Objective
Assess the accuracy and precision of the Aktiia initialization oscillometric upper-arm cuff device (Aktiia SA, Neuchâtel, Switzerland) for home blood pressure (BP) monitoring in the general population according to the American National Standards Institute / Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2013 standard.
Methods
Three trained observers validated BP measurements performed using the Aktiia cuff versus BP measurements performed using a standard mercury sphygmomanometer. Two ISO 81060-2 criteria were used to validate the Aktiia cuff. Criterion 1 evaluated, for both SBP and DBP, whether the mean error between BP readings performed by the Aktiia cuff and auscultation was ≤±5 mmHg, and whether the SD of the error was ≤8 mmHg. Criterion 2 assessed whether, for the SBP and DBP of each individual subject, the SD of the averaged paired determinations per subject of the Aktiia cuff and of the auscultation met the criteria listed in the table of Averaged Subject Data Acceptance.
Results
Mean differences between the Aktiia cuff and the standard mercury sphygmomanometer (criterion 1) were 1.3 ± 7.11 mmHg for SBP and −0.2 ± 5.46 mmHg for DBP. The SD of the averaged paired differences per subject (criterion 2) was 6.55 mmHg for SBP and 5.15 mmHg for DBP.
Conclusion
Aktiia initialization cuff complies with the requirements of the ANSI/AAMI/ISO guidelines and can be safely recommended for BP measurements in the adult population.
Keywords: blood pressure, calibration, clinical investigation, cuffless blood pressure monitor, initialization, optical signals, validation
Introduction
Hypertension is a major cause of preventable death in the world [1–3]. Blood pressure (BP) measurement is recommended for diagnostics and long-term hypertension management [1–3]. Accordingly, the validation of BP measurement devices is critical to support accurate hypertension diagnostic and medication titration [4,5]. Currently, many BP devices are commercialized prior to validation, which hinders adequate hypertension management and consumers’ access to validated BP devices [6,7].
Aktiia SA (Neuchâtel, Switzerland) has developed a Conformité Européenne-marked, non-invasive, cuffless BP monitor (Fig. 1). The Aktiia monitor requires a once-a-month initialization procedure that recalibrates measurements using BP readings performed by the Aktiia cuff (Fig. 1a). Although the Aktiia monitor has been thoroughly investigated in previous studies [8–13] and the Aktiia cuff has been fully validated according to the International Organization for Standardization (ISO) 81060-2 protocol, the publication of the results for the Aktiia cuff validation is still pending [5].
Fig. 1.
Illustration of the Aktiia monitor with (a) the Aktiia cuff, (b) the Aktiia bracelet and (c) the associated smartphone application to visualize SBP and DBP.
In the present work, we sought to validate the Aktiia cuff for upper-arm BP measurements in accordance with the American National Standards Institute / Association for the Advancement of Medical Instrumentation / International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2013 protocol [5].
Methods
The Aktiia cuff
The Aktiia cuff is manufactured by Guangdong Transtek Medical Electronics Co., Ltd (Zhongshan, China). Transtek was responsible for data collection and protocol implementation during the study. The Aktiia cuff is designed for BP measurements at the upper arm. It is provided with a USB cable to recharge its internal battery and is equipped with Bluetooth technology that supports communication with the Aktiia smartphone application. The Aktiia cuff performs BP measurements in the range of 0–299 mmHg, and heart rate (HR) within 40–199 bpm. It is a one-size-fits-all cuff, which can be adjusted around the user’s arm (arm circumference between 22 and 42 cm).
Study population
The Aktiia cuff was tested on 99 subjects following the ANSI/AAMI/ISO protocol, 2013. All subjects were volunteers recruited at Zhongshan’s City People Hospital (Zhongshan, China). Subjects with any two reference SBP values that differ by more than 12 mmHg or any two reference DBP values that differ by more than 8 mmHg were excluded from the study (14 subjects excluded). The demographic and clinical characteristics of the study population included in this study (85 subjects) are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study population (N = 85)
| Age, years | 43.2 ± 17.2 [18–77] |
| Prevalence of | |
| The elderly (>60 years) | 36% |
| Obesity (BMI ≥ 30 kg/m2) | 48% |
| Male/female | 38/47 [45%/55%] |
| Arm circumference, cm | 32.8 ± 5.4 [22–42] |
| Arm circumference, cuff specified range | |
| Lower half | 41.2% |
| Upper half | 58.8% |
| Lower quarter | 22.4% |
| Upper quarter | 22.4% |
| Lower octal | 5.9% |
| Upper octal | 9.4% |
| SBP, mmHg | 126.6 ± 16.0 [84–162] |
| ≤100 | 5.9% |
| ≥140 | 20.0% |
| ≥160 | 5.5% |
| DBP, mmHg | 79.2 ± 12.3 [53–120] |
| ≤60 | 6.7% |
| ≥85 | 29.0% |
| ≥100 | 5.1% |
Numbers represented as [range].
This study was approved by the Zhongshan People’s Hospital Ethics Committee with written informed consent obtained from each subject.
Study protocol
Study subjects were seated in a quiet room at a comfortable temperature and instructed not to move or speak during the procedure. Subjects were requested to place their feet flat on the floor. The arm circumference of each subject was measured before the start of the procedure. BP measurements started at least 5 min after the participants were seated. All BP measurements were performed on the participants’ left arm at the heart level. Accordingly, the Aktiia cuff was placed on the subjects’ left arm at heart level, and the double stethoscope was placed on the inner part of the elbow joint for recording Korotkoff sounds.
Blood pressure measurements validation
BP measurements were conducted by three observers experienced in BP measurement. BP measurements were conducted following the same-limb sequential procedure described in the ISO 81060-2:2013 protocol [5], with alternating measurements performed with the Aktiia cuff and a mercury sphygmomanometer. Simultaneous auscultatory measurements were performed by two observers blinded from each other using a double stethoscope (T piece) via the Korotkoff method. The third observer recorded the device readings and supervised the BP readings made by the first two observers.
Data analysis
Data analyses were performed as delineated in the ANSI/AAMI/ISO protocol. Auscultation BP measurements were computed as the average between the readings performed by the two observers before and after each device reading. The Aktiia cuff performed automated BP readings by oscillometry. The two criteria for ISO 81060-2 validation were investigated [5].
Statistical analysis
The results of the comparisons are expressed as mean ± SD .
Bland–Altman plots were created to highlight individual-level measurements. Additionally, regions of interest (ROIs) within ±5 mmHg, ±10 mmHg and ±15 mmHg were included to assess levels of agreement [14].
Additionally, results extending the ISO 81060-2 requirements are shown in the Supplementary materials, Supplemental digital content 1, http://links.lww.com/BPMJ/A186.
Results
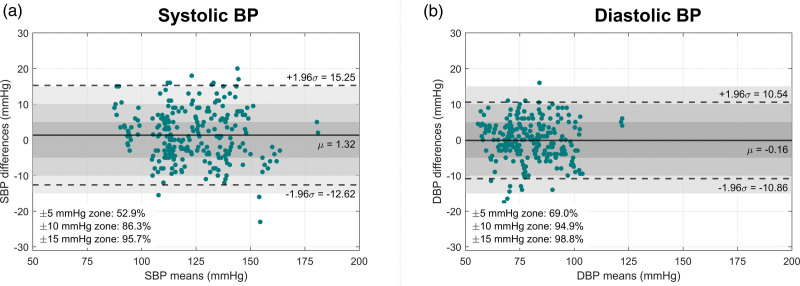
Table 2 and Fig. 2 summarize the results for criteria 1 and 2 of ISO 81060-2. For criterion 1, the bias and SD found between Aktiia cuff and reference double-auscultation for SBP were 1.3 ± 7.11. For DBP, the bias and SD were −0.2 ± 5.46. For criterion 2, the SD of the averaged paired differences per subject was 6.55 mmHg for SBP and 5.15 mmHg for DBP – also within the limits delineated by ISO 81060-2.
Table 2.
Mean and SD of the differences between Aktiia cuff and reference
| Criterion 1 | Criterion 2 | ||||||
|---|---|---|---|---|---|---|---|
| Mean error | ISO target | SD | ISO target | SD of averaged differences per subject | ISO target | Result | |
| SBP (mmHg) | 1.3 | ≤±5.0 | 7.11 | ≤8.00 | 6.55 | ≤6.82 | Pass |
| DBP (mmHg) | −0.2 | ≤±5.0 | 5.46 | ≤8.00 | 5.15 | ≤6.95 | Pass |
Fig. 2.
Bland–Altman plots comparing (a) SBP and (b) DBP measurements performed by the Aktiia cuff and the reference double-auscultation. ROIs within ±5 mmHg, ±10 mmHg and ±15 mmHg are highlighted in dark grey and light grey, respectively. The percentage of agreement for each region is shown on the bottom left of the Bland–Altman plots. The solid black line denotes the average mean of differences, while the dotted lines denote the limits of agreement (±1.96σ). ROIs, regions of interest.
Additionally, the two modalities resulted in SBP agreements of 53%, 86% and 96% within the ±5 mmHg, ±10 mmHg and the ±15 mmHg ROIs, respectively (Fig. 2). Similarly, the two modalities resulted in DBP agreements of 69%, 95% and 99% within the ±5/±10/±15 mmHg ROIs, respectively.
Discussion
In the present study, we investigate the accuracy of the Aktiia initialization oscillometric upper-arm cuff device for home BP monitoring in the general population according to the ANSI/AAMI/ISO 81060-2:2013 standard.
Our study demonstrates that the accuracy of SBP and DBP values measured by the Aktiia cuff satisfying the criteria defined by ISO 81060-2:2013 [5]. The tested oscillometric device fulfils the same requirements as any validated upper-arm device available on the market. Consequently, the Aktiia cuff is validated according to international standards and is thus recommended for use in clinical practice.
Cuffless blood pressure monitors
The Aktiia cuff will be integrated into the Aktiia System and will be used to initialize Aktiia’s cuffless BP monitor, allowing users to perform on-demand readings. The Aktiia cuffless monitor comprises a small bracelet (Fig. 1b) that contains optical sensors to collect photoplethysmography signals acquired on the user’s wrist. Pulse wave analysis is applied to the photoplethysmography signals to estimate SBP, DBP and HR, which are displayed in a smartphone application (Fig. 1c). This over-the-counter fully automated device is ideal for long-term continual BP monitoring in daily conditions.
Cuffless BP devices offer a new perspective and strategy for continual BP monitoring. The validation of the Aktiia cuff in the sitting position is a first step towards this paradigm change in the management of hypertension.
Limitations
The present validation was performed in early 2019 following the recommendations of the ISO 81060-2:2013 standard that was applicable then, and its data supported the immediate commercialization of the Aktiia System across Europe. In November of the same year, a new version of the ISO 81060-2 was released (EN ISO 81060-2:2019). The Aktiia cuff also complies with the procedures described by this new version of the standard, except for the lower and upper octal arm circumferences found in the present cohort (Table 1). The presented results, however, support that the Aktiia Initialization cuff can be safely recommended for BP measurements in the adult population.
The Aktiia cuff is produced and tested by Guangdong Transtek Medical Electronics, which might lead to potential conflicts of interest. We believe, however, that the results shown in the present work are representative since Transtek implemented all methods in accordance with the guidelines described in the ISO 81060-2 standard. Additionally, all data collected during the validation study have been recorded and stored and can be accessed upon request for further verification.
Conclusion
In the present work, we validated the Aktiia cuff for upper-arm BP measurements following the ANSI/AAMI/ISO 81060-2:2013 protocol. Our results show that the Aktiia cuff complies with the requirements of the ANSI/AAMI/ISO guidelines and can be safely recommended for BP measurements in the adult population. This is of paramount importance for an accurate once-a-month initialization procedure that recalibrates measurements using the Aktiia cuffless monitor. Therefore, the Aktiia monitor can be effectively used for non-invasive, self-triggered, intermittent SBP and DBP monitoring in adult patients when the user is sitting and the device is calibrated.
Acknowledgements
We thank Abrie Coertze for the support in preparing the figures. The present study was conducted with funding from Aktiia SA.
Conflicts of interest
The Aktiia cuff is produced (and tested) by Guangdong Transtek Medical Electronics. B.S.A. serves as a consultant for Aktiia SA. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.bpmonitoring.com.
Bruce S. Alpert retired.
References
- 1.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension 2020; 75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al.; Authors/Task Force Members:. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 4.Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. J Hypertens 2018; 36:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association for the Advancement of Medical Instrumentation. American National Standards Institute. International Organization for Standardization. AAMI/ANSI/ISO 81060-2:2013, non-invasive sphygmomanometers - part 2: clinical investigation of automated measurement type Arlington, VA, USA. AAMI; 2013. [Google Scholar]
- 6.Picone DS, Deshpande RA, Schultz MG, Fonseca R, Campbell NRC, Delles C, et al. Nonvalidated home blood pressure devices dominate the online marketplace in australia: major implications for cardiovascular risk management. Hypertension 2020; 75:1593–1599. [DOI] [PubMed] [Google Scholar]
- 7.Picone DS, Padwal R, Campbell NRC, Boutouyrie P, Brady TM, Olsen MH, et al. How to check whether a blood pressure monitor has been properly validated for accuracy. J Clin Hypertens 2020; 22:2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellaton C, Vybornova A, Fallet S, Marques L, Grossenbacher O, De Marco B, et al. Accuracy testing of a new optical device for noninvasive estimation of systolic and diastolic blood pressure compared to intra-arterial measurements. Blood Press Monit 2020; 25:105–109. [DOI] [PubMed] [Google Scholar]
- 9.Sola J, Bertschi M, Krauss J. Measuring pressure: introducing oBPM, the optical revolution for blood pressure monitoring. IEEE Pulse 2018; 9:31–33. [DOI] [PubMed] [Google Scholar]
- 10.Sola J, Cortes M, Perruchoud D, De Marco B, Lobo MD, Pellaton C, et al. Guidance for the interpretation of continual cuffless blood pressure data for the diagnosis and management of hypertension. Front Med Technol 2022; 4:899143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sola J, Vybornova A, Fallet S, Polychronopoulou E, Wurzner-Ghajarzadeh A, Wuerzner G. Validation of the optical Aktiia bracelet in different body positions for the persistent monitoring of blood pressure. Sci Rep 2021; 11:20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vybornova A, Polychronopoulou E, Wurzner-Ghajarzadeh A, Fallet S, Sola J, Wuerzner G. Blood pressure from the optical Aktiia bracelet: a 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press Monit 2021; 26:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuerzner G, Vybornova A, Wurzner-Ghajarzadeh A, Polychronopoulou E, Fallet S, Sola J, et al. Is auscultation an issue when validating 24-h blood pressure monitoring devices? Blood Press Monit 2020; 25:301–302. [DOI] [PubMed] [Google Scholar]
- 14.Manual, electronic, or automated sphygmomanometers. Association for the Advancement of Medical Instrumentation; 2003. ISBN 1–57020–183–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.