Abstract
Aims: The Academic Research Consortium (ARC) has proposed international criteria to standardize the definition of high bleeding risk (HBR) in patients undergoing percutaneous coronary intervention (PCI). In this context, Japan has also established its own guidelines, that is, the Japanese version of HBR (J-HBR) criteria. However, the J-HBR criteria have not been fully validated, especially in patients with acute myocardial infarction (MI).
Methods: This bi-center registry included 1079 patients with acute MI undergoing primary PCI in a contemporary setting. Patient bleeding risks were evaluated using the ARC-HBR and J-HBR criteria. The primary endpoint was rates of major bleeding events (Bleeding Academic Research Consortium type 3 or 5) at 1 year.
Results: Of the 1079 patients, 505 (46.8%) and 563 (52.2%) met the ARC-HBR and J-HBR criteria, respectively. Patients who met the J-HBR criteria were found to have a higher rate of major bleeding events at 1 year than those who did not (12.8% vs. 3.3%,p<0.001). When patients were scored and stratified using the J-HBR major and minor criteria, risks of major bleedings were progressively increased with the increase in the number of J-HBR criteria. In the receiver operating characteristic curve analysis, the ARC-HBR and J-HBR significantly predicted subsequent major bleedings after PCI, with ARC-HBR having greater predictive ability than J-HBR.
Conclusions: More than half of the patients with acute MI undergoing primary PCI in Japan met the J-HBR criteria. Although the J-HBR criteria successfully identified patients who were likely to develop major bleeding events after primary PCI, the superiority of J-HBR to ARC-HBR in predicting bleeding outcomes warrants further investigation.
Keywords: High bleeding risk, Acute myocardial infarction, Percutaneous coronary intervention
Introduction
Owing to advances in medical therapy and early reperfusion strategies, especially with primary percutaneous coronary intervention (PCI), the prognosis of acute myocardial infarction (MI) has significantly improved during the past decades 1) . While rates of ischemic events including recurrent MI and stent thrombosis have declined, a risk of major bleeding has been increased in patients with acute MI undergoing PCI 2 , 3) . The international criteria by the Academic Research Consortium for high bleeding risk (ARC-HBR) have been proposed to define HBR patients undergoing PCI 4) . However, East Asian patients including Japanese reportedly have different risk profiles for bleeding events as compared with those in Western countries 5) , which probably prevents the direct application of ARC-HBR to Japanese populations. In this context, the recent guidelines by the Japanese Circulation Society proposed the Japanese version of the HBR (J-HBR) criteria, in which Japanese-specific factors associated with HBR such as low body weight, frailty, heart failure, and peripheral artery disease were added to the original ARC-HBR criteria 6) . The ARC-HBR has been well validated in several previous studies 7 - 10) , but the applicability of J-HBR has been tested in only one large-scale all-comers PCI registry in Japan 11) . In addition, a recent report indicated that the performance of ARC-HBR to discriminate bleeding risks was lower in patients with acute coronary syndrome than those with chronic coronary syndrome 12) . In this present study, we aimed to examine the validity of the J-HBR, the domestically modified ARC-HBR, in Japanese patients with acute MI undergoing primary PCI.
Methods
Study Design and Population
This was a retrospective, bi-center, observational study at two tertiary referral hospitals, namely, Chiba University Hospital and Eastern Chiba Medical Center. Between January 2012 and March 2020, 1128 patients with acute MI underwent primary PCI. Study details were described in previous reports 13 - 16) . Briefly, acute MI, including both ST-segment elevation and non-ST-segment elevation MI, was defined based on the fourth universal definition of MI 17) . All PCI procedures were done according to local standard practice and guideline recommendations, including dual antiplatelet therapy, intracoronary imaging, and contemporary drug-eluting stents 1 , 6 , 18 - 21) . Duplicated patients (n=26) and those with missing information for calculating the ARC-HBR and J-HBR (n=23) were excluded. Thus, in total, 1079 patients with acute MI undergoing primary PCI were included in this present study. Informed consent was obtained in the form of opt-out. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical committee of Chiba University Hospital and Eastern Chiba Medical Center.
Definitions of ARC-HBR and J-HBR
Supplementary Table 1 lists the definitions of ARC-HBR and J-HBR criteria. The ARC-HBR and J-HBR have major and minor criteria, and a patient is defined as having HBR when one major criterion or two minor criteria were met 4 , 6) . Patients with HBR were estimated to have a risk of major bleeding defined as Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding of ≥ 4% or intracranial hemorrhage of ≥ 1% at 1 year after PCI 4 , 22) . The J-HBR included low body weight, frailty, heart failure, and peripheral artery disease as major criteria, in addition to the original ARC-HBR. In this present study, some ARC-HBR and J-HBR criteria were modified, as shown in Supplementary Table 1 11 , 23) . Scores were calculated by allocating one point for each major criterion and 0.5 points for each minor criterion (i.e., HBR was defined as ≥ 1 point) 23) . Patients were divided into two groups according to the presence or absence of J-HBR.
Supplementary Table 1. High bleeding risk definitions.
| J-HBR criteria | Criteria in the present study | Category | Comments |
|---|---|---|---|
| Age ≥ 75 years | Age ≥ 75 years | Minor | Identical |
| Anticipated use of long-term OAC | OAC use at discharge | Major | Modified |
| Severe CKD (eGFR <30 ml/min) | eGFR <30 ml/min | Major | Identical |
| Moderate CKD (eGFR 30-59 ml/min) | eGFR 30-<60 ml/min | Minor | Identical |
| Hemoglobin <11 g/dl | Hemoglobin <11 g/dl | Major | Identical |
| Hemoglobin 11-12.9 g/dl for men | Hemoglobin 11-12.9 g/dl for men | Minor | Identical |
| Hemoglobin 11-11.9 g/dl for women | Hemoglobin 11-11.9 g/dl for women | ||
| Bleedings requiring hospitalization or transfusion in the past 6 months | N/A | Major | N/A |
| Bleedings requiring hospitalization or transfusion in the past 6 months not meeting the major criterion | Prior gastrointestinal bleeding at any time | Minor | Modified |
| Thrombocytopenia (Plt <100×109/l) | Thrombocytopenia (Plt <100×109/l) | Major | Identical |
| Chronic bleeding diathesis | N/A | Major | N/A |
| LC with portal hypertension | LC | Major | Modified |
| Long-term use of NSAIDs or steroids | NSAIDs or steroids use at discharge | Minor | Modified |
| Active malignancy (excluding non-melanoma skin cancer) within the past 12 months | Active malignancy at baseline | Major | Modified |
| Previous spontaneous intracranial hemorrhage (at any time); Traumatic intracranial hemorrhage within the past 12 months; Brain arteriovenous malformation; Moderate or severe ischemic stroke within the past 6 months | History of intracranial hemorrhage at any time | Major | Modified |
| Any ischemic stroke at any time not meeting the major criterion | History of ischemic stroke without intracranial hemorrhage at any time | Minor | Identical |
| Non-deferrable major surgery on DAPT | N/A | Major | N/A |
| Recent major surgery or trauma within 30 days before PCI | N/A | Major | N/A |
|
Low body weight (<55 kg for men and <50 kg for women) or frailty |
Low body weight (<55 kg for men and <50 kg for women) |
Major | Modified |
| Peripheral vascular disease | Peripheral artery disease | Major | Modified |
| Heart failure | Heart failure | Major | Identical |
CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; J-HBR, Japanese version of the high bleeding risk; LC, liver cirrhosis; NSAIDs, non-steroidal anti-inflammatory drugs; N/A, not applicable; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; Plt, platelet count.
Endpoint and Statistical Analysis
Follow-up data were obtained from medical records at Chiba University Hospital and Eastern Chiba Medical Center. The primary endpoint of this present study was the rates of major bleeding (BARC type 3 or 5) events at 1 year 22) . Major bleedings were further divided into gastrointestinal, intracranial, access site-related, and other bleeding events. The prevalence of J-HBR, impact of J-HBR and each component on bleeding outcomes, and diagnostic ability of J-HBR as compared with ARC-HBR were evaluated.
Statistical analysis was performed using JMP Pro 15.0.0 (SAS Institute, Cary, USA). All data are expressed as mean±standard deviation or frequency (%). Continuous variables were compared with Student’s t-test, while categorical variables were assessed using Fisher’s exact test. The Kaplan–Meier analysis was used to calculate the time to major bleeding events and to estimate major bleeding event rates at 1 year, and the log-rank test was applied for between-group comparisons. The receiver operating characteristics (ROC) curve analysis was performed based on bleeding events. The area under the curve (AUC) of the ROC curve was compared using the Delong method. A p-value <0.05 was considered statistically significant.
Results
Of the 1079 patients with acute MI undergoing primary PCI, 505 (46.8%) and 563 (52.2%) met the ARC-HBR and J-HBR, respectively. Table 1 lists the baseline characteristics. Patients who met the J-HBR were older and were likely to have more cardiovascular risk factors and comorbidities; however, they were less likely to receive medications for secondary prevention such as angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, β-blocker, and statin ( Table 1 ) . In terms of proton pump inhibitor, the prescription rate was not significantly different between the two groups in patients who recovered and were discharged ( Supplementary Table 2 ) . Fig.1 displays the prevalence of criteria included in the J-HBR. The common major criteria were low body weight, oral anticoagulation, and severe anemia ( Fig.1 ) . In-hospital mortality was noted to be significantly higher in patients with J-HBR than those without (11.6% vs. 4.7%, p<0.001). During the median follow-up period of 418 days, 91 (8.4%) patients experienced major bleeding events, in which gastrointestinal bleedings were most frequently observed ( Table 2 ) . Fig.2 shows that patients with J-HBR had a significantly increased risk of major bleedings than those without. The cumulative incidence of major bleeding events at 1 year was stratified by the J-HBR criteria in Fig.3 , in which oral anticoagulation, severe anemia, moderate and severe chronic kidney disease (CKD), active malignancy, thrombocytopenia, and prior ischemic stroke were identified as significant factors associated with major bleedings. When patients were scored and stratified by J-HBR major and minor criteria, risks of major bleeding events were progressively increased with the increase in the number of J-HBR criteria ( Fig.4 ) . In the ROC curve analysis, the ARC-HBR (AUC 0.73, p<0.001) and J-HBR (AUC 0.71, p<0.001) were able to significantly predict subsequent major bleedings after PCI, with ARC-HBR having greater diagnostic ability than J-HBR (p=0.004). Supplementary Table 3 shows sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of ARC-HBR and J-HBR for predicting major bleeding events.
Table 1. Baseline characteristics.
| Variable | J-HBR (n= 563) | No J-HBR (n= 516) | p value |
|---|---|---|---|
| Age (years) | 72.9±10.8 | 61.3±10.5 | <0.001 |
| Men | 376 (66.8%) | 451 (87.4%) | <0.001 |
| Body mass index (kg/m2) | 22.9±3.6 | 25.6±3.3 | <0.001 |
| Hypertension | 404 (71.8%) | 328 (63.7%) | 0.005 |
| Diabetes | 208 (36.9%) | 198 (38.4%) | 0.63 |
| Dyslipidemia | 310 (55.1%) | 359 (69.7%) | <0.001 |
| Current smoker | 104 (18.5%) | 255 (49.5%) | <0.001 |
| Prior myocardial infarction | 45 (8.0%) | 27 (5.2%) | 0.07 |
| Prior heart failure | 29 (5.2%) | 0 (0%) | <0.001 |
| Atrial fibrillation | 67 (11.9%) | 4 (0.8%) | <0.001 |
| Peripheral artery disease | 29 (5.2%) | 0 (0%) | <0.001 |
| Hemodialysis | 39 (6.9%) | 0 (0%) | <0.001 |
| eGFR (ml/min/1.73 m2) | 53.4±24.3 | 74.2±19.4 | <0.001 |
| Hemoglobin (g/dl) | 12.6±2.2 | 14.9±1.5 | <0.001 |
| Platelet (104/μl) | 21.4±8.5 | 23.2±6.4 | <0.001 |
| LVEF (%) | 45.5±13.8 | 49.0±12.5 | <0.001 |
| Active malignancy | 59 (10.5%) | 0 (0%) | <0.001 |
| Prior intracranial hemorrhage | 14 (2.5%) | 0 (0%) | <0.001 |
| Prior ischemic stroke | 70 (12.4%) | 10 (1.9%) | <0.001 |
| Prior GI bleeding | 19 (3.4%) | 5 (1.0%) | 0.007 |
| Liver cirrhosis | 9 (1.6%) | 0 (0%) | 0.004 |
| Clinical presentation | 0.76 | ||
| STEMI | 378 (67.1%) | 351 (68.0%) | |
| NSTEMI | 185 (32.9%) | 165 (32.0%) | |
| Killip class on admission | <0.001 | ||
| I | 337 (59.9%) | 404 (78.3%) | |
| II | 64 (11.4%) | 29 (5.6%) | |
| III | 47 (8.3%) | 15 (2.9%) | |
| IV | 115 (20.4%) | 68 (13.2%) | |
| Mechanical circulatory support | 83 (14.7%) | 45 (8.7%) | 0.003 |
| IABP | 69 (12.3%) | 41 (7.9%) | 0.02 |
| ECMO | 31 (5.5%) | 24 (4.7%) | 0.58 |
| Impella | 3 (0.5%) | 0 (0%) | 0.25 |
| Arterial access site | <0.001 | ||
| Radial | 458 (81.3%) | 471 (91.3%) | |
| Brachial | 18 (3.2%) | 7 (1.4%) | |
| Femoral | 87 (15.5%) | 38 (7.4%) | |
| Medication at discharge | |||
| Antiplatelet therapy | |||
| Aspirin | 489 (86.9%) | 513 (99.4%) | <0.001 |
| Clopidogrel | 287 (51.0%) | 197 (38.2%) | <0.001 |
| Prasugrel | 221 (39.3%) | 307 (59.5%) | <0.001 |
| Oral anticoagulation | 124 (22.0%) | 0 (0%) | <0.001 |
| ACE-I/ARB | 405 (71.9%) | 441 (85.5%) | <0.001 |
| β-blocker | 356 (63.2%) | 400 (77.5%) | <0.001 |
| Statin | 462 (82.1%) | 475 (92.1%) | <0.001 |
| NSAIDs/steroids | 53 (9.4%) | 15 (2.9%) | <0.001 |
| PPI | 511 (90.8%) | 490 (95.0%) | 0.009 |
| H2-blocker | 17 (3.0%) | 7 (1.4%) | 0.10 |
Mechanical circulatory support includes intra-aortic balloon pump (IABP), extracorporeal membrane oxygenation (ECMO), and percutaneous transvalvular microaxial flow pump (Impella; Abiomed, Danvers, USA).
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; J-HBR, the Japanese version of the high bleeding risk; LVEF, left ventricular ejection fraction; NSAIDs, non-steroidal anti-inflammatory drugs; NSTEMI, non ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; PPI, proton pump inhibitor.
Supplementary Table 2. Medication at discharge in patients who survived to discharge.
| Variable | J-HBR (n= 498) | No J-HBR (n= 492) | p value |
|---|---|---|---|
| Antiplatelet therapy | |||
| Aspirin | 441 (88.6%) | 491 (99.8%) | <0.001 |
| Clopidogrel | 260 (52.2%) | 187 (38.0%) | <0.001 |
| Prasugrel | 199 (40.0%) | 295 (60.0%) | <0.001 |
| Oral anticoagulation | 112 (22.5%) | 0 (0%) | <0.001 |
| ACE-I/ARB | 391 (78.5%) | 434 (88.2%) | <0.001 |
| β-blocker | 336 (67.5%) | 389 (79.1%) | <0.001 |
| Statin | 435 (87.4%) | 464 (94.3%) | <0.001 |
| NSAIDs/steroids | 49 (9.8%) | 15 (3.1%) | <0.001 |
| PPI | 468 (94.0%) | 473 (96.1%) | 0.14 |
| H2-blocker | 14 (2.8%) | 7 (1.4%) | 0.18 |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; J-HBR, the Japanese version of the high bleeding risk; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor.
Fig.1. Prevalence of major and minor criteria in the J-HBR.
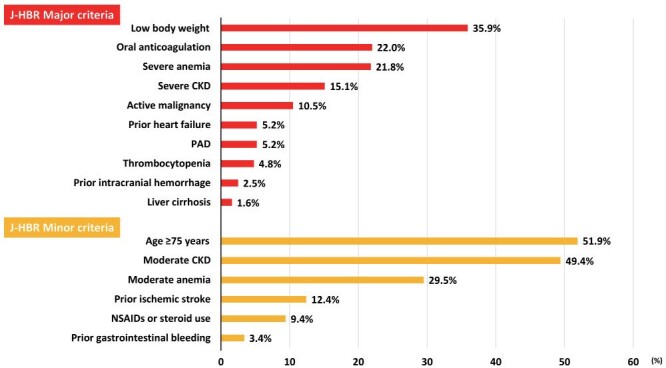
CKD, chronic kidney disease; J-HBR, the Japanese version of the high bleeding risk; PAD, peripheral artery disease.
Table 2. Major bleeding events.
| Variable | All (n= 1079) | J-HBR (n= 563) | No J-HBR (n= 516) | p value |
|---|---|---|---|---|
| Major bleeding events | 91 (8.4%) | 71 (12.6%) | 20 (3.9%) | <0.001 |
| BARC 3 | 83 (7.7%) | 64 (11.4%) | 19 (3.7%) | <0.001 |
| BARC 5 | 8 (0.7%) | 7 (1.2%) | 1 (0.2%) | 0.07 |
| Gastrointestinal bleeding | 30 (2.8%) | 26 (4.6%) | 4 (0.8%) | <0.001 |
| Access site-related bleeding | 27 (2.5%) | 19 (3.4%) | 8 (1.6%) | 0.08 |
| Intracranial bleeding | 11 (1.0%) | 10 (1.8%) | 1 (0.2%) | 0.01 |
| Others | 23 (2.1%) | 16 (2.8%) | 7 (1.4%) | 0.14 |
BARC, Bleeding Academic Research Consortium; J-HBR, Japanese version of the high bleeding risk.
Fig.2. Probability free from major bleeding events in patients with and without J-HBR.
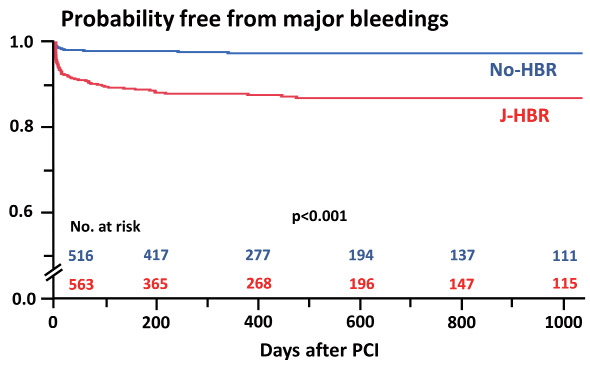
J-HBR, the Japanese version of the high bleeding risk; PCI, percutaneous coronary intervention.
Fig.3. Cumulative incidence of major bleedings at 1 year by each J-HBR criterion.
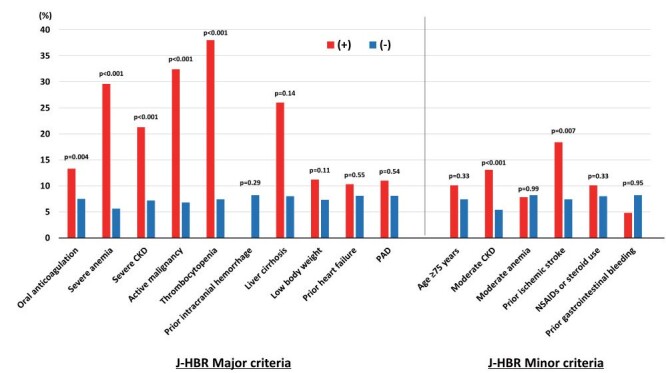
CKD, chronic kidney disease; J-HBR, the Japanese version of the high bleeding risk; PAD, peripheral artery disease.
Fig.4. Probability free from major bleeding events by J-HBR score categories.
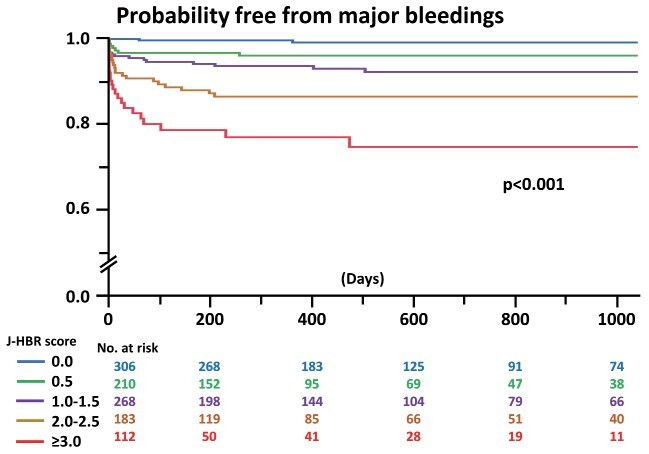
J-HBR, the Japanese version of the high bleeding risk; PCI, percutaneous coronary intervention.
Supplementary Table 3. Diagnostic ability of ARC-HBR and J-HBR for major bleeding events.
| Variable | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| ARC-HBR | 76.9% | 56.0% | 13.9% | 96.3% | 57.7% |
| J-HBR | 78.0% | 50.2% | 12.6% | 96.1% | 52.5% |
ARC-HBR, Academic Research Consortium for high bleeding risk; J-HBR, Japanese version of the high bleeding risk; NPV, negative predictive value; PPV, positive predictive value.
Discussion
This present study demonstrated the applicability of J-HBR, the domestically modified ARC-HBR, in patients with acute MI undergoing primary PCI in Japan. More than half of patients with acute MI were defined as having J-HBR, and those with J-HBR had a higher rate of major bleeding events at 1 year than those without. Although the J-HBR criteria were useful to identify patients at HBR after PCI for acute MI, whether the J-HBR criteria were clinically superior to the ARC-HBR criteria remains unclear.
ARC-HBR and J-HBR
Both ischemic and bleeding events have a similar prognostic impact on subsequent mortality after PCI 4) . However, the definitions of bleeding outcomes in previous clinical studies have varied widely 7) , and in 2019, the ARC proposed a set of HBR criteria to globally standardize the definitions 4) . In 2020, the J-HBR criteria were domestically proposed in the Japanese guidelines to better discriminate patient bleeding risks, because of different bleeding risk profiles between patients in Western countries and East Asia 5 , 11) . While the ARC-HBR has been well investigated inside and outside of Japan 7) , the J-HBR was not fully validated.
According to previous reports in Japan, the rate of HBR by the ARC criteria ranged from 40% to 50% 7) , which is in line with our results (i.e., 46.8%). In the CREDO-Kyoto Registry Cohort-3, which is the only previous large-scale study to evaluate the J-HBR criteria, patients having J-HBR were found in 64.1% 11) . Based on the definition that the J-HBR includes additional components to the ARC-HBR criteria (e.g., low body weight, frailty, heart failure, and peripheral artery disease), the number of patients with J-HBR is inevitably greater than those with ARC-HBR. While the J-HBR criteria were significantly associated with a risk of major bleeding events after PCI in the CREDO-Kyoto Registry Cohort-3, the prognostic impact of J-HBR in patients with acute MI remained unclear. In this present study, patients with J-HBR had a three- to four-fold higher risk of major bleedings at 1 year than those without (12.8% vs. 3.3%, p<0.001). Given that the original ARC-HBR was arbitrarily defined as a risk of major bleeding of ≥ 4% at 1 year after PCI 4) , it is deemed conceivable that the J-HBR criteria successfully identified patients at HBR. In addition, when the HBR criteria were considered as a scoring system with allocating one point for major criterion and 0.5 points for minor criterion, the J-HBR further stratified patient bleeding risks. Although the ROC curve analyses indicated that the predictivity of ARC-HBR was slightly better than that of J-HBR, the AUCs (i.e., 0.71–0.73) in this present study were numerically better than that in previous reports (i.e., 0.68–0.69) 7) . Taking into account that the sensitivity of J-HBR criteria was numerically higher than that of ARC-HBR criteria (78.0% vs. 76.9%), the J-HBR might be a better screening tool to identify HBR patients. Further investigations are needed to evaluate the diagnostic ability of ARC-HBR and J-HBR and whether individualized therapeutic strategies under J-HBR guidance are superior to no risk score guidance.
Impact of Component of J-HBR on Bleedings
In the J-HBR criteria, oral anticoagulation, severe anemia, moderate and severe CKD, active malignancy, thrombocytopenia, and prior ischemic stroke were associated with an increased risk of major bleeding events, all of which were included in the original ARC-HBR major or minor criteria. Although 35.9% of patients were found to be of low body weight (i.e., <55 kg for men and <50 kg for women) in this present study, this major criterion in the J-HBR was not significantly related to major bleedings after primary PCI. The prevalence of low body weight in this present study was higher as compared with the previous CREDO-Kyoto Registry Cohort-3 (i.e., 22.8%) 11) , suggesting that the prognostic impact of the HBR criteria may differ in different populations 12) . Interestingly, the PARIS bleeding risk score, a risk stratifying system derived from a large PCI cohort from Western countries, includes low body mass index as a criterion to predict bleeding outcomes 24) , while the Japanese CREDO-Kyoto bleeding risk score does not 25) . The prevalence of other Japanese-specific factors associated with HBR including heart failure and peripheral artery disease was low in this present study. Thus, larger sample size studies may elucidate the prognostic impact of these factors.
Oral anticoagulation is a significant factor in determining antithrombotic regimens in the guidelines and was shown to have an impact on bleeding events in this present study. Beyond oral anticoagulation, severe anemia and CKD, active malignancy, thrombocytopenia, and prior ischemic stroke were identified as significant factors associated major bleedings. Although gastrointestinal bleeds were the most common, accounting for more than 30%, prior gastrointestinal bleeding was not associated with an increased risk of major bleeding events. Given the high risks of having severe anemia and CKD, active malignancy, thrombocytopenia, and prior ischemic stroke for major bleedings after PCI for acute MI, patients with those factors should be followed up with a caution on bleeding events. In addition, thrombotic risks must be balanced against bleeding complications as the guidelines recommend 6) . Risk stratification using risk-predicting models such as the PARIS and CREDO-Kyoto scores may be deemed useful 15) .
Study Limitations
This study has several limitations. For one, this was a retrospective study with a moderate sample size. Because some J-HBR criteria were modified in this present study ( Supplementary Table 1 ) , HBR was underestimated in the present study as well as many previous studies validating the ARC-HBR criteria 7 , 11) . In particular, J-HBR has the frailty criterion in addition to low body weight, which was not included in the present analysis. Given that clinically assessed frailty was reportedly associated with an increased risk of bleeding outcomes in patients with acute MI 26 , 27) , the additional information on frailty may improve the diagnostic ability of J-HBR. All medical therapies were left to treating physicians due to the retrospective nature. Thus, therapeutic strategies including antithrombotic regimens may have affected the results. Furthermore, data on medications including antithrombotic therapy during follow-up period were not available.
Conclusions
More than half of the patients with acute MI undergoing PCI met the J-HBR. The J-HBR criteria have successfully identified patients at HBR after primary PCI.
Disclosures
None
References
- 1).Ozaki Y, Hara H, Onuma Y, Katagiri Y, Amano T, Kobayashi Y, Muramatsu T, Ishii H, Kozuma K, Tanaka N, Matsuo H, Uemura S, Kadota K, Hikichi Y, Tsujita K, Ako J, Nakagawa Y, Morino Y, Hamanaka I, Shiode N, Shite J, Honye J, Matsubara T, Kawai K, Igarashi Y, Okamura A, Ogawa T, Shibata Y, Tsuji T, Yajima J, Iwabuchi K, Komatsu N, Sugano T, Yamaki M, Yamada S, Hirase H, Miyashita Y, Yoshimachi F, Kobayashi M, Aoki J, Oda H, Katahira Y, Ueda K, Nishino M, Nakao K, Michishita I, Ueno T, Inohara T, Kohsaka S, Ismail TF, Serruys PW, Nakamura M, Yokoi H, Ikari Y; Task Force on Primary Percutaneous Coronary Intervention (PCI) of the Japanese Cardiovascular Interventional Therapeutics (CVIT). CVIT expert consensus document on primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) update 2022. Cardiovasc Interv Ther, 2022; 37: 1-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Simonsson M, Wallentin L, Alfredsson J, Erlinge D, Hellström Ängerud K, Hofmann R, Kellerth T, Lindhagen L, Ravn-Fischer A, Szummer K, Ueda P, Yndigegn T, Jernberg T. Temporal trends in bleeding events in acute myocardial infarction: insights from the SWEDEHEART registry. Eur Heart J, 2020; 41: 833-843 [DOI] [PubMed] [Google Scholar]
- 3).Takeji Y, Shiomi H, Morimoto T, Yoshikawa Y, Taniguchi R, Mutsumura-Nakano Y, Yamamoto K, Yamaji K, Tazaki J, Kato ET, Watanabe H, Yamamoto E, Yamashita Y, Fuki M, Suwa S, Inoko M, Takeda T, Shirotani M, Ehara N, Ishii K, Inada T, Tamura T, Onodera T, Shinoda E, Yamamoto T, Watanabe H, Yaku H, Nakatsuma K, Sakamoto H, Ando K, Soga Y, Furukawa Y, Sato Y, Nakagawa Y, Kadota K, Komiya T, Minatoya K, Kimura T; CREDO-Kyoto AMI Registry Wave-1 and the CREDO-Kyoto AMI Registry Wave-2 Investigators. Changes in demographics, clinical practices and long-term outcomes of patients with ST segment-elevation myocardial infarction who underwent coronary revascularisation in the past two decades: cohort study. BMJ Open, 2021; 11: e043683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J, 2019; 40: 2632-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, Taubert K, Smith SC Jr. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol, 2014; 11: 597-606 [DOI] [PubMed] [Google Scholar]
- 6).Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, Kosuge M, Shinke T, Nakagawa Y, Natsuaki M, Yasuda S, Akasaka T, Kohsaka S, Haze K, Hirayama A. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients With Coronary Artery Disease. Circ J, 2020; 84: 831-865 [DOI] [PubMed] [Google Scholar]
- 7).Saito Y, Kobayashi Y. Academic Research Consortium Definition of High Bleeding Risk in Clinical Practice - Validation and Beyond. Circ J, 2021; 85: 806-807 [DOI] [PubMed] [Google Scholar]
- 8).Silverio A, Di Maio M, Buccheri S, De Luca G, Esposito L, Sarno G, Vecchione C, Galasso G. Validation of the academic research consortium high bleeding risk criteria in patients undergoing percutaneous coronary intervention: A systematic review and meta-analysis of 10 studies and 67,862 patients. Int J Cardiol, 2022; 347: 8-15 [DOI] [PubMed] [Google Scholar]
- 9).Tsukizawa T, Fujihara M. Relationship between in-hospital event rates and high bleeding risk score in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Cardiovasc Interv Ther, 2021. doi:10.1007/s12928-021-00805-3 [DOI] [PubMed] [Google Scholar]
- 10).Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T; STOPDAPT-2 investigators. Details on the effect of very short dual antiplatelet therapy after drug-eluting stent implantation in patients with high bleeding risk: insight from the STOPDAPT-2 trial. Cardiovasc Interv Ther, 2021; 36: 91-103 [DOI] [PubMed] [Google Scholar]
- 11).Natsuaki M, Morimoto T, Shiomi H, Ehara N, Taniguchi R, Tamura T, Tada T, Suwa S, Kaneda K, Watanabe H, Tazaki J, Watanabe S, Yamamoto E, Saito N, Fuki M, Takeda T, Eizawa H, Shinoda E, Mabuchi H, Shirotani M, Uegaito T, Matsuda M, Takahashi M, Inoko M, Tamura T, Ishii K, Onodera T, Sakamoto H, Aoyama T, Sato Y, Ando K, Furukawa Y, Nakagawa Y, Kadota K, Kimura T; CREDO-Kyoto PCI/CABG Registry Cohort-3 Investigators. Application of the Modified High Bleeding Risk Criteria for Japanese Patients in an All-Comers Registry of Percutaneous Coronary Intervention - From the CREDO-Kyoto Registry Cohort-3. Circ J, 2021; 85: 769-781 [DOI] [PubMed] [Google Scholar]
- 12).Gragnano F, Spirito A, Corpataux N, Vaisnora L, Galea R, Gargiulo G, Siontis GCM, Praz F, Lanz J, Billinger M, Hunziker L, Stortecky S, Pilgrim T, Bär S, Ueki Y, Capodanno D, Urban P, Pocock SJ, Mehran R, Heg D, Windecker S, Räber L, Valgimigli M. Impact of clinical presentation on bleeding risk after percutaneous coronary intervention and implications for the ARC-HBR definition. EuroIntervention, 2021; 17: e898-e909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Sato T, Saito Y, Matsumoto T, Yamashita D, Saito K, Wakabayashi S, Kitahara H, Sano K, Kobayashi Y. Impact of CADILLAC and GRACE risk scores on short- and long-term clinical outcomes in patients with acute myocardial infarction. J Cardiol, 2021; 78: 201-205 [DOI] [PubMed] [Google Scholar]
- 14).Matsumoto T, Saito Y, Yamashita D, Sato T, Wakabayashi S, Kitahara H, Sano K, Kobayashi Y. Impact of Active and Historical Cancer on Short- and Long-Term Outcomes in Patients With Acute Myocardial Infarction. Am J Cardiol, 2021; 159: 59-64 [DOI] [PubMed] [Google Scholar]
- 15).Yamashita D, Saito Y, Sato T, Matsumoto T, Saito K, Wakabayashi S, Kitahara H, Sano K, Kobayashi Y. Impact of PARIS and CREDO-Kyoto Thrombotic and Bleeding Risk Scores on Clinical Outcomes in Patients With Acute Myocardial Infarction. Circ J, 2022; 86: 622-629 [DOI] [PubMed] [Google Scholar]
- 16).Sato T, Saito Y, Matsumoto T, Yamashita D, Saito K, Wakabayashi S, Kitahara H, Sano K, Kobayashi Y. In-hospital adverse events in low-risk patients with acute myocardial infarction - Potential implications for earlier discharge. J Cardiol, 2022; 79: 747-751 [DOI] [PubMed] [Google Scholar]
- 17).Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol, 2018; 72: 2231-2264 [DOI] [PubMed] [Google Scholar]
- 18).Saito Y, Kobayashi Y, Fujii K, Sonoda S, Tsujita K, Hibi K, Morino Y, Okura H, Ikari Y, Honye J. Clinical expert consensus document on intravascular ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics (2021). Cardiovasc Interv Ther, 2022; 37: 40-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Fujii K, Kubo T, Otake H, Nakazawa G, Sonoda S, Hibi K, Shinke T, Kobayashi Y, Ikari Y, Akasaka T. Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography. Cardiovasc Interv Ther, 2022; 37: 248-254 [DOI] [PubMed] [Google Scholar]
- 20).Saito Y, Kobayashi Y. Contemporary coronary drug-eluting and coated stents: a mini-review. Cardiovasc Interv Ther, 2021; 36: 20-22 [DOI] [PubMed] [Google Scholar]
- 21).Yamashita T, Sakamoto K, Tabata N, Ishii M, Sato R, Nagamatsu S, Motozato K, Yamanaga K, Sueta D, Araki S, Arima Y, Yamamoto E, Takashio S, Fujisue K, Fujimoto K, Shimomura H, Tsunoda R, Maruyama H, Nakamura N, Sakaino N, Nakamura S, Yamamoto N, Matsumura T, Kajiwara I, Tayama S, Sakamoto T, Nakao K, Oshima S, Kaikita K, Hokimoto S, Tsujita K; Kumamoto Intervention Conference Study (KICS) Investigators. Imaging-guided PCI for event suppression in Japanese acute coronary syndrome patients: community-based observational cohort registry. Cardiovasc Interv Ther, 2021; 36: 81-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation, 2011; 123: 2736-2747 [DOI] [PubMed] [Google Scholar]
- 23).Nakamura M, Kadota K, Nakao K, Nakagawa Y, Shite J, Yokoi H, Kozuma K, Tanabe K, Iijima R, Harada A, Kuroda T, Murakami Y. High bleeding risk and clinical outcomes in East Asian patients undergoing percutaneous coronary intervention: the PENDULUM registry. EuroIntervention, 2021; 16: 1154-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J Am Coll Cardiol, 2016; 67: 2224-2234 [DOI] [PubMed] [Google Scholar]
- 25).Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Akasaka T, Hanaoka KI, Kozuma K, Tanabe K, Morino Y, Muramatsu T, Kimura T; CREDO‐Kyoto PCI/CABG Registry Cohort 2, RESET, and NEXT trial investigators. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J Am Heart Assoc, 2018; 7: e008708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Nishihira K, Yoshioka G, Kuriyama N, Ogata K, Kimura T, Matsuura H, Furugen M, Koiwaya H, Watanabe N, Shibata Y. Impact of frailty on outcomes in elderly patients with acute myocardial infarction who undergo percutaneous coronary intervention. Eur Heart J Qual Care Clin Outcomes, 2021; 7: 189-197 [DOI] [PubMed] [Google Scholar]
- 27).Kurobe M, Uchida Y, Ishii H, Yamashita D, Yonekawa J, Satake A, Makino Y, Hiramatsu T, Mizutani K, Mizutani Y, Ichimiya H, Amano T, Watanabe J, Kanashiro M, Matsubara T, Ichimiya S, Murohara T. Impact of the clinical frailty scale on clinical outcomes and bleeding events in patients with ST-segment elevation myocardial infarction. Heart Vessels, 2021; 36: 799-808 [DOI] [PubMed] [Google Scholar]


