Abstract
Background
Stereotactic ablative radiotherapy (SABR) is now the standard of care for patients with inoperable early‐stage lung cancer. Many of these patients are elderly. EGFR (epidermal growth factor receptor) mutation is also common in the Asian population.
Methods
To evaluate the effects of old age and EGFR mutation on treatment outcomes and toxicity, we reviewed the medical records of 71 consecutive patients with inoperable early‐stage non‐small cell lung cancer (NSCLC) who received SABR at Taipei Veterans General Hospital between 2015 and 2021.
Results
The study revealed that median age, follow‐up, Charlson comorbidity index, and ECOG score were 80 years, 2.48 years, 3, and 1, respectively. Of these patients, 37 (52.1%) were 80 years or older, and 50 (70.4%) and 21 (29.6%) had T1 and T2 diseases, respectively. EGFR mutation status was available for 33 (46.5%) patients, of whom 16 (51.5%) had a mutation. The overall survival rates at 1, 3, and 5 years were 97.2, 74.9, and 58.3%, respectively. The local control rate at 1, 3, and 5 years was 97.1, 92.5, and 92.5%, respectively. Using Cox proportional hazards regression we found that male sex was a risk factor for overall survival (p = 0.036, 95% CI: 1.118–26.188). Two patients had grade 2 pneumonitis, but no other grade 2 or higher toxicity was observed. We did not find any significant differences in treatment outcomes or toxicity between patients aged 80 or older and those with EGFR mutations in this cohort.
Conclusion
These findings indicate that age and EGFR mutation status do not significantly affect the effectiveness or toxicity of SABR for patients with inoperable early‐stage NSCLC.
Keywords: EGFR, lung cancer, old age, SABR
We reviewed 71 consecutive early‐stage inoperable NSCLC patients who received SABR at our institute, and there were no significant differences in patients aged 80 or older, or with EGFR mutation.
INTRODUCTION
Lung cancer is a major global health burden, ranking as the second most common cancer worldwide with 2.2 million new cases in 2020. It is also the leading cause of cancer deaths, with approximately 1.8 million deaths in 2020. 1 In Taiwan, lung cancer is the leading cause of cancer deaths, accounting for around 10 000 deaths each year. 2 Effective screening tools are needed for the early diagnosis of lung cancer. Low‐dose computed tomography (LDCT) has been tested in smokers in randomized controlled trials (RCTs) and has shown survival benefits. 3 LDCT has become increasingly popular in health examinations in the general population, even though the results of RCTs are still pending. This has led to an increase in the incidence of early‐stage lung cancer, as more cases of the disease are diagnosed at an early stage. 4 The standard treatment for early‐stage lung cancer is surgical resection, typically a lobectomy, accompanied by radical lymph node dissection. For patients who are not candidates for surgery, radiotherapy is the standard alternative. Conventional fractionated external beam radiotherapy (EBRT) typically takes 5–7 weeks to complete. Stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy (SABR) offers higher precision and higher doses per fraction, allowing the entire course of treatment to be completed in 1–2 weeks. Randomized controlled trials have shown that SABR has lower toxicity compared to conventional EBRT, with similar or better treatment outcomes. 5 , 6 SABR has become the standard of care for inoperable patients with early‐stage lung cancer. Many of these patients are elderly, and it is of interest to determine whether there are differences in treatment outcomes for older patients. Taipei Veterans General Hospital (TVGH) is known for caring for one of the oldest populations in Taiwan. We reviewed the medical records of inoperable patients who received SABR at TVGH to determine if there were any differences in treatment outcomes for elderly patients. We also evaluated the presence of EGFR mutations in this population, as EGFR mutations are common in Asian patients with lung adenocarcinoma, particularly in nonsmoking female patients. 7 The prognostic role of EGFR mutation status in early‐stage non‐small cell lung cancer (NSCLC) after surgery is still controversial. 8 , 9 Previous studies have examined the impact of EGFR mutations on treatment outcomes after SABR for early‐stage NSCLC, but the results have been mixed. Nakamura et al. found that patients with EGFR mutations had a similar local control rate, but a higher rate of out‐of‐field progression compared to patients without EGFR mutations, with five EGFR‐mutated patients in the study. 10 Other studies have also examined the impact of EGFR mutations on treatment outcomes after SABR for early‐stage NSCLC. These studies, which included seven and 24 EGFR‐mutated patients, respectively, did not find a difference in treatment outcomes between patients with and without EGFR mutations. 11 , 12 However, the small sample sizes of these studies may be insufficient to draw definitive conclusions. Here, we analyzed the role of EGFR mutation and old age to determine their roles in prognosis and toxicity.
METHODS
The cancer registry of Taipei Veterans General Hospital (TVGH) was reviewed using the following inclusion criteria: (1) aged 20 years or older, (2) pathological proof of non‐small cell lung cancer, (3) clinical stage cT1‐2N0M0, and (4) received SABR between 2015 and 2021 at TVGH. The exclusion criteria were: (1) tumors larger than 5 cm, and (2) distant metastasis of lung cancer or other synchronous cancer noted before SABR. The tumors were staged according to the AJCC seventh edition for patients diagnosed between 2015 and 2017, and according to the AJCC eighth edition for patients diagnosed in 2018 or later. Since this study only considered T1 or T2 stages and excluded tumors larger than 5 cm, the change in staging criteria did not affect the statistical analysis. IBM SPSS version 22 was used for statistical analysis. Associations between categorical and continuous variables were detected using two‐tailed Chi‐square tests and two‐tailed Student's t‐tests, respectively. Statistical significance between factors was determined using the log‐rank test. A p‐value less than 0.05 was considered statistically significant. The Cox proportional hazards model was applied to estimate hazard ratios and 95% confidence intervals (CIs). Toxicity was reported according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.03. Survival duration was calculated from the last day of SABR. Progression‐free survival was defined as patients surviving without radiographic evidence of disease progression. Disease control was defined as patients without radiographic evidence of disease progression. Distant control was calculated until radiographic evidence of distant metastasis. Lung cancer‐specific survival was calculated until the patient died after radiographic evidence of lung cancer recurrence. Local control was calculated until radiographic evidence of local recurrence. The Charlson comorbidity index (CCI) was calculated according to the original definition without age‐adjustment. 13
RESULTS
Patient characteristics
A total of 71 consecutive patients were reviewed. The demographics are shown below. The median age was 80 years old, median ECOG score was 1, median CCI score was 3, and median follow‐up time was 2.48 years. The patient characteristics are listed in Table 1.
TABLE 1.
Patient characteristics
| Characteristic | All N = 71 | <80 y/o N = 34 | ≥80 y/o N = 37 | p‐value |
|---|---|---|---|---|
| Age, median (range) | 80 (42–93) | 74.5 (42–79) | 86 (80–93) | ‐ |
| Male | 38 (53.5%) | 17 (50%) | 21 (56.8%) | 0.57 |
| Smoking | 35 (49.3%) | 16 (47.1%) | 19 (51.4%) | 0.718 |
| ECOG (%) | 0.926 | |||
| 0 | 6 (8.5%) | 3 (8.8%) | 3 (8.1%) | |
| 1 | 47 (66.2%) | 22 (64.7%) | 25 (67.6%) | |
| 2 | 15 (21.1%) | 7 (20.6%) | 8 (21.6%) | |
| 3 | 3 (4.2%) | 2 (5.9%) | 1 (2.7%) | |
| EGFR mutation | 0.401 | |||
| Mutated | 17 (23.9%) | 7 | 10 | |
| Wild | 16 (22.5%) | 6 | 10 | |
| Unknown | 38 (53.5%) | 21 | 17 | |
| Pathology | 0.401 | |||
| Adenocarcinoma | 57 (80.3%) | 27 | 30 | |
| Mucinous adenocarcinoma | 2 (2.8%) | 0 | 2 | |
| Adenosquamous | 1 (1.4%) | 1 | 0 | |
| SqCC | 10 (14.1%) | 5 | 5 | |
| NSCLC | 1 (1.4%) | 1 | 0 | |
| Charlson comorbidity index | 0.067 | |||
| 2 | 17 | 4 | 13 | |
| 3 | 26 | 13 | 13 | |
| 4 | 9 | 4 | 5 | |
| 5 | 8 | 6 | 2 | |
| 6 | 6 | 5 | 1 | |
| 7 | 3 | 2 | 1 | |
| 8 | 2 | 0 | 2 | |
| Synchronous other cancer | 10 | 5 | 5 | 0.885 |
| NSCLC diagnosed in past 5 years | 18 | 9 | 9 | 0.836 |
| T stage | 0.623 | |||
| 1 | 50 | 23 | 27 | |
| 2 | 21 | 11 | 10 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor status; SqCC, squamous cell carcinoma; NSCLC, non‐small cell lung cancer.
The dose‐fractionation of SABR performed is shown in Table 2. A total of 67 (94.3%) patients received a biological equivalent dose with α/β = 10(BED10) ≧ 100 Gy. More than 60% of the patients received 50 Gy in five fractions.
TABLE 2.
Radiation dose, fraction number, and biologically equivalent dose
| Total physical dose (Gy) | Fraction number | BED10 (Gy) | Number | (%) |
|---|---|---|---|---|
| 45 | 6 | 78.8 Gy | 1 | 1.4% |
| 45 | 5 | 85.5 Gy | 1 | 1.4% |
| 48 | 6 | 86.4 Gy | 2 | 2.8% |
| 50 | 5 | 100 Gy | 43 | 60.6% |
| 53.1 | 6 | 100.1 Gy | 4 | 5.6% |
| 54 | 6 | 102.6 Gy | 4 | 5.6% |
| 55 | 5 | 115.5 Gy | 2 | 2.8% |
| 60 | 6 | 120 Gy | 6 | 8.5% |
| 34 | 1 | 149.6 Gy | 8 | 11.3% |
Abbreviations: BED, biologically effective dose; Gy, Gray.
Treatment results
Among the patients who suffered from disease failure, six (8.5%) patients had local failure, two (2.8%) patients had regional failure, and 10 (14.1%) patients had distant failure.
Data on the EGFR mutation status was available for 33 (46.4%) patients. Among them, 17 (51.5%) patients were found to have EGFR mutation. The pattern of mutation is listed in Table 3. L858R (41.2%) and exon 19 deletion (35.3%) were the most common mutation sites.
TABLE 3.
EGFR mutation status
| Mutation site | Patient number (%) |
|---|---|
| Exon19 deletion | 6 (35.3%) |
| L858R | 7 (41.2%) |
| L861Q | 2 (11.8%) |
| G719X | 2 (11.8%) |
Overall survival at 1, 3, and 5 years was 97.2%, 74.9%, and 58.3%. There were 29 patients who died during follow‐up. Of these, 17 patients were noted to have lung cancer progression before death and were determined to have died of the disease (Figure 1). Noncancer death was then considered for the other 12 patients (41.4%). Progression‐free survival at 1, 3, and 5 years was 87.2, 57.7, and 40.8%. Disease control rate at 1, 3, and 5 years was 89.7, 68.9, and 59.3%. Distant control rate at 1, 3, and 5 years was 97, 82.9, and 72.2%. Lung cancer‐specific survival at 1, 3, and 5 years was 95.6, 85.6, and 85.6%. Local control rate at 1, 3, and 5 years was 97.1%, 92.5, and 92.5%.
FIGURE 1.
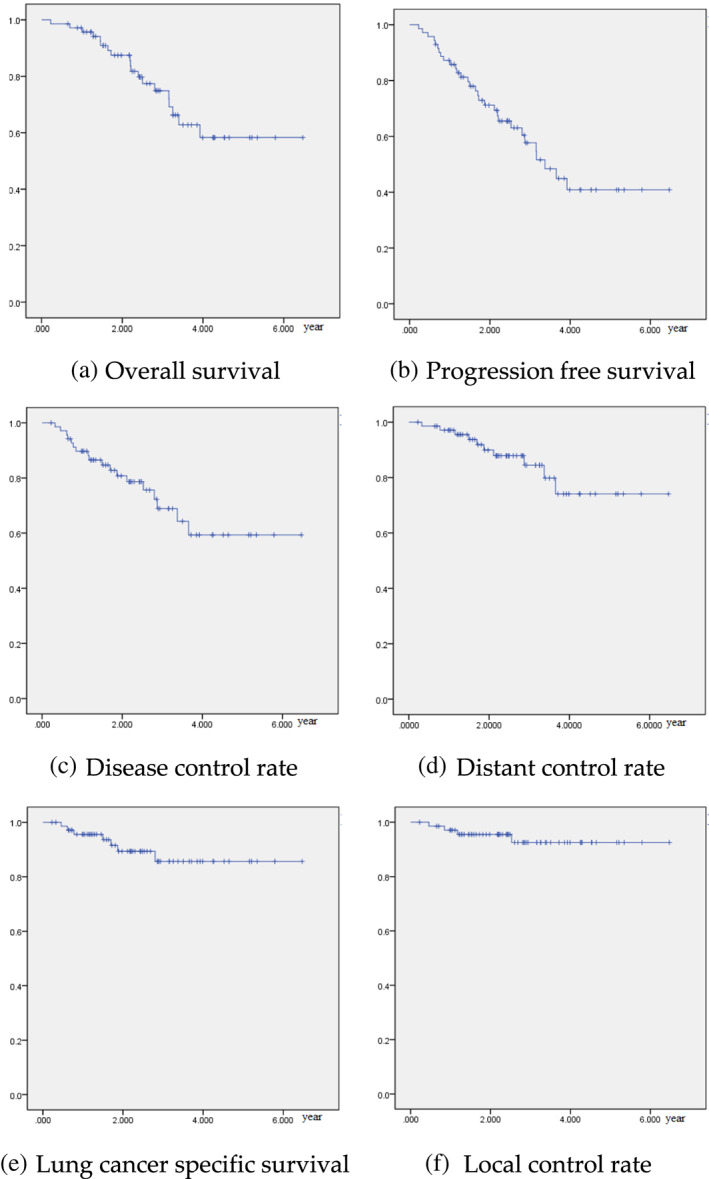
(a) Overall survival, (b) progression‐free survival, (c) disease control rate, (d) distant control rate, (e) lung cancer specific survival and (f) local control rate
We compared the treatment outcomes of patients aged 80 or more to patients less than 80 years old and found no significant differences in overall survival (p = 0.458), progression‐free survival (p = 0.216), disease control rate (p = 0.137), distant control rate (p = 0.116), lung cancer‐specific survival (p = 0.220), and local control rate (p = 0.217) (Figure 2).
FIGURE 2.
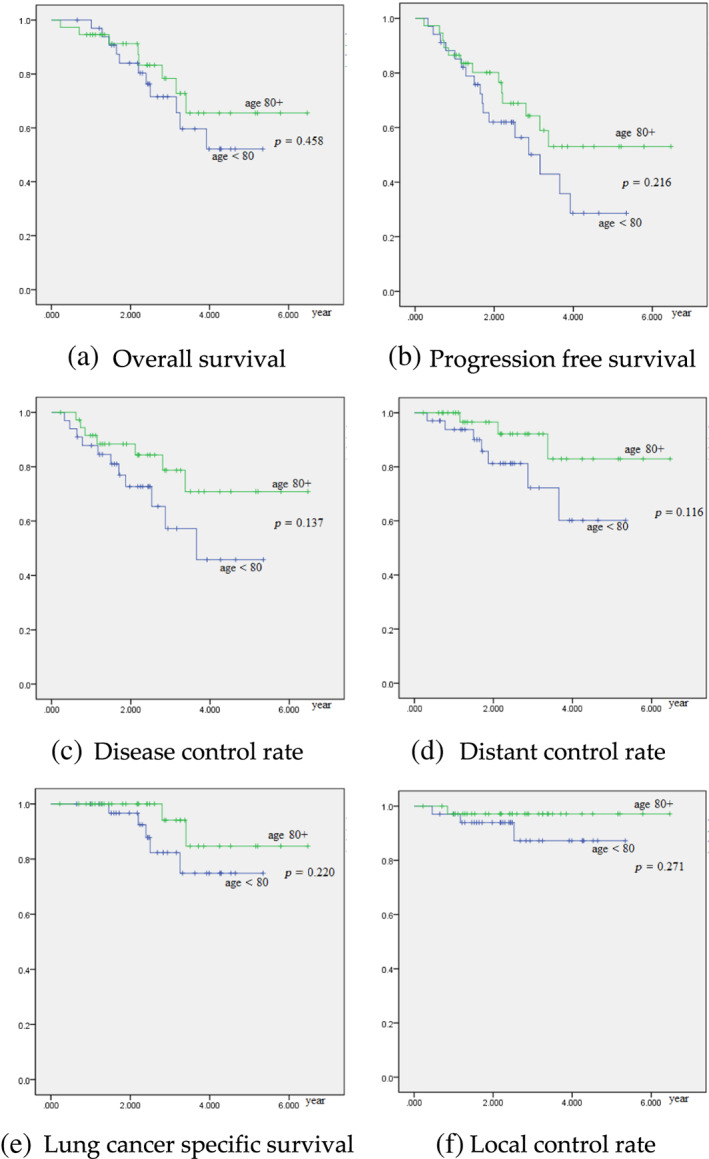
Univariate analysis on age 80 or more. (a) Overall survival, (b) progression‐free survival, (c) disease control rate, (d) distant control rate, (e) lung cancer specific survival and (f) local control rate
Among patients with EGFR status, there were no significant differences between patients with mutated EGFR and those with EGFR wild‐type in terms of overall survival (p = 0.781), progression‐free survival (p = 0.712), disease control rate (p = 0.688), distant control rate (p = 0.629), lung cancer‐specific survival (p = 0.905), or local control rate (p = 0.317) (Figure 3).
FIGURE 3.
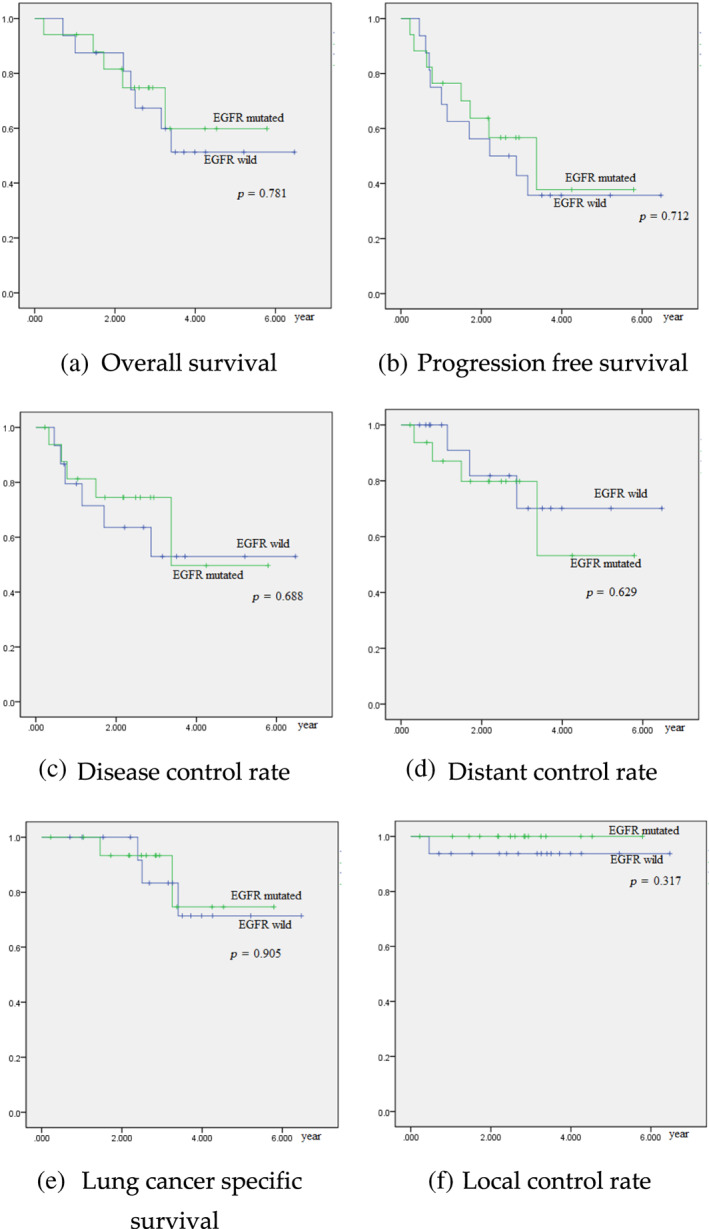
Univariate analysis on EGFR mutation: (a) overall survival, (b) progression‐free survival, (c) disease control rate, (d) distant control rate, (e) lung cancer specific survival and (f) local control
Because EGFR status and squamous cell carcinoma are highly colinear, we used Cox proportional regression with pathology type as a categorical variable for overall survival analysis.
Cox proportional regression on overall survival showed that there was a significant difference in sex, with females showing better overall survival than men (hazard ratio = 4.613, p = 0.041). Other factors, such as smoking status, T stage, recurrence, age over 80 years old, synchronous with other cancer, EGFR status, and pathological type, did not indicate significant differences in overall survival (Table 4).
TABLE 4.
Cox proportional regression on overall survival
| Risk factor | p‐value | Hazard ratio | 95% confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Sex | 0.041 | 4.613 | 1.062 | 20.029 |
| Smoking | 0.851 | 1.130 | 0.318 | 4.017 |
| T stage | 0.550 | 1.366 | 0.491 | 3.798 |
| Recurrence | 0.773 | 1.173 | 0.396 | 3.469 |
| Age over 80 years old | 0.359 | 0.623 | 0.227 | 1.712 |
| synchronous cancer | 0.416 | 1.748 | 0.456 | 6.704 |
| Adenocarcinoma c/unknown EGFR | 0.365 | n/a | n/a | n/a |
| Adenocarcinoma c/EGFR mutation | 0.277 | 0.273 | 0.026 | 2.833 |
| Adenocarcinoma c/EGFR wild | 0.977 | 1.035 | 0.101 | 10.653 |
| Squamous cell carcinoma | 0.834 | 0.773 | 0.070 | 8.556 |
| others: one adenosquamous carcinoma and one NSCLC | 0.296 | 0.252 | 0.019 | 3.352 |
Abbreviations: EGFR, epidermal growth factor receptor status; n/a, not available; NSCLC, non‐small cell lung cancer.
Cox proportional regression on progression‐free survival, disease control rate, distant control rate, lung cancer‐specific survival, or local control did not find significant risk factors.
Toxicity
In 34 patients aged less than 80, there were four with grade 1 pneumonitis (defined by CTCAE vs. 4.03 as asymptomatic; clinical or diagnostic observations only; intervention not indicated). There were no patients with toxicity grade 2 or more in the cohort. In 37 patients aged 80 or more, there were eight with grade 1 pneumonitis and one patient with grade 1 malaise (defined by CTCAE vs. 4.03 as uneasiness or lack of wellbeing). Two patients were noted with grade 2 pneumonitis (defined by CTCAE vs. 4.03 as symptomatic; medical intervention indicated; limiting instrumental ADL). There was no grade 3 or more toxicity in the cohort.
Using a 2 x 3 chi‐square test among the two cohorts, we did not find a significant difference in toxicity (p = 0.1294).
The toxicities profile by EGFR mutation status are shown in Table 5. There was no significant difference among EGFR mutation status noted using a 2 x 3 chi‐square test (p = 0.322).
TABLE 5.
Toxicity by EGFR mutation status
| Toxicity | Grade 0 | Grade 1 | Grade 2 |
|---|---|---|---|
| EGFR (+) | 12 | 5 | 0 |
| EGFR (−) | 10 | 4 | 2 |
Abbreviation: EGFR, epidermal growth factor receptor status.
DISCUSSION
In this study, we reviewed 71 consecutive patients at TVGH to investigate risk factors affecting oncological outcomes and toxicities for inoperable early‐stage NSCLC patients receiving SABR, including age ≧ 80 and EGFR mutation status. A previous study on 772 elderly patients did not show evident differences in progression‐free survival, lung cancer‐specific survival, or toxicity in patients aged 75 or more. 14 Another study on 197 patients did not show an evident difference in treatment outcomes and toxicity between elderly (75–85 years old) and very elderly patients (>85 years old). 15 However, one study on 335 patients found that age >75 years old was a risk factor for worse survival after adjusting with the Charlson comorbidity index (CCI). 16 SABR has become essential in treating elderly patients with early‐stage NSCLC. For patients aged 75 or more, the population‐based in Netherland noted SABR introduction was associated with a 16% absolute increase in radiotherapy use, a decline in the proportion of untreated elderly patients, and an improvement in overall survival. 17 For age ≧ 80, we also did not find any significant difference in oncological outcomes and toxicities in the current study. While whether old age would affect outcomes remains controversial, 14 , 15 , 16 the current study could provide some evidence in treating patients age ≧ 80. Although Nakamura et al. found a higher out‐of‐field progression rate for EGFR‐mutant patients receiving SABR, 10 in this cohort, we did not find a significant difference in disease control and local control rate across EGFR mutation status.
Males have been noted to have a shorter average life expectancy in the general population. As announced by the government, the average life expectancy in Taipei City for age 80 is 10.09 years and 12.34 years for males and females, respectively. 18 The difference of sex on overall survival noted in the current cohort could be contributed by the intrinsic difference of the general population. Similarly, female gender has been noted as the only favorable variable on overall survival in another study on patients with potentially operable stage I NSCLC receiving SABR. 19
The overall survival rate of early‐stage NSCLC patients receiving SABR is highly affected by comorbidities and general condition. For operable patients enrolled in the revised STARS trial, 5‐year OS has been reported as 87%. 20 Another study using a population‐based database of Taiwan found that nonoperated NSCLC receiving SABR had 5‐year OS of only 31%. 21 The 5‐year OS of 58.3% noted in this study is between the previous two studies and may reveal the current situation of a medical center in Taiwan. The local recurrence rate of 7.5% at 5‐years in the current study is comparable to the 6.3% noted in the revised STARS trial. 20
In this study, we found a distant control rate of only 72.2% at 5 years. Effective systemic treatment is needed to prevent distant metastasis. For surgical patients, adjuvant osimertinib has shown survival benefits in EGFR‐mutated stage IB–IIIA patients in the ADAURA trial. 22 For SABR patients with EGFR mutation, adjuvant osimertinib may also improve survival after SABR. Since no significant difference in survival was noted based on EGFR mutation status in this study, an improved prognosis may be noted with adjuvant osimertinib in patients with EGFR mutation.
There were some strengths in this study. With EGFR mutation status available in 33 (46.5%) patients, to the best of our knowledge, we analyzed the second largest cohort of EGFR‐mutated early‐stage NSCLC patients receiving SABR currently available in the literature. Additionally, the BED of SABR was relatively uniform, with 67 (94.3%) patients receiving BED10 ≥ 100 Gy, while the dose coverage has previously been noted to be associated with better local control and survival. 23
Our study had a number of limitations. First, it was a retrospective analysis and there may have been some selection bias. Second, because the patient group was not large enough, our study may not have enough statistical power to analyze the impact of other factors. Third, because the dose‐fractionation was tailored to their clinical condition, the prescribed radiation dose was not uniform.
In conclusion, in this study we reviewed 71 patients with inoperable early‐stage non‐small cell lung cancer receiving SABR at TVGH. Male gender was noted as a risk factor for poor overall survival. No significant differences were noted in treatment outcomes or toxicity for patients age ≧80 or with EGFR mutation.
AUTHOR CONTRIBUTIONS
Conceptualization, Yuan‐Hung Wu; methodology, Yuan‐Hung Wu; software, Yu‐Wen Hu; validation, Yu‐Mei Kang, Yu‐Wen Hu, Heng‐sheng Chao, Chi‐Lu Chiang; formal analysis, Yuan‐Hung Wu, Yu‐Mei Kang, Yu‐Wen Hu; resources, Yuan‐Hung Wu, Yu‐Mei Kang, Yu‐Wen Hu, Keng‐Li Lan, Sang‐Hue Yen, Tzu‐Yu Lai, Tien‐Li Lan, Yuh‐Min Chen, Chao‐Hua Chiu, Yung‐Hung Luo, Heng‐sheng Chao, Chi‐Lu Chiang, Tsu‐Hui Shiao, Chao‐Neng Yang, Wen‐Hu Hsu, Yu‐Chung Wu, Han‐Shui Hsu, Jung‐Jyh Hung, Chien‐Sheng Huang, Po‐Kuei Hsu, Yi‐Wei Chen; writing—original draft preparation, Yuan‐Hung Wu; writing—review and editing, Yuan‐Hung Wu, Yu‐Mei Kang; supervision, Yi‐Wei Chen. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This research received no funding.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Part of the study data is based on Cancer Registry Data Base, Taipei Veterans General Hospital. ChatGPT was consulted for English grammar.
Wu Y‐H, Kang Y‐M, Hu Y‐W, Lan K‐L, Yen S‐H, Lai T‐Y, et al. Old age and EGFR mutation status in inoperable early‐stage non‐small cell lung cancer patients receiving stereotactic ablative radiotherapy: A single institute experience of 71 patients in Taiwan. Thorac Cancer. 2023;14(7):654–661. 10.1111/1759-7714.14786
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Center TCR. Taiwan Cancer Registry Center. 2022. https://twcr.tw/?page_id=1843&lang=en.
- 3. Moyer VA. Force* UPST. Screening for lung cancer: US preventive services task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. [DOI] [PubMed] [Google Scholar]
- 4. Gao W, Wen CP, Wu A, Welch HG. Association of computed tomographic screening promotion with lung cancer overdiagnosis among Asian women. JAMA Intern Med. 2022;182:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nyman J, Hallqvist A, Lund J‐Å, Brustugun O‐T, Bergman B, Bergström P, et al. Space–a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. [DOI] [PubMed] [Google Scholar]
- 6. Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non‐small‐cell lung cancer (TROG 09.02 CHISEL): a phase 3, open‐label, randomised controlled trial. Lancet Oncol. 2019;20(4):494–503. [DOI] [PubMed] [Google Scholar]
- 7. Zhou F, Zhou C. Lung cancer in never smokers—the east Asian experience. Transl Lung Cancer Res. 2018;7(4):450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X‐N, Yan H‐H, Wang J, Chu X‐Y, Liu Z‐D, Shen Y, et al. Real‐world survival outcomes based on EGFR mutation status in Chinese patients with lung adenocarcinoma after complete resection: results from the ICAN study. J Thorac Oncol. 2022;3(1):100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takamochi K, Oh S, Matsunaga T, Suzuki K. Prognostic impacts of EGFR mutation status and subtype in patients with surgically resected lung adenocarcinoma. J Thorac Cardiovasc Surg. 2017;154(5):1768–74. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura M, Nishikawa R, Mayahara H, Uezono H, Harada A, Hashimoto N, et al. Pattern of recurrence after CyberKnife stereotactic body radiotherapy for peripheral early non‐small cell lung cancer. J Thorac Dis. 2019;11(1):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cassidy RJ, Zhang X, Patel PR, Shelton JW, Escott CE, Sica GL, et al. Next‐generation sequencing and clinical outcomes of patients with lung adenocarcinoma treated with stereotactic body radiotherapy. Cancer. 2017;123(19):3681–90. [DOI] [PubMed] [Google Scholar]
- 12. Lee I‐H, Chen G‐Y, Chien C‐R, Cheng JC‐H, Chen JL‐Y, Yang W‐C, et al. A retrospective study of clinicopathologic and molecular features of inoperable early‐stage non‐small cell lung cancer treated with stereotactic ablative radiotherapy. J Formos Med Assoc. 2021;120(12):2176–85. [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 14. Brooks ED, Sun B, Zhao L, Komaki R, Liao Z, Jeter M, et al. Stereotactic ablative radiation therapy is highly safe and effective for elderly patients with early‐stage non‐small cell lung cancer. International journal of radiation oncology* biology*. Phys Ther. 2017;98(4):900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mihai A, Santos A, Armstrong J, Kennedy A, Hickey D, McDermott R, et al. Outcomes of elderly and very elderly patients treated by dose‐adapted SABR for early stage non‐small cell lung cancer or lung Oligometastatic disease. Int J Radiat Oncol Biol Phys. 2017;99(2):E481. [Google Scholar]
- 16. Holmes OE, MacRae R, Cook G, Cross P, Nair V, Marginean H, et al. Age‐not Charlson Co‐morbidity index‐predicts for mortality after stereotactic ablative radiotherapy for medically inoperable stage I non‐small cell lung cancer. Clin Transl Radiat Oncol. 2017;5:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non–small‐cell lung cancer: a population‐based time‐trend analysis. J Clin Oncol. 2010;28(35):5153–9. [DOI] [PubMed] [Google Scholar]
- 18. Ministry of Interior RoCT . https://www.moi.gov.tw/cl.aspx?n=3087.
- 19. Lagerwaard FJ, Verstegen NE, Haasbeek CJ, Slotman BJ, Paul MA, Smit EF, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non‐small cell lung cancer. International journal of radiation oncology* biology*. Phys Ther. 2012;83(1):348–53. [DOI] [PubMed] [Google Scholar]
- 20. Chang JY, Mehran RJ, Feng L, Verma V, Liao Z, Welsh JW, et al. Stereotactic ablative radiotherapy for operable stage I non‐small‐cell lung cancer (revised STARS): long‐term results of a single‐arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021;22(10):1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tu C‐Y, Hsia T‐C, Fang H‐Y, Liang J‐A, Yang S‐T, Li C‐C, et al. A population‐based study of the effectiveness of stereotactic ablative radiotherapy versus conventional fractionated radiotherapy for clinical stage I non‐small cell lung cancer patients. Radiol Oncol. 2018;52(2):181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Y‐L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR‐mutated non–small‐cell lung cancer. N Engl J Med. 2020;383(18):1711–23. [DOI] [PubMed] [Google Scholar]
- 23. Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high‐dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101(7):1623–31. [DOI] [PubMed] [Google Scholar]


