Abstract
The principal treatment modalities for esophageal cancer are radiation, chemotherapy and surgery or a combination of them. In some sense, technological advances have tremendously heightened patients’ survival rates. Nevertheless, the debate on the prognostic value of postoperative radiotherapy (PORT) has never ceased. On that account, this study made an effort to probe deep into the effects of PORT and surgery on the prognosis of stage III esophageal cancer. Our study included patients diagnosed with stage III esophageal cancer between 2004 and 2015 through the Surveillance, Epidemiology, and End Results (SEER) program. We performed propensity score matching (PSM) on the basis of whether surgery was carried out and whether PORT conducted. We identified the independent risk factors by multivariate Cox regression and constructed a nomogram model. In this research, we included 3940 patients, and the median follow-up is 14 months: 1932 cases without surgery; 2008 cases with surgery, and 322 cases of them underwent PORT. In the postPSM patient cohort, patients who underwent surgery had a median overall survival rate (OS) of 19.0 (95% confidence interval [CI] 17.2–20.8) and a median cancer-specific survival rate (CSS) of 23.0 (95% CI 20.6–25.3) months, which were remarkably higher than those without surgery (P < .001). The OS(P < .05)and CSS(P < .05)of the patients who underwent PORT were lower than those who did not. Similar results were obtained in the groups of N0 and N1. This study revealed surgery can heighten patients’ survival rate, while PORT could not elevate patients’ survival rate in stage III esophageal cancer patients.
Keywords: esophageal cancer, prognosis, radiotherapy, SEER program, surgery
1. Introduction
As the 10th most common cancer worldwide, 604,100 (3.1%) new esophageal cancer cases and 544,076 (5.5%) esophageal cancer deaths were reported in 2020.[1] The first choice method of treatment for patients with resectable stage III esophageal cancer is surgery. Nevertheless, survival rate for patients who underwent surgery alone was far from satisfactory.[2–4]{Smyth, 2017 #3} Symptoms of stenosis may not appear until the tumor reaches a relatively advanced or even locally metastatic stage.[3,5] Endoscopic resection is feasible in the early stage, chemotherapy can be considered in the advanced stage, and chemotherapy, radiochemotherapy, surgery and combined therapy are recommended in the middle. Surgery alone is less effective and prone to recurrence, therefore needs to be combined with a variety of other adjuvant treatments.[6–8] Postoperative radiotherapy (PORT) is one of the extensively used methods. PORT has been employed in the treatment of esophageal cancer since 1969.[9] Nonetheless, some studies have reported conflicting results regarding the role of PORT.10–12 Just as evidently indicated by the results of a randomized controlled trial, PORT elevated patients’ survival rate in stage III esophageal cancer compared with a control group (P = .0027).[10] In contrast, in another randomized controlled trial, esophageal cancer patients who underwent PORT had markedly shorter survival rate times than nonPORT patients (P = .02).[11] A meta-analysis on 3 randomized controlled trials and 7 retrospective studies persuasively illustrates that PORT can ameliorate overall survival rate (OS) (P = .0004) and disease-free survival rate (P = .004) in esophageal cancer compared with surgery alone.[12] These studies demonstrates that the role of PORT in esophageal cancer still remains controversial. As a consequence, it remains of interest to evaluate the prognostic value of surgery and PORT. By comparing the role of surgery and PORT, providing clear evidence for the clinical decision-making of clinicians was our primary aim in conducting this study.
2. Methods
2.1. Study population
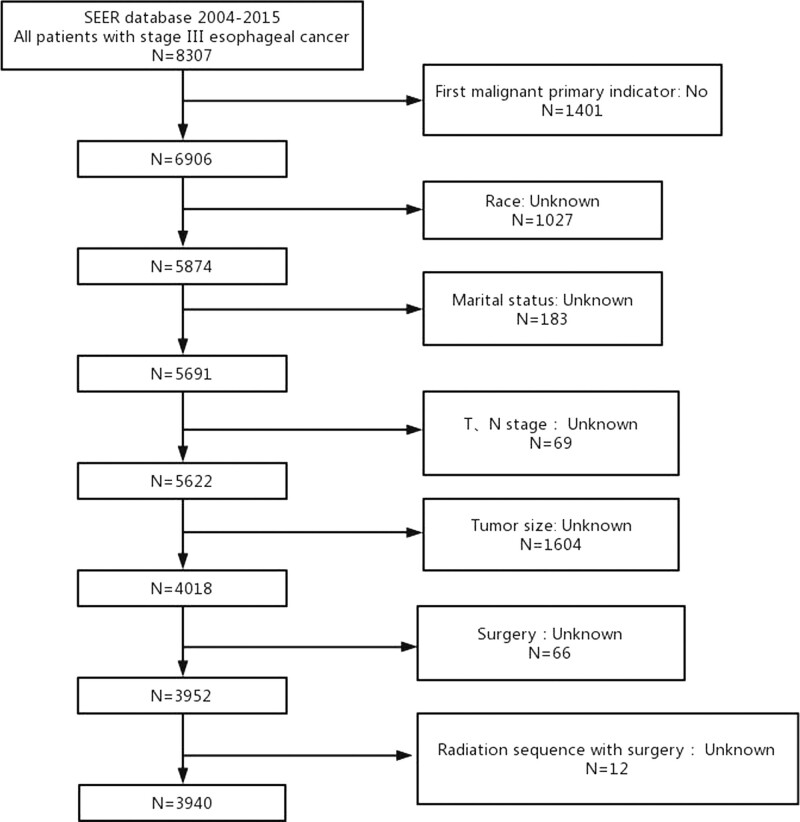
Surveillance, Epidemiology, and End Results (SEER) 18 Regs custom Data (1975–2016) is the data source for this retrospective study. We identified patients diagnosed stage III esophageal cancer from 2004 to 2015 (Fig. 1). Patients included in this exploration had to meet all of the criteria over 18 years of age, tumor size <600 mm, diagnosed with stage III esophageal cancer. The exclusion criteria were as follows not first malignant primary indicator, complete data could not be obtained, patients diagnosed by autopsy. We extracted the following data from the SEER database: age, primary site of tumor, histologic, race, gender, chemotherapy history, T stage, N stage, surgery history, radiation history, tumor size, marital status, radiation sequence with surgery, and follow-up information. We employed OS and cancer-specific survival rate (CSS) as survival rate times analyzed in this investigation. OS is the survival rate time from the day of diagnosis to the day of death from any cause or last follow-up. CSS is an OS measure excluding other causes of death. In this research, we adopted the AJCC 6th edition TNM staging. We use data from public databases and was exempt from institutional review board approval.
Figure 1.
Patient screening flowchart. This figure contains how we screened 3940 stage III esophageal cancer patients from the SEER database. SEER = the Surveillance, Epidemiology, and End Results.
2.2. Nomogram construction
Univariate and multivariate Cox proportional hazards regression analyses were performed on the postpropensity score matching (postPSM) cohort. On the basis of the above results, we constructed a nomogram using R version 4.1.3.
2.3. Statistical analysis and the optimal cutoff value
We compared categorical variables using chi-square or Fisher’s exact test. 1:1 PSM of surgery and PORT were performed separately to eliminate possible effects of other variables. The log-rank test was adopted to evaluate Kaplan–Meier survival rate curves and reported hazard ratios (HR) with 95% confidence interval (CI). In accordance with the results of univariate analysis, we included factors with P < .05 into multivariate analysis. SPSS v26.0 (SPSS Inc) and GraphPad Prism v8.0.2 (GraphPad Software, Inc.) were used for Statistical analysis. The optimal cutoffs for tumor size and age were determined in line with X-tile v3.6.1 (Yale University). P < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
In this exploration, 3940 stage III esophageal cancer patients were screened from SEER database, of whom 51.0% (n = 2008) underwent surgery and 49.0% (n = 1932) did not. Table 1 summarized the clinical characteristics before and after PSM in line with whether the surgery is performed was conducted. Most surgical and nonsurgical patients were between the ages of 23 and 66 (64.9%; 48.8%) and were both male (84.6%;76.3%), and were both married (68.5%;55.4%); and were both the white (89.9%;77.9%); and were both Poorly differentiated (Grade III) (55.1%;48.4%); and were both T3 stage (81.6%;61.2%); and were both N1 stage (95.7%;83.4%); and all received radiotherapy (77.1%;80.5%) and chemotherapy (83.8%;78.9%). We then compared the clinical characteristics of patients before and after PSM in accordance with whether the PORT is carried out (Table 2). Although most variables did not exhibit statistical differences between PORT(+) and PORT(−) (P > .05), we still performed PSM to remove potential effects of other variables. Table 3 displays the clinical characteristics of all stage III esophageal cancer in different N stages.
Table 1.
Characteristics of patients before and after PSM according to whether or not surgery.
| Characteristics | Entire patients | Propensity-matched patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery (+) | Surgery (−) | P value | Surgery (+) | Surgery (−) | P value | |||||
| (n = 2008) | % | (n = 1932) | % | (n = 1192) | % | (n = 1192) | % | |||
| Age at diagnosis | <.001 | .343 | ||||||||
| 23–66 | 1304 | 64.9 | 944 | 48.8 | 660 | 55.37 | 661 | 55.45 | ||
| 67–74 | 473 | 23.5 | 481 | 24.8 | 329 | 27.60 | 305 | 25.59 | ||
| 75–97 | 231 | 11.5 | 507 | 26.2 | 203 | 17.03 | 226 | 18.96 | ||
| Gender | <.001 | .756 | ||||||||
| Female | 308 | 15.3 | 456 | 23.6 | 234 | 19.63 | 227 | 19.04 | ||
| Male | 1700 | 84.6 | 1476 | 76.3 | 958 | 80.37 | 965 | 80.96 | ||
| Tumor size (mm) | <.001 | .799 | ||||||||
| 1–44 | 926 | 46.1 | 647 | 33.4 | 462 | 38.76 | 472 | 39.60 | ||
| 45–70 | 764 | 38.0 | 807 | 41.7 | 484 | 40.60 | 468 | 39.26 | ||
| 71–550 | 318 | 15.8 | 478 | 24.7 | 246 | 20.64 | 252 | 21.14 | ||
| Marital status | <.001 | .313 | ||||||||
| Unmarried | 632 | 31.4 | 861 | 44.5 | 475 | 39.85 | 450 | 37.75 | ||
| Married | 1376 | 68.5 | 1071 | 55.4 | 717 | 60.15 | 742 | 62.25 | ||
| Race | <.001 | .573 | ||||||||
| White | 1806 | 89.9 | 1506 | 77.9 | 1021 | 85.65 | 1005 | 84.31 | ||
| Black | 114 | 5.6 | 283 | 14.6 | 95 | 7.97 | 109 | 9.14 | ||
| Other | 88 | 4.3 | 143 | 7.4 | 76 | 6.38 | 78 | 6.54 | ||
| Grade | <.001 | .857 | ||||||||
| Well differentiated; Grade I | 82 | 4 | 116 | 6.0 | 66 | 5.54 | 64 | 5.37 | ||
| Moderately differentiated; Grade II | 779 | 38.7 | 843 | 43.6 | 493 | 41.36 | 485 | 40.69 | ||
| Poorly differentiated; Grade III | 1108 | 55.1 | 936 | 48.4 | 605 | 50.76 | 620 | 52.01 | ||
| Undifferentiated; anaplastic; Grade IV | 39 | 1.9 | 37 | 1.9 | 28 | 2.35 | 23 | 1.93 | ||
| T-stage | <.001 | .372 | ||||||||
| T3 | 1730 | 81.6 | 1184 | 61.2 | 923 | 77.43 | 942 | 79.03 | ||
| T4 | 278 | 13.8 | 748 | 38.7 | 269 | 22.57 | 250 | 20.97 | ||
| N-stage | <.001 | .464 | ||||||||
| N0 | 86 | 4.2 | 320 | 16.5 | 86 | 7.21 | 76 | 6.38 | ||
| N1 | 1922 | 95.7 | 1612 | 83.4 | 1106 | 92.79 | 1116 | 93.62 | ||
| Radiation recode | .010 | .046 | ||||||||
| YES | 1549 | 77.1 | 1556 | 80.5 | 914 | 76.68 | 955 | 80.12 | ||
| None/Unknown | 459 | 22.8 | 376 | 19.4 | 278 | 23.32 | 237 | 19.88 | ||
| Chemotherapy recode | <.001 | .08 | ||||||||
| YES | 1684 | 83.8 | 1526 | 78.9 | 957 | 80.29 | 991 | 83.14 | ||
| No/Unknown | 324 | 16.1 | 406 | 21.0 | 235 | 19.71 | 201 | 16.86 | ||
| Primary_site | <.001 | <.001 | ||||||||
| Upper | 31 | 1.5 | 186 | 9.6 | 25 | 2.10 | 69 | 5.79 | ||
| Middle | 198 | 9.8 | 417 | 21.5 | 171 | 14.35 | 199 | 16.69 | ||
| Lower | 1583 | 78.8 | 967 | 50.0 | 875 | 73.41 | 695 | 58.31 | ||
| Other | 196 | 9.7 | 362 | 18.7 | 121 | 10.15 | 229 | 19.21 | ||
| Histologic | <.001 | <.001 | ||||||||
| Adenocarcinoma | 1381 | 68.7 | 777 | 40.2 | 682 | 57.21 | 603 | 50.59 | ||
| Squamous cell carcinoma | 371 | 18.4 | 947 | 49.0 | 305 | 25.59 | 480 | 40.27 | ||
| Other | 256 | 12.7 | 208 | 10.7 | 205 | 17.20 | 109 | 9.14 | ||
The optimal cutoffs for age and tumor size were determined according to X-tile v3.6.1. Most variables did not show remarkably statistical differences between surgery and nonsurgery (P > .05) in the postPSM cohort.
PSM = propensity score matching.
Table 2.
Characteristics of patients before and after PSM according to whether or not PORT.
| Characteristics | Entire patients | Propensity-matched patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PORT(+) | PORT(−) | P value | PORT (+) | PORT(−) | P value | |||||
| (n = 322) | % | (n = 1686) | % | (n = 318) | % | (n = 318) | % | |||
| Age | .236 | .231 | ||||||||
| 23–66 | 219 | 68.0 | 1085 | 64.35 | 216 | 67.92 | 212 | 66.67 | ||
| 67–74 | 64 | 19.88 | 409 | 24.26 | 64 | 20.13 | 78 | 24.53 | ||
| 75–97 | 39 | 12.11 | 192 | 11.39 | 38 | 11.95 | 28 | 8.81 | ||
| Gender | .311 | .651 | ||||||||
| Female | 43 | 13.35 | 265 | 15.72 | 43 | 13.52 | 48 | 15.09 | ||
| Male | 279 | 86.65 | 1421 | 84.28 | 275 | 86.48 | 270 | 84.91 | ||
| Tumor size (mm) | .858 | .688 | ||||||||
| 1–44 | 153 | 47.52 | 773 | 45.85 | 152 | 47.80 | 142 | 44.65 | ||
| 45–70 | 119 | 37.0 | 645 | 38.26 | 116 | 36.48 | 120 | 37.74 | ||
| 71–550 | 50 | 15.53 | 268 | 15.90 | 50 | 15.72 | 56 | 17.61 | ||
| Marital status | .964 | .796 | ||||||||
| Unmarried | 101 | 31.37 | 531 | 31.49 | 98 | 30.82 | 94 | 29.56 | ||
| Married | 221 | 68.63 | 1155 | 68.51 | 220 | 69.18 | 224 | 70.44 | ||
| Race | .064 | .132 | ||||||||
| White | 283 | 87.89 | 1523 | 90.33 | 280 | 88.05 | 277 | 87.11 | ||
| Black | 27 | 8.39 | 87 | 5.16 | 26 | 8.18 | 19 | 5.97 | ||
| Other | 12 | 3.73 | 76 | 4.51 | 12 | 3.77 | 22 | 6.91 | ||
| Grade | .131 | .169 | ||||||||
| Grade I | 16 | 4.97 | 66 | 3.9 | 16 | 5.03 | 6 | 1.89 | ||
| Grade II | 117 | 36.34 | 662 | 39.26 | 115 | 36.16 | 117 | 36.79 | ||
| Grade III | 178 | 55.28 | 930 | 55.16 | 176 | 55.35 | 186 | 58.49 | ||
| Grade IV | 11 | 3.42 | 28 | 1.66 | 11 | 3.46 | 9 | 2.83 | ||
| T stage | .112 | .826 | ||||||||
| T3 | 268 | 83.23 | 1462 | 86.71 | 268 | 84.28 | 271 | 85.22 | ||
| T4 | 54 | 16.77 | 224 | 13.29 | 50 | 15.72 | 47 | 14.78 | ||
| N stage | .132 | .317 | ||||||||
| N0 | 19 | 5.90 | 67 | 3.97 | 16 | 5.03 | 10 | 3.14 | ||
| N1 | 303 | 94.10 | 1619 | 96.03 | 302 | 94.97 | 308 | 96.86 | ||
| Chemotherapy recode | .005 | .796 | ||||||||
| Yes | 287 | 89.13 | 1397 | 82.86 | 283 | 88.99 | 286 | 89.94 | ||
| No | 35 | 10.87 | 289 | 17.1 | 35 | 11.01 | 32 | 10.06 | ||
| Primary_site | .06 | .190 | ||||||||
| Upper | 8 | 2.48 | 23 | 1.36 | 8 | 2.52 | 3 | 0.94 | ||
| Middle | 28 | 8.70 | 170 | 10.08 | 28 | 8.81 | 36 | 11.32 | ||
| Lower | 244 | 75.78 | 1339 | 79.4 | 241 | 75.79 | 248 | 77.99 | ||
| Other | 42 | 13.04 | 154 | 9.13 | 41 | 12.89 | 31 | 9.75 | ||
| Histologic | .215 | .726 | ||||||||
| Adenocarcinoma | 209 | 64.91 | 1172 | 69.51 | 207 | 65.09 | 199 | 62.58 | ||
| Squamous cell carcinoma | 64 | 19.88 | 307 | 18.2 | 63 | 19.81 | 64 | 20.13 | ||
| Other | 49 | 15.22 | 207 | 12.28 | 48 | 15.09 | 55 | 17.30 | ||
Although most variables did not show statistical differences between PORT(+) and PORT(−) (P > .05), we still performed PSM to remove potential effects of other variables.
PORT = postoperative radiotherapy, PSM = propensity score matching.
Table 3.
Characteristics of different N stages.
| Characteristics | N0 | N1 | |||
|---|---|---|---|---|---|
| (n = 406) | % | (n = 3534) | % | P value | |
| Age | <.001 | ||||
| 23–66 | 219 | 53.94 | 2029 | 57.41 | |
| 67–74 | 75 | 18.47 | 879 | 24.87 | |
| 75–97 | 112 | 27.59 | 626 | 17.71 | |
| Tumor size(mm) | <.001 | ||||
| 1–44 | 124 | 30.54 | 1449 | 41.00 | |
| 45–70 | 176 | 43.35 | 1395 | 39.47 | |
| 71–550 | 106 | 26.11 | 690 | 19.52 | |
| Chemotherapy | <.001 | ||||
| No | 150 | 36.95 | 580 | 16.41 | |
| Yes | 256 | 63.05 | 2954 | 83.59 | |
| Radiation | <.001 | ||||
| No | 137 | 33.74 | 698 | 19.75 | |
| Yes | 269 | 66.26 | 2836 | 80.25 | |
| Surgery | <.001 | ||||
| No | 320 | 78.82 | 1612 | 45.61 | |
| Yes | 86 | 21.18 | 1922 | 54.39 | |
| PORT | <.001 | ||||
| Nonsurgery | 320 | 78.82 | 1612 | 45.61 | |
| Yes | 67 | 16.50 | 303 | 8.57 | |
| No | 19 | 4.68 | 1619 | 45.81 | |
| Grade | .001 | ||||
| I | 30 | 7.39 | 168 | 4.75 | |
| II | 184 | 45.32 | 1438 | 40.69 | |
| III | 179 | 44.09 | 1865 | 52.77 | |
| IV | 13 | 3.20 | 63 | 1.78 | |
| Sex | <.001 | ||||
| Female | 115 | 28.33 | 649 | 18.36 | |
| Male | 291 | 71.67 | 2885 | 81.64 | |
| Race | <.001 | ||||
| White | 298 | 73.40 | 3014 | 85.29 | |
| Black | 81 | 19.95 | 316 | 8.94 | |
| Other | 27 | 6.65 | 204 | 5.77 | |
| Histologic | <.001 | ||||
| Adenocarcinoma | 139 | 34.24 | 2019 | 57.13 | |
| Squamous cell carcinoma | 218 | 53.69 | 1100 | 31.13 | |
| Other | 49 | 12.07 | 415 | 11.74 | |
| Primary site | <.001 | ||||
| Upper | 49 | 12.07 | 168 | 4.75 | |
| Middle | 89 | 21.92 | 526 | 14.88 | |
| Lower | 184 | 45.32 | 2366 | 66.95 | |
| Other | 84 | 20.69 | 474 | 13.41 | |
| Marital status | <.001 | ||||
| Unmarried | 207 | 50.99 | 1286 | 36.39 | |
| Married | 199 | 49.01 | 2248 | 63.61 | |
All variables show statistical differences between N0 stage and N1 stage.
PORT = postoperative radiotherapy.
3.2. The role of surgery and PORT
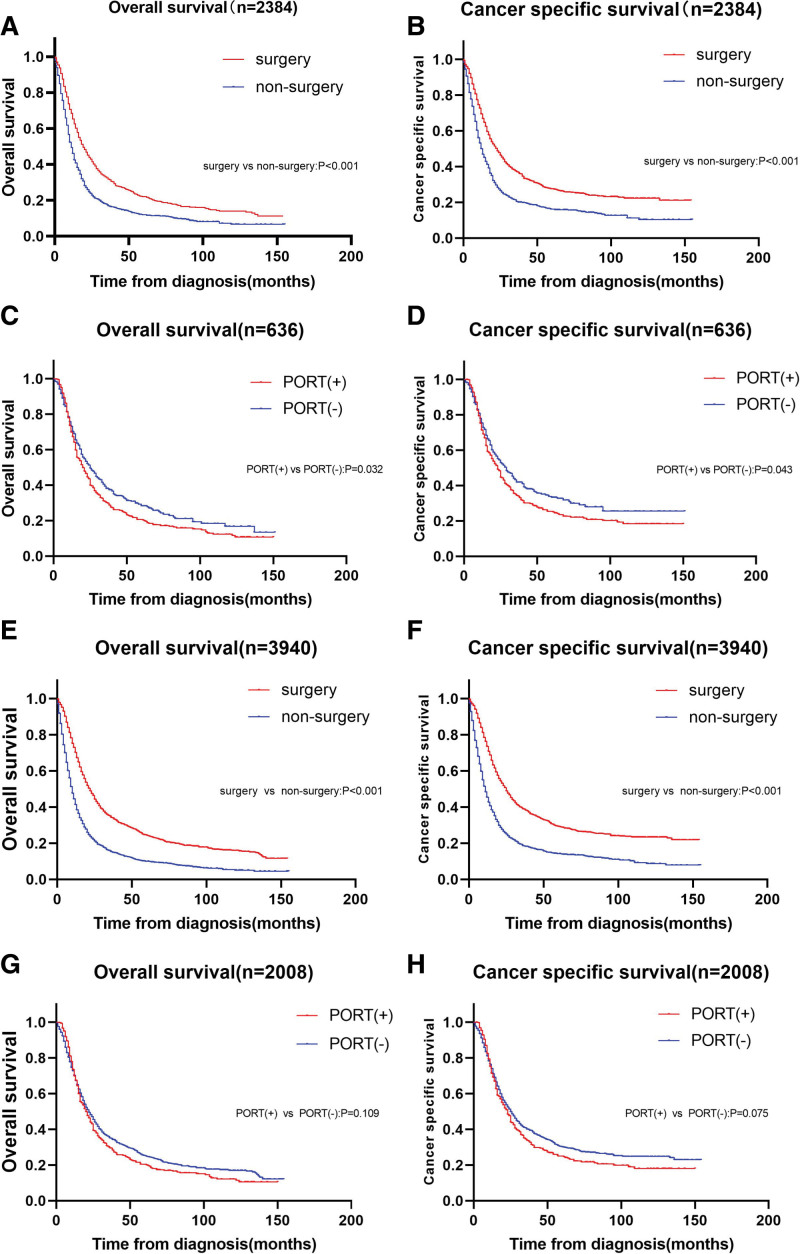
The median follow-up in our study was 14 months (range 1–155). During the follow-up period, 3158 cases (80.1%) died, including 2743 cases (69.6%) of esophageal cancer deaths. In the whole cohort, half of patients (n = 2008,51.0%) received surgery, and 16.0% (n = 322)of those undergoing surgery received PORT. In an effort to minimize the influence of other variables, we conducted 1:1 PSM analysis according to surgery or nonsurgery and whether the PORT is conducted. Tables 1 and 2 illustrates the balance of variables before and after PSM in accordance with whether the surgery is conducted and whether the PORT is performed. 1192 patients who underwent surgery were matched with 1192 patients who underwent nonsurgery, and 316 patients who underwent PORT were matched with 316 patients. There were no striking differences in clinical characteristics were observed for most variables in the matched population. In the postPSM population in line with whether the surgery is carried out, the median OS of surgery and nonsurgery were respectively 19.0 (95% CI: 17.2–20.8) and 11.0 (95% CI: 10.2–11.8) months, and the median CSS were respectively 23.0 (95% CI: 20.7–25.3) and 12.0 (95% CI: 10.9–13.0) months. We observed that OS and CSS were evidently better in surgical patients than in nonsurgical patients (Fig. 2a and b). In the postPSM population in accordance with whether the PORT is conducted, patients who received PORT had strikingly lower median OS and CSS than those who did not (OS 20.0, 95% CI:16.5–23.5 vs 25.0, 95% CI:20.4–29.6, P = .032; CSS 23.0, 95% CI:19.8–26.2 vs 29.0, 95% CI:23.4–34.6, P = .043) (Fig. 2c and d).We also probed into pre-PSM population in line with surgery or nonsurgery and whether the PORT is carried out, found that The median OS and CSS were conspicuously better in patients undergoing surgery than those did not (OS 22.0, 95% CI:20.6–23.4 vs 10.0, 95% CI:9.4–10.6, P < .001; CSS 25.0, 95% CI: 23.2–26.8 vs 11.0, 95% CI: 10.3–11.7, P < .001) (Fig. 2e and f) and the median OS and CSS were lower in patients undergoing PORT than those did not (OS 20.0, 95% CI:16.7–23.3 vs 22.0, 95% CI:20.4–23.6,P = .109; CSS 23.0, 95% CI: 20.0–26.0 vs 25.0, 95% CI: 23.0–27.0, P = .075) (Fig. 2g and h). Hence, our results demonstrated that surgery can augment patients’ survival rate, whereas PORT did not heighten patients’ survival rate and even decreased OS and CSS.
Figure 2.
Kaplan–Meier curves before and after PSM in line with whether the surgery is conducted and whether the PORT is carried out. This figure contains (a) overall survival rate (OS) and (b) cancer specific survival rate (CSS) in postPSM cohort in line with whether the surgery is carried out; (c) OS and (d) CSS in postPSM cohort in accordance with whether the PORT is carried out; (e) OS and (f) CSS in pre-PSM cohort according to whether the surgery is carried out; (g) OS and (h) CSS in pre-PSM cohort according to whether the PORT is conducted. PORT = postoperative radiotherapy, PSM = propensity score matching.
3.3. Stratified analysis on OS and CSS
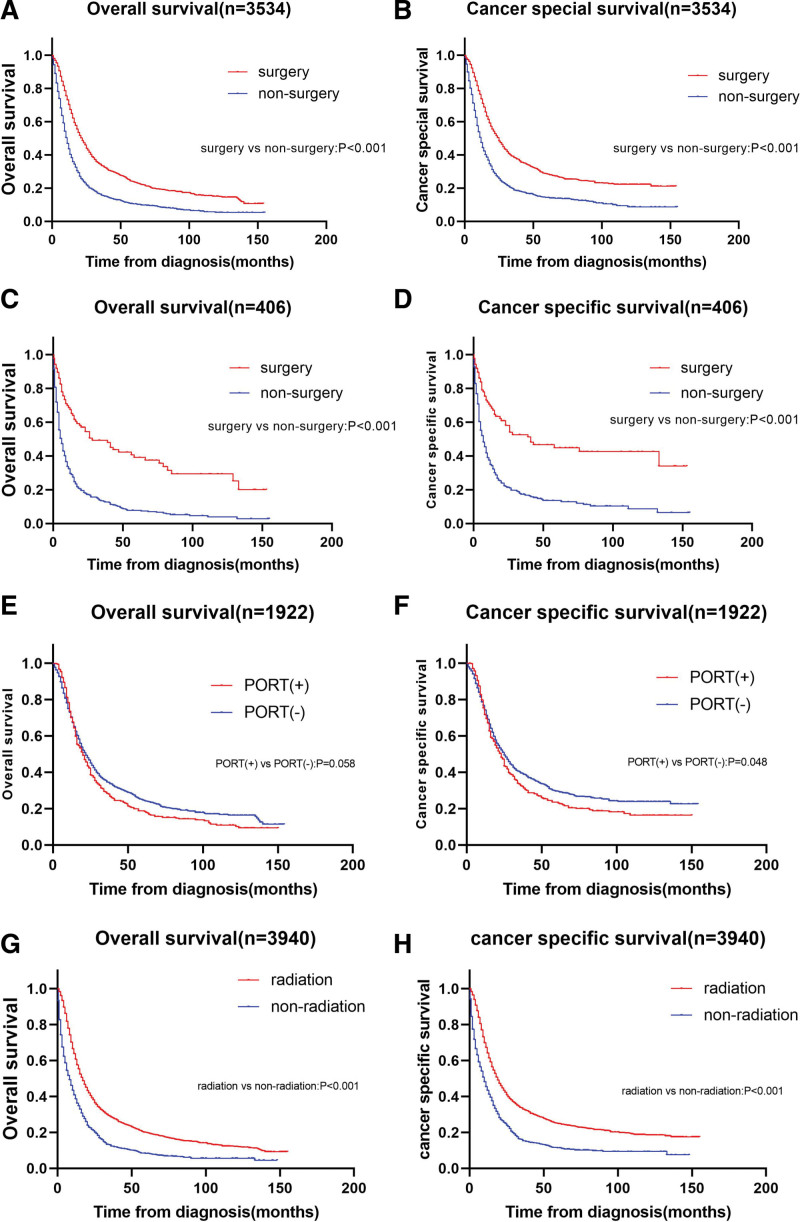
In stratified analysis on the basis of N stage, surgery exhibited OS and CSS benefit for N1 stage (OS HR = 0.548, 95% CI: 0.508–0.590, P < .001; CSS HR = 0.534, 95% CI: 0.493–0.579, P < .001) (Fig. 3a and b) and N0 stage (OS HR = 0.376, 95% CI: 0.282–0.502, P < .001; CSS HR = 0.377, 95% CI: 0.274–0.518, P < .001) (Fig. 3c and d). For patients with N1 stage, although the Kaplan–Meier curve demonstrated that the CSS of the nonPORT group was longer than that of the PORT group (P = .048), there was no statistically significant difference in CSS between the PORT and nonPORT groups (HR = 1.156, 95% CI: 0.999–1.338, P = .051) (Fig. 3f). There are not statistically significant in OS between PORT and nonPORT groups (P = .058) (Fig. 3e). In stratified analysis in accordance with whether or not they received radiotherapy, radiotherapy illustrated OS and CSS benefit in patients (OS HR = 0.559, 95% CI: 0.515–0607, P < .001; CSS HR = 0.554, 95% CI:0.508–0.605, P < .001) (Fig. 3g and h).
Figure 3.
Kaplan–Meier curves for stratified analysis. This figure contains (a) overall survival rate (OS) and (b) cancer specific survival rate (CSS) in esophageal cancer patients with and without surgery in stage N1; (c) OS and (d) CSS in esophageal cancer patients with and without surgery in stage N0; (e) OS and (f) CSS in esophageal cancer patients with and without PORT in stage N1; (g) OS and (h) CSS in all 3940 esophageal cancer patients with and without radiation. PORT = postoperative radiotherapy.
3.4. Univariate and multivariate analysis
Univariate and multivariate analyses were performed on the postPSM cohort in line with whether the surgery is performed. Univariate analysis manifested that OS and CSS were markedly correlated with age, tumor size, chemotherapy, radiation, surgery, grade and marital status, gender, Primary site and T stage (Table 4). The multivariate analysis on OS revealed that age, tumor size, chemotherapy, radiation, surgery, T stage, grade, gender, marital status independently affected OS (Table 4). The multivariate analysis on CSS revealed that age, tumor size, chemotherapy, radiation, surgery, T stage, grade, gender, marital status and primary site independently affected CSS (Table 4).
Table 4.
Univariate and multivariate analyses on the postPSM cohort according to whether or not surgery.
| Characteristics | OS | CSS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | 95%CI lower | 95%CI upper | P | Multivariate analysis | 95%CI lower | 95%CI upper | P | Univariate analysis | 95%CI lower | 95%CI upper | P | Multivariate analysis | 95%CI lower | 95%CI upper | P | ||
| Age | 23–66 | 1 | 1 | 1 | 1 | ||||||||||||
| 67–74 | 1.071 | 0.963 | 1.192 | .207 | 1.122 | 1.005 | 1.252 | .040 | 1.015 | 0.904 | 1.139 | .806 | 1.041 | 0.925 | 1.172 | .501 | |
| 75–97 | 1.565 | 1.391 | 1.761 | <.001 | 1.406 | 1.242 | 1.591 | <.001 | 1.475 | 1.298 | 1.676 | <.001 | 1.295 | 1.134 | 1.480 | <.001 | |
| Tumor size(mm) | 1–44 | 1 | 1 | 1 | 1 | ||||||||||||
| 45–70 | 1.023 | 0.924 | 1.132 | .661 | 1.027 | 0.928 | 1.138 | .604 | 1.095 | 0.981 | 1.222 | .105 | 1.094 | 0.979 | 1.222 | .111 | |
| 71–550 | 1.266 | 1.123 | 1.428 | <.001 | 1.354 | 1.198 | 1.531 | <.001 | 1.361 | 1.196 | 1.549 | <.001 | 1.431 | 1.255 | 1.633 | <.001 | |
| Chemotherapy | No | 1 | 1 | 1 | 1 | ||||||||||||
| Yes | 0.441 | 0.395 | 0.492 | <.001 | 0.573 | 0.498 | 0.660 | <.001 | 0.441 | 0.392 | 0.497 | <.001 | 0.557 | 0.479 | 0.648 | <.001 | |
| Radiation | NO | 1 | 1 | 1 | 1 | ||||||||||||
| Yes | 0.497 | 0.448 | 0.552 | <.001 | 0.631 | 0.553 | 0.72 | <.001 | 0.499 | 0.446 | 0.558 | <.001 | 0.638 | 0.553 | 0.735 | <.001 | |
| Surgery | NO | 1 | 1 | 1 | 1 | ||||||||||||
| Yes | 0.638 | 0.583 | 0.699 | <.001 | 0.585 | 0.533 | 0.72 | <.001 | 0.615 | 0.558 | 0.678 | <.001 | 0.562 | 0.509 | 0.622 | <.001 | |
| N stage | N0 | 1 | 1 | ||||||||||||||
| N1 | 1.035 | 0.865 | 1.238 | .708 | 1.024 | 0.843 | 1.243 | .843 | |||||||||
| T stage | T3 | 1 | 1 | 1 | 1 | ||||||||||||
| T4 | 1.123 | 1.009 | 1.249 | .034 | 1.140 | 1.022 | 1.272 | .019 | 1.159 | 1.034 | 1.300 | .011 | 1.172 | 1.043 | 1.317 | .008 | |
| Grade | I | 1 | 1 | 1 | <.001 | 1 | |||||||||||
| II | 1.228 | 0.992 | 1.52 | .060 | 1.296 | 1.045 | 1.607 | .018 | 1.438 | 1.122 | 1.843 | .004 | 1.512 | 1.178 | 1.941 | .001 | |
| III | 1.448 | 1.173 | 1.787 | .001 | 1.523 | 1.232 | 1.884 | <.001 | 1.735 | 1.358 | 2.217 | <.001 | 1.809 | 1.413 | 2.315 | <.001 | |
| IV | 1.693 | 1.184 | 2.421 | .004 | 1.634 | 1.141 | 2.342 | .007 | 2.125 | 1.439 | 3.137 | <.001 | 2.035 | 1.376 | 3.001 | <.001 | |
| Sex | Female | 1 | 1 | 1 | 1 | ||||||||||||
| Male | 1.215 | 1.080 | 1.366 | .001 | 1.347 | 1.191 | 1.522 | <.001 | 1.228 | 1.081 | 1.395 | .002 | 1.335 | 1.169 | 1.525 | <.001 | |
| Race | White | 1 | 1 | 1 | |||||||||||||
| Black | 1.228 | 1.051 | 1.435 | .010 | 1.217 | 1.034 | 1.431 | .1018 | 1.223 | 1.034 | 1.447 | .019 | |||||
| Other | 0.995 | 0.825 | 1.201 | .962 | 0.982 | 0.812 | 1.1186 | .848 | 1.068 | 0.878 | 1.301 | .510 | |||||
| Histologic | Adenocarcinoma | 1 | 1 | ||||||||||||||
| Squamous cell carcinoma | 0.990 | 0.896 | 1.093 | .843 | 0.970 | 0.871 | 1.080 | .576 | |||||||||
| Other | 0.929 | 0.809 | 1.066 | .294 | 0.944 | 0.814 | 1.094 | .445 | |||||||||
| Primary site | Upper | 1 | 1 | 1 | 1 | ||||||||||||
| Middle | 1.254 | 0.967 | 1.626 | .087 | 1.346 | 1.036 | 1.749 | .026 | 1.347 | 1.1012 | 1.792 | .041 | 1.492 | 1.119 | 1.990 | .006 | |
| Lower | 1.083 | 0.851 | 1.377 | .517 | 1.157 | 0.906 | 1.477 | .243 | 1.152 | 0.883 | 1.504 | .297 | 1.243 | 0.949 | 1.628 | .114 | |
| Other | 1.213 | 0.934 | 1.574 | .148 | 1.192 | 0.916 | 1.551 | .191 | 1.279 | 0.959 | 1.706 | .094 | 1.283 | 0.960 | 1.714 | .092 | |
| Marital status | Unmarried | 1 | 1 | 1 | 1 | ||||||||||||
| Married | 0.847 | 0.733 | 0.929 | <.001 | 0.812 | 0.739 | 0.893 | <.001 | 0.881 | 0.798 | 0.973 | .012 | 0.839 | 0.758 | 0.929 | .001 | |
According to the results of univariate analysis, we included factors with P < .05 into multivariate analysis. We found that the independent factors including age, tumor size, chemotherapy, radiation, surgery, T stage, grade, gender and marital status.
CI = confidence interval, PSM = propensity score matching.
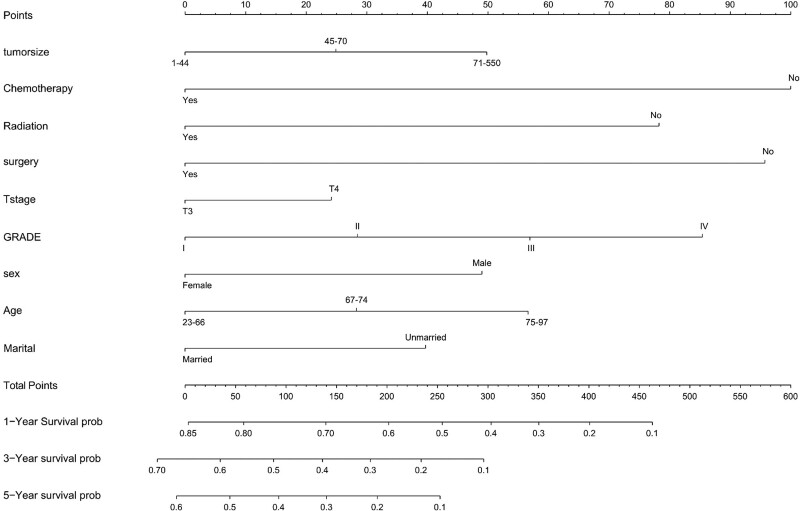
3.5. Nomogram construction
The nomogram was constructed in accordance with the independent factors including age, tumor size, chemotherapy, radiation, surgery, T stage, grade, gender and marital status (Fig. 4). In the nomogram, chemotherapy exerted the most conspicuous influence on prognosis, followed by surgery and grade.
Figure 4.
Nomogram for predicting OS in patients with stage III esophageal cancer. We developed a nomogram to predict 5-year survival rate in stage III esophageal cancer patients. The OS nomogram had c-index values of 0.663, indicating low discriminative ability. OS = overall survival rate.
4. Discussion
When investigating the data from the SEER database for stage III esophageal cancer diagnosed between 2004 and 2015, we found that surgery noticeably prolonged patients’ survival rate time. Similarly, we also arrived at the same conclusion in N0 and N1 patients. Meanwhile, we found that radiotherapy can better the OS and CSS of patients, but PORT will not ameliorate patients’ survival rate and even lower OS as well as CSS. The value of PORT in patients with esophageal cancer remains controversial. In several studies of patients with PORT, reporting an improvement in the 5-year survival rate in stage III esophageal cancer,[10] noticeably heightened disease-free survival rate in patients with thoracic esophageal squamous cell carcinoma (P = .030),[13] an improvement in the 5-year OS (P < .001)[14] and an improvement in OS in lymph node-positive patients (P < .001).[15] Nonetheless, relevant studies also suggested that PORT did not confer any survival rate benefit compared to nonPORT. As conspicuously revealed by a prospective study, compared with the control group, PORT had shorter overall median survival rate.[11] In another prospective randomized controlled study, there was no statistically significant difference in survival rate between the PORT group and surgery alone.[16] Just as conspicuously illustrated by a meta-analysis on 13 retrospective studies and 6 randomized controlled trials, PORT can ameliorate the OS only in retrospective studies.[17] Nevertheless, most of the aforementioned studies did not stratify patients by stage or had insufficient data or did not take into account the influence of other factors. As a consequence, there may be errors in the detection of the effect of PORT on stage III esophageal cancer. As a result, we conducted this PSM study and found that PORT did not ameliorate patients’ survival rate and even decreased OS and CSS. We matched 318 patients who underwent surgery alone with 318 patients who underwent PORT in an attempt to eliminate the influence of other factors. Apart from that, we obtained similar results when we investigated patients with stage N1 in accordance with whether PORT or not. We did not investigate it due to the deficiency of enough data on patients with stage N0. To investigate whether radiotherapy was responsible for this unusual outcome or PORT, we also explored the effect of radiotherapy on prognosis. Surprisingly, we found that radiotherapy lengthened patients’ survival rate time, while PORT did not elevate or even decreased patients’ survival rate.
One of the most common method for judging the prognosis of cancer is the nomogram.[18] In this retrospective study, we constructed an OS nomogram integrating available information such as age, tumor size, chemotherapy, radiation, surgery, T stage, grade, gender and marital status to predict OS. Previous scholars have constructed a variety of nomogram models for the prognosis of esophageal cancer.[19–21] Nonetheless, a nomogram for stage III esophageal cancer has not yet been constructed, which was the reason why we constructed this nomogram model. The Concordance Index, also known as the c-index, is adopted to evaluate the accuracy of the nomogram model. The c-index >0.7 is considered to have satisfactory predictive ability of nomogram model.[22] The c-index values of this OS nomogram was 0.663, indicating low discriminative ability. Hence, we did not validate the nomogram model. And the model can be employed as a reference tool for decision-making.
It’s essential to discuss several limitations existing in this study. First and foremost, the treatment information in the SEER database is incomplete. The deficiency of crucial information such as radiation dose, chemotherapy dose, patient performance status, and radiation field hindered a more in-depth analysis on the prognostic value of PORT for stage III esophageal cancer. Likewise, although our study revealed that age, tumor size, surgery, T stage, grade, gender and marital status are independent risk factors, the shortage of relevant specific information made further analysis more difficult. Aside from that, the SEER database covers no more than a subset of the entire US patient population, which may not be representative of the entire population.
5. Conclusion
As evidently exhibited by the retrospective analysis, surgery can heighten OS and CSS, whereas PORT did not. Nonetheless, given the various limitations of this analysis, caution must be exercised before these findings are universally employed in clinical practice until more randomized trials confirm these results.
Acknowledgments
The authors thank the participants and their families in this investigation.
Author contributions
Conceptualization: Wenwen Yang, Yanjiang Yang.
Data curation: Wenwen Yang, Xiang Ma, Minjie Ma, Biao Han.
Formal analysis: Wenwen Yang, Minjie Ma.
Funding acquisition: Minjie Ma.
Investigation: Wenwen Yang, Yanjiang Yang, Minjie Ma.
Methodology: Wenwen Yang, Yanjiang Yang.
Project administration: Wenwen Yang.
Resources: Wenwen Yang, Yanjiang Yang, Biao Han.
Software: Wenwen Yang, Biao Han.
Supervision: Wenwen Yang, Biao Han.
Validation: Wenwen Yang, Biao Han.
Visualization: Wenwen Yang.
Writing – original draft: Wenwen Yang.
Writing – review & editing: Wenwen Yang, Xiang Ma.
Abbreviations:
- CI
- confidence interval
- CSS
- cancer-specific survival rate
- HR
- hazard ratios
- OS
- overall survival rate
- PORT
- postoperative radiotherapy
- PSM
- propensity score matching
- SEER
- the Surveillance, Epidemiology, and End Results
WY, YY, XM, and MM contributed equally to this work.
This study was supported by Natural Science Foundation of Gansu Province (21JR1RA118) and Gansu Provincial Youth Science and Technology Fund (21JR1RA107, 18JR3RA305).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Yang W, Yang Y, Ma X, Ma M, Han B. Surgery and postoperative radiotherapy affect the prognosis of esophageal cancer: A SEER analysis. Medicine 2023;102:9(e32925).
Contributor Information
Wenwen Yang, Email: 934637615@qq.com.
Yanjiang Yang, Email: 934637615@qq.com.
Xiang Ma, Email: maminjie24@sina.com.
Minjie Ma, Email: maminjie24@sina.com.
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [2].Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet (London, England). 2017;390:2383–96. [DOI] [PubMed] [Google Scholar]
- [3].Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the American Joint Committee on Cancer/International Union against cancer staging manuals. Cancer. 2010;116:3763–73. [DOI] [PubMed] [Google Scholar]
- [5].Anandavadivelan P, Langergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13:185–98. [DOI] [PubMed] [Google Scholar]
- [6].Chen G, Wang Z, Liu XY, et al. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg. 2007;31:1107–14. [DOI] [PubMed] [Google Scholar]
- [7].Hsu PK, Wang BY, Huang CS, et al. Prognostic factors for post-recurrence survival in esophageal squamous cell. J Gastrointest Surg. 2011;15:558–65. [DOI] [PubMed] [Google Scholar]
- [8].Miyata H, Yamasaki M, Kurokawa Y, et al. Survival factors in patients with recurrence after curative resection of esophageal. Ann Surg Oncol. 2011;18:3353–61. [DOI] [PubMed] [Google Scholar]
- [9].Goodner JT. Surgical and radiation treatment of cancer of the thoracic esophagus. Am J Roentgenol Radium Ther Nucl Med. 1969;105:523–8. [DOI] [PubMed] [Google Scholar]
- [10].Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg. 2003;75:331–6. [DOI] [PubMed] [Google Scholar]
- [11].Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized. Surgery. 1993;113:138–47. [PubMed] [Google Scholar]
- [12].Lin HN, Chen LQ, Shang QX, et al. A meta-analysis on surgery with or without postoperative radiotherapy to treat. Int J Surg. 2020;80:184–91. [DOI] [PubMed] [Google Scholar]
- [13].Deng W, Yang J, Ni W, et al. Postoperative radiotherapy in pathological T2-3N0M0 thoracic esophageal squamous. Oncologist. 2020;25:e701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang X, Deng W, Ni W, et al. Comparison of two major staging systems in predicting survival and recommendation of postoperative radiotherapy based on the 11th Japanese classification for esophageal carcinoma after curative resection: a propensity score-matched analysis. Ann Surg Oncol. 2021;28:7076–86. [DOI] [PubMed] [Google Scholar]
- [15].Chen J, Pan J, Zheng X, et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:475–82. [DOI] [PubMed] [Google Scholar]
- [16].Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg. 1995;19:444–9. [DOI] [PubMed] [Google Scholar]
- [17].Liu T, Liu W, Zhang H, et al. The role of postoperative radiotherapy for radically resected esophageal squamous cell carcinoma: a systemic review and meta-analysis. J Thorac Dis. 2018;10:4403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. [DOI] [PubMed] [Google Scholar]
- [19].Shi M, Tang JW, Cao ZR. Nomograms for predicting survival in early-onset esophageal cancer. Expert Rev Gastroenterol Hepatol. 2021;15:437–46. [DOI] [PubMed] [Google Scholar]
- [20].Jia R, Xiao W, Zhang H, et al. Comparative study of treatment options and construction nomograms to predict survival for early-stage esophageal cancer: a population-based study. Scand J Gastroenterol. 2021;56:635–46. [DOI] [PubMed] [Google Scholar]
- [21].Zhang DY, Huang GR, Ku JW, et al. Development and validation of a prognostic nomogram model for Chinese patients with primary small cell carcinoma of the esophagus. World J Clin Cases. 2021;9:9011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nie Z, Zhao P, Shang Y, et al. Nomograms to predict the prognosis in locally advanced oral squamous cell carcinoma after curative resection. BMC Cancer. 2021;21:021–08106. [DOI] [PMC free article] [PubMed] [Google Scholar]