Abstract
Background
The term central sleep apnoea (CSA) encompasses diverse clinical situations where a dysfunctional drive to breathe leads to recurrent respiratory events, namely apnoea (complete absence of ventilation) and hypopnoea sleep (insufficient ventilation) during sleep. Studies have demonstrated that CSA responds to some extent to pharmacological agents with distinct mechanisms, such as sleep stabilisation and respiratory stimulation. Some therapies for CSA are associated with improved quality of life, although the evidence on this association is uncertain. Moreover, treatment of CSA with non‐invasive positive pressure ventilation is not always effective or safe and may result in a residual apnoea‐hypopnoea index.
Objectives
To evaluate the benefits and harms of pharmacological treatment compared with active or inactive controls for central sleep apnoea in adults.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 30 August 2022.
Selection criteria
We included parallel and cross‐over randomised controlled trials (RCTs) that evaluated any type of pharmacological agent compared with active controls (e.g. other medications) or passive controls (e.g. placebo, no treatment or usual care) in adults with CSA as defined by the International Classification of Sleep Disorders 3rd Edition. We did not exclude studies based on the duration of intervention or follow‐up. We excluded studies focusing on CSA due to periodic breathing at high altitudes.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were central apnoea‐hypopnoea index (cAHI), cardiovascular mortality and serious adverse events. Our secondary outcomes were quality of sleep, quality of life, daytime sleepiness, AHI, all‐cause mortality, time to life‐saving cardiovascular intervention, and non‐serious adverse events. We used GRADE to assess certainty of evidence for each outcome.
Main results
We included four cross‐over RCTs and one parallel RCT, involving a total of 68 participants. Mean age ranged from 66 to 71.3 years and most participants were men. Four trials recruited people with CSA associated with heart failure, and one study included people with primary CSA. Types of pharmacological agents were acetazolamide (carbonic anhydrase inhibitor), buspirone (anxiolytic), theophylline (methylxanthine derivative) and triazolam (hypnotic), which were given for between three days and one week.
Only the study on buspirone reported a formal evaluation of adverse events. These events were rare and mild. No studies reported serious adverse events, quality of sleep, quality of life, all‐cause mortality, or time to life‐saving cardiovascular intervention.
Carbonic anhydrase inhibitors versus inactive control
Results were from two studies of acetazolamide versus placebo (n = 12) and acetazolamide versus no acetazolamide (n = 18) for CSA associated with heart failure. One study reported short‐term outcomes and the other reported intermediate‐term outcomes. We are uncertain whether carbonic anhydrase inhibitors compared to inactive control reduce cAHI in the short term (mean difference (MD) −26.00 events per hour, 95% CI −43.84 to −8.16; 1 study, 12 participants; very low certainty). Similarly, we are uncertain whether carbonic anhydrase inhibitors compared to inactive control reduce AHI in the short term (MD −23.00 events per hour, 95% CI −37.70 to 8.30; 1 study, 12 participants; very low certainty) or in the intermediate term (MD −6.98 events per hour, 95% CI −10.66 to −3.30; 1 study, 18 participants; very low certainty). The effect of carbonic anhydrase inhibitors on cardiovascular mortality in the intermediate term was also uncertain (odds ratio (OR) 0.21, 95% CI 0.02 to 2.48; 1 study, 18 participants; very low certainty).
Anxiolytics versus inactive control
Results were based on one study of buspirone versus placebo for CSA associated with heart failure (n = 16). The median difference between groups for cAHI was −5.00 events per hour (IQR −8.00 to −0.50), the median difference for AHI was −6.00 events per hour (IQR −8.80 to −1.80), and the median difference on the Epworth Sleepiness Scale for daytime sleepiness was 0 points (IQR −1.0 to 0.00).
Methylxanthine derivatives versus inactive control
Results were based on one study of theophylline versus placebo for CSA associated with heart failure (n = 15). We are uncertain whether methylxanthine derivatives compared to inactive control reduce cAHI (MD −20.00 events per hour, 95% CI −32.15 to −7.85; 15 participants; very low certainty) or AHI (MD −19.00 events per hour, 95% CI −30.27 to −7.73; 15 participants; very low certainty).
Hypnotics versus inactive control
Results were based on one trial of triazolam versus placebo for primary CSA (n = 5). Due to very serious methodological limitations and insufficient reporting of outcome measures, we were unable to draw any conclusions regarding the effects of this intervention.
Authors' conclusions
There is insufficient evidence to support the use of pharmacological therapy in the treatment of CSA. Although small studies have reported positive effects of certain agents for CSA associated with heart failure in reducing the number of respiratory events during sleep, we were unable to assess whether this reduction may impact the quality of life of people with CSA, owing to scarce reporting of important clinical outcomes such as sleep quality or subjective impression of daytime sleepiness. Furthermore, the trials mostly had short‐term follow‐up. There is a need for high‐quality trials that evaluate longer‐term effects of pharmacological interventions.
Keywords: Adult; Aged; Female; Humans; Male; Acetazolamide; Apnea; Buspirone; Carbonic Anhydrase Inhibitors; Disorders of Excessive Somnolence; Heart Failure; Hypnotics and Sedatives; Sleep Apnea, Central; Sleep Apnea, Central/drug therapy; Theophylline; Triazolam
Plain language summary
Medicines for central sleep apnoea in adults
Key messages
Studies in this area are small and we were unable to conclude whether any of the medicines studied helped people with central sleep apnoea (CSA) compared with dummy treatment.
What is central sleep apnoea and how is it treated?
CSA is a disorder in which breathing repeatedly stops and starts during sleep because the brain does not send proper signals to the muscles that control breathing. CSA mainly affects men and people with heart disease. The condition is different from and less common than obstructive sleep apnoea, where breathing is interrupted by blocked or narrowed airways. The treatment of CSA can involve using devices to help breathing, but people with CSA do not always like using them. Treatment with medicines, such as hypnotics (used to reduce tension and induce calm) and respiratory modulators (used to stimulate breathing), may be an alternative for adults with CSA.
What did we want to find out?
The aim of this review was to find out whether medicines can improve the following outcomes in people with CSA.
• Central apnoea‐hypopnoea index (a key indicator of CSA that measures the number of times someone's breathing pauses per hour of sleep) • Death related to heart disease • Quality of sleep • Quality of life • Daytime sleepiness • Apnoea‐hypopnoea index (another score of apnoea events) • Death from any cause • Time to a life‐saving heart‐related intervention (such as a transplant)
We also wanted to know whether these medicines had any unwanted effects.
What did we do?
We searched for studies that investigated the use of medicines for CSA compared with a different treatment (such as the breathing devices commonly used to treat CSA), dummy treatment (placebo), no treatment or usual care. Participants had to be at least 18 years of age. We only included randomised controlled trials (RCTs), which allocate people to treatment groups at random.
What did we find?
We found five studies involving a total of 66 adults with CSA and an average age of 66 to 71 years. The included studies used four different medicines and mostly looked at men who had CSA together with heart disease. In one study, five men received either triazolam (a medicine to help sleep) or placebo. In another study, 16 men received either buspirone (a medicine to help reduce anxiety) or placebo. In a third study, 15 men received either theophylline (a medicine to help wheezing, shortness of breath and chest tightness) or placebo. Thirty adults in the two remaining studies received either acetazolamide (a medicine to help stimulate breathing) or placebo/no acetazolamide.
The studies that provided information on the length of treatment tested the medicines for between three days and one week.
We are uncertain about the effects of the medicines on the central apnoea‐hypopnoea index, death related to heart disease, the apnoea‐hypopnoea index and daytime sleepiness. The studies also could not tell us whether unwanted events were more common with medicines than with placebo. No studies reported our other outcomes of interest.
What are the limitations of the evidence?
Our confidence in the evidence is very low, mainly because it comes from small studies with very few participants.
How up to date is the evidence?
The evidence is up to date to 30 August 2022.
Summary of findings
Summary of findings 1. Carbonic anhydrase inhibitors versus inactive control for central sleep apnoea associated with heart failure in adults .
| Acetazolamide 250 mg or 3.5 mg/kg (once daily) compared to placebo/no acetazolamide for central sleep apnoea in adults | |||||
| Patient or population: adults with central sleep apnoea associated with heart failure Setting: outpatients Intervention: acetazolamide 250 mg or 3.5 mg/kg (once daily) Comparison: placebo/no acetazolamide | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo/no acetazolamide | Risk with acetazolamide | ||||
|
cAHI Follow‐up: mean 4 weeks (short‐term) |
Mean cAHI was 49 events/hour | MD 26.00 events/hour lower (43.84 lower to 8.16 lower) |
— | 12 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
|
Cardiovascular mortality Follow‐up: mean 12 months |
Study population | OR 0.21 (0.02 to 2.48) | 18 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c |
|
| 40 per 100 | 12 per 100 (1 to 62) | ||||
| Serious adverse events | Not reported | ||||
| Quality of sleep | Not reported | ||||
| Quality of life | Not reported | ||||
| AHI Follow‐up: mean 4 weeks (short‐term) | Mean AHI was 57 events/hour | MD 23.00 events/hour lower (37.70 lower to 8.30 lower) | — | 12 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
| AHI Follow‐up: mean 12 months (intermediate‐term) | Mean AHI was 21.68 events/hour | MD 6.98 events/hour lower (10.66 lower to 3.30 lower) | — | 18 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c |
| All‐cause mortality | Not reported | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
a Downgraded one level due to serious study limitations (risk of selection bias, performance bias, detection bias and reporting bias. b Downgraded two levels due to very serious imprecision (very small sample size). c Downgraded two levels due to very serious imprecision (few events and wide CI, including both null effect and appreciable benefit).
Summary of findings 2. Anxiolytics compared to inactive control for central sleep apnoea associated with heart failure in adults.
| Buspirone 15 mg (3 times daily) compared to inactive control for central sleep apnoea in adults | |||||
| Patient or population: adults with central sleep apnoea associated with heart failure Setting: outpatients Intervention: buspirone 15 mg (3 times daily) Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with buspirone | ||||
|
cAHI Follow‐up: mean 1 week (short‐term) |
Median difference between groups was −5.00 events/hour (IQR −8.00 to −0.50). The study reported median and IQRs due to skewed data distribution, so did not compare differences between groups. |
16 (1 RCT) | — | ||
| Cardiovascular mortality | Not reported | ||||
| Serious adverse events | Not reported | ||||
| Quality of sleep | Not reported | ||||
| Quality of life | Not reported | ||||
| AHI Follow‐up: 1 week (short‐term) | Median difference between groups was −6.00 (IQR −8.80 to −1.80). The study reported median and IQRs due to skewed data distribution, so did not compare differences between groups. |
16 (1 RCT) | — | ||
| All‐cause mortality | Not reported | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CI: confidence interval; IQR: interquartile range. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 3. Methylxanthine derivatives versus inactive control for central sleep apnoea associated with heart failure in adults.
| Theophylline 3.3 mg/kg (twice daily)compared to placebo for central sleep apnoea in adults | |||||
| Patient or population: adults with central sleep apnoea associated with heart failure in adults Setting: outpatients Intervention: theophylline 3.3 mg/kg (twice daily) Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with theophylline | ||||
|
cAHI Follow‐up: mean 3 weeks (short‐term) |
Mean cAHI was 26 events/hour | MD 20.00 events/hour lower (32.15 lower to 7.85 lower) |
— | 15 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
| Cardiovascular mortality | Not reported | ||||
| Serious adverse events | Not reported | ||||
| Quality of sleep | Not reported | ||||
| Quality of life | Not reported | ||||
| AHI Follow‐up: mean 3 weeks (short‐term) | Mean AHI was 37 events/hour | MD 19.00 events/hour lower (30.27 lower to 7.73 lower) | — | 15 (1 RCT) | ⊕⊝⊝⊝ Very lowa |
| All‐cause mortality | Not reported | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
a Downgraded three levels due to serious study limitations (unclear risk of selection bias and reporting bias) and very serious imprecision (very small sample size and wide CI).
Summary of findings 4. Hypnotics compared to inactive control for primary central sleep apnoea in adults.
| Triazolam 0.125 mg or 0.250 mg (once daily)compared to placebo for primary central sleep apnoea in adults | |||||
| Patient or population: adults with primary central sleep apnoea Setting: outpatients Intervention: triazolam 0.125 mg or 0.250 mg (once daily) Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with triazolam | ||||
|
cAHI Follow‐up: 3 days (short‐term) |
Mean cAHI was 9.40 events/hour in participants who received triazolam 0.125 mg versus 8.00 events/hour in those who received triazolam 0.250 mg versus 16.30 events/hour in those who received placebo. We could not compare the groups because the study did not report SDs for the treatment effects. |
5 (1 RCT) | — | ||
| Cardiovascular mortality | Not reported | ||||
| Serious adverse events | Not reported | ||||
| Quality of sleep | Not reported | ||||
| Quality of life | Not reported | ||||
| AHI Follow‐up: 3 days (short‐term) | Mean AHI was 13.50 events/hour in the participants who received triazolam 0.125 mg versus 11.00 events/hour in those who received triazolam 0.25 mg versus 20.90 events/hour in those who received placebo. We could not compare the groups because the study did not report SDs for the treatment effects. |
5 (1 RCT) | — | ||
| All‐cause mortality | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CI: confidence interval; RCT: randomised controlled trial; SD: standard deviation. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Background
Description of the condition
The term central sleep apnoea (CSA) encompasses a diversity of clinical situations where a dysfunctional drive to breathe leads to recurrent respiratory events, namely apnoea (complete absence of ventilation) and hypopnoea (insufficient ventilation) during sleep (Eckert 2007). Central respiratory events may emerge from distinct conditions such as chronic heart failure (CHF), chronic abuse of opioids, idiopathic disease, and high altitude. However, CSA due to periodic breathing at high altitudes is usually triggered by environmental exposure and is not a chronic health condition comparable to the other types of CSA. The International Classification of Sleep Disorders – Third Edition (ICSD‐3) identifies the following six types of CSA in adults (American Academy of Sleep Medicine 2014).
CSA with Cheyne‐Stokes Breathing (CSB)
CSA due to a medical disorder without CSB
CSA due to high altitude periodic breathing
CSA due to a medication or substance
Primary CSA
Treatment‐emergent CSA
CSA is far less common than obstructive sleep apnoea (OSA), which affects an estimated 936 million adults worldwide (Benjafield 2019). Less than 5% of people referred to a sleep clinic present with CSA (Malhotra 2004). Although there are no precise estimates of CSA prevalence in the general population, reported prevalences in special populations, such as people with CHF, range from 38% to 70% (Naughton 2016; Peer 2010; Vazir 2007)
The pathophysiology of CSA may involve abnormally increased chemosensitivity of respiratory centres located in the brainstem, with small changes in partial pressure of carbon dioxide in arterial blood (PCO2) generating a hyperreactive response and ultimately culminating in unstable ventilatory patterns (Eckert 2007). Peripheral chemoreceptor sensitisation is also involved, and the risk of CSA seems particularly high when both receptors (peripheral and central) are oversensitive (Giannoni 2009; Nakayama 2003). Alternatively, structural lesions or genetic or substance‐induced dysfunction of respiratory nuclei may lead to blunted ventilatory responses and central apnoeas.
Description of the intervention
The treatment of CSA with different types of non‐invasive positive pressure ventilation, such as continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP) or adaptive servo‐ventilation (ASV), is not always effective or safe (Bradley 2005; Cowie 2015), and may be associated with residual apnoea‐hypopnoea index (AHI; Aurora 2012). Unlike OSA, CSA appears to respond to pharmacological agents such as zolpidem (Quadri 2009), triazolam (Bonnet 1990), acetazolamide (Javaheri 2006) and theophylline (Javaheri 1996). These drugs may reach the therapeutical goal of mitigating central apnoeas through very distinct mechanisms of action, such as sleep stabilisation and respiratory stimulation. Of note, in people with CSA associated with heart failure, optimised treatment may include the association of drugs for treating CSA with other options for treating heart failure, such as sacubitril‐valsartan and cardiac resynchronisation therapy (Simantirakis 2008; Fala 2015).
How the intervention might work
Pharmacological agents with very distinct mechanisms of action may act on ventilatory control, chemoreflex modulation, and sleep stability. Hypnotics such as triazolam and zolpidem consolidate the sleep state by reducing fluctuations between wakefulness and unstable sleep. This may exert a protective action, as frequent arousals are associated with increased chemoresponsiveness, which leads to the pattern of hyperventilation and subsequent hypoventilation (Bonnet 1990). In fact, periodic breathing predominates during light non‐rapid eye movement (NREM) sleep and disappears during rapid eye movement (REM) sleep (Berssenbrugge 1983).
Respiratory stimulants may also exert a beneficial action by mitigating central apnoeas. Metabolic acidosis induced by acetazolamide increases the apnoeic threshold of PCO2, leading to a reduction in central apnoeas (Nakayama 2002). The mechanism of action of theophylline on central apnoeas is not completely understood. Theophylline competes with adenosine, which depresses ventilatory function (Müller 2011). It is reasonable to attribute the ventilatory stimulation caused by theophylline to adenosine blockage to some extent (Javaheri 1996).
Serotonin (5‐hydroxytryptamine or 5‐HT) neurons play an important role in central chemoreception. Buspirone is a 5‐HT receptor agonist that decreases central chemosensitivity to carbon dioxide (CO2) in a dose‐dependent manner, leading to ventilatory stability and central apnoea disappearance (Giannoni 2020a).
Why it is important to do this review
CSA associated with CSB (CSA‐CSB) in the context of CHF is considered a severity marker and indicative of poor prognosis (Emdin 2017; Hanly 1996; Wilcox 1998). It is not entirely clear whether treating CSA‐CSB in this population improves survival, which would be of utmost importance. Additionally, sleep fragmentation due to CSA may lead to difficulty maintaining sleep and daytime sleepiness, impacting negatively on quality of life. Central apnoeas are also observed in the daytime and in the upright position. Of note, upright central apnoea in people with heart failure is associated with worse clinical conditions and a greater risk of cardiac death (Giannoni 2020b). Although some therapies for CSA are associated with improved quality of life, it remains unclear whether and to what extent pharmacological therapies might improve quality of life in this population (Sasayama 2009). Some experts have hypothesised that implantable phrenic nerve stimulation devices may provide a novel treatment approach; however, to date, no studies have drawn solid conclusions on this intervention (Schwartz 2021). In contrast to CPAP/BiPAP/ASV used only at night, pharmacological treatment could also be useful for daytime central apnoea.
Much of the evidence on the effectiveness of pharmacological agents for CSA is derived from non‐randomised studies or from randomised studies with methodological limitations. A comprehensive search of the literature and a critical appraisal of the quality of studies following the recommendations proposed by Cochrane will provide a reliable summary of the available evidence to guide decision making.
Objectives
To evaluate the benefits and harms of pharmacological treatment compared with active or inactive controls for central sleep apnoea in adults.
Methods
Criteria for considering studies for this review
Types of studies
We had originally planned to include only randomised controlled trials (RCTs) with a parallel design and exclude cross‐over trials. However, the initial search results indicated that most relevant trials for this review used cross‐over methodology. Hence, with the approval of the editorial board, we made a post‐hoc protocol change to include randomised cross‐over trials.
We included studies published as full‐text articles, studies published as abstract only, and studies with unpublished data.
Types of participants
We included studies that enrolled participants aged 18 years or older diagnosed with one of the following CSA syndromes, as defined by the ICSD‐3 (American Academy of Sleep Medicine 2014).
CSA with CSB
CSA due to a medical disorder without CSB
CSA due to a medication or substance
Primary CSA
Treatment‐emergent CSA
We had originally planned to include studies of CSA due to periodic breathing at high altitudes but later decided not to include this group because the condition is usually triggered by environmental exposure and is not considered a chronic health condition.
Types of interventions
We included studies that evaluated any type of pharmacological agent aimed primarily at the mitigation of CSA, regardless of drug class, compared with active controls (e.g. non‐invasive positive pressure ventilation) or inactive controls (e.g. placebo, no treatment or usual care, defined as the treatment of underlying diseases applied to intervention and control groups).
Eligible pharmacological agents anticipated in the protocol for this review included sleep stabilising agents (e.g. zolpidem, temazepam) and respiratory stimulants that increase the apnoeic threshold (e.g. acetazolamide, theophylline). Based on the types of pharmacological agents included in the identified evidence, we used the following intervention classifications as the basis for our analysis.
Carbonic anhydrase inhibitors (acetazolamide)
Anxiolytics (buspirone)
Methylxanthine derivatives (theophylline)
Hypnotics (triazolam)
We did not include pharmacological treatment of underlying diseases associated with CSA, such as beta‐blockers for people with CHF.
Eligible comparisons included any drug versus any comparator. We expected to include the following comparisons.
Agents stabilising sleep versus inactive control
Respiratory stimulants versus inactive control
Another class of pharmacological agent versus inactive control
Any drug class versus any type of non‐invasive positive pressure ventilation (e.g. CPAP, automatic positive airway pressure (APAP), BiPAP or ASV)
Based on the studies and intervention classes identified in the search, we were able to conduct the following comparisons.
Hypnotics (e.g. triazolam) versus any type of active control or inactive control
Carbonic anhydrase inhibitors (e.g. acetazolamide) versus any type of active control or inactive control
Methylxanthine derivatives (e.g. theophylline) versus any type of active control or inactive control
Anxiolytics (e.g. buspirone) versus any type of active control or inactive control
Three comparisons were in people with CSA‐CSB due to heart failure and one comparison was in people with primary CSA.
Types of outcome measures
We assessed outcomes at all time points reported in primary studies and classified follow‐up duration as follows.
Short‐term: less than three months
Intermediate‐term: three months to one year
Long‐term: longer than one year
Primary outcomes
Central apnoea‐hypopnoea index (cAHI), defined as the number of central apnoea and hypopnoea events per hour of sleep, measured objectively by polysomnography
Cardiovascular mortality, defined as the number of deaths attributable to myocardial ischaemia and infarction, heart failure, cardiac arrest because of other or unknown cause, or cerebrovascular accident (Carrero 2011)
Serious adverse events, defined as those leading to death, life‐threatening events, hospitalisation, disability or permanent damage, congenital anomaly, or intervention to prevent permanent impairment or damage
Secondary outcomes
Quality of sleep, measured with validated scales or questionnaires, such as the Pittsburgh Sleep Quality Index (Buysse 1989)
Quality of life, measured with validated scales or questionnaires, such as the Medical Outcomes Study 36‐Item Short Form Health Survey (SF‐36; Jenkinson 1996)
Daytime sleepiness, measured with validated scales or questionnaires, such as the Epworth Sleepiness Scale (Johns 1991)
Apnoea‐hypopnoea index (AHI), defined as the number of obstructive, mixed, and central apnoea‐hypopnoea events per hour of sleep, measured objectively by polysomnography
All‐cause mortality, defined as number of deaths from any cause
Time to life‐saving cardiovascular intervention (e.g. cardiac transplantation, implantation of cardioverter‐defibrillator)
Non‐serious adverse events (e.g. nasal congestion, upper airway dryness, mask‐induced pressure ulcer)
Search methods for identification of studies
Electronic searches
We searched the following bibliographic databases from their inception, applying no language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org), which incorporates the Cochrane Airways Trials Register
MEDLINE OvidSP (1946 to 30 August 2022)
Embase OvidSP (1974 to 30 August 2022)
Scopus (from 2004 to 30 August 2022)
The Information Specialist searched CENTRAL, MEDLINE and Embase, and the review authors searched Scopus. Appendix 1 presents the MEDLINE strategy, which we adapted for use in the other databases. We handsearched conference abstracts and grey literature through the CENTRAL database.
We also searched the following trials registries.
US National Institutes of Health (NIH) ongoing trials register ClinicalTrials.gov (clincialtrials.gov)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch)
Searching other resources
We checked the reference lists of all included studies and relevant review articles for additional references. We also examined any errata or retraction notices for included studies and looked for additional information on ongoing trials on manufacturers' websites.
Data collection and analysis
Selection of studies
Two review authors (AR and ACP) independently screened titles and abstracts of all references returned by the searches using the predefined inclusion criteria, coding them as 'retrieve' (eligible/potentially eligible) or 'do not retrieve' (clearly ineligible). We retrieved the full‐text reports of all eligible and potentially eligible studies, and two review authors (AR and ACP) independently screened them against our inclusion criteria, recording the reasons for exclusion of ineligible studies. We resolved any disagreements through discussion or by involving another review author (DP) when necessary. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Page 2020).
Data extraction and management
Two review authors (AR and ACP) independently extracted study characteristics and outcome data using the Cochrane data collection form as a template (EPOC 2017). We piloted the form on at least one included study. We assigned each study a unique study identifier and double‐checked for eligibility before data extraction.
We extracted the following data.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, date of study
Participants: number, mean age, age range, sex, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria
Interventions: intervention, comparison, concomitant medications, excluded medications
Outcomes: primary and secondary outcomes specified and collected, time points reported
Notes: funding for studies, notable conflicts of interest of trial authors
We noted in the Characteristics of included studies table if a study had not reported outcome data in a usable way. We resolved disagreements by consensus or by involving a third review author (DP). One review author (AR) entered data into Review Manager Web (RevMan Web) software for analysis (RevMan Web 2022). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (DP) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (AR and ACP) independently assessed the risk of bias of all included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (DP). We assessed risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We judged each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. When considering the effects of treatment, we took into account the risk of bias for the studies that contributed to that outcome. We considered the domains 'blinding of participants and personnel' and 'blinding of outcome assessment' differently according to the type of outcome: for subjective outcomes, such as daytime sleepiness, quality of life and quality of sleep, we considered any deviation of blinding procedures indicative of high risk of bias; but for objective outcomes such as mortality, we did not consider the absence or inadequacy of blinding to impose a risk of bias. If any information on risk of bias had come from unpublished data or correspondence with a trialist, we would have noted this in the risk of bias table.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs). If we had been able to combine studies that had used different scales for the same outcome, we would have analysed them with standardised mean differences (SMDs). Had we combined data from rating scales in a meta‐analysis, we would have ensured they were entered with a consistent direction of effect (e.g. lower scores always indicating improvement). We described skewed data narratively (e.g. as medians and interquartile ranges (IQRs) for each group).
Where a single study included multiple trial arms, we included only the relevant arms. Where there were two comparisons (e.g. drug A versus placebo and drug B versus placebo), we had planned to either combine the active arms or halve the control group to avoid double‐counting. Where adjusted analyses were available (ANOVA or ANCOVA), we had planned to use them in our meta‐analyses. Where studies included more than two arms with the same medication but different doses, we had planned to combine these arms.
If both change from baseline and endpoint scores had been available for continuous data, we would have used change from baseline unless there was low correlation between measurements in individuals. If a study had reported outcomes at multiple time points, we would have used data from all time points. We had planned to use intention‐to‐treat (ITT) or 'full analysis set' analyses where possible (i.e. where studies had imputed data for participants who were randomised but did not complete the study) instead of completer or per protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of participants admitted to hospital, rather than number of admissions per participant). If a study had reported rate ratios, we would have analysed them on this basis.
Dealing with missing data
We contacted the authors of included studies to verify key study characteristics and obtain any missing numerical outcome data. If this had not been possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. Where there were missing outcome data (e.g. standard deviations (SDs) or correlation coefficients) that could not be obtained from the study authors, we attempted to calculate them from other available statistics such as P values according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). Where this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
Had we been able to combine data from different studies in a meta‐analysis, we would have investigated statistical heterogeneity using the Chi² test (considering a P value below 0.1 to represent heterogeneity of intervention effects) and quantified statistical heterogeneity using the I² statistic (considering a value of 50% of greater to represent substantial heterogeneity (Higgins 2011), although we recognise that there is uncertainty in the I² measurement when a meta‐analysis includes few studies). If we had identified substantial heterogeneity, we would have reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we had pooled more than 10 studies in the same meta‐analysis, we would have created and examined a funnel plot to explore possible small‐study effects and publication biases.
Data synthesis
We had planned to pool data from studies judged to be clinically homogeneous using Review Manager software. Had more than one study provided data for any comparison, we would have performed meta‐analysis. We would have used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
As we were unable to pool any data, we used RevMan Web to calculate the effect size from raw data reported in individual studies (RevMan Web 2022). We estimated the MDs between groups in cross‐over RCTs, separately for each pharmacological comparison, using the data type generic inverse variance. For the parallel RCT, we estimated the MDs between groups using the inverse variance method. For dichotomous variables we estimated the ORs between groups using the Mantel‐Haenszel method.
Subgroup analysis and investigation of heterogeneity
We had planned to carry out the following subgroup analyses on the outcomes AHI and cardiovascular mortality.
Severity of CSA based on cAHI, with two prespecified subgroups, namely mild central apnoea, defined as less than 15 central apnoea events per hour of sleep, and moderate to severe central apnoea, defined as more than 15 central apnoea events per hour of sleep. The rationale for this subgroup analysis is based on the assumption that treatment of CSA may have different effects according to the severity of CSA.
Severity of CHF based on the New York Heart Association (NYHA) functional classification (Class IV versus Class I to III; New York Heart Association 1994) or based on left ventricular ejection fraction (LVEF; below 30% versus above 30%). The rationale for scrutinising intervention effects regarding severity of CHF is based on the assumption that people with severe CHF may respond differently.
Aetiology of CSA as defined by the ICSD‐3 for comparisons involving heterogeneous populations
Pharmacological agents for comparisons involving multiple agents within the same drug class
However, due to scarcity of studies, subgroup analysis was not possible. Had it been possible, we would have used the formal test for subgroup interactions in RevMan Web (RevMan Web 2022).
Sensitivity analysis
We had planned to carry out sensitivity analyses, removing the following studies from the primary outcome analyses.
Studies that fulfilled criteria for high risk of bias or unclear risk of bias in at least two domains of the risk of bias table
Studies in which the source of funding may have influenced the results (industry sponsorship)
We would have compared the results from a fixed‐effect model with the random‐effects model, if we had been able to conduct meta‐analyses.
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro GDT to prepare summary of findings tables for the following outcomes (GRADEpro GDT).
cAHI
Cardiovascular mortality
Serious adverse events
Quality of sleep
Quality of life
AHI
All‐cause mortality
Time to life‐saving cardiovascular intervention
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess certainty of the body of evidence (Schumemann 2013). We provided justifications for downgrading certainty of the evidence in footnotes.
Results
Description of studies
Results of the search
The electronic database search run on 30 August 2022 retrieved 5046 references; a handsearch of reference lists and manufacturers’ websites identified no further studies. After excluding duplicate publications and irrelevant reports, we assessed 55 potentially eligible studies (63 reports). No studies were eligible for inclusion according to the original protocol of this review, as we had excluded cross‐over RCTs. However, a post‐hoc revision of the protocol, that considered cross‐over RCTs eligible for the review, enabled us to include five studies (6 reports). Figure 1 shows the selection process.
1.
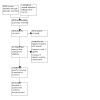
Study flow diagram.
We found two ongoing studies (2015‐003119‐39; NCT04118387). The record for 2015‐003119‐39 was last updated in February 2016, but the lead investigator did not respond to our email requests for information.
NCT04118387 is entitled 'Central Sleep Apnea: Physiologic Mechanisms to Inform Treatment (CSA)', and it aims identify mechanistic pathways to guide future therapeutic interventions for central sleep apnoea based on the strong premise that multimodality therapy will normalise respiration and hence mitigate adverse long‐term consequences of CSA. It compares positive airway pressure (PAP), pharmacological treatment (acetazolamide, zolpidem and buspirone) and supplemental oxygen to reduce cAHI and the CO2 reserve during sleep in people with CEA. The trial is still recruiting and is scheduled to finish in December 2024.
Included studies
Methods
We included four cross‐over RCTs (Bonnet 1990; Giannoni 2020a; Javaheri 1996; Javaheri 2006) and one parallel RCT (Sorokina 2019a). Three studies were conducted in the USA (Bonnet 1990; Javaheri 1996; Javaheri 2006), one in Russia (Sorokina 2019a) and one in Italy (Giannoni 2020a). All were single‐centre studies and only one reported the period of recruitment (2016 to 2018; Giannoni 2020a). Table 5 provides an overview of the characteristics of the included studies.
1. Study characteristics.
| Study design (publication type) | Participants | Age (years) | Definition of CSA | Comparators | Duration of intervention period (washout) |
|
Bonnet 1990 Cross‐over (full report) |
5 men with primary CSA | Average 70 (range 65–74) | Central apnoeas were scored when there was an event of complete absence of chest movement from both chest leads and a complete absence of airflow throughout the event (lasting >10 seconds). | Triazolam 0.125 mg versus triazolam 0.250 mg (once daily) versus placebo |
3 days (≥ 1 week) |
|
Giannoni 2020a Cross‐over (full report) |
16 men with systolic heart failure (LVEF < 50%) and moderate‐severe CSA |
Mean 71.3 (SD 5.8) | Echocardiographic evidence of LVEF < 50% and nocturnal AHI ≥ 15 events/hour | Buspirone 15 mg (3 times daily) versus placebo | 1 week (1 week) |
|
Javaheri 1996 Cross‐over (full report) |
15 men with compensated heart failure (LVEF ≤ 45%) and AHI > 10 events/hour | Not reported (adults) |
Polysomnograms showed periodic breathing, with > 10 episodes of apnoea and hypopnoea/hour. | Theophylline 3.3 mg/kg (twice daily) versus placebo | 5 days (1 week) |
|
Javaheri 2006 Cross‐over (full report) |
12 men with systolic heart failure whose initial polysomnograms showed CSB with AHI > 15 events/hour | Mean 66 (SD 6) | Systolic heart failure (initial polysomnograms showed CSB with an AHI > 15 events/hour) | Acetazolamide 3.5 mg/kg (once daily) versus placebo | 6 days (2 weeks) |
|
Sorokina 2019a RCT (abstract) |
18 people (mostly men) with CSA associated with CHF | Not reported (adults) |
AHI > 15 events/hour by echocardiographic evidence were considered as living with CSA. CHF ranged from II to III NYHA functional classes. | Standard medical treatment + acetazolamide 250 mg versus standard medical treatment without acetazolamide |
Not reported; follow‐up was 12 months |
AHI: apnoea‐hypopnoea index; CHF: chronic heart failure; CSA: central sleep apnoea; CSB: Cheyne‐Stokes breathing; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SD: standard deviation.
Participants
The five studies randomised a total of 68 participants. Sixty‐three participants, from four studies, had heart failure in addition to CSA (Giannoni 2020a; Javaheri 1996; Javaheri 2006; Sorokina 2019a). Bonnet 1990 included people with primary CSA. The studies included participants aged 18 years and older. All participants were men in four studies (Bonnet 1990; Giannoni 2020a; Javaheri 1996; Javaheri 2006), and most participants were men in Sorokina 2019a. The smallest study included five adults (Bonnet 1990), and the largest included 18 adults (Sorokina 2019a).
Interventions
In the studies that reported the duration of the intervention, it ranged from three days to one week, and the wash‐out period ranged from one to two weeks. Sorokina 2019a did not report the duration of the intervention or wash‐out period. Javaheri 2006 and Sorokina 2019a compared acetazolamide (a carbonic anhydrase inhibitor) with placebo or no acetazolamide (inactive control), Giannoni 2020a compared buspirone (an anxiolytic) with placebo (inactive control), Javaheri 1996 compared theophylline (a methylxanthine derivative) with placebo (inactive control), and Bonnet 1990 compared triazolam (a hypnotic) with placebo (inactive control).
Outcomes
All included RCTs evaluated AHI. Four studies evaluated cAHI (Bonnet 1990; Giannoni 2020a; Javaheri 1996; Javaheri 2006). Only Sorokina 2019a evaluated cardiovascular mortality. Two studies measured daytime sleepiness using the Epworth Sleepiness Scale (Bonnet 1990; Giannoni 2020a). Giannoni 2020a reported non‐serious adverse events, but no other studies reported a formal evaluation of adverse events.
Conflicts of interest and study funding
Only Javaheri 2006 reported no conflicts of interest. Giannoni 2020a received funding from the US NIH and Javaheri 1996 was supported by Merit Review grants from the US Department of Veterans Affairs. Javaheri 2006 reported no financial support.
Excluded studies
Of the studies excluded during full‐text assessment, we provided justification for the exclusion of 28 (34 references) in the Characteristics of excluded studies table. Fifteen studies had an ineligible population, 12 studies were not RCTs, and one study had an ineligible intervention.
Risk of bias in included studies
Full details of risk of bias judgements can be found in the Characteristics of included studies table. Figure 2 provide a graph and summary of the results.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only Giannoni 2020a was at low risk of selection bias. The remaining RCTs were at unclear risk for this domain because they did not describe the method of generating or concealing the random sequence.
Blinding
For Javaheri 1996 and Sorokina 2019a), it was unclear whether the placebo was indistinguishable from the intervention, which may have compromised the blinding of participants and personnel. We considered Bonnet 1990 and Sorokina 2019a at unclear risk of detection bias, because they provided insufficient information about blinding of the outcome assessors. Bonnet 1990 and Giannoni 2020a reported subjective outcomes (daytime sleepiness): we considered Bonnet 1990 at unclear risk of detection bias for this outcome owing to lack of information, while Giannoni 2020a was at low risk of bias because the outcome assessors were blinded.
Incomplete outcome data
Bonnet 1990 had only five participants, one of whom dropped out. As the study provided no information about the imputation of data, we judged it at unclear risk of attrition bias. All other studies had no attrition, and we judged them at low risk of bias.
Selective reporting
All studies appeared to be free of selective outcome reporting; however, no trial registration information was available for four studies, and we judged them at unclear risk of reporting bias (Bonnet 1990; Javaheri 1996; Javaheri 2006; Sorokina 2019a).
Other potential sources of bias
All studies appeared to be free of other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We had planned to perform sensitivity analyses considering risk of bias (excluding studies with high risk of bias) and industry sponsorship. We also planned to analyse subgroups to scrutinise differences of intervention effects according to aetiology. However, we were unable to perform any of the planned subgroup or sensitivity analyses for cAHI or cardiovascular mortality, owing to the small number of studies.
Carbonic anhydrase inhibitors versus inactive control
Javaheri 2006 and Sorokina 2019a compared acetazolamide with placebo/no acetazolamide in adults with CSA and heart failure.
Primary outcomes
Central apnoea‐hypopnoea index
Only Javaheri 2006 reported cAHI. We are uncertain whether acetazolamide compared to placebo reduces cAHI after six nights (MD −26.00 events per hour, 95% CI −43.84 to −8.16; 12 participants; very low‐certainty evidence due to risk of bias and imprecision; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Carbonic anhydrase inhibitors versus inactive control , Outcome 1: Central apnoea‐hypopnoea index (short‐term)
Cardiovascular mortality
Only Sorokina 2019a reported cardiovascular mortality. We are uncertain about the effects of acetazolamide compared to no acetazolamide on cardiovascular mortality after 12 months (OR 0.21, 95% CI 0.02 to 2.48; 18 participants; very low‐certainty evidence due to risk of bias and imprecision; Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1: Carbonic anhydrase inhibitors versus inactive control , Outcome 2: Cardiovascular mortality (intermediate‐term)
Serious adverse events
No studies reported a formal evaluation of serious adverse events.
Secondary outcomes
Quality of sleep
No studies reported quality of sleep.
Quality of life
No studies reported quality of life.
Daytime sleepiness
No studies reported daytime sleepiness.
Apnoea‐hypopnoea index
Javaheri 2006 reported API at six nights (short‐term) and Sorokina 2019a reported API at six months (intermediate term). We are uncertain whether acetazolamide compared to placebo/no acetazolamide can reduce API in the short term (MD −23.00 events per hour, 95% CI −37.70 to −8.30; 12 participants; very low−certainty evidence due to risk of bias and imprecision; Analysis 1.3; Table 1) or in the intermediate term (MD −6.98 events per hour, 95% CI −10.66 to −3.30; 18 participants; very low‐certainty evidence due to risk of bias and imprecision; Analysis 1.4; Table 1).
1.3. Analysis.

Comparison 1: Carbonic anhydrase inhibitors versus inactive control , Outcome 3: Apnoea‐hypopnoea index (short‐term)
1.4. Analysis.

Comparison 1: Carbonic anhydrase inhibitors versus inactive control , Outcome 4: Apnoea‐hypopnoea index (intermediate‐term)
All‐cause mortality
No studies reported all‐cause mortality.
Time to life‐saving cardiovascular intervention
No studies reported time to life‐saving cardiovascular intervention.
Non‐serious adverse events
No studies reported a formal evaluation of non‐serious adverse events.
Anxiolytics compared to inactive control
Giannoni 2020a compared buspirone with placebo in adults with CSA and heart failure.
Primary outcomes
Central apnoea‐hypopnoea index
The median difference between groups for cAHI was −5.00 events per hour (IQR −8.00 to −0.50; 16 participants; Table 2). Giannoni 2020a presented the median and IQR due to data skew; therefore, we considered that estimating the mean and SD would not have given an accurate result, and we were unable to compare differences between groups.
Cardiovascular mortality
Giannoni 2020a did not report cardiovascular mortality.
Serious adverse events
Giannoni 2020a listed serious adverse events as an outcome measure, but reported none.
Secondary outcomes
Quality of sleep
Giannoni 2020a did not report quality of sleep.
Quality of life
Giannoni 2020a did not report quality of life
Daytime sleepiness
The median difference between groups for daytime sleepiness using the Epworth Sleepiness Scale was 0.00 (IQR −1.00 to 0.00; 16 participants).
Apnoea‐hypopnoea index
The median difference between groups for AHI was −6.00 (IQR −8.80 to −1.80; 16 participants; Table 2).
All‐cause mortality
Giannoni 2020a did not report all‐cause mortality.
Time to life‐saving cardiovascular intervention
Giannoni 2020a did not report time to life‐saving cardiovascular intervention.
Non‐serious adverse events
Three participants reported mild and transient buspirone‐related side effects, including lower limb tingling (n = 1; 6%) and dizziness (n = 2; 12%).
Methylxanthine derivatives versus inactive control
Javaheri 1996 compared theophylline with placebo in adults with CSA and heart failure.
Primary outcomes
Central apnoea‐hypopnoea index
We are uncertain whether theophylline compared with placebo reduces cAHI after five days (MD −20.00 events per hour, 95% CI −32.15 to −7.85; 15 participants, very low‐certainty evidence due to risk of bias and imprecision; Analysis 2.1; Table 3).
2.1. Analysis.

Comparison 2: Methylxanthine derivative agents versus inactive control , Outcome 1: Central apnoea‐hypopnoea index (short‐term)
Cardiovascular mortality
Javaheri 1996 did not report cardiovascular mortality.
Serious adverse events
Javaheri 1996 did not report a formal evaluation of serious adverse events.
Secondary outcome
Quality of sleep
Javaheri 1996 did not report quality of sleep.
Quality of life
Javaheri 1996 did not report quality of life.
Daytime sleepiness
Javaheri 1996 did not report daytime sleepiness.
Apnoea‐hypopnoea index
We are uncertain whether theophylline compared to placebo decreases AHI after five days (MD −19.00 events per hour, 95% CI −30.27 to −7.73; 15 participants; very low‐certainty evidence due to risk of bias and imprecision; Analysis 2.2; Table 3).
2.2. Analysis.

Comparison 2: Methylxanthine derivative agents versus inactive control , Outcome 2: Apnoea‐hypopnoea index (short‐term)
All‐cause mortality
Javaheri 1996 did not report all‐cause mortality.
Time to life‐saving cardiovascular intervention
Javaheri 1996 did not report time to life‐saving cardiovascular intervention.
Non‐serious adverse events
Javaheri 1996 did not report a formal evaluation of non‐serious adverse events.
Hypnotics versus inactive control
Bonnet 1990 analysed triazolam 0.125 mg versus triazolam 0.250 mg versus placebo in adults with primary CSA. We were unable to perform comparisons between groups, as the study did not report SDs for the treatment effects. Had it provided SDs, we would have combined the groups that received triazolam versus placebo. Furthermore, several other features of Bonnet 1990 limit the external validity of the results; for example, the participants refrained from alcohol and caffeine consumption prior to the study and during the laboratory sessions. Most people with CSA are unlikely to follow these recommendations in the long term.
Primary outcomes
Central apnoea‐hypopnoea index
The mean cAHI was 9.40 events per hour in participants who received triazolam 0.125 mg versus 8.00 events per hour in those who received triazolam 0.250 mg versus 16.30 events per hour in those who received placebo (Table 4).
Cardiovascular mortality
Bonnet 1990 did not report cardiovascular mortality.
Serious adverse events
Bonnet 1990 did not report a formal evaluation of serious adverse events.
Secondary outcome
Quality of sleep
Bonnet 1990 did not report quality of sleep.
Quality of life
Bonnet 1990 did not report quality of life.
Daytime sleepiness
Bonnet 1990 assessed daytime sleepiness using the Epworth Sleepiness Scale. Mean daytime sleepiness was 1.50 points in participants who received triazolam 0.125 mg versus 2.00 points in those who received triazolam 0.250 mg versus 2.20 points in those who received placebo.
Apnoea‐hypopnoea index
The mean AHI was 13.50 events per hour in the participants who received triazolam 0.125 mg versus 11.00 events per hour in those who received triazolam 0.25 mg versus 20.90 events per hour in those who received placebo (Table 4).
All‐cause mortality
Bonnet 1990 did not report all‐cause mortality.
Time to life‐saving cardiovascular intervention
Bonnet 1990 did not report time to life‐saving cardiovascular intervention.
Non‐serious adverse events
Bonnet 1990 did not report a formal evaluation of non‐serious adverse events.
Discussion
Summary of main results
In this review, we evaluated the available evidence on pharmacological treatment for CSA in adults. We included four cross‐over RCTs and one parallel RCT. Sample sizes were very small; the largest study enrolled 18 adults with CSA associated with heart failure. We evaluated four different pharmacological agents (acetazolamide, theophylline, buspirone and triazolam). We were unable to perform a meta‐analysis because of the clinical heterogeneity between the studies. In the four trials that reported treatment duration, it lasted one week or less.
It is unclear whether carbonic anhydrase inhibitors (acetazolamide) and methylxanthine derivatives (theophylline) compared with inactive control can reduce cAHI and AHI in adults with CSA associated with heart failure, as we found only very low‐certainty evidence. We are uncertain about the effects of carbonic anhydrase inhibitors on cardiovascular mortality, as the estimated effect included the possibility of both increasing and reducing cardiovascular mortality, and the evidence was of very low certainty. Only one RCT assessed the effects of hypnotics in primary CSA. Due to several methodological limitations and the insufficient reporting of outcome results in this study, a number of questions still persist on the effects of this intervention. Only the study on the anxiolytic reported adverse events: these events were rare and mild (dizziness, sweating), but the safety of this drug should be evaluated in larger and longer multicentre trials before considering it a reasonable treatment option in people with CSA associated with heart failure. No studies reported serious adverse events, quality of sleep, quality of life, all‐cause mortality or time to life‐saving cardiovascular intervention.
Overall completeness and applicability of evidence
As most participants in the included studies were men with CSA associated with heart failure, the generalisability of our results is limited. The studies did not report whether the abnormal respiratory pattern could be classified as CSB or if it was associated with non‐periodic CSA. This may have important implications for practice because people with CSB have poor prognosis (Lanfranchi 1999; Lorenzi‐Filho 2005). In addition, no RCTs assessed clinically relevant outcome measures related to treatment efficacy, such as quality of sleep, quality of life, all‐cause mortality, or time to life‐saving cardiovascular intervention. Only one RCT reported daytime sleepiness, a commonly disabling symptom related to sleep disorders. Daytime sleepiness is less easily identified in people with CSA and heart failure than in those with CSA without heart failure, as those with heart failure have several symptoms that may strongly overlap, limiting the validity of sleepiness metrics. All included RCTs reported AHI, cAHI or both. However, it is unclear whether the treatment effect in any individual study was clinically meaningful because, to the best of our knowledge, the minimally important difference of AHI or cAHI remains to be established by studies applying anchoring methods.
No studies included participants with treatment‐emergent CSA or CSA due to a medication or substance. Our findings should not be extrapolated to these populations, given the varying pathophysiological aspects of CSA syndromes.
Quality of the evidence
We judged four of the five included RCTs at unclear risk of bias for random sequence generation, blinding of outcome assessment and participants, and selective reporting. Because these domains were poorly described, we downgraded the certainty of evidence by one level due to study limitations for all outcomes. We also downgraded the certainty of evidence for imprecision due to the small sample sizes and wide CIs in some cases. The certainty of the evidence was very low for all outcomes.
Potential biases in the review process
We conducted this review in accordance with the Methodological Expectations of Cochrane Intervention Reviews guidelines (Higgins 2022b). Except for some minor deviations (see Differences between protocol and review), we followed our protocol (Riera 2018). We conducted sensitive searches in the most important databases and clinical trial registers and manual searches to identify and collect all relevant RCTs. Although we identified several trials, some studies lacked sufficient data to permit inclusion or exclusion; we assessed these trials as awaiting classification. When studies reported skewed data (e.g. medians and IQRs for each group), we chose to describe them narratively instead of transforming them into means and SDs. We were also unable to design a funnel plot to assess publication bias due to an insufficient number of eligible RCTs to perform meta‐analyses.
Agreements and disagreements with other studies or reviews
The effectiveness of pharmacological agents such as respiratory stimulants, anxiolytics and sedatives are uncertain because of the important methodological issues of the includes studies. To our knowledge, no other systematic reviews have specifically examined the effects of pharmacological medications for central apnoea in adults. One previous systematic review and meta‐analysis evaluating acetazolamide versus no acetazolamide for CSA and OSA in adults showed improved OSA and CSA in the intervention group (Schmickl 2020a). However, it combined data from different study populations (CSA‐CHF; CSA‐high altitude; CSA‐idiopathic) and different study designs (randomised and non‐randomised). The RCT evaluating acetazolamide for CSA that was included in that review was also identified in the present review (Javaheri 2006).
Authors' conclusions
Implications for practice.
There is insufficient evidence to recommend the use of pharmacological therapy in the treatment of central sleep apnoea (CSA). Although small studies found that certain agents reduced the number of respiratory events during sleep in people with CSA associated with heart failure, they could not evaluate whether this reduction affected participants' quality of life as they did not measure important clinical outcomes such as sleep quality or subjective impression of daytime sleepiness. Furthermore, the trials were mostly short term, so more trials evaluating longer‐term effects are needed.
Implications for research.
Most participants of the included randomised controlled trials (RCTs) in this review were older men with heart failure. Central apnoea can be observed in up to 60% of people with heart failure at night and 30% during the day (Brack 2007; Emdin 2017). Of note, central apnoeas are also observed at daytime and in the upright position, which is associated with worse clinical conditions and with a greater risk of cardiac death (Giannoni 2020b). Therefore, it is important to evaluate the effect of pharmacological treatment in people with apnoea in upright position and on central apnoeas over 24 hours, considering the prognostic significance of daytime apnoeas. In addition, future studies should report the time with oxygen saturation below 90% in each group at baseline, as hypoxaemic burden is a robust and independent predictor of all‐cause mortality in people with heart failure (Oldenburg 2015).
The number of older adults with heart failure may increase in the future, and this condition can lead to difficulty maintaining sleep and daytime sleepiness, reducing quality of life. Moreover, the overall drugs safety profile in adults with CSA is not yet consolidated in the literature. One study evaluated the safety of acetazolamide under different conditions, showing that adverse events of acetazolamide are rare and that some common side effects, such as paraesthesia, are dose‐dependent (Schmickl 2020b). However, studies evaluating other pharmacological agents, such as those included in our review, are still scarce, and questions about the safety of sleep apnoea treatment remain unanswered.
Most trials included in this review measured outcomes in the short term only, so the long‐term sustainability of treatment effects remains unclear. There is a need for larger multicentre RCTs that explore relevant clinical outcomes, including quality of life metrics and survival, after a longer follow‐up period, to clarify the effectiveness of pharmacological treatment for adults with CSA.
What's new
| Date | Event | Description |
|---|---|---|
| 28 March 2023 | Amended | Affiliation amended for Luciano F Drager |
History
Protocol first published: Issue 1, 2018 Review first published: Issue 2, 2023
Acknowledgements
We would like to thank Elizabeth Stovold for her valuable assistance in developing search strategies and the Cochrane Airways Editorial group for assistance in the development of this review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
The authors and Cochrane Airways Editorial Team are grateful to the following peer reviewers for their time and comments: Alberto Giannoni, Sant'Anna School of Advanced Studies (Italy); and Jeremy Orr, University of California San Diego (USA).
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. Sleep Apnea, Central/
2. (central adj2 sleep adj2 (apnea$ or apnoea$)).tw.
3. (central adj2 alveolar adj2 hypoventilation$).tw.
4. (central adj2 sleep disordered breathing).tw.
5. (ondine$ adj2 (syndrome or curse)).tw.
6. Cheyne‐Stokes Respiration/
7. Cheyne$ Stokes.tw.
8. (periodic adj2 (breathing or respiration)).tw.
9. or/1‐8
10. (controlled clinical trial or randomised controlled trial).pt.
11. (randomised or randomised).ab,ti.
12. placebo.ab,ti.
13. dt.fs.
14. randomly.ab,ti.
15. trial.ab,ti.
16. groups.ab,ti.
17. or/10‐16
18. Animals/
19. Humans/
20. 18 not (18 and 19)
21. 17 not 20
22. 9 and 21
Appendix 2. Scopus search strategy
#1 "central sleep apnoea*” OR “central sleep apnea*"
#2 "central alveolar hipoventilation*"
#3 "central sleep disordered breathing"
#4 "ondine* syndrome” OR “ondine curse"
#5 "cheyne‐stokes respiration"
#6 "cheyne* stokes"
#7 "periodic breathing” OR “periodic respiration"
#8 OR/1‐7
#9 TITLE‐ABS (controlled)
#11 TITLE‐ABS (randomised or randomised)
#12 TITLE‐ABS (placebo)
#13 TITLE‐ABS (randomly)
#14 TITLE‐ABS (trial)
#15 TITLE‐ABS (groups)
#16 OR/10‐16
# 17 #8 AND #16
Data and analyses
Comparison 1. Carbonic anhydrase inhibitors versus inactive control .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Central apnoea‐hypopnoea index (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Cardiovascular mortality (intermediate‐term) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Apnoea‐hypopnoea index (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Apnoea‐hypopnoea index (intermediate‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Methylxanthine derivative agents versus inactive control .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Central apnoea‐hypopnoea index (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Apnoea‐hypopnoea index (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonnet 1990.
| Study characteristics | ||
| Methods | 3‐arm RCT with cross‐over design | |
| Participants |
Number randomised/analysed: 5/4 or 5 (unclear) Sex (% male): 100% Age: average 70 years (range 65–74) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention group 1: triazolam (benzodiazepine) 0.125 mg once daily for 3 days Intervention group 2: triazolam (benzodiazepine) 0.250 mg once daily for 3 days Control group: placebo (inactive control) once daily for 3 days Wash‐out period: at least 1 week Details of intervention: on 4 nights of 3 nonconsecutive laboratory weeks, participants took a pill 30 minutes before bedtime (between 10 PM and 12 AM). All participants received placebo on the first night, then their allocated treatment for the next 3 consecutive nights. Participants could not use tranquillisers or hypnotics during the study. They were asked not to consume alcohol for 2 days prior to study initiation and during the laboratory sessions, or to consume caffeine during the study. Co‐interventions: none reported |
|
| Outcomes |
|
|
| Notes |
Study funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to judge selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to judge selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "On each night, a pill was taken 30 min prior to a standard 10 pm to 12 am bedtime. The pill was matched placebo, 0.125 mg, triazolam, or 0.25 mg triazolam."; "The assignment of medication and placebo conditions to subjects was random and double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge the measures used to blind outcome assessors. |
| Blinding of outcome assessment (detection bias) (subjective outcomes) | Unclear risk | Insufficient information to judge the measures used to blind outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One subject did not participate in the 0.25 mg condition." Comment: there is no information about data imputation. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the study protocol to judge reporting bias. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Giannoni 2020a.
| Study characteristics | ||
| Methods | RCT with a cross‐over design | |
| Participants |
Number randomised/analysed: 16/16 Sex (% male): 100% Age: mean 71.3 (SD 5.8) years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention group: buspirone (anxiolytic) 15 mg 3 times daily for 1 week Control group: placebo (inactive control) 3 times daily for 1 week Wash‐out period: 1 week Details of intervention: to minimise adverse reactions, the cumulative dose was titrated every 2 days from 15 mg/day (5 mg 3 times daily), to 30 mg/day (10 mg 3 times daily), to 45 mg/day (15 mg 3 times daily) in participants with eGFR ≥ 70 mL/min/1.73 m2. For participants with eGFR of 50–69 mL/min/1.73 m2, the drug was titrated to a maximum dose of 30 mg/day; and for those with eGFR of 30–49 mL/min/1.73 m2, the drug was kept at 15 mg/day. Co‐interventions: optimal medical therapy (not described) |
|
| Outcomes |
|
|
| Notes |
Study funding source: 1 study author (GBR) received funding from the US National Institutes of Health (U01NS090414). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...who created a computerized randomisation list 0 = placebo, 1 = buspirone) and delivered to the study staff the masked complete treatments (I and II) in sealed opaque envelopes. The randomisation list remained hidden from patients and investigators (double blind) until the conclusion of the study." |
| Allocation concealment (selection bias) | Low risk | Quote: "a hospital pharmacist [...] delivered to the study staff the masked complete treatments (I and II) in sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The randomisation list remained hidden from patients and investigators (double blind) until the conclusion of the study."; "The galenic preparation (tablets with identical appearance) of the experimental drugs (buspirone or placebo)"; "To maintain masking, similar procedures related to drug titration were used for placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The randomisation list remained hidden from patients and investigators (double blind) until the conclusion of the study." |
| Blinding of outcome assessment (detection bias) (subjective outcomes) | Low risk | Quote: "The randomisation list remained hidden from patients and investigators (double blind) until the conclusion of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient withdrew from the study, two patients were intolerant to the chemoreflex test, while all other measurements were available in all patients."; "The primary effectiveness endpoint was evaluated in all patients who were randomised to either placebo or buspirone in the intention‐to‐treat (ITT) analysis. Patients randomised without chemoreflex data (intolerance to the test) or who withdrew from the study were imputed as treatment failures. A per‐protocol (PP) analysis was also performed for the primary outcome. Finally, imputation analysis by median substitution was also performed. In order to test the primary endpoint, a two‐sided McNemar's exact test was used with a type I error rate of 0.05." |
| Selective reporting (reporting bias) | Low risk | Study was registered (EudraCT 2015‐005383‐42). All the primary outcomes were reported. |
| Other bias | Low risk | Study funding source was reported and possible conflicts of interest were declared. |
Javaheri 1996.
| Study characteristics | ||
| Methods | RCT with a cross‐over design | |
| Participants |
Number randomised/analysed: 15 Sex (% male): 100% Age: not reported (adults) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention group: theophylline (methylxanthine derivative) 3.3 mg/kg orally twice daily for 5 days Control group: placebo (inactive control) for 5 days Wash‐out period: 1 week Details of intervention: no further details Co‐interventions: optimal therapy, which included angiotensin‐converting‐enzyme inhibitors (in 13 participants), hydralazine (in 2 participants), digoxin (in 11 participants), isosorbide dinitrate (in 7 participants) and furosemide (in all 15 participants) |
|
| Outcomes |
|
|
| Notes |
Study funding source: supported by Merit Review grants from the US Department of Veterans Affairs Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients received placebo or theophylline orally twice daily for five days, in a randomised, double‐blind fashion." Comment: no further details; insufficient information to judge selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients received placebo or theophylline orally twice daily for five days, in a randomised, double‐blind fashion." Comment: no further details; insufficient information to judge selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "To maintain blinding, the patients were monitored during both the theophylline and the placebo phases of the study." Comment: no further details of how blinding was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The polysomnograms were scored blindly." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information about the study protocol to judge reporting bias. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Javaheri 2006.
| Study characteristics | ||
| Methods | RCT with a cross‐over design | |
| Participants |
Number randomised/analysed: 14/12 Sex (% male): 100% Age: mean 66 (SD 6) years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention group: acetazolamide (carbonic anhydrase inhibitor) 3.5 mg/kg orally (once daily) 1 hour before bedtime for 6 nights Control group: placebo (inactive control) for 6 nights Wash‐out period: 2 weeks Details of intervention: participants received 3 identical capsules of either placebo or one acetazolamide and two potassium chloride (to compensate for acetazolamide‐induced urinary potassium loss). To induce mild metabolic acidosis and decrease total CO2 by about 5 mmol/L, a venous blood sample was obtained on the morning of the third day after randomisation, and the dose of acetazolamide was increased to 4 mg/kg to achieve the target concentration of total CO2. Co‐interventions: potassium chloride (total, 30 mEq). |
|
| Outcomes |
|
|
| Notes |
Study funding source: not reported Conflicts of interest: no relationship with any commercial entity that could be interested in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised to a double‐blind cross‐over protocol with acetazolamide or placebo." Comment: no further details; insufficient information to judge selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomised to a double‐blind cross‐over protocol with acetazolamide or placebo." Comment: no further details; insufficient information to judge selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only a researcher in pharmacology and a physician, both of whom were monitoring the patients, were not blind to randomization. The patients, the principal investigator, and the technicians who performed various tests were unaware of randomization. The procedures were identical for placebo and acetazolamide arms." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[...] the technicians who performed various tests were unaware of randomisation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient refused to take part in the study (because of long travel distance) and one patient did not complete the study." Comment: data loss was low; reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the study protocol to judge reporting bias. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Sorokina 2019a.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
Number randomised/analysed: 18/18 Sex (% male): not reported (mostly men) Age: not reported (adults) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention group: acetazolamide (carbonic anhydrase inhibitor) 250 mg plus standard medical treatment (n = 8) Control group: standard medical treatment (inactive control; n = 10) Wash‐out period: not applicable (not a cross‐over trial) Details of intervention: no further details Co‐interventions: no description of standard medical treatment |
|
| Outcomes |
|
|
| Notes |
Study funding source: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[...] patients with CSA were randomised into two groups." Comment: no further details; insufficient information to judge selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[...] patients with CSA were randomised into two groups." Comment: no further details; insufficient information to judge selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information about measures used to blind participants and professionals. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about measures used to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the study protocol to judge reporting bias. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
AHI: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CHF: chronic heart failure; CSA: central sleep apnoea; CSB: Cheyne‐Stokes breathing; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; OSA: obstructive sleep apnoea; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belyavskiy 2010 | Wrong population (obstructive sleep apnoea). |
| Caravita 2022 | Wrong population (mixed population without individual data). |
| Connaughton 1984 | Non‐randomised study. |
| DeBacker 1995 | Non‐randomised study. |
| Dubowitz 1998 | Wrong study design (case‐control). |
| Ginter 2020 | Wrong population. |
| Jaffuel 2021 | Non‐randomised study. |
| Javed 2020 | Wrong intervention. |
| Murray 1977 | Non‐randomised study. |
| Naghan 2019 | Wrong population. |
| Naghan 2020 | Wrong population. |
| NCT00746954 | Wrong population. |
| NCT01500473 | Non‐randomised study. |
| NCT02670096 | Wrong population. |
| Obernadorfer 2000 | Wrong population. |
| Passino 2021 | Wrong population. |
| Prowting 2021 | Wrong population. |
| Rastogi 2020 | Wrong population. |
| Saletu 1999 | Wrong population. |
| Schumacher 2014 | Wrong population. |
| Schwarz 2020 | Non‐randomised study. |
| Shore 1983 | Non‐randomised study. |
| Sin 1999 | Non‐randomised study. |
| Sorokina 2019b | Non‐randomised study. |
| Ulrich 2013 | Wrong population. |
| Ulrich 2015 | Wrong population. |
| Westwood 2012 | Non‐randomised study. |
| Yasuma 2006 | Non‐randomised study. |
Characteristics of studies awaiting classification [ordered by study ID]
Ahmad 2022.
| Methods | RCT with cross‐over design |
| Participants | People with CSA, defined as an AHI ≥ 15 and a cAHI ≥ 5 (n = 11) |
| Interventions | Zolpidem 5/10 mg versus placebo for 2 nights |
| Outcomes |
|
| Notes | Only abstract available. We contacted the study authors to ask for the full text. |
Guo 2003.
| Methods | Prospective double‐blind placebo‐controlled clinical study |
| Participants | 40 people with sleep apnoea‐hypopnoea syndrome (20 obstructive and 20 central) and 40 healthy people |
| Interventions | Zolpidem 5mg versus zolpidem 10 mg versus placebo |
| Outcomes |
|
| Notes | Only abstract available. |
NCT02569970.
| Methods | Randomised parallel assignment |
| Participants | People with CSA and seizure |
| Interventions | Fluoxetine 20mg versus placebo 20 mg |
| Outcomes |
|
| Notes | Study with status 'completed' in ClinicalTrials.gov but without results. We contacted the study authors to check the availability of data. |
Sorokina 2022.
| Methods | Randomised parallel assignment |
| Participants | People with Cheyne‐Stokes respiration and CHF |
| Interventions | Standard medical therapy in combination with acetazolamide 250 mg once daily (n =10) versus standard therapy (n = 11) |
| Outcomes |
|
| Notes | Only abstract available. We contacted the study authors to ask for the full text. |
API: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CHF: chronic heart failure; CSA: central sleep apnoea; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
2015‐003119‐39.
| Study name | Effects of a short therapy of zolpidem with support servo‐ventilation versus placebo in patients with central sleep apnoea with chronic heart failure |
| Methods | Prospective double‐blind placebo‐controlled clinical study |
| Participants | People with CSA and CHF |
| Interventions | Zolpidem versus placebo |
| Outcomes |
|
| Starting date | 25 February 2016 |
| Contact information | MCoutard@chu‐grenoble.fr |
| Notes |
NCT04118387.
| Study name | CSA: physiologic mechanisms to inform treatment |
| Methods | RCT with a cross‐over design |
| Participants | People with CSA, defined as AHI >15 events/hour with cAHI > 5 events/hour |
| Interventions | Acetazolamide + supplemental oxygen + PAP therapy versus zolpidem + PAP therapy versus buspirone + PAP therapy |
| Outcomes | cAHI |
| Starting date | 7 January 2021 |
| Contact information | sbadr@med.wayne.edu |
| Notes | Recruitment status: recruiting |
AHI: apnoea‐hypopnoea index; cAHI: central apnoea‐hypopnoea index; CHF: chronic heart failure; CSA: central sleep apnoea; PAP: positive airway pressure; RCT: randomised controlled trial.
Differences between protocol and review
In the protocol we planned to exclude cross‐over trials but decided to include them in the full review as most of the studies we identified used this design, and potential carry‐over effects were considered minimal (Riera 2018). To be included, the study should have a sufficient wash‐out period, based on the drug half‐life (from two or three hours to 11 hours) to avoid the risk of carry‐over effect.
We did not include studies focusing on central sleep apnoea (CSA) due to periodic breathing at high altitudes because this is usually triggered by environmental exposure and is not a chronic health condition comparable to the other types of CSA included in the review.
We initially planned to include seven outcomes in the summary of findings table: apnoea‐hypopnoea index (AHI), cardiovascular mortality, quality of sleep, quality of life, all‐cause mortality, time to cardiovascular intervention, and serious adverse events. However, we were unable to find studies that evaluated some of these outcomes, such as time to lifesaving cardiovascular intervention. Nonetheless, we were able to assess the primary outcome of this review, which is central apnoea‐hypopnoea (cAHI). Therefore, we revised our selection of outcomes to be included in the summary of findings table in line with Cochrane methodology, which recommends presenting a maximum of seven outcomes (Schumemann 2013): we added cAHI, defined as the number of central apnoeas and hypopnoeas per hour of sleep, objectively measured by polysomnography; and we removed time to lifesaving cardiovascular intervention. We made this minor change to improve the presentation of outcome results reported in the studies included in this review.
We made the following changes to the specified team members performing different roles within the review:
AR and ACP screened the titles and abstracts and full texts (instead of DP, COCL, RLP or ALCM). DP acted as the third person to resolve disagreements (instead of RR). AR and ACP conducted data extraction (instead of DP and COCL). AR transferred data to Review Manager (instead of DP). DP spot‐checked study characteristics for accuracy against the study report (instead of RR) AR and ACP assessed studies for risk of bias (instead of DP and COCL).
Contributions of authors
AR: conception of the review; design of the review; co‐ordination of the review; search and selection of studies for inclusion in the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; interpretation of data; writing of the review. ACP: conception of the review; design of the review; search and selection of studies for inclusion in the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; interpretation of data; writing of the review. DP: conception of the review; design of the review; search and selection of studies for inclusion in the review. GLF: writing of the review. AA: co‐ordination of the review; writing of the review. LD: writing of the review.
Contributions of editorial team
Sally Spencer (Co‐ordinating Editor): edited the review; advised on methodology, interpretation, and content; approved the review prior to publication. Teresa Anna Cantisani (Contact Editor): edited the review; advised on methodology, interpretation, and content. Rebecca Fortescue (Co‐ordinating Editor): checked the data entry prior to the full write‐up of the review. Emma Dennett (Deputy Co‐ordinating Editor): advised on methodology, interpretation, and content; edited the review. Emma Jackson (Managing Editor): co‐ordinated the editorial process; conducted peer review; obtained translations; edited the plain language summary and reference sections of the review. Kayleigh Kew (Freelance Editor): advised on methodology, interpretation, and content; edited the review. Elizabeth Stovold (Information Specialist): designed the search strategy and ran the searches. Vittoria Lutje (Freelance Information Specialist): ran the updated search and edited the search methods section.
Sources of support
Internal sources
-
Cochrane Brazil, Brazil
Institutional support for DVP, ALCM, COCL, RLP, RR
-
Universidade Federal de São Paulo (UNIFESP), Brazil
Institutional support for DVP, ALCM, COCL, RLP, RR
-
Universidade de São Paulo – Instituto do Coração (USP – INCOR), Brazil
Institutional support for LD, GLF
External sources
-
National Institute for Health and Care Research, UK
This project was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
AR: none known ACP: none known DP: none known GLF: owns stocks in Biologix (a start‐up company producing a simple device for sleep apnoea diagnosis) AA: none known LD: paid consultant for ResMed Foundation
Edited (no change to conclusions)
References
References to studies included in this review
Bonnet 1990 {published data only}
- Bonnet MH, Dexter JR, Arand DL. The effect of triazolam on arousal and respiration in central sleep apnea patients. Sleep 1990;13(1):31-41. [DOI] [PubMed] [Google Scholar]
Giannoni 2020a {published data only}
- Giannoni A, Borrelli G, Mirizzi G, Richerson GB, Emdin M, Passino C. Benefit of buspirone on chemoreflex and central apnoeas in heart failure: a randomized controlled crossover trial. European Journal of Heart Failure 2020;23(2):312-20. [DOI] [PubMed] [Google Scholar]
Javaheri 1996 {published data only}
- Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. New England Journal of Medicine 1996;335(8):562-7. [DOI] [PubMed] [Google Scholar]
Javaheri 2006 {published data only}
- Javaheri S, Sands SA, Edwards BA. Acetazolamide attenuates Hunter-Cheyne-Stokes breathing but augments the hypercapnic ventilatory response in patients with heart failure. Annals of the American Thoracic Society 2014;11:80-6. [DOI] [PubMed] [Google Scholar]
- Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. American Journal of Respiratory and Critical Care Medicine 2006;173(2):234-7. [DOI] [PubMed] [Google Scholar]
Sorokina 2019a {published data only}
- Sorokina K, Poltavskaya M, Palman A, Kuklina M, Kharkevich K. Central sleep apnea in patients with chronic heart failure and its treatment with acetazolamide. European Journal of Heart Failure 2019;21(suppl.S1):P1678. [Google Scholar]
References to studies excluded from this review
Belyavskiy 2010 {published data only}
- Belyavskiy E, Kalinkin A, Mareev V. Acetazolamide improves severity of chronic heart failure in patients with obstructive sleep apnea syndrome. European Journal of Heart Failure 2010;9(Suppl 1):S196. [Google Scholar]
- Belyavskiy E, Litvin A, Mareev V. Effects of acetazolamide in patients with obstructive sleep apnea syndrome and chronic heart failure. European Heart Journal 2010;31(Suppl 1):857 [P4878]. [Google Scholar]
- Belyavskiy E, Mareev V. Acetazolamide decreases obstructive sleep apnea severity in patients with chronic heart failure. European Journal of Heart Failure 2011;10:S30-1. [Google Scholar]
Caravita 2022 {published data only}
- Caravita S, Faini A, Vignati C, Pelucchi S, Salvioni E, Cattadori G, et al. Intravenous iron therapy improves the hypercapnic ventilatory response and sleep disordered breathing in chronic heart failure. European Journal of Heart Failure 2022;24(10):1940-9. [DOI: 10.1002/ejhf.2628] [DOI] [PMC free article] [PubMed]
Connaughton 1984 {published data only}
- Connaughton JJ, Morgan AD, Douglas NJ. Central sleep apnoeas are not reduced by almitrine. Clinical Science 1984;66:4P. [Google Scholar]
DeBacker 1995 {published data only}
- DeBacker W, Verbraecken J, Willemen M, Wittsaele W, DeCock W, de Hayning P. Central apnea index decreases after prolonged treatment with acetazolamide. American Journal of Respiratory and Critical Care Medicine 1995;151(1):87-91. [DOI] [PubMed] [Google Scholar]
Dubowitz 1998 {published data only}
- Dubowitz G. Effect of temazepam on oxygen saturation and sleep quality at high altitude: randomised placebo controlled crossover trial. BMJ 1998;316(7131):587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginter 2020 {published data only}
- Ginter G, Sankari A, Eshraghi M, Obiakor H, Yarandi H, Chowdhuri S, et al. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. Journal of Applied Physiology 2020;128(4):960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffuel 2021 {published data only}
- Jaffuel D, Nogue E, Berdague P, Galinier M, Fournier P, Dupuis M, et al. Sacubitril-valsartan initiation in chronic heart failure patients impacts sleep apnea: the ENTRESTO-SAS study. ESC Heart Failure 2021;8(4):2513-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Javed 2020 {published data only}
- Javed F, Tamisier R, Pepin JL, Cowie MR, Wegscheider K, Angermann C, et al. Association of serious adverse events with Cheyne-Stokes respiration characteristics in patients with systolic heart failure and central sleep apnoea: a SERVE-Heart Failure substudy analysis. Respirology 2020;25(3):305-11. [DOI] [PubMed] [Google Scholar]
Murray 1977 {published data only}
- Murray GB. Carbamazepine for central sleep apnea. JAMA 1977;238(3):212-3. [DOI] [PubMed] [Google Scholar]
Naghan 2019 {published data only}
- Naghan PA, Raeisi K, Khoundabi B, Foroughi M, Malekmohammad M, Mohebbi M, et al. The effect of acetazolamide on the improvement of central apnea caused by abusing opioid drugs in the clinical trial. Sleep and Breathing 2019;24:1-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Naghan 2020 {published data only}
- Naghan PA, Raeisi K, Khoundabi B, Foroughi M, Malekmohammad M, Mohebbi M, et al. The effect of acetazolamide on the improvement of central apnea caused by abusing opioid drugs in the clinical trial. Sleep and Breathing 2020;24(4):1417-25. [DOI] [PubMed] [Google Scholar]
NCT00746954 {published data only}
- NCT00746954. Buspirone as a potential treatment for recurrent central apnea (CSA treatment). clinicaltrials.gov/ct2/show/NCT00746954 (first received 4 September 2008).
NCT01500473 {published data only}
- NCT01500473. Therapeutic effect of desogestrel on ventilatory control in patients with congenital central hypoventilation syndrome. clinicaltrials.gov/ct2/show/NCT01500473 (first received 28 December 2011).
NCT02670096 {published data only}
- NCT02670096. A single-center pilot study evaluating the immediate effects of low-dose acetazolamide on respiratory control in subjects with treatment emergent sleep disordered breathing. www.clinicaltrials.gov/ct2/show/NCT02670096 (first received 1 February 2016).
Obernadorfer 2000 {published data only}
- Obernadorfer S, Saletu B, Gruber G, Anderer P, Saletu M, Mandl M, et al. Theophylline in snoring and sleep-related breathing disorders: sleep laboratory investigations on subjective and objective sleep and awakening quality. Methods and Findings in Experimental and Clinical Pharmacology 2000;22(4):237-45. [DOI] [PubMed] [Google Scholar]
Passino 2021 {published data only}
- Passino C, Sciarrone P, Vergaro G, Borrelli C, Spiesshoefer J, Gentile F, et al. Sacubitril-valsartan treatment is associated with decrease in central apneas in patients with heart failure with reduced ejection fraction. International Journal of Cardiology 2021;330:112-9. [DOI] [PubMed] [Google Scholar]
Prowting 2021 {published data only}
- Prowting J, Maresh S, Vaughan S, Kruppe E, Alsabri B, Badr MS, et al. Mirtazapine reduces susceptibility to hypocapnic central sleep apnea in males with sleep-disordered breathing: a pilot study. Journal of Applied Physiology 2021;131(1):414-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rastogi 2020 {published data only}
- Rastogi R, Bakkila KA, Badr M, Chowdhuri S, Dingell JD. Effect of acetazolamide on pathophysiology of sleep disordered breathing in older adults. American Journal of Respiratory and Critical Care Medicine 2020;201:A6454. [Google Scholar]
Saletu 1999 {published data only}
- Saletu B, Oberndorfer S, Anderer P, Gruber G, Divos H, Lachner A, et al. Efficiency of continuous positive airway pressure versus theophylline therapy in sleep apnea: comparative sleep laboratory studies on objective and subjective sleep and awakening quality. Neuropsychobiology 1999;39:151-9. [DOI] [PubMed] [Google Scholar]
Schumacher 2014 {published data only}
- Schumacher DS, Müller-Mottet S, Hasler ED, Hildenbrand FF, Keusch S, Speich R, et al. Effect of oxygen and acetazolamide on nocturnal cardiac conduction, repolarization, and arrhythmias in precapillary pulmonary hypertension and sleep-disturbed breathing. Chest 2014;146(5):1226-36. [DOI] [PubMed] [Google Scholar]
Schwarz 2020 {published data only}
- Schwarz EI, Kohler M. Randomized controlled trials on the comparative effect of treatment modalities of central sleep apnea with Cheyne-Stokes Respiration on cardiovascular outcomes and physiology studies required. Journal of Clinical Sleep Medicine 2020;16(4):653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shore 1983 {published data only}
- Shore T, Millman P. Central sleep apnea and acetazolamide therapy. Archives of Internal Medicine 1983;143:1278. [DOI: 10.1001/archinte.1983.00350060210040] [DOI] [PubMed] [Google Scholar]
Sin 1999 {published data only}
- Sin D, Bradley D. Theophylline therapy for near-fatal Cheyne-Stokes respiration. Annals of Internal Medicine 1999;131(9):713-4. [DOI] [PubMed] [Google Scholar]
Sorokina 2019b {published data only}
- Sorokina KV, Palman AD, Brovko M, Poltavskaya MG. Central sleep apnea in patients with chronic heart failure. Journal of Neurology and Psychiatry 2019;119:99-104. [DOI] [PubMed] [Google Scholar]
Ulrich 2013 {published data only}
- Ulrich S, Keusch S, Hildenbrand F, Huber LC, Speich R, Tanner F, et al. Nocturnal oxygen therapy improves exercise performance in patients with pulmonary hypertension and sleep disturbed breathing compared to acetazolamide and placebo. Randomized, double-blind, cross-over trial. Respiration 2013;34:527. [DOI] [PubMed] [Google Scholar]
Ulrich 2015 {published data only}
- Ulrich S, Keusch S, Hildenbrand FF, Lo Cascio C, Huber LC, et al. Effect of nocturnal oxygen and acetazolamide on exercise performance in patients with pre-capillary pulmonary hypertension and sleep-disturbed breathing: randomized, double-blind, cross-over trial. European Heart Journal 2015;36(10):615-22. [DOI] [PubMed] [Google Scholar]
Westwood 2012 {published data only}
- Westwood AJ, Vendrame M, Montouris G, Auerbach SH. Pearls & oy-sters: treatment of central sleep apnea with topiramate. Neurology 2012;78(16):e97-9. [DOI] [PubMed] [Google Scholar]
Yasuma 2006 {published data only}
- Yasuma F, Murohara T, Hayano J. Long-term efficacy of acetazolamide on Cheyne-Stokes respiration in congestive heart failure. American Journal of Respiratory and Critical Care Medicine 2006;174(4):479; author reply 479-80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ahmad 2022 {published data only}
- Ahmad B, Aldwaikat A, Eshraghi M, Carroll SW, Lasisi O, Al-Maktar T, et al. Effect of zolpidem on susceptibility to develop central sleep apnea. American Journal of Respiratory and Critical Care Medicine 2021;203:A4748.
- Ahmad B, Sankari A, Eshraghi M, Aldwaikat A, Yarandi H, Zeineddine S, et al. Effect of zolpidem on nocturnal arousals and susceptibility to central sleep apnea. Sleep and Breathing 2022 Mar 14 [Epub ahead of print]. [DOI: 10.1007/s11325-022-02593-3] [PMID: ] [DOI] [PMC free article] [PubMed]
Guo 2003 {published data only}
- Guo X, Chen W, Hongyu Z, Weimin K, Li A, Li L, et al. Effects of zolpidem on nocturnal breathing and sleep in normal and SAHS subjects. Sleep Medicine 2003;4(Suppl 1):S15. [Google Scholar]
NCT02569970 {published data only}
- NCT02569970. Efficacy of fluoxetine against seizure-induced central apneas (FLUOXETINE) [Efficacy of fluoxetine against seizure-induced central apneas: a randomized placebo-controled double-blind trial]. clinicaltrials.gov/ct2/show/NCT02569970 (first received 7 October 2015).
Sorokina 2022 {published data only}
- Sorokina KV, Poltavskaya MG, Palman AD, Kuklina MD, Kharkevich KY, Andreev AD, et al. Acetazolamide in the Cheyne Stokes respiration therapy in patients with chronic heart failure: a pilot randomized study. Human Physiology 2022;48(1):78-85. [Google Scholar]
References to ongoing studies
2015‐003119‐39 {published data only}
- 2015-003119-39. Effects of a short therpay of zolpidem with support servo-ventilation versus placebo in patients with central sleep apnea with chronic heart failure. www.clinicaltrialsregister.eu/ctr-search/search?query=2015-003119-39 (first received 29 October 2015).
NCT04118387 {published data only}
- NCT04118387. Central sleep apnea: physiologic mechanisms to inform treatment. clinicaltrials.gov/ct2/show/NCT04118387 (first received 8 October 2022).
Additional references
American Academy of Sleep Medicine 2014
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
Aurora 2012
- Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep 2012;35(1):17-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjafield 2019
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet 2019;7(8):687-98. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berssenbrugge 1983
- Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. Journal of Physiology 1983;343:507-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brack 2007
- Brack T, Thüer I, Clarenbach CF, Senn O, Noll G, Russi EW, et al. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest 2007;135:1463-71. [DOI] [PubMed] [Google Scholar]
Bradley 2005
- Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, et al. Continuous positive airway pressure for central sleep apnea and heart failure. New England Journal of Medicine 2005;19:2025-33. [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193-213. [DOI] [PubMed] [Google Scholar]
Carrero 2011
- Carrero JJ, Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clinical Journal of the American Society of Nephrology 2011;6(7):1722-30. [DOI] [PubMed] [Google Scholar]
Cowie 2015
- Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. New England Journal of Medicine 2015;373(12):1095-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eckert 2007
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007;131(2):595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emdin 2017
- Emdin M, Mirizzi G, Giannoni A, Poletti R, Iudice G, Bramanti F, et al. Prognostic significance of central apneas throughout a 24-hour period in patients with heart failure. Journal of the American College of Cardiology 2017;70(11):1351-64. [DOI] [PubMed] [Google Scholar]
EPOC 2017
- EPOC. Cochrane Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors, 2017. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/good_practice_data_extraction_form.doc (accessed prior to 21 September 2022).
Fala 2015
- Fala L. Entresto (Sacubitril/Valsartan): first-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. American Health & Drug Benefits 2015;6:330-4. [PMC free article] [PubMed] [Google Scholar]
Giannoni 2009
- Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. Journal of the American College of Cardiology 2009;21:1975-80. [DOI] [PubMed] [Google Scholar]
Giannoni 2020b
- Giannoni A, Gentile F, Sciarrone P, Borrelli C, Pasero G, Mirizzi G, et al. Upright central apnea in patients with heart failure is associated with worse clinical conditions and with a greater risk of cardiac death. Journal of the American College of Cardiology 2020;23:2934-46. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 24 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hanly 1996
- Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. American Journal of Respiratory and Critical Care Medicine 1996;153:272-6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2022a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Higgins 2022b
- Higgins J, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates. Available from community.cochrane.org/mecir-manual.
Jenkinson 1996
- Jenkinson C, Layte R, Wright L, Coulter A. Manual and Interpretation Guide for the UK SF-36. Oxford: Health Services Research Unit, 1996. [Google Scholar]
Johns 1991
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14(6):540-5. [DOI] [PubMed] [Google Scholar]
Lanfranchi 1999
- Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation 1999;11:1435-40. [DOI] [PubMed] [Google Scholar]
Lorenzi‐Filho 2005
- Lorenzi-Filho G, Genta PR, Figueiredo AC, Inoue D. Cheyne-Stokes respiration in patients with congestive heart failure: causes and consequences. Clinics 2005;60(4):333-44. [DOI] [PubMed] [Google Scholar]
Malhotra 2004
- Malhotra A, Berry RB, White DP. Central sleep apnea. In: Carney PR, Berry RB, Geyer JD, editors(s). Clinical Sleep Disorders. Philadelphia, PA: Lippincott Williams and Wilkins, 2004:331-46. [Google Scholar]
Müller 2011
- Müller CE, Jacobson KA. Xanthines as adenosine receptor antagonists. Handbook of Experimental Pharmacology 2011;200:151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakayama 2002
- Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. American Journal of Respiratory and Critical Care Medicine 2002;165(9):1251-9. [DOI] [PubMed] [Google Scholar]
Nakayama 2003
- Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Carotid body denervation eliminates apnea in response to transient hypocapnia. Journal of Applied Physiology 2003;94:155-64. [DOI] [PubMed] [Google Scholar]
Naughton 2016
- Naughton MT. Epidemiology of central sleep apnoea in heart failure. International Journal of Cardiology 2016;206:S4-S7. [DOI] [PubMed] [Google Scholar]
New York Heart Association 1994
- New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. Boston, MA: Lippincott Williams and Wilkins, 1994. [Google Scholar]
Oldenburg 2015
- Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. European Heart Journal 2016;21:1695-703. [DOI] [PubMed] [Google Scholar]
Page 2020
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:71. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peer 2010
- Peer A, Lorber A, Suraiya S, Malhotra A, Pillar G. The occurrence of Cheyne-Stokes respiration in congestive heart failure: The effect of age. Frontiers in Psychiatry 2010;1:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quadri 2009
- Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. Journal of Clinical Sleep Medicine 2009;5(2):122-9. [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.16.1. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Sasayama 2009
- Sasayama S, Izumi T, Matsuzaki M, Matsumori A, Asanoi H, Momomura S, et al. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circulation Journal 2009;73(7):1255-62. [DOI] [PubMed] [Google Scholar]
Schmickl 2020a
- Schmickl CN, Landry SA, Orr JE, Chin K, Murase K, Verbraecken J, et al. Acetazolamide for OSA and central sleep apnea: a comprehensive systematic review and meta-analysis. Chest 2020;158(8):2632-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmickl 2020b
- Schmickl CN, Owens RL, Orr JE, Edwards BA, Malhotra A. Side effects of acetazolamide: a systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respiratory Research 2020;7(1):e000557. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumemann 2013
- Schumemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE Handbook. Available from gdt.gradepro.org/app/handbook/handbook.html 2013.
Schwartz 2021
- Schwartz AR, Goldberg LR, McKane S, Morgenthaler TI. Transvenous phrenic nerve stimulation improves central sleep apnea, sleep quality, and quality of life regardless of prior positive airway pressure treatment. Sleep and Breathing 2021;25(4):2053-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simantirakis 2008
- Simantirakis EN, Schiza SE, Siafakas NS, Vardas PE. Sleep-disordered breathing in heart failure and the effect of cardiac resynchronization therapy. EP Europace 2008;10:1029-33. [DOI] [PubMed] [Google Scholar]
Vazir 2007
- Vazir A, Hastings PC, Dayer M, et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. European Journal of Heart Failure 2007;9:243-50. [DOI] [PubMed] [Google Scholar]
Wilcox 1998
- Wilcox I, McNamara SG, Wessendorf T, Willson GN, Piper AJ, Sullivan CE. Prognosis and sleep disordered breathing in heart failure. Thorax 1998;53 Suppl 3:S33-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Riera 2018
- Riera R, Latorraca CO, Martimbianco AL, Pacheco RL, Drager LF, Lorenzi-Filho G, et al. Pharmacological treatment for central sleep apnoea in adults. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012922. [DOI: 10.1002/14651858.CD012922] [DOI] [PMC free article] [PubMed] [Google Scholar]


