Abstract
Cytokinesis in eukaryotic cells requires the inactivation of mitotic cyclin-dependent kinase complexes. An apparent exception to this relationship is found in Schizosaccharomyces pombe mutants with mutations of the anaphase-promoting complex (APC). These conditional lethal mutants arrest with unsegregated chromosomes because they cannot degrade the securin, Cut2p. Although failing at nuclear division, these mutants septate and divide. Since septation requires Cdc2p inactivation in wild-type S. pombe, it has been suggested that Cdc2p inactivation occurs in these mutants by a mechanism independent of cyclin degradation. In contrast to this prediction, we show that Cdc2p kinase activity fluctuates in APC cut mutants due to Cdc13/cyclin B destruction. In APC-null mutants, however, septation and cutting do not occur and Cdc13p is stable. We conclude that APC cut mutants are hypomorphic with respect to Cdc13p degradation. Indeed, overproduction of nondestructible Cdc13p prevents septation in APC cut mutants and the normal reorganization of septation initiation network components during anaphase.
Cytokinesis in all eukaryotes is coordinated with the nuclear division cycle such that sister chromosomes are distributed equally to daughter cells. In many eukaryotes, cell separation is achieved through contraction of an actomyosin-based ring that forms around the cell cortex between the divided sets of chromosomes (reviewed in reference 28). The fission yeast, Schizosaccharomyces pombe, has proven to be an excellent model organism for studying the events and regulation of eukaryotic cytokinesis. S. pombe cells have a well-characterized mitotic cell cycle, and they divide using an actomyosin ring. Furthermore, a large number of mutants have been isolated with mutations that affect various aspects of cell division, allowing a detailed understanding of these events to emerge (22, 24).
Key to S. pombe cytokinesis is the activity of a signaling cascade termed the septation initiation network (SIN) (reviewed in reference 24). The SIN is required for the final steps in cell division including contraction of the actomyosin ring and formation of the septum. Mutations in the SIN give rise to the septation initiation defective (sid) phenotype, in which cells become highly elongated and multinucleate. The SIN is triggered by the activity of Spg1p (32), a small Ras superfamily GTPase that resides at the spindle pole bodies (SPBs) throughout the cell cycle (33). During interphase, Spg1p is in an inactive GDP-bound form. During metaphase, it becomes activated at both SPBs (GTP-bound form), but during anaphase B, it becomes inactivated at only one pole, giving rise to a poorly understood asymmetric state (33). The GTP-bound form of Spg1p recruits the Cdc7p protein kinase, resulting in Cdc7p localization to both SPBs during metaphase and just one SPB during anaphase B (33). The Sid1p protein kinase, in a complex with Cdc14p, is then recruited to the SPB that contains Cdc7p and activated Spg1p at this time (16). The Sid2p protein kinase is also found constitutively at SPBs (20, 29, 34). Following recruitment of the Sid1p-Cdc14p complex to a single SPB during anaphase, Sid2p is activated and recruited to the medial ring (34).
In addition to being required for the initiation of cytokinesis, the SIN is probably what is regulated to achieve the proper timing of cell division with respect to other events in the cell cycle. Recently, it was shown that inactivation of the mitotic cyclin-dependent kinase Cdc2p in metaphase allows the recruitment of Sid1p-Cdc14p to an SPB and subsequent septation (16). This result suggests that the final steps of the SIN are normally entrained to the state of Cdc2p activity, a situation that would provide an ideal coupling between nuclear and cell division. However, there is a class of S. pombe mutants whose phenotype indicates that high Cdc2p kinase activity might not prevent septation. These mutations are in components of the anaphase-promoting complex (APC) (reviewed in reference 43).
The APC is an E3 ubiquitin ligase that was first identified based on its role in facilitating the multiubiquitination of A- and B-type cyclins, thereby targeting them for proteasome-mediated destruction during mitosis (reviewed in references 27 and 44). The APC is a ∼20S multisubunit complex that has been conserved throughout evolution. In S. pombe, seven components of the APC have been identified to date: Cut4p (APC1) (42). Cut9p (homologous to Saccharomyces cerevisiae Cdc16p) (30, 37), Lid1p/Cut20p (APC4) (5, 41), Nuc2p (homologous to S. cerevisiae Cdc27p) (19), Cut23p (APC8) (41), Hcn1p (homologous to S. cerevisiae Cdc26p) (37), and Apc10p (21). Temperature-sensitive cut4, cut9, nuc2, lid1/cut20, cut23, and apc10 mutants display a cut phenotype at the restrictive temperature. In these mutants, chromosome segregation and spindle elongation fail to occur, such that subsequent cytokinesis bisects the nucleus or results in segregation of DNA to only one daughter cell.
While M-phase cyclins were the first targets known, other APC target proteins have subsequently been identified. One of the most important for cell cycle progression is the securin, Cut2p (homologous to S. cerevisiae Pds1p), whose destruction is required for chromosome segregation (7, 12, 38). Since Cdc13p, the only essential S. pombe cyclin B, is ubiquitinated in an APC-dependent manner (39), the assumption has been made that in APC cut mutants, Cdc13p levels and Cdc2p kinase activity remain elevated (see, for example reference 44). In light of the model for septation discussed above, it is difficult to reconcile the ability of APC cut mutants to form septa and divide, since these events should require Cdc2p inactivation. Here, we present an investigation of this apparent paradox using synchronous cultures of APC cut mutants. Our results demonstrate that Cdc2p activity oscillates in the conditional APC cut mutants and that, contrary to expectation, this is due to Cdc13p degradation. Cdc13p degradation is not observed in APC null mutants, however, and neither are septation and “cutting,” indicating that the APC cut mutants are hypomorphic with respect to Cdc13p degradation. In reciprocal experiments, we used a nondestructible form of Cdc13p to probe the consequences of high Cdc2p kinase activity to APC cut mutants and to the SIN. We present evidence that elevated Cdc2p kinase activity prevents Cdc7p from becoming asymmetrically localized to one SPB in anaphase B and all subsequent relocalization events in the SIN.
MATERIALS AND METHODS
Yeast methods and strains.
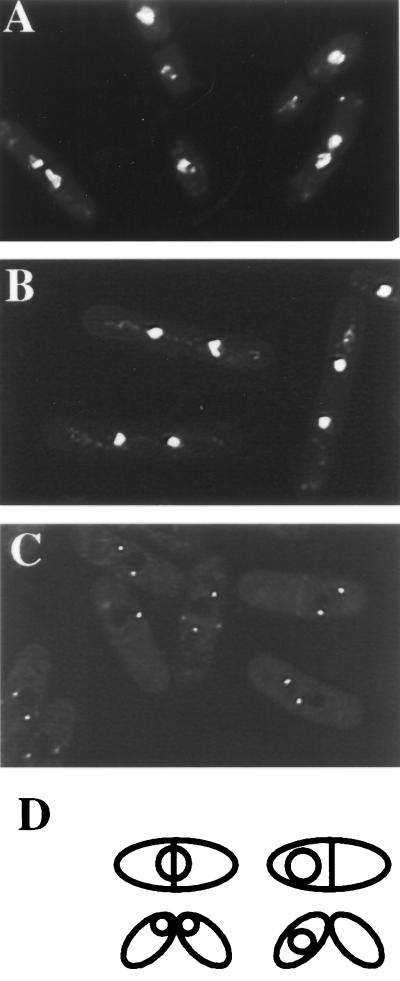
S. pombe strains used in this study (Table 1) were grown in yeast extract medium or minimal medium with appropriate supplements (26). Strains were constructed by tetrad analysis. To obtain synchronous cultures of cells, small cells in early G2 phase were isolated from 4-liter cultures that had been grown in YE at 25°C to mid-log phase by centrifugal elutriation using a Beckman JE 5.0 rotor. The isolated, small cells were filtered immediately and resuspended in YE medium prewarmed to 36°C. Cell cycle synchrony was then monitored at 15 to 20-min intervals for at least two cell cycles by determining the septation index and DNA content. DNA content was determined by flow cytometric analysis following ethanol fixation as detailed previously (6, 31). Fluorescence-activated cell sorter (FACS) data were graphed on a linear scale. The “cut” cell phenotype was defined as illustrated in Fig. 1 and determined after ethanol fixation and 4′,6-diamidino-2-phenylindole (DAPI) staining. For experiments involving genes under control of the nmt promoter (23), cells were grown in minimal medium in the presence of 5 μg of thiamine per ml to repress expression. Induction was achieved by washing twice and resuspending the cells in thiamine-free medium.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| KGY246 | h−ade6-M210 ura4-D18 leu1-32 | Laboratory stock |
| KGY352 | h−nuc2-663 | P. Nurse |
| KGY1164 | h−cut9-665 leu1-32 | R. McIntosh |
| KGY1165 | h+lid1-6 | 5 |
| KGY1167 | h+lid1-6 cdc11-119 | This study |
| KGY1292 | h−rum1::ura4+ura4-D18 leu1-32 ade6-M21X | T. Kelly |
| KGY1570 | h−cut2::cut2-myc::Kanr | 5 |
| KGY1573 | h−cut2::cut2-myc::Kanrcdc10-V50 | This study |
| KGY2474 | h+cdc7-HA::ura4+leu1-32::pJK148nmt1cdc13ΔDB ura4-D18 ade6-M210 | This study |
| KGY2675 | h−lid1::ura4+leu1-32::pJK148nmt1-T81lid1+ura4-D18 ade6-M210 | This study |
| KGY2780 | h−lid1-6 sid2-GFP::ura4+leu1-32 ura4-D18 ade6-M210 | This study |
| KGY2908 | h−cut9-665 rum1::ura4+ | This study |
| KGY2909 | h−lid1-6 rum1::ura4+ | This study |
| KGY2985 | h−sid2-GFP::ura4+leu1-32::pJK148nmt1cdc13ΔDB ura4-D18 ade6-M210 | This study |
| KGY2990 | h−GFP-sid1::ura4+leu1-32::pJK148nmt1cdc13ΔDB ura4-D18 ade6-M210 | This study |
| KGY3115 | h−nuc2-663 leu1-32::pJK148nmt1cdc13ΔDB | This study |
| KGY3493 | h−cut2::cut2-myc::Kanrleu1-32::pJK148nmt1cdc13ΔDB leu1-32 cdc7-HA::ura4+ura4-D18 ade6-M210 | This study |
FIG. 1.
Septation in APC cut mutants proceeds through the SIN. The lid1-6 (A) and lid1-6 cdc11-119 (B) strains were grown in YE medium at 25°C to mid-log phase and shifted to 36°C for 4 h. Cells were collected, fixed with ethanol, and stained with aniline blue to visualize the cell wall and with DAPI to visualize DNA. (C) The lid1-6 sid2-GFP strain was grown at 25°C to mid-log phase, and cells in the G2 phase were collected by centrifugal elutriation. The cells were then shifted to 36°C, and pictures were taken 220 min into the temperature shift. (D) Illustration of the cut phenotype scored in subsequent experiments.
Protein kinase assays and immunoblotting.
Pellets of approximately 108 cells were collected from each time point of the cell synchronization experiments. For histone H1 kinase assays, the cells were lysed in NP-40 buffer and protein concentrations were determined by the bicinchoninic acid assay (Pierce Chemical, Rockford, Ill.). Equal amounts of proteins were subject to immunoprecipitation of Cdc2p as described previously (15). Four-fifths of each immunoprecipitate was used for histone H1 kinase assays in HB5 buffer (25). Histone H1 phosphorylation was quantified by determining the Cerenkov counts associated with dried gel slices containing radioactive histone H1. The remaining fifth of each immunoprecipitate was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting with anti-PSTAIR monoclonal antibody (40) as described previously (6). This antibody recognizes not only Cdc2p but also another, minor Cdc2p-related kinase (35), as well as a Cdc2p-specific degradation product. For determination of Cdc2p tyrosine phosphorylation and Cdc13p levels, cell pellets were lysed in SDS lysis buffer (15) and protein concentrations were determined. Equal amounts of protein were resolved by SDS-PAGE and analyzed by immunoblotting with anti-Cdc2 pTyr polyclonal antibody (Cell Signaling Technologies, Beverly, Mass.), anti-PSTAIR monoclonal antibody (Sigma, St. Louis, Mo.) or affinity-purified rabbit polyclonal anti-Cdc13p antibodies (GJG56). All immunoblots were developed by enhanced chemiluminescence.
Construction of cdc13ΔDB and lid1+ shutoff strains.
From an S. pombe cDNA library (a gift of Chris Norbury and Bruce Edgar) constructed in pREP3X (11), a cdc13+ cDNA was obtained that rescued the cdc13-113 allele. To produce Cdc13p that lacked its destruction box, this cDNA was digested with XhoI and religated to remove the promoter and N-terminal sequences encoding the destruction box. From the resultant plasmid, a PstI-SmaI fragment, including the nmt1 promoter and cdc13ΔDB, was subcloned into PstI- and HincII-digested pJK148. This construct was then linearized within the leu1+ gene with NruI and integrated at the leu1-32 locus to create strain KGY2474.
NdeI and BamHI sites were introduced into the lid1+ cDNA at its initiating methionine and after its termination codon, respectively, by site-directed mutagenesis. The cDNA was then subcloned into pREP81 (4). A PstI-BamHI fragment containing the nmt1-T81 promoter and lid1+ cDNA was then subcloned into pJK148. The resulting plasmid was linearized within the leu1+ gene with NruI and integrated at the leu1-32 locus to create strain KGY2573. This strain was then crossed to the heterozygous diploid lid1+/lid1::ura4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 h+/h+. From a random spore preparation, haploid Ura+ Leu+ colonies were selected to generate strain KGY2675.
Microscopy.
All fluorescence microscopy was performed on a Zeiss microscope (Axioskop; Carl Zeiss, Thornwood, N.Y.), and images were captured with a cooled charge-coupled device camera (Optronics ZVS47DEC). To visualize DNA, cells were fixed with ethanol and stained with DAPI (3). For immunofluorescence, cells were fixed with methanol or ethanol and processed as described previously (3). Following incubation with the anti-alpha-tubulin TAT-1 antibody (36) to detect microtubules, the 12CA5 antibody to detect Cdc7p-HA, or affinity-purified anti-Cdc13p serum, cells were incubated with Alexa594 goat anti-mouse immunoglobulin G (IgG) or Alexa488 goat anti-rabbit IgG (Molecular Probes). The localization of green fluorescent protein-tagged proteins was determined in live cells.
RESULTS
Cdc2p kinase activity fluctuates in APC cut mutants.
To demonstrate that septum formation in APC cut mutants occurs through activation of the SIN, the lid1-6 mutant (Fig. 1A) (Lid1p/Cut20p is the S. pombe APC4 homolog [5, 41]) was combined with a SIN mutant, cdc11-119. Septum formation in lid1-6, as in wild-type cells and the cut1 and cut2 mutants (18), required the SIN since lid1-6 cdc11-119 mutant cells did not display a cut phenotype at restrictive temperature (Fig. 1B). Instead, they arrested in their second mitosis with condensed chromosomes, as observed previously (5), and no septa. Furthermore, Sid2p was able to localize normally to the medial ring in lid1-6 cells (Fig. 1C), indicating that the SIN is activated normally in the cut mutants.
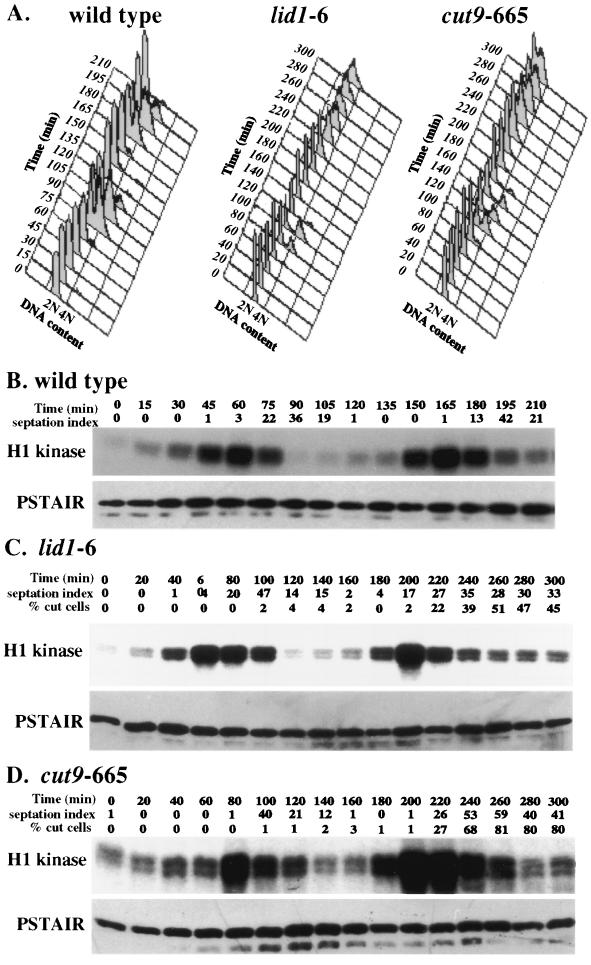
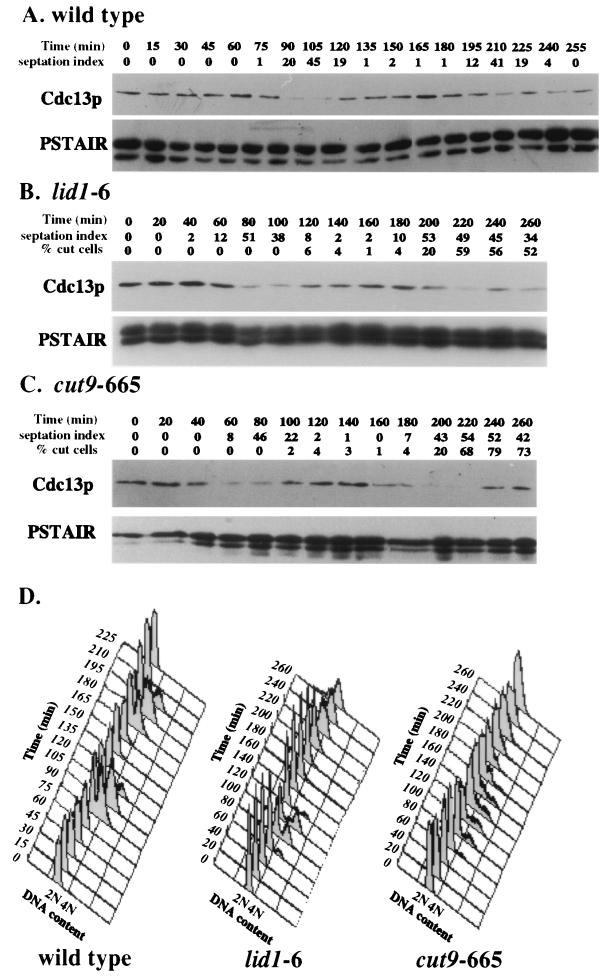
Next, the state of Cdc2p kinase activity in wild-type and two different temperature-sensitive APC mutants (lid1-6 and cut9-665) (5, 37) was determined in cells that had been synchronized in G2 at 25°C and released to 36°C, the nonpermissive temperature for the mutants. In these synchronization experiments and those whose results are shown in subsequent figures, there was slight variability between experiments in the size of the cell population selected by centrifugal elutriation and hence slight variability in the relative time between isolation of the cells (time = 0) and their entry into mitosis. However, each selected cell population was in the G2 phase of the cell cycle (see the FACS analyses) and progressed very synchronously through the cell cycle (see the septation peaks). Samples were collected at regular intervals after isolation and shift to the nonpermissive temperature and examined for DNA content by FACS analysis (Fig. 2A), septation index, percentage of cut cells in the APC mutants, and Cdc2p-associated protein kinase activity (Fig. 2B to D). The cut phenotype was defined as illustrated in Fig. 1D. Separated daughter cells produced from a cutting event were not included in this tally. Cdc2p-associated kinase activity peaked and declined in all three strains (Fig. 2B to D). In each case, the peak of Cdc2p kinase activity preceded the peak of septation and the onset of cutting in the APC mutants. Similar results were also obtained with the nuc2-663 and cut4-553 mutants (data not shown). Thus, there appears to be no uncoupling of the dependency between Cdc2p inactivation and septation in S. pombe APC cut mutants.
FIG. 2.
Cdc2p kinase activity cycles in APC cut mutants. Wild-type (A and B), lid1-6 (A and C), and cut9-665 (A and D) cells were grown to mid-log phase at 25°C, synchronized in early G2 phase by centrifugal elutriation, filtered, and released into prewarmed medium at 36°C. (A) Cells were collected at 15-min (for wild type) or 20-min (for APC mutants) intervals and processed for DNA content by flow cytometry. (B to D) The septation index and percentage of cut cells (in APC mutants) were also determined for each sample. The definition of the cut cell phenotype is provided in the Materials and Methods and shown in Fig. 1D. Cell samples were processed for H1 kinase activity, which was visualized by autoradiography. The amount of Cdc2p in the assayed immunoprecipitates was determined by immunoblotting using PSTAIR monoclonal antibodies.
Cdc13p levels fluctuate in APC cut mutants.
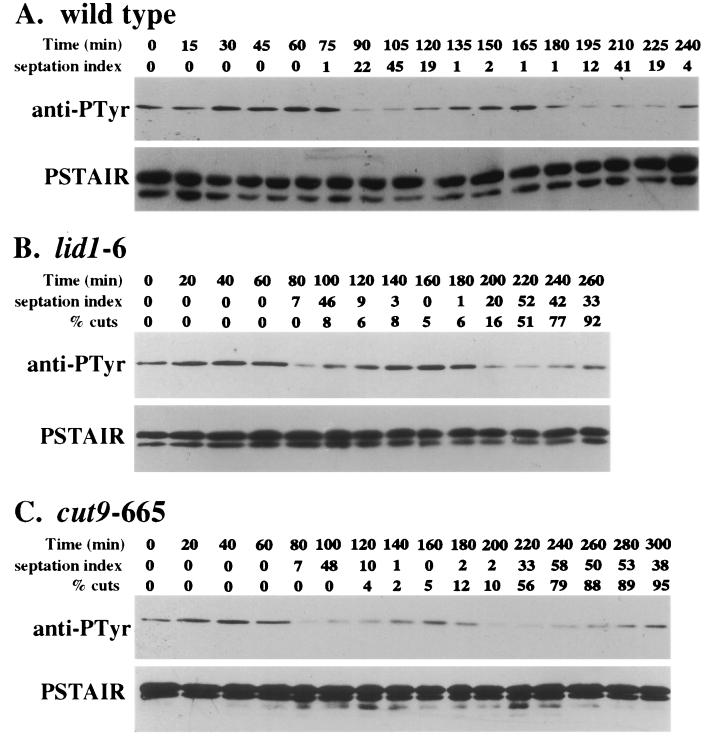
We next examined the reason for the decline in Cdc2p activity observed in the APC cut mutants. There are three known mechanisms to inhibit the mitotic form of Cdc2p kinase: tyrosine 15 phosphorylation, inhibition by the Rum1p inhibitor, and cyclin destruction (reviewed in reference 14). We examined Tyr15 phosphorylation first, again in synchronous cultures of wild-type and APC mutant cells. In each case, Tyr15 phosphorylation declined prior to the increase in septation (Fig. 3). These data suggest that the kinetics of Tyr15 phosphorylation-dephosphorylation is unaltered by mutations in the APC and that Tyr15 rephosphorylation is not responsible for the decline in Cdc2p activity observed in the APC mutants just prior to cutting.
FIG. 3.
Cdc2p phospho-Tyr15 levels continue to cycle in the APC cut mutants. Wild-type (A), lid1-6 (B), and cut9-665 (C) cells were grown to mid-log phase at 25°C in YE medium, synchronized in early G2 by centrifugal elutriation, filtered, and then released into prewarmed medium at 36°C. The cells were collected at 15-min (for wild-type cells) or 20-min (for APC mutants) intervals, and the septation index and percentage of cut cells were determined in each sample. Protein lysates were also prepared from each sample. Cdc2p levels and the Cdc2p-Tyr15 phosphorylation state were determined by immunoblotting using PSTAIR monoclonal and phospho-Cdc2 (Tyr15) polyclonal antibodies, respectively. anti-PTyr, antiphosphotyrosine.
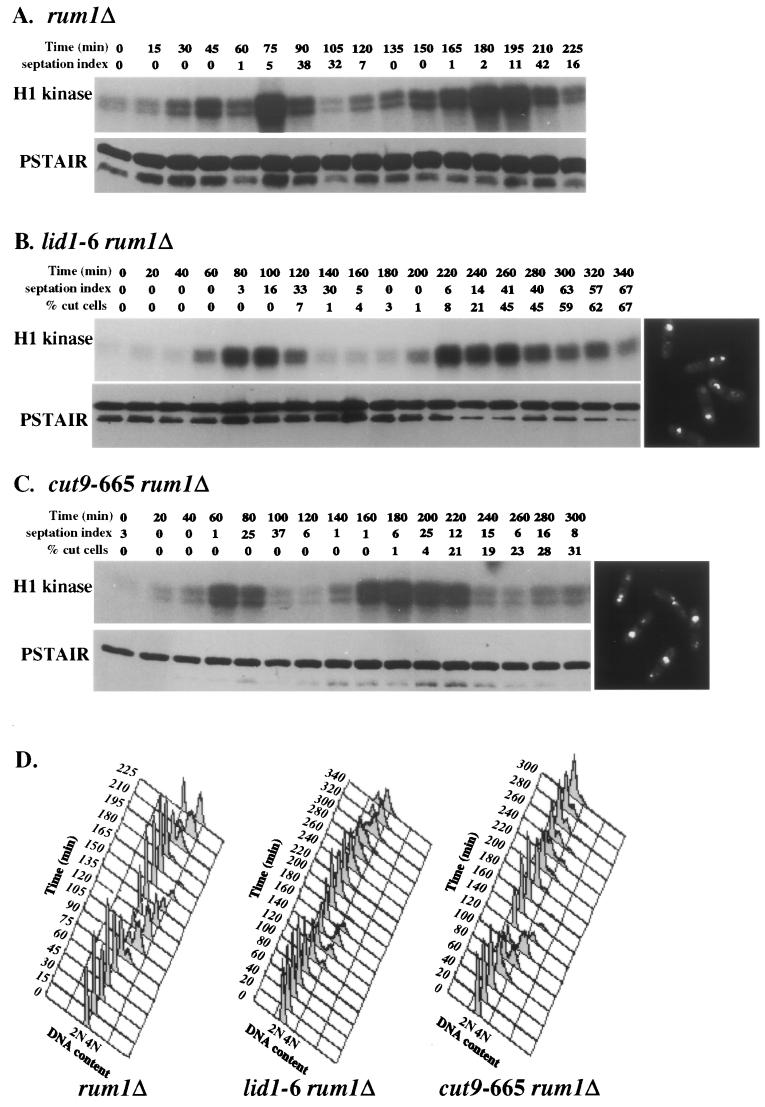
To determine whether Rum1p was responsible for the observed decrease in Cdc2p activity that preceded septation and cutting in the APC mutants, the rum1 deletion allele was combined with mutations in the APC. Again, Cdc2p activity was examined in synchronous cultures of these strains. In rum1Δ and the rum1Δ double mutants, Cdc2p kinase activity persisted longer during each cell cycle than in wild-type cells (Fig. 4). This observation is consistent with the inhibitory role of Rum1p on Cdc2p activity as cells proceed into the G1 phase (9) and its role in promoting Cdc13p destruction (8). Importantly, however, the absence of Rum1p did not preclude Cdc2p inactivation in the APC mutants. These double-mutant cells still displayed a cut phenotype (Fig. 4B and C).
FIG. 4.
Cdc2p kinase activity cycles in APC cut mutants in the absence of Rum1p. (A to C) rum1Δ (A), lid1-6 rum1Δ (B), and cut9-665 rum1Δ (C) cells were grown to mid-log phase at 25°C in YE medium, synchronized in early G2 phase by centrifugal elutriation, filtered, and then released to 36°C. Samples were collected every 15 min (for the rum1Δ single mutant) or 20 min (for APC mutants) and analyzed for H1 kinase activity, Cdc2p levels by immunoblotting using PSTAIR antibodies, and DNA content by FACS. The septation index and the percentage of cut cells were also measured at each time point. Representative images of DAPI-stained lid1-6 rum1Δ and cut9-665 rum1Δ cells at 300 min are shown at the right-hand ends of panels B and C. (D) Histograms of DNA content for rum1Δ, lid1-6 rum1Δ, and cut9-665rum1Δ cells from the experiments in panels A to C. Cells from each time point were fixed in ethanol and stained with Sytox green, and DNA content was measured by FACS analysis.
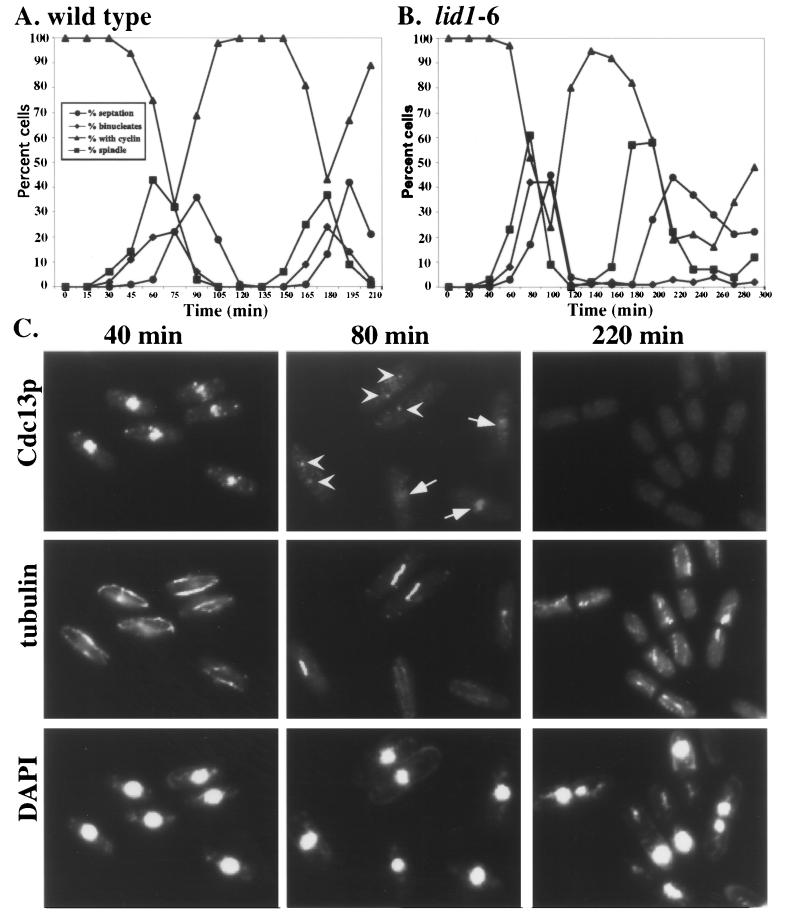
Lastly, Cdc13p levels were determined in synchronous cultures of wild-type, lid1-6, and cut9-665 cells by immunoblotting. As observed previously (10, 17), in wild-type cells Cdc13p exhibited a slow decrease in abundance and was not completely destroyed between cell cycles. Unexpectedly, we observed fluctuations of Cdc13p in APC mutant strains very similar to those observed in wild-type cells (Fig. 5). Cdc13p abundance declined in each case prior to the peaks of septation and cutting. Because the visual detection of Cdc13p can also be used as a measure of its abundance, we examined synchronous cultures of wild-type and lid1-6 cells for the presence of Cdc13p by indirect immunofluorescence (Fig. 6). Cdc13p can be detected during interphase in both the nucleolar and chromatin compartments of the nucleus and during metaphase along the spindle and at the SPBs. The ability to detect Cdc13p by indirect immunofluorescence is lost during anaphase and cytokinesis (1, 2, 13, 16). As with immunoblotting, we observed decreases in Cdc13p abundance prior to septation and cutting in both wild-type and lid1-6 cells (Fig. 6A and B). Importantly, we were unable to detect Cdc13p in any cell containing a septum (Fig. 6C), a finding consistent with its degradation prior to cell division.
FIG. 5.
Cdc13p is degraded in APC cut mutants. (A to C) Wild-type (A), lid1-6 (B), and cut9-665 (C) cells were grown to mid-log phase at 25°C in YE medium, synchronized in early G2 phase by centrifugal elutriation, and then shifted to 36°C. Cells were collected at 15-min (for wild-type cells) or 20-min (for APC mutants) intervals, and the septation index and percentage of cut cells were determined in each sample. Protein lysates were also prepared from each sample. Cdc2p and Cdc13p levels were determined by immunoblotting with PSTAIR monoclonal antibodies and affinity-purified anti-Cdc13p antibodies, respectively. (D) The DNA content of each sample was also determined by flow cytometry.
FIG. 6.
Analysis of Cdc13p localization in APC cut mutants. (A and B) Wild-type (A) and lid1-6 (B) cells were grown to mid-log phase at 25°C in YE medium, synchronized in early G2 phase by centrifugal elutriation, and then shifted to 36°C. Cells were collected at 15-min (for wild-type cells) or 20-min (for lid1-6 cells) intervals and processed for indirect immunofluorescence. The percentage of binucleate cells and septated cells was determined by DAPI staining. The percentage of cells containing spindles was determined by staining cells with the anti-tubulin TAT-1 monoclonal antibody, and the percentage of Cdc13p-positive cells was determined with affinity-purified anti-Cdc13p. (C) Representative fields of lid1-6 cells at the times indicated stained with affinity-purified anti-Cdc13p, TAT-1, or DAPI. Arrows indicate cells containing nuclear Cdc13p, and arrowheads indicate cells with Cdc13p at SPBs.
APC cut mutants are hypomorphic with respect to cyclin degradation.
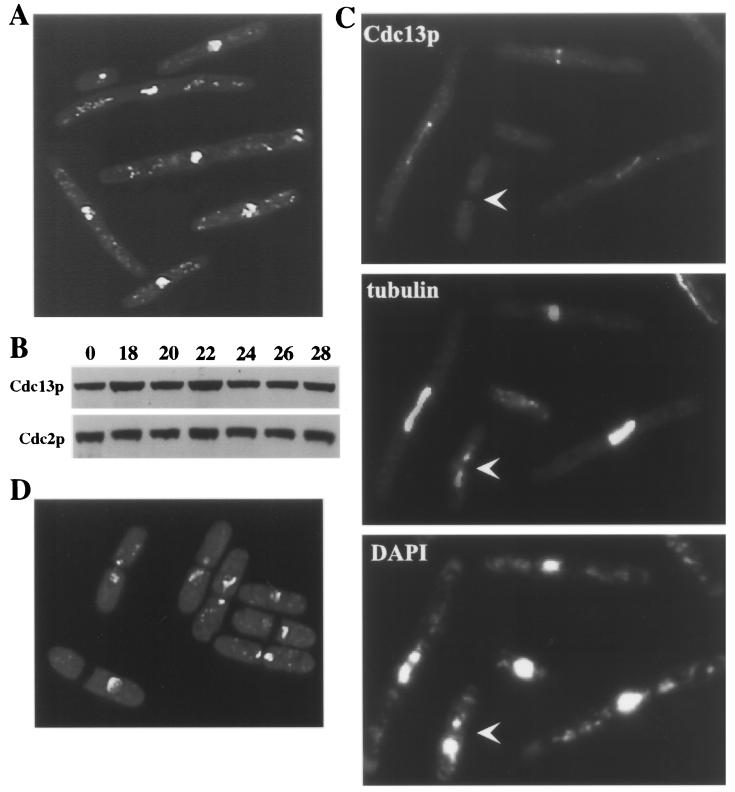
That Cdc13p-cyclin B is degraded in temperature-sensitive APC mutants is at first glance irreconcilable with the established role of the APC in degrading mitotic cyclins. Moreover, APC cut mutants have been shown previously to be unable to support multiubiquitination and degradation of Cut2p (5, 12), consistent with the inability of these mutants to undergo anaphase. We reasoned, however, that these alleles might be hypomorphic in their ability to degrade Cdc13p. This led to the prediction that null alleles of genes encoding APC components would lack the ability to degrade Cdc13p and would not be able to form septa. To test this hypothesis, we examined lid1 null cells conditionally expressing the lid1+ cDNA under control of the nmt1-T81 promoter. In the absence of thiamine, these cells grew at a wild-type rate and displayed normal morphology (data not shown). When thiamine was added to the culture medium, several rounds of cell division occurred normally and then abnormal phenotypes began to arise at 18 to 22 h, at which time cell division ceased. More than 42% of the cells were elongated at this arrest point and contained condensed chromosomes but no septum (Fig. 7A). This phenotype was never observed in the lid1-6 mutant (Fig. 7D). A significant percentage of the cells in the lid1 shutoff strain had died as a result of cutting, while Lid1p levels began to fall and before Lid1p function was completely absent. As predicted and despite the large number of dead cells contributing to the population, Cdc13p levels did not decrease during lid1 repression (Fig. 7B), suggesting that it actually increased among the elongated cell population. Consistent with this interpretation, Cdc13p could be readily detected at the SPBs and spindles of these elongated cells but not within any cut cells in the population (Fig. 7C). These data support our hypothesis that Cdc13p degradation and subsequent septation can occur in temperature-sensitive APC mutants but does not occur in the absence of APC function.
FIG. 7.
APC cut mutants are hypomorphic with respect to Cdc13p degradation. (A to C) The lid1::ura4+ allele was covered by an integrated version of the lid1+ cDNA under control of the nmt1-T81 promoter (KGY2675). Cells were grown to mid-log phase in the absence of thiamine. Thiamine was then added to repress lid1+ expression (time = 0). (A) DAPI-stained cells 27 h later. (B) Samples were examined for Cdc13p and Cdc2p abundance by immunoblotting at the indicated number of hours following thiamine addition. (C) Samples were examined for microtubule structures with TAT-1 antibody and Cdc13p localization using affinity-purified anti-Cdc13p at the same time point as in panel A. The arrowhead indicates a cut cell in which Cdc13p is no longer detectable. (D) lid1-6 cells were grown to mid-log phase at 25°C, shifted to 36°C for 4 h, and stained with DAPI.
Overproduction of nondestructible Cdc13p prevents septation in APC cut mutants.
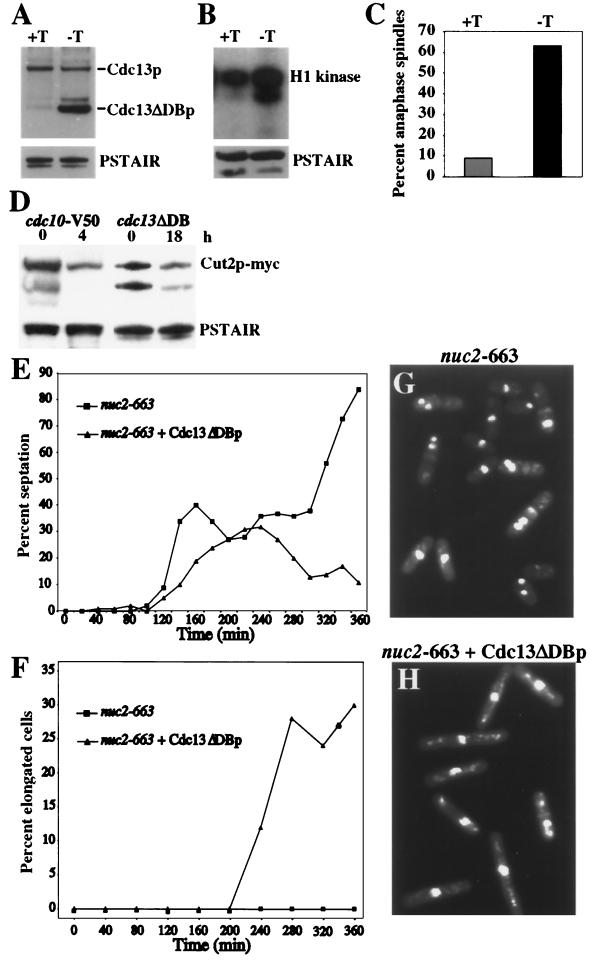
Consistent with Cdc2p inactivation preceding cell division, it has been shown previously that overproduction of a nondestructible form of Cdc13p inhibits septum formation in wild-type cells (39). We therefore tested whether overproduction of a version of Cdc13p lacking its destruction box, Cdc13ΔDBp, would prevent septation in an APC cut mutant, and we chose nuc2-663 as our example because it accumulates a significant percentage of cut cells in the first mitosis following synchronization. As shown previously (39), Cdc13ΔDBp overproduction in wild-type cells (Fig. 8A) led to high levels of Cdc2p kinase activity (Fig. 8B), and an accumulation of cells in anaphase (Fig. 8C). In these cells, Cut2p-myc levels were as low as in cdc10 mutant cells that arrest in G1 with active APC and low levels of Cut2p (12) (Fig. 8D). This result indicates that the APC is active in anaphase cells overproducing Cdc13ΔDBp. To test its effect on septum formation in nuc2-663 cells, nuc2-663 cells or nuc2-663 cells just beginning to overproduce Cdc13ΔDBp at 25°C were subjected to centrifugal elutriation and cells in G2 phase were isolated. These cells were then shifted to 36°C, and the percentage of septated cells was determined. In nuc2 mutant cells overproducing Cdc13ΔDBp, the peak of septation was delayed in the first mitosis and there was no second peak of septation (Fig. 8E). Moreover, elongated cells lacking a septum accumulated in the population (Fig. 8F and H), a phenotype never observed in the nuc2 mutant alone (Fig. 8F and G). We conclude from these data that by delaying Cdc2p inactivation in nuc2-663 using Cdc13ΔDBp, septation and cutting are similarly prevented. There was not a complete block to septation, because it is technically impossible to obtain a pure population of G2 cells expressing high levels of Cdc13ΔDBp (data not shown).
FIG. 8.
High Cdc2p activity delays septation in an APC cut mutant. cdc7-HA cells with an integrated copy of cdc13ΔDB (Cdc13p lacking the destruction box) under the control of the nmt1 promoter (KGY2474) were grown at 32°C in the presence or absence of thiamine, and samples were collected 18 h later. (A) Overproduction of Cdc13ΔDBp. Cells were collected before induction (+T) or 18 h following induction (−T), and Cdc13p and Cdc13ΔDBp levels were detected by immunoblotting using anti-Cdc13p antibodies. Cdc2p, as detected by PSTAIR antibodies, was used as a loading control. (B) Parallel samples to those used in panel A were processed for H1 kinase activity. Cdc2p, as detected by PSTAIR antibodies, was used as a loading control. (C) Samples collected in parallel to those used in panels A and B were fixed and stained with TAT-1 antibodies and Alexa-conjugated goat anti-mouse secondary antibodies. The percentage of cells containing an anaphase spindle was determined by microscopic examination before (+T) and after (−T) induction of Cdc13ΔDBp. (D) The APC is active in cells overexpressing Cdc13ΔDBp. The level of Cut2p-myc was determined in cdc10-V50 cells (KGY1573) 0 or 4 h after the shift to 36°C and in cells in which Cdc13ΔDBp was overproduced for 0 or 18 h (KGY3493). The lower band represents a degradation product. (E to H) The nuc2-663 mutant (KGY352) or the nuc2-663 mutant just beginning to express Cdc13ΔDBp (KGY3115) (19 h following thiamine removal) was synchronized in G2 at 25°C by centrifugal elutriation and shifted to 36°C. The percentage of cells with septa (E) and the percentage of elongated cells containing a single nucleus and no septum (F) were determined following the temperature shift. Photomicrographs of nuc2-663 (G) and nuc2-663-overproducing Cdc13ΔDBp (H) at the 280-min time point are shown.
Overproduction of nondestructible Cdc13p prevents the relocalization of SIN components during anaphase.
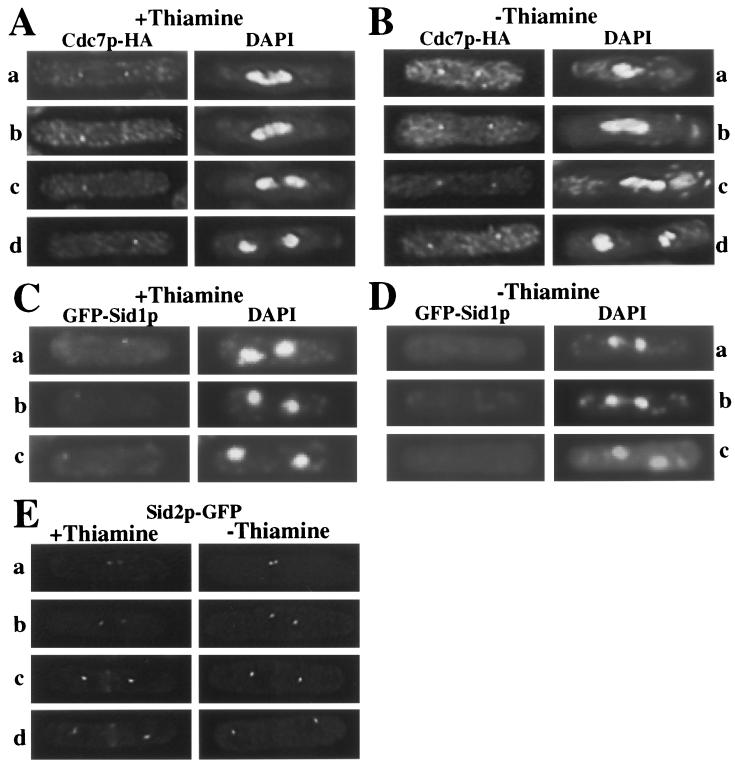
It has been shown that Cdc2p inactivation in metaphase-arrested cells allows recruitment of the SIN pathway component Sid1p to a single SPB that contains activated Spg1p and Cdc7p (16). We therefore tested whether overproduction of Cdc13ΔDBp, which leads to high Cdc2p kinase activity and an accumulation of cells in anaphase (Fig. 8B and C) (39) would interfere with Sid1p localization to the SPB or other relocalization events typical of SIN components during anaphase. As shown previously (33), the Cdc7p-HA protein kinase is localized at both SPBs when cells are in metaphase (Fig. 9A, row a), and as the spindle elongates in anaphase B, Cdc7p-HA is detected at only a single SPB (rows b to d). Sid1p joins the SPB containing Cdc7p-HA at this time (16). In Cdc13ΔDBp-overproducing cells, Cdc7p-HA remained at both SPBs in 27 of 34 cells delayed in anaphase (Fig. 9B), whereas it was asymmetrically localized in all 30 wild-type anaphase cells examined (Fig. 9A). Green fluorescent protein-Sid1p was not localized to either SPB in these cells (59 of 63 anaphase cells examined) (Fig. 9C and D), and Sid2p-GFP was not detected at the medial ring (27 of 29 anaphase cells examined) (Fig. 9E). These data indicate that high Cdc2p kinase activity prevents the final relocalization events in the SIN (a model is shown in Fig. 10).
FIG. 9.
Cdc13ΔDBp overproduction delays the relocalization of SIN components. (A and B) Cdc7p remains on both SPBs under conditions of high Cdc2p kinase activity. cdc7-HA cells with an integrated copy of cdc13ΔDB under the control of the nmt1 promoter (KGY2474) were grown at 32°C in the presence (A) or absence (B) of thiamine, and samples were collected 18 h later. Cells were fixed in methanol and stained with anti-HA (12CA5) monoclonal antibodies followed by Alexa-conjugated goat anti-mouse secondary antibodies. Rows: a, cells in metaphase or early anaphase; b to d, cells in later stages of anaphase. (C and D) Cells producing endogenously tagged GFP-Sid1p and containing the integrated copy of cdc13ΔDB were grown in the presence (C) or absence (D) of thiamine for 18 h and fixed in methanol, and the localization of GFP-Sid1p and DNA was examined. (E) Cells producing endogenously tagged Sid2p-GFP and containing an integrated copy of cdc13ΔDB were grown in the presence or absence of thiamine for 18 h, and the localization of Sid2p-GFP was examined. Rows: a and b, cells in metaphase; c and d, cells in anaphase.
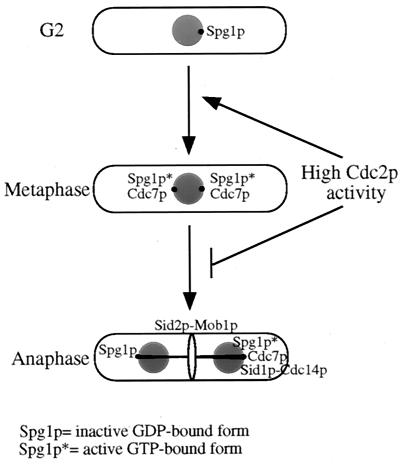
FIG. 10.
Model of SIN component rearrangements during the cell cycle.
DISCUSSION
This study is the first to examine the kinetics of Cdc2p kinase activation-inactivation in synchronous cultures of S. pombe APC cut mutants. We have determined that Cdc2p kinase activity fluctuates in these mutants due to cyclin degradation and that a decrease in Cdc2p kinase activity always precedes the formation of division septa. Thus, these mutants are not exceptions to the rule that Cdc2p inactivation precedes cell division in eukaryotic cells. Moreover, our results show that the APC plays a critical role in the initiation of cytokinesis, most probably by eliminating mitotic Cdk activity.
Why has it been thought that Cdc2p kinase activity remains elevated in temperature-sensitive APC mutants? Similar to what has been observed previously, we noted that when asynchronous cultures of APC cut mutants were shifted to the nonpermissive temperature, Cdc2p activity was higher in some of the mutants than in G2-arrested cells (data not shown). This observation can be reconciled with the periodicity of Cdc2p activity in these mutants if Cdc2p remained active longer in each cell cycle in certain APC cut mutants due to inefficient degradation of the cyclin B, Cdc13p. This could result in a higher percentage of mitotic cells in APC mutants than in wild-type cultures and, hence, could result in relatively higher Cdc2p activity. We obtained evidence for a somewhat longer period of Cdc2p activity in the analysis of the cut mutants relative to wild-type cells, a result consistent with this explanation (Fig. 2).
It was somewhat surprising that Cdc13p-cyclin B levels fluctuated in APC cut mutants since it is well established biochemically and genetically that these mutants are defective in degrading the securin, Cut2p. Cut2p fails to become efficiently multiubiquitinated and degraded in lid1-6 and cut9-665 cells (5, 12), and all APC cut mutants arrest with unsegregated chromosomes (43), which is expected if Cut2p cannot be degraded. In contrast to the situation with Cut2p, Cdc13p levels fall in the lid1-6 mutant as determined by both immunoblotting and indirect immunofluorescence. However, consistent with a requirement for APC in Cdc13p destruction, in cells lacking lid1+ function altogether (lid1::ura4+), the level of Cdc13p remains unchanged, Cdc13p is easily detected at the spindle and SPBs, and septa do not form. The difference between APC target degradation in the temperature-sensitive mutants might reflect a differential sensitivity of the cells to their inefficient degradation. For example, it might be that Cut2p is also degraded to some extent in the cut mutants but that residual Cut2p has a more profound effect on cell cycle progression than residual Cdc13p does. Unfortunately, due to synthetic interactions between APC cut mutants and Cut2p-myc, we are unable to test this possibility directly (data not shown). In any case, our results are consistent with the results of Yamashita et al. (42), who reported that the cut4-533 temperature-sensitive mutant displayed a high percentage of cut cells whereas the cut4 null mutant had a much lower percentage of cut cells and a high percentage of elongated cells containing condensed chromosomes.
It was shown previously that overproduction of nondegradable Cdc13ΔDBp delayed cells in anaphase and prevented septum formation and exit from mitosis (39). Hence, we used this reagent to show that septum formation in the nuc2-663 APC cut mutant was similarly inhibited. This is consistent with the idea that high levels of Cdc2p kinase activity prevent the latter signal transduction events in the SIN (16). We explored this possibility further and found that overproduction of nondestructible Cdc13p in wild-type cells blocked the relocalization events of SIN components that occur during a normal anaphase. Cdc7p-HA remained at both SPBs, Sid1p was not recruited to either SPB, and Sid2p did not relocalize to the medial ring. Since Sid1p is thought to act upstream of Sid2p (16), it is likely that Cdc2p inactivation directly or indirectly affects components of the SPB involved in the formation of an asymmetric state of Cdc7p localization and Sid1p recruitment (a model is shown in Fig. 10). Interestingly, overproduction of Cdc13ΔDBp did not prevent APC activation as measured by the decline in Cut2p-myc in these anaphase cells. Hence, the role of the APC in cytokinesis is most probably inactivation of mitotic Cdk, although it is possible that it plays a second role in the degradation of a protein protected from APC-mediated destruction by Cdk phosphorylation. It will be of interest to determine the identity of the presumed Cdk targets involved in the final steps of the SIN. It will also be of interest to learn how an asymmetric state of the SIN is established prior to cell division and whether, as this work suggests, it is critical for cytokinesis.
ACKNOWLEDGMENTS
We thank Mohan Balasubramanian, Dan McCollum, and all members of the Gould laboratory for useful discussions, critical comments on the manuscript, and encouragement. We are also grateful to K. Gull for the TAT-1 monoclonal antibody.
This work was supported by the Howard Hughes Medical Institute, of which K.L.G. is an associate investigator.
L.C., J.L.M., and A.F. contributed equally to this work.
REFERENCES
- 1.Alfa C E, Booher R, Beach D, Hyams J S. Fission yeast cyclin: subcellular localisation and cell cycle regulation. J Cell Sci Suppl. 1989;12:9–19. doi: 10.1242/jcs.1989.supplement_12.2. [DOI] [PubMed] [Google Scholar]
- 2.Alfa C E, Ducommun B, Beach D, Hyams J S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian M K, McCollum D, Gould K L. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- 4.Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- 5.Berry L D, Feoktistova A, Wright M D, Gould K L. The Schizosaccharomyces pombe dim1(+) gene interacts with the anaphase-promoting complex or cyclosome (APC/C) component lid1(+) and is required for APC/C function. Mol Cell Biol. 1999;19:2535–2546. doi: 10.1128/mcb.19.4.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breeding C S, Hudson J, Balasubramanian M K, Hemmingsen S M, Young P G, Gould K L. The cdr2(+) gene encodes a regulator of G2/M progression and cytokinesis in schizosaccharomyces pombe. Mol Biol Cell. 1998;9:3399–3415. doi: 10.1091/mbc.9.12.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Fix O, Peters J M, Kirschner M W, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 8.Correa-Bordes J, Gulli M P, Nurse P. p25rum1 promotes proteolysis of the mitotic B-cyclin p56cdc13 during G1 of the fission yeast cell cycle. EMBO J. 1997;16:4657–4664. doi: 10.1093/emboj/16.15.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa-Bordes J, Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 10.Creanor J, Mitchison J M. The kinetics of the B cyclin p56cdc13 and the phosphatase p80cdc25 during the cell cycle of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1996;109:1647–1653. doi: 10.1242/jcs.109.6.1647. [DOI] [PubMed] [Google Scholar]
- 11.Forsburg S L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid seperation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher I M, Alfa C E, Hyams J S. p63cdc13, a B-type cyclin, is associated with both the nucleolar and chromatin domains of the fission yeast nucleus. Mol Biol Cell. 1993;4:1087–1096. doi: 10.1091/mbc.4.11.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould K L. Cyclin dependent kinases. In: Woodgett J, editor. Protein kinase functions. Oxford, United Kingdom: Oxford University Press; 2000. pp. 277–302. [Google Scholar]
- 15.Gould K L, Moreno S, Owen D J, Sazer S, Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guertin D A, Chang L, Irshad F, Gould K L, McCollum D. The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 2000;19:1803–1815. doi: 10.1093/emboj/19.8.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayles J, Nurse P. A pre-start checkpoint preventing mitosis in fission yeast acts independently of p34cdc2 tyrosine phosphorylation. EMBO J. 1995;14:2760–2771. doi: 10.1002/j.1460-2075.1995.tb07276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano T, Funahashi S-i, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block nuclear division but not cytokinesis. EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano T, Hiraoka Y, Yanagida M. A temperature-sensitive mutation of the Schizosaccharomyces pombe gene nuc2+ that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1988;106:1171–1183. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou M C, Salek J, McCollum D. Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr Biol. 2000;10:619–622. doi: 10.1016/s0960-9822(00)00492-9. [DOI] [PubMed] [Google Scholar]
- 21.Kominami K, Seth-Smith H, Toda T. Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell-cycle arrest in fission yeast. EMBO J. 1998;17:5388–5399. doi: 10.1093/emboj/17.18.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Goff X, Utzig S, Simanis V. Controlling septation in fission yeast: finding the middle, and timing it right. Curr Genet. 1999;35:571–584. doi: 10.1007/s002940050455. [DOI] [PubMed] [Google Scholar]
- 23.Maundrell K. nmt1 of fission yeast. J Biol Chem. 1991;265:10857–10864. [PubMed] [Google Scholar]
- 24.McCollum D, Gould K L. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 2001;11:89–95. doi: 10.1016/s0962-8924(00)01901-2. [DOI] [PubMed] [Google Scholar]
- 25.Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- 26.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 27.Morgan D O. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 28.Robinson D N, Spudich J A. Towards a molecular understanding of cytokinesis. Trends Cell Biol. 2000;10:228–237. doi: 10.1016/s0962-8924(00)01747-5. [DOI] [PubMed] [Google Scholar]
- 29.Salimova E, Sohrmann M, Fournier N, Simanis V. The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signalling the onset of septum formation. J Cell Sci. 2000;113:1695–1704. doi: 10.1242/jcs.113.10.1695. [DOI] [PubMed] [Google Scholar]
- 30.Samejima I, Yanagida M. Bypassing anaphase by fission yeast cut9 mutation: requirement of cut9+ to initiate anaphase. J Cell Biol. 1994;127:1655–1670. doi: 10.1083/jcb.127.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sazer S, Sherwood S W. Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J Cell Sci. 1990;97:509–516. doi: 10.1242/jcs.97.3.509. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt S, Sohrmann M, Hofmann K, Woollard A, Simanis V. The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev. 1997;11:1519–1534. doi: 10.1101/gad.11.12.1519. [DOI] [PubMed] [Google Scholar]
- 33.Sohrmann M, Schmidt S, Hagan I, Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparks C A, Morphew M, McCollum D. Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J Cell Biol. 1999;146:777–790. doi: 10.1083/jcb.146.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka K, Okayama H. A pcl-like cyclin activates the Res2p-Cdc10p cell cycle “Start” transcriptional factor complex in fission yeast. Mol Biol Cell. 2000;11:2845–2862. doi: 10.1091/mbc.11.9.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods A, Sherwin T, Sasse R, MacRae T H, Baines A J, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- 37.Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto A, Guacci V, Koshland D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita M, Fukada S, Yoshikuni M, Bulet P, Hirai T, Yamaguchi A, Yasuda H, Ohba Y, Nagahama Y. M-phase-specific histone H1 kinase in fish oocytes. Purification, components and biochemical properties. Eur J Biochem. 1992;205:537–543. doi: 10.1111/j.1432-1033.1992.tb16810.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita Y M, Nakaseko Y, Kumada K, Nakagawa T, Yanagida M. Fission yeast APC/cyclosome subunits, Cut20/Apc4 and Cut23/Apc8, in regulating metaphase-anaphase progression and cellular stress responses. Genes Cells. 1999;4:445–463. doi: 10.1046/j.1365-2443.1999.00274.x. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita Y M, Nakaseko Y, Samejima I, Kumada K, Yamada H, Michaelson D, Yanagida M. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature. 1996;384:276–279. doi: 10.1038/384276a0. [DOI] [PubMed] [Google Scholar]
- 43.Yanagida M. Fission yeast cut mutations revisited: control of anaphase. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/s0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]
- 44.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]