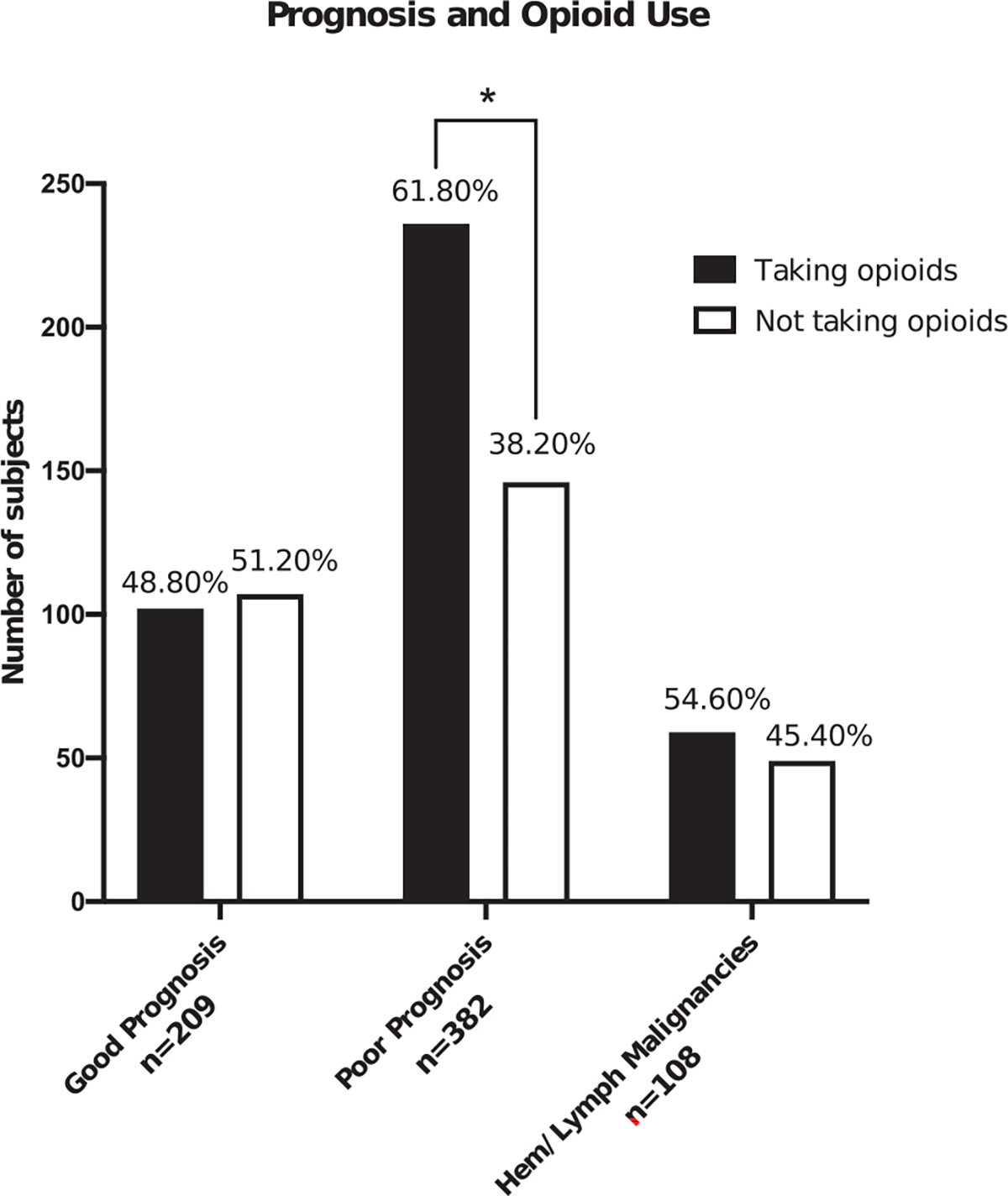
Frequency of opioid use in patients with chronic pain and cancer. Hem indicates hematologic.
* indicates significance P ≤.05.
Figure 2.
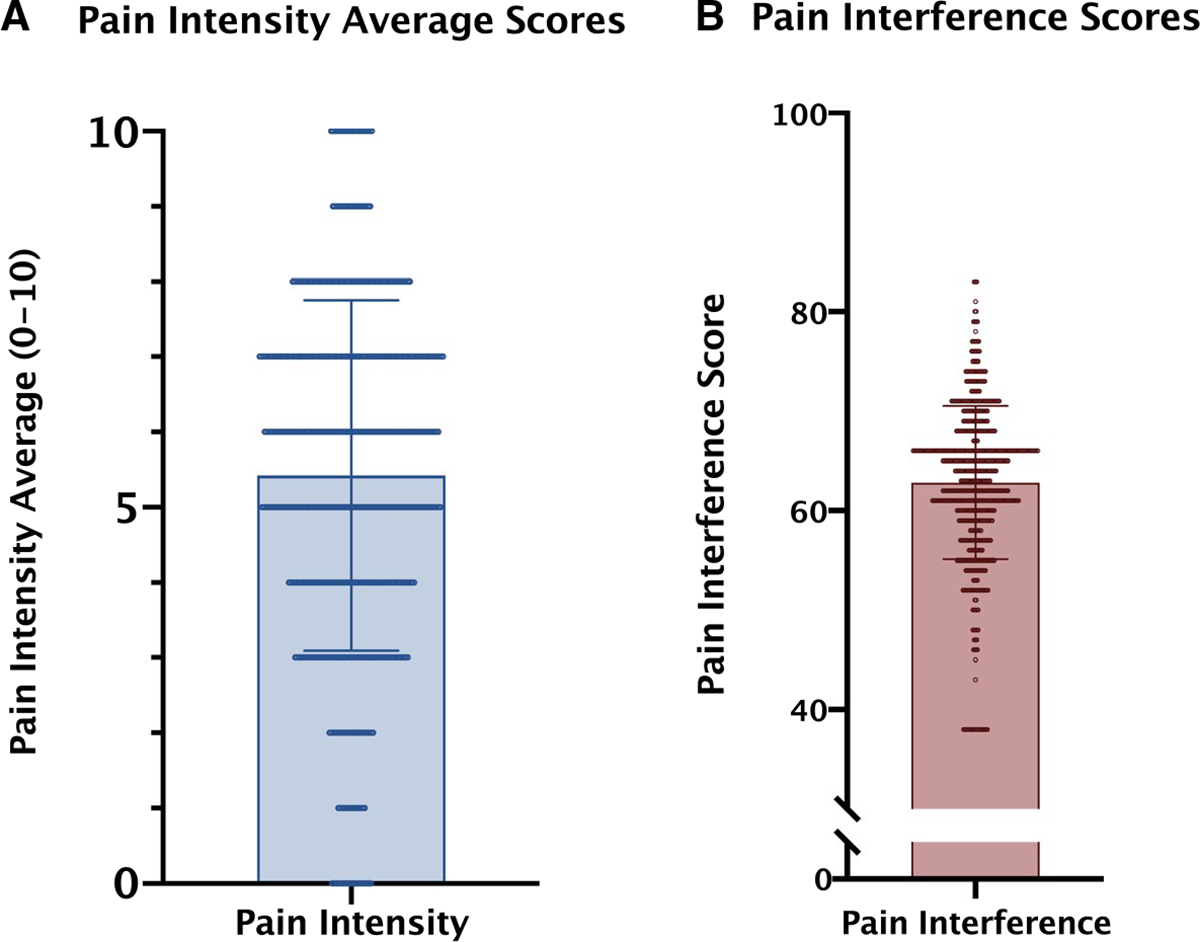
t scores of patients’ self-reported pain scores: (A) pain severity (mean score = 5.42; SD = 2.329; 11-point Likert scale [0–10]) and (B) pain interference (PROMIS Pain Interference measure; mean score = 62.82; SD = 7.721). PROMIS indicates Patient-Reported Outcomes Measurement Information System; SD, standard deviation.
Pain Intensity
Hierarchical linear regression analysis indicated that sociodemographic, disease, social, and psychological variables significantly explained 22% of the total variance in average pain intensity (F(5,657) = 22.45; R2 change = 0.10, 0.01, and 0.13, respectively; P ≤ .05), whereas the cancer prognosis did not significantly contribute to this model (Table 2). Psychological factors uniquely explained 13% of the variance in average pain intensity.
TABLE 2.
Hierarchical Linear Regression Model Summaries
| Dependent Variable | Model No. | Model Variables | Adjusted R2 | R2 Δ | F Change (df) | P |
|---|---|---|---|---|---|---|
|
| ||||||
| Pain intensity | 1 | Sociodemographics | 0.082 | 0.095 | 7.028 (10,668) | .000 |
| 2 | Clinical/medications | 0.078 | 0.004 | 0.532 (5,663) | .752 | |
| 3 | Social factors | 0.084 | 0.008 | 3.059 (2,661) | .048 | |
| 4 | Psychological factors | 0.216 | 0.125 | 21.293 (5,656) | .000 | |
| Pain interference | 1 | Sociodemographics | 0.020 | 0.034 | 2.355 (10,668) | .010 |
| 2 | Clinical/medications | 0.266 | 0.249 | 38.344 (6,662) | .000 | |
| 3 | Social factors | 0.325 | 0.060 | 29.885 (2,660) | .000 | |
| 4 | Psychological factors | 0.442 | 0.118 | 28.749 (5,655) | .000 | |
In the final model, significant individual contributors to pain intensity included older age, African American or “other” race, higher sleep disturbance, and higher catastrophizing. (Table 3). Sex, prognostic status, and use of gabapentinoids, antidepressants, or anxiolytics were not significantly associated with pain intensity, nor were specific psychosocial factors, including emotional support, social isolation, depression, anxiety, and anger (P > .05).
TABLE 3.
Final Hierarchical Linear Regressions for Pain Intensity and Pain Interference Outcomes
| Dependent Variable | Final Model Variables | Standardized b | t Value |
|---|---|---|---|
|
| |||
| Pain intensity | Sociodemographics | ||
| Age | 0.082a | 2.168 | |
| Female | 0.056 | 1.580 | |
| Education | |||
| College | 0.024 | 0.445 | |
| Bachelor’s degree | −0.100 | −1.859 | |
| Master’s degree | −0.054 | −1.085 | |
| Doctorate | −0.103a | −2.207 | |
| Race/ethnicity | |||
| African American | 0.102b | 2.881 | |
| Asian | −0.049 | −1.346 | |
| Other race | 0.081a | 2.259 | |
| Hispanic | 0.061 | 1.596 | |
| Clinical/medication factors | |||
| Prognosis | |||
| Poor prognosis | 0.033 | 0.817 | |
| Hematologic/lymph | 0.011 | 0.285 | |
| Gabapentinoid use | −0.005 | −0.139 | |
| Antidepressant use | −0.013 | −0.370 | |
| Anxiolytic use | −0.002 | −0.065 | |
| Social factors | |||
| Emotional support | −0.027 | −0.686 | |
| Social isolation | −0.074 | −1.461 | |
| Psychological factors | |||
| Depression | −0.043 | −0.655 | |
| Anxiety | 0.059 | 0.873 | |
| Sleep disturbance | 0.148c | 3.811 | |
| Anger | −0.024 | −0.481 | |
| Pain catastrophizing | 0.338c | 7.572 | |
| Pain interference | Sociodemographics | ||
| Age | 0.014 | 0.453 | |
| Female | −0.052 | −1.769 | |
| Education | |||
| Some college | 0.044 | 0.981 | |
| Bachelor’s degree | 0.015 | 0.328 | |
| Master’s degree | 0.01 | 0.25 | |
| Doctorate | 0.019 | 0.488 | |
| Race/ethnicity | |||
| African American | 0.003 | 0.094 | |
| Asian | −0.049 | −1.607 | |
| Other | −0.03 | −0.991 | |
| Hispanic | −0.022 | −0.668 | |
| Clinical/medication factors | |||
| Prognosis | |||
| Poor prognosis | 0.043 | 1.275 | |
| Hematologic/lymph | 0.013 | 0.386 | |
| Gabapentinoid use | 0.094b | 3.178 | |
| Antidepressant use | 0.02 | 0.666 | |
| Anxiolytic use | −0.027 | −0.912 | |
| Pain intensity (average) | 0.348c | 10.641 | |
| Social factors | |||
| Emotional support | 0.095b | 2.848 | |
| Social isolation | 0.048 | 1.128 | |
| Psychological factors | |||
| Depression | 0.284c | 5.091 | |
| Anxiety | 0.023 | 0.407 | |
| Sleep disturbance | 0.147c | 4.447 | |
| Anger | −0.069 | −1.619 | |
| Pain catastrophizing | 0.163c | 4.181 | |
The reference groups were a high school degree or lower for education, White race for Race, and a good prognosis for current prognosis.
P ≤ .05.
P ≤ .01.
P ≤ .001.
Pain Interference
Hierarchical linear regression analysis indicated that sociodemographic, disease, social, and psychological variables significantly explained 44% of the variance in pain interference (F(5,653) = 9.47; R2 change = 0.4, 0.25, 0.06, and 0.12, respectively; P ≤ .05, P ≤ .001, P ≤ .001, and P ≤ .001, respectively; Table 2). Prognostic status and medication use accounted for 25% of the variance, and psychological factors uniquely explained 12% of the variance in pain interference.
Greater pain intensity, depression, sleep disturbance, pain catastrophizing, and emotional support as well as gabapentinoid use were significantly associated with greater pain interference (Table 3). Age, sex, education, race, prognostic status, using antidepressants or anxiolytics, social isolation, anxiety, and anger were not significantly associated with pain interference (P > .05).
Opioid Use
In multivariable logistic regression analysis, race, clinical characteristics, and sleep were significantly related to higher odds of taking opioid medications. Specifically, having a poor prognosis (Exp(B) = 1.62) versus a good prognosis, taking antidepressants (Exp(B) = 1.77), and greater sleep disturbance (Exp(B) = 1.02) were associated with higher odds of taking opioids (see Table 4). Whites (χ2(df = 25) = 97.87; P < .001) had 2.03 (Exp(B) = 0.474; CI, 0.284–0.791) and 2.04 (Exp(B) = 0.456; CI, 0.26–0.801) times higher odds of using opioids than Asians and Hispanics, respectively. The odds of opioid use were 2% higher per unit increase in sleep disturbance, and those with a poor prognosis had 1.6 times the odds of taking opioids than those with a good prognosis. Education, sex, social isolation, and other psychological factors were not significantly related to current opioid use (P ≥ .05).
TABLE 4.
Multivariable Logistic Regression for Current Opioid Use
| 95% Exp(B) CI |
|||||
|---|---|---|---|---|---|
| Wald | Exp(B) | Lower | Upper | Standard Error | |
|
| |||||
| Sociodemographics | |||||
| Age | 1.839 | 0.992 | 0.980 | 1.004 | 0.006 |
| Female | 2.966 | 0.734 | 0.517 | 1.044 | 0.179 |
| Education | |||||
| College | 1.958 | 1.489 | 0.853 | 2.601 | 0.285 |
| Master’s degree | 0.507 | 0.802 | 0.438 | 1.471 | 0.309 |
| Doctorate | 1.409 | 0.657 | 0.328 | 1.315 | 0.354 |
| Bachelor’s degree | 0.171 | 1.13 | 0.633 | 2.016 | 0.295 |
| Race | |||||
| African American | 2.285 | 0.486 | 0.191 | 1.238 | 0.477 |
| Asian | 8.178 | 0.474a | 0.284 | 0.791 | 0.261 |
| Other | 0.227 | 0.832 | 0.39 | 1.775 | 0.387 |
| Hispanic | 7.456 | 0.456a | 0.26 | 0.801 | 0.287 |
| Clinical/medication factors | |||||
| Prognosis | |||||
| Poor prognosis | 6.027 | 1.616b | 1.102 | 2.37 | 0.195 |
| Hematologic/lymph | 0.293 | 1.152 | 0.69 | 1.926 | 0.262 |
| Gabapentinoid | 1.206 | 1.245 | 0.842 | 1.841 | 0.200 |
| Antidepressant | 7.734 | 1.772a | 1.184 | 2.651 | 0.206 |
| Anxiolytic | 2.397 | 1.443 | 0.907 | 2.296 | 0.237 |
| Pain intensity average | 3.306 | 1.077 | 0.994 | 1.166 | 0.041 |
| Social factors | |||||
| Emotional support | 2.025 | 1.015 | 0.994 | 1.036 | 0.010 |
| Social isolation | 0.005 | 0.999 | 0.972 | 1.026 | 0.014 |
| Psychological factors | |||||
| Depression | 2.683 | 1.028 | 0.995 | 1.062 | 0.017 |
| Anxiety | 0.457 | 0.988 | 0.955 | 1.022 | 0.017 |
| Sleep disturbance | 4.249 | 1.022b | 1.001 | 1.043 | 0.01 |
| Anger | 2.134 | 1.018 | 0.994 | 1.044 | 0.013 |
| Pain catastrophizing | 0.285 | 1.005 | 0.987 | 1.023 | 0.009 |
The reference groups were a high school degree or lower for education, White race for Race, and a good prognosis for Prognosis.
P ≤ .01.
P ≤ .05.
DISCUSSION
Among a diverse sample of 700 patients with chronic pain and cancer, we explored biopsychosocial correlates of pain and opioid use. Sociodemographic, psychological, and clinical factors explained some of the interindividual variation in pain intensity (22%) and a greater proportion of the variance in pain interference (44%). Sociodemographic and clinical factors significantly predicted which individuals used opioids, whereas psychological symptoms did not significantly contribute to this association. Patients with poor prognoses had a higher odds of using opioids; this was not unexpected in light of current prescribing recommendations for advanced cancer.15 Notably, sleep disturbance was the only variable consistently independently associated with pain severity, pain interference, and opioid use, and this indicated a strong relationship between sleep disturbance and pain treatment.
Using a hierarchical approach, we were able to differentiate the relative contributions of psychological factors in comparison with the impact of sociodemographic and clinical variables. Psychological symptoms, specifically pain catastrophizing and sleep disturbance, were consistently associated with increased pain symptoms. Pain catastrophizing sensitizes pain perception after mastectomy in cancer,18 yet few studies have evaluated its impact in large, diverse cancer pain samples as demonstrated in our study. Our findings varied somewhat from previous smaller studies identifying depression or anxiety16,18,20–23,26,30,31 as being importantly associated with cancer pain severity. Although we found that depression was significantly related to interference, anxiety was not independently associated with either pain severity or interference, and this indicated that pain catastrophizing may be more influential for patients with cancer. Our findings implicate psychosocial factors as being uniquely associated with pain interference above and beyond demographic/clinical characteristics, and they suggest that the impact of pain on function may rely heavily on patients’ psychological well-being. This is similar to noncancer pain settings in which psychological treatments that target pain catastrophizing often lead to meaningful improvements in pain-related interference.43,44
Few studies have evaluated the role of sleep disturbance in cancer pain,16 although sleep disturbance is a common experience with cancer treatment.45 We found that when we concurrently evaluated biopsychosocial associations, sleep disturbance was the sole factor associated with pain intensity, interference, and opioid use. Undergoing cancer treatment often results in noteworthy sleep disruption.46 The significant impact of psychological factors and sleep on pain intensity and interference suggests that cancer pain treatment could benefit from integrated treatments such as cognitive behavioral therapy for pain and insomnia that concurrently address psychological and sleep symptoms.5,47 To better define pain intervention targets, future investigations can explore the complex interrelationships between pain, psychological symptoms, and sleep symptoms that are commonly exacerbated during cancer treatment trajectories.
This sample endorsed higher rates of opioid use in comparison with patients with chronic pain seen at Stanford’s pain clinic. Approximately 28% of the total clinic population reported active opioid use (4226 of 14,875 total patients in 2016–2019), whereas 57% did in our current sample. Unsurprisingly, higher rates of opioid use were seen in patients with poor prognoses, and this corresponds to current treatment guidelines for cancer pain.10 Such use may not be distinctly problematic because patients did not report elevated opioid misuse behaviors, and patients with worse prognoses did not seem to have significantly worse pain; this indicates that opioids may be helpful in managing pain. In this racially diverse sample, our findings also showed that minorities reported significantly greater pain severity but lower rates of opioid use. Although variations in patient-provider dynamics may explain barriers to opioid prescribing,48,49 there are striking disparities in pain symptoms and opioid use. It may be the case that minorities in this sample were more hesitant to use opioids19,31,50 or that there was an insufficient supply of opioids in their neighborhoods. Psychological symptoms were not directly related to opioid use, and this suggests that psychological and social symptoms may not be as important in determining opioid use for cancer populations as observed in noncancer pain cohorts.51 Patients with cancer, especially those with better prognoses and longer life expectancies, may, however, benefit from lower risk therapies. To effectively improve undertreated pain with cancer,1 pain treatments must acknowledge patient-specific needs and disparities to ensure optimal opioid prescribing and cancer pain care.
An important strength of this study is being one of the first to comprehensively compare the roles of sociodemographic, clinical, and psychosocial factors in pain and opioid use in a large sample of patients with cancer. The diverse demographic and disease makeup of this sample is another strength that allows for greater generalizability in comparison with other evaluations. Additionally, this study used highly validated brief patient-reported outcomes pertinent to clinical pain care.
This study also has several limitations. First, the cross-sectional nature precludes making causal inferences about the reported associations. Many patients are seen for a pain consultation, but not all receive longitudinal care; this limits our ability to evaluate longitudinal outcomes and directionality of effect. For example, it is not clear if being prescribed opioids is a consequence of the identified factors or itself causes the elevation of the identified factors or whether patients have benefitted from opioids. Additionally, disease comorbidities and cancer treatments were not included in our analysis. Such treatments may improve pain, worsen pain, and cause other side effects that increase overall pain expression and opioid use. Future longitudinal studies are required to assess the relationship of sociodemographic and psychosocial factors with changes in pain symptoms, opioid use, and current treatments over time. Importantly, although all patients in this sample had a cancer diagnosis and were being treated for chronic pain, chronicity of pain was not diagnostically confirmed, and these symptoms may not be solely related to their cancer. However, cancer pain management is often complicated by underlying pain disorders predating or unrelated to an individual’s cancer diagnosis, and this potentially makes the distinction less important. Determining optimal pain interventions in the presence of multiple pain disorders and a cancer diagnosis remains challenging and may increase the complexity of a patient’s pain/opioid management plan. As such, the diversity of our sample acknowledges real-world clinical complexities and can inform such clinical nuances in managing cancer pain. Lastly, other relevant psychosocial factors, including financial, spiritual, and racial distress, were not specifically evaluated, and the rate of African Americans in this sample was low.
Our findings emphasize the need to assess patients’ sociodemographic and psychosocial profiles when we are treating chronic pain in cancer because they are related to clinically meaningful differences in pain and opioid use. Providing informed, personalized pain management may be an important step toward reducing disparities in cancer pain treatment. Addressing modifiable psychosocial pain symptoms through targeted behavioral interventions may allow for improved quality of life for patients with cancer.
Acknowledgments
We acknowledge the patients for their participation and the Collaborative Health Outcomes Information Registry team.
FUNDING SUPPORT
This study was funded by the Redlich Pain Endowment and the National Institutes of Health (K24DA029262 and R01DA047236).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Desiree R. Azizoddin reports grants or contracts from the National Palliative Care Research Center and the David Lynch Foundation to her institution. Kristin Schreiber reports a National Institutes of Health/National Institute of General Medical Sciences grant to study pain and opioid use in postsurgical and chronic pain patients; support for attending meetings and/or travel from the American Society of Regional Anesthesiologists, the American Academy of Pain Medicine, the National Institutes of Health (educational seminar), and the Veterans Administration (MERIT grant review study section member); chairmanship of the International Anesthesia Research Society Mentored Research Award Study Section; and membership on the travel grant selection committee of the International Association for the Study of Pain. Beth D. Darnall reports a research grant and a research award from Stanford University; royalties for books; and consulting payments from Applied VR. The other authors made no disclosures.
REFERENCES
- 1.Van Den Beuken-Van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070–1090.e9. doi: 10.1016/j.jpainsymman.2015.12.340 [DOI] [PubMed] [Google Scholar]
- 2.O’Mahony S, Goulet J, Kornblith A, et al. Desire for hastened death, cancer pain and depression: report of a longitudinal observational study. J Pain Symptom Manage. 2005;29:446–457. doi: 10.1016/j.jpainsymman.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Weiss SC, Emanuel LL, Fairclough DL, Emanuel EJ. Understanding the experience of pain in terminally ill patients. Lancet. 2001;357:1311–1315. doi: 10.1016/S0140-6736(00)04515-3 [DOI] [PubMed] [Google Scholar]
- 4.Syrjala KL, Jensen MP, Elena Mendoza M, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32:1703–1711. doi: 10.1200/JCO.2013.54.4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorin SS, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30:539–547. doi: 10.1371/journal.pmed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Psychological distress of patients with advanced cancer: influence and contribution of pain severity and pain interference. Cancer Nurs. 2006;29:400–405. doi: 10.1097/00002820-200609000-00009 [DOI] [PubMed] [Google Scholar]
- 7.Bruera E Relieving physical and psychosocial pain in patients with cancer—the search for enlightened academic medical leaders. JAMA Oncol. 2019;5:1401–1402. doi: 10.1002/cncr.30912 [DOI] [PubMed] [Google Scholar]
- 8.Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19:1985–1991. doi: 10.1093/annonc/mdn419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705–713. doi: 10.14694/edbk_180469 [DOI] [PubMed] [Google Scholar]
- 10.Swarm R, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw. 2010;8:1046–1086. doi: 10.6004/jnccn.2010.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang C, Wang H, Wang Q, Luo Y, Sidlow R, Zuesong H. Prevalence of chronic pain and high-impact chronic pain in cancer survivors in the United States. JAMA Oncol. 2019;5:122–1228. doi: 10.1155/2015/589301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/nejmra1507771 [DOI] [PubMed] [Google Scholar]
- 13.Luckett T, Newton-John T, Phillips J, et al. Risk of opioid misuse in people with cancer and pain and related clinical considerations: a qualitative study of the perspectives of Australian general practitioners. BMJ Open. 2020;10:1–11. doi: 10.1136/bmjopen-2019-034363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett MI, Eisenberg E, Ahmedzai SH, et al. Standards for the management of cancer-related pain across Europe—a position paper from the EFIC Task Force on Cancer Pain. Eur J Pain. 2019;23:660–668. doi: 10.1002/ejp.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarm RA, Paice JA, Anghelescu DL, et al. Adult cancer pain, version 3.2019. J Natl Compr Canc Netw. 2019;17:977–1007. doi: 10.6004/jnccn.2019.0038 [DOI] [PubMed] [Google Scholar]
- 16.Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain. 2013;14:1185–1195. doi: 10.1016/j.jpain.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Schreiber KL, Zinboonyahgoon N, Xu X, et al. Preoperative psychosocial and psychophysical phenotypes as predictors of acute pain outcomes after breast surgery. J Pain. 2019;20:540–556. doi: 10.1016/j.jpain.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154:660–668. doi: 10.1016/j.pain.2012.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2012;18:242–253. doi: 10.1016/j.ejon.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen K, Kehlet K. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Edmond SN, Shelby RA, Keefe FJ, et al. Persistent breast pain among women with histories of breast-conserving surgery for breast cancer compared with women without histories of breast surgery or cancer. Clin J Pain. 2017;33:51–56. doi: 10.1097/AJP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalton JA, Higgins MK, Miller AH, Keefe FJ, Khuri FR. Pain intensity and pain interference in patients with lung cancer: a pilot study of biopsychosocial predictors. Am J Clin Oncol. 2015;38:457–464. doi: 10.1097/COC.0b013e3182a79009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai Y-H, Guo S-L, Keefe FJ, et al. Effects of brief pain education on hospitalized cancer patients with moderate to severe pain. Support Care Cancer. 2003;12:645–652. doi: 10.1007/s00520-004-0626-1 [DOI] [PubMed] [Google Scholar]
- 24.Badr H, Laurenceau JP, Schart L, Basen-Engquist K, Turk D. The daily impact of pain from metastatic breast cancer on spousal relationships: a dyadic electronic diary study. Pain. 2010;151:644–654. doi: 10.1016/j.pain.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 25.Lemay K, Wilson KG, Buenger U, et al. Fear of pain in patients with advanced cancer or in patients with chronic noncancer pain. Clin J Pain. 2011;27:116–124. doi: 10.1097/AJP.0b013e3181f3f667 [DOI] [PubMed] [Google Scholar]
- 26.Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manage. 2002;24:526–542. doi: 10.1016/S0885-3924(02)00497-9 [DOI] [PubMed] [Google Scholar]
- 27.Poulin PA, Solomon BK, Smyth CE, et al. The relationship between mindfulness, pain intensity, pain catastrophizing, depression, and quality of life among cancer survivors living with chronic neuropathic pain. Support Care Cancer. 2016;24:4167–4175. doi: 10.1007/s00520-016-3243-x [DOI] [PubMed] [Google Scholar]
- 28.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–634. doi: 10.1016/j.jpain.2006.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155:232–243. doi: 10.1016/j.pain.2013.09.028 [DOI] [PubMed] [Google Scholar]
- 30.Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2013;18:242–253. doi: 10.1016/j.ejon.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevilacqua LA, Dulak D, Schofield E, et al. Prevalence and predictors of depression, pain, and fatigue in older- versus younger-adult cancer survivors. Psychooncology. 2018;27:900–907. doi: 10.1002/pon.4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturgeon JA, Darnall BD, Kao MCJ, MacKey SC. Physical and psychological correlates of fatigue and physical function: a Collaborative Health Outcomes Information Registry (CHOIR) study. J Pain. 2015;16:291–298.e1. doi: 10.1016/j.jpain.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan JS, Hah JM, Mackey SC. Effects of smoking on patients with chronic pain. Pain. 2019;160:2374–2379. doi: 10.1097/j.pain.0000000000001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allsop MJ, Ziegler LE, Mulvey MR, Russell S, Taylor R, Bennett MI. Duration and determinants of hospice-based specialist palliative care: a national retrospective cohort study. Palliat Med. 2018;32:1322–1333. doi: 10.1177/0269216318781417 [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine. Appendix C: clarification of cancer groupings used in reporting results, with correspondence to National Institute for Occupational Safety and Health cause-of-death codes and International Classification of Diseases codes for cancers. In: Veterans and Agent Orange: Update 2012. National Academies Press; 2014:1067–1076. [Google Scholar]
- 36.Enzinger A, Ghosh K, Keating N, Cutler D, Landrum M, Wright A. US trends and racial/ethnic disparities in opioid access among patients with poor prognosis cancer at the end of life (EOL). J Clin Oncol. 2020;38(suppl):7005. [Google Scholar]
- 37.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121 [DOI] [PubMed] [Google Scholar]
- 38.Obermeyer Z, Powers BW, Makar M, Keating NL, Cutler DM. Physician characteristics strongly predict patient enrollment in hospice. Health Aff (Millwood). 2015;34:993–1000. doi: 10.1377/hlthaff.2014.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cella D, Yount S, Rothrock N, et al. Developing the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007;45(suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 41.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 42.Passik SD, Kirsh KL, Whitcomb L, et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy. Clin Ther. 2004;26:552–561. doi: 10.1016/S0149-2918(04)90057-4 [DOI] [PubMed] [Google Scholar]
- 43.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- 44.Sturgeon JA. Psychological therapies for the management of chronic pain. Psychol Res Behav Manag. 2014;7:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulls HW, Hoogland AI, Small BJ, et al. Lagged relationships among chemotherapy-induced peripheral neuropathy, sleep quality, and physical activity during and after chemotherapy. Ann Behav Med. Published online November 16, 2020. doi: 10.1093/abm/kaaa101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309–1321. doi: 10.1016/S0277-9536(01)00043-0 [DOI] [PubMed] [Google Scholar]
- 47.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the Lifestyles Randomized Controlled Trial. J Am Geriatr Soc. 2013;61:947–956. doi: 10.1111/jgs.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol. 2014;32:1727–1733. doi: 10.1200/JCO.2013.52.4827 [DOI] [PubMed] [Google Scholar]
- 49.Agarwal A, Roberts A, Dusetzina SB, Royce TJ. Changes in opioid prescribing patterns among generalists and oncologists for Medicare Part D beneficiaries from 2013 to 2017. JAMA Oncol. 2020;6:1271–1274. doi: 10.1001/jamaoncol.2020.2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meghani SH, Thompson AML, Chittams J, Bruner DW, Riegel B. Adherence to analgesics for cancer pain: a comparative study of African Americans and Whites using an electronic monitoring device. J Pain. 2015;16:825–835. doi: 10.1016/j.jpain.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martel MO, Wasan AD, Jamison RN, Edwards RR. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol Depend. 2013;132:335–341. doi: 10.1016/j.drugalcdep.2013.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]


