Abstract
Signals from the extracellular matrix are essential for the survival of many cell types. Dominant-negative mutants of two members of Rho family GTPases, Rac1 and Cdc42, mimic the loss of anchorage in primary mouse fibroblasts and are potent inducers of apoptosis. This pathway of cell death requires the activation of both the p53 tumor suppressor and the extracellular signal-regulated mitogen-activated protein kinases (Erks). Here we characterize the proapoptotic Erk signal and show that it differs from the classically observed survival-promoting one by the intensity of the kinase activation. The disappearance of the GTP-bound forms of Rac1 and Cdc42 gives rise to proapoptotic, moderate activation of the Raf-MEK-Erk cascade via a signaling pathway involving the kinases phosphatidlyinositol 3-kinase and Akt. Moreover, concomitant activation of p53 and inhibition of Akt are both necessary and sufficient to signal anoikis in primary fibroblasts. Our data demonstrate that the GTPases of the Rho family control three major components of cellular signal transduction, namely, p53, Akt, and Erks, which collaborate in the induction of apoptosis due to the loss of anchorage.
Elimination by apoptosis is a frequent cellular destiny: essential in normal physiology, its deregulation is associated with numerous pathologies, including cancer (56). In the initial stages of oncogenesis, the loss of cell cycle controls typically results in the activation of the cellular apoptotic program (13). Certain cells nevertheless survive and progress to more tumorigenic stages that, for some cell types, result in the ability to survive without signals normally provided by contacts with the extracellular matrix (ECM). Indeed, survival and proliferation of normal adherent cells are possible only in the presence of sustained signaling by both soluble factors and those emanating from the ECM (recently reviewed in reference 51). Several pathways participate in transducing survival signals from the ECM (16, 19, 21). Moreover, these different signal transduction cascades engage in extensive cross talk (16, 19, 21, 49). One important and well-studied pathway involves phosphatidlyinositol 3-kinase [PI(3)K] and its downstream target, Akt (15). Numerous Akt substrates have been identified that are directly related to the control of cellular survival (reviewed in references 11 and 14). Under some physiological conditions, Akt represses the Erk mitogen-activated protein kinase (MAPK) cascade by inactivating phosphorylation of the MAPK kinase kinases Raf-1 (46, 59) and B-Raf (20). On the other hand, PI(3)K can activate Erk by stimulatory phosphorylation of Raf through a pathway which involves the small GTPase Rac1 and one of its effectors, the serine/threonine kinase PAK (8). The relationship between PI(3)K and Rac1 is complex, since the kinase can apparently act both upstream and downstream of the GTPase (3, 45).
It was recently reported that two Rho family GTPases, Rac1 and Cdc42, are intimately involved in the control of survival of murine fibroblasts linked to adherence to the ECM (30). Inhibition of either Rac1 or Cdc42 signaling in adherent cells mimics the loss of anchorage and efficiently induces apoptosis in both immortalized and primary cells. This process is dependent on the wild-type p53 tumor suppressor and requires Erk activation. Here, we report that the inhibition of Rac1 or Cdc42 signaling leads to Erk activation via a pathway involving PI(3)K, Akt, Raf, and MEK but not Ras. These results delineate a novel pathway of apoptotic signaling initiated as a consequence of the loss of the GTP-bound forms of Rho family GTPases.
MATERIALS AND METHODS
Plasmids.
The plasmids encoding the constitutively active and the dominant-negative forms of Rac1 and Cdc42, dominant-negative N-WASP, wild-type p53, and truncated rat CD2 have been previously described (30), as have those encoding hemagglutinin (HA)-tagged myristoylated Akt and Akt K179M (15). pcDNA3 HA-AKT T308AS473A was kindly provided by M. Vandromme, pcDNA3-RasN17 was provided by S. Roche, Raf-1–CAAX was provided by C. Marshall (35), pDCR-RasV12 was provided by M. White, and MKP3C/S (mutated form of MAPK phosphatase 3) was provided by P. Lenormand (4). In all of the above plasmids, expression is under the control of the early cytomegalovirus promoter.
The pcDNA3/HA1-KsrCA5 construct was obtained by subcloning the EcoRI fragment containing the kinase domain of mKsr-1 (CA5) from the pGBT-9/KsrCA5 construct (12) into the EcoRI site of the pcDNA3-HA1 vector.
The pEF/myc-B-Raf construct was obtained by subcloning the BamHI-HindIII fragment of pcDNA3/B-Raf (41) into a derivative of the pEFpLink2 vector (35) partially digested with BamHI and HindIII.
The pcDNA3/HA1-NB-Raf construct, encoding the N terminus of quail B-Raf from Met 1 to Arg 443, was obtained as described previously (6).
Cell culturing and transfection.
Early-passage mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS) in 5% CO2 at 37°C. Transfections into subconfluent cells were performed using Fugene 6 as recommended by the supplier (Boehringer Mannheim).
For experiments involving placing cells in suspension, exponentially growing MEFs were trypsinized, centrifuged, resuspended in complete culture medium at 105 cells/ml, and placed for various times in culture dishes over a layer of 1% tissue culture agar (Gibco-BRL), preequilibrated overnight with complete culture medium. Cell viability was assayed by trypan blue exclusion.
Apoptosis assay.
Apoptotic cells in transfected subpopulations were quantified basically as previously described (29).
Briefly, MEFs were cotransfected with the appropriate expression plasmids and a plasmid encoding a truncated form of rat CD2 antigen, lacking the intracytoplasmic domain. The cells were cultured for various times in medium supplemented with 10% FCS, after which floating and adherent cells were harvested, pooled, and centrifuged. The pellets were taken up in Annexin V binding buffer and incubated with Annexin V-FLUOS (Boehringer Mannheim) as specified by the manufacturer. The cells were then fixed in 3.7% formaldehyde (Sigma), rinsed with phosphate-buffered saline (PBS), incubated at room temperature for 2 h with biotinylated anti-rat CD2 antibody (OX-34; Cedarlane), rinsed with PBS, and incubated for 30 min with Streptavidin-Tri Color (Caltag). The quantification of apoptosis in transfected-cell populations was done by immunofluorescence microscopy. All assays were performed a minimum of three times.
Immunoblotting.
Cells were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, and proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and incubated with primary antibodies as recommended by the supplier (New England Biolabs) and rabbit peroxidase-conjugated secondary antibody (Sigma). The immune complexes were detected by enhanced chemiluminescence.
In vitro kinase assays.
Erk2 and Akt assays were performed as previously described (47). B-Raf kinase activity was assayed by a coupled in vitro assay (1). Briefly, cells transfected with myc epitope-tagged B-Raf and dominant-negative GTPases were starved in medium containing 0.5% FCS for 16 h and lysed. myc–B-Raf was immunoprecipitated with anti-myc monoclonal antibody (9E10) and incubated with 0.1 μg of glutathione S-transferase (GST)–MEK1 (Upstate Biotechnology) for 20 min at room temperature. The resin was removed by centrifugation, the supernatant was incubated sequentially with 0.1 μg of GST-Erk (Upstate Biotechnology) for 10 min and then with 3 μg of myelin basic protein (MBP) and [γ-32P]ATP for 20 min. Reactions were stopped with SDS-PAGE sample buffer, and the products were resolved by gel electrophoresis.
Signal intensities were quantified with a PhosphorImager (Molecular Dynamics) and normalized by the levels of expression of the transfected tagged kinases, as assayed by immunoblotting with the anti-HA (12CA5) or anti-myc (9E10) monoclonal antibody.
Kinase assays were performed with serum-starved cells in order to improve the sensitivity of the assays. We verified that both anoikis and RacN17- or CdcN17-induced apoptosis occur similarly in the presence and absence of serum, although cell death is accelerated in the latter case. All assays were performed at least three times, and representative experiments are shown.
In-gel kinase assay.
Erk activity was assayed as described by Chao et al. (7). Briefly, cells were lysed in buffer containing 20 mM Tris (pH 7.5), 137 mM NaCl, 2 mM sodium pyrophosphate, 1% Triton X-100, 10% glycerol, 1 mM Na3VO4, 25 mM β-glycerophosphate, and a cocktail of protease inhibitors. Proteins were separated on SDS–10% polyacrylamide gels containing 0.5 mg of rabbit MBP/ml. Following electrophoresis, the gels were fixed in 20% isopropanol in 50 mM HEPES (pH 7.4)–5 mM 2-mercaptoethanol and rinsed. The proteins were denatured by two incubations for 30 min each in 6 M guanidine hydrochloride in 50 mM HEPES (pH 7.4)–5 mM 2-mercaptoethanol. The renaturation was carried out at 4°C over 20 h with two changes of buffer containing 50 mM HEPES (pH 7.4)–5 mM 2-mercaptoethanol–0.04% Tween 20. The gels were preincubated for 30 min at room temperature in kinase buffer (25 mM HEPES [pH 7.4], 10 mM MgCl2, 5 mM 2-mercaptoethanol, 90 μM Na3VO4), and the reaction was performed with 10 ml of the same buffer containing 125 μCi of [γ-32P]ATP for 30 min at room temperature. After extensive washing in 5% trichloroacetic acid (TCA)–10 mM sodium pyrophosphate, the gels were dried and radioactivity was revealed by PhosphorImager analysis.
GTPase loading assays.
Ras family GTPase activity assays were performed as described previously (40). In brief, 3 × 105 cells were grown in 10-cm dishes for 24 h; when needed, they were further cultured in the absence of adhesion on agar-coated dishes. The cells were then lysed, and the supernatants were incubated at 4°C for 30 min with GST-PAK fusion protein containing the Rac binding domain of human PAK1B (amino acids 56 to 272) coupled to glutathione-Sepharose beads (Pharmacia Biotech) for Rac1 and Cdc42 assays or a fusion protein containing GST and the Ras binding domain of RalGDS (RalGDSRBD) for Ras assays. Precipitated complexes were washed three times with lysis buffer (40), eluted in SDS-PAGE sample buffer, immunoblotted, and analyzed with antibodies specific for Rac1, Cdc42, or Ras (Transduction Laboratories). Aliquots taken from supernatants prior to precipitation with GST fusion proteins were used to quantify total GTPases present in cell lysates.
Immunofluorescence.
Indirect immunofluorescence was performed as described by Brunet et al. (4). Briefly, cells were cultured and transfected on coverslips. For detection of Erk, cells were fixed and permeabilized by incubation with methanol-acetone (70:30) at −20°C for 15 min. For phosphorylated Erk (phospho-Erk) detection, cells were fixed in 10% paraformaldehyde at room temperature for 15 min, followed by permeabilization with methanol (10 min at −20°C). Both treatments allowed efficient detection of myc epitope-tagged MKP3C/S. The myc epitope was detected with a monoclonal antibody (9E10) and a secondary antibody conjugated to fluorescein isothiocyanate. Erk and phospho-Erk were detected with polyclonal antibodies (New England Biolabs) followed by biotin-conjugated secondary antibody and Texas red-labeled streptavidin (Amersham).
RESULTS
Erk is transiently activated following detachment of MEFs from the extracellular matrix.
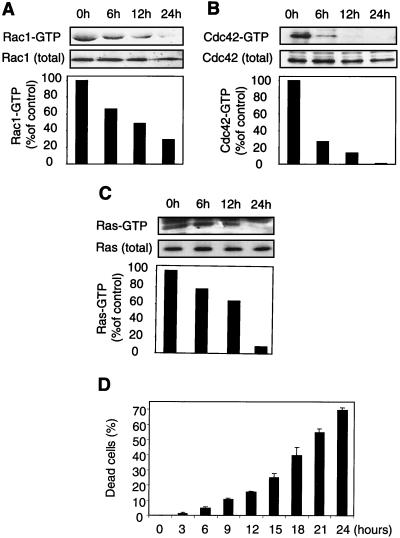
Mouse primary fibroblasts enter apoptosis upon loss of contact with the ECM. The cells can be rescued by activated forms of either Rac1 or Cdc42 GTPases (30). Consistent with this finding, cells placed in suspension lose the GTP-bound form of Rac1 and Cdc42 (Fig. 1A and B). This effect is not limited to Rho family GTPases, since the levels of GTP-bound Ras also decrease in anchorage-deprived cells (Fig. 1C). We observed a substantial decrease in the proportions of active GTPases after 12 h of culturing in suspension (85, 55, and 35% inhibition for Cdc42, Rac1, and Ras, respectively, in the experiment shown in Fig. 1) and a further decrease after 24 h relative to the values observed for cells grown attached to the substratum (0-h control point). As expected, the loss of GTP-bound forms of the Rho GTPases preceded a late manifestation of death of cells in suspension (15.5% ± 0.5% [mean and standard deviation {SD}] trypan blue-permeable cells at the 12-h time point) (Fig. 1D).
FIG. 1.
Loss of anchorage leads to inactivation of Ras family GTPases and apoptosis. Mouse primary fibroblasts were kept in suspension over a layer of 1% agar in culture medium supplemented with 10% FCS. At the indicated times, cells were collected and lysed, and the GTP-bound form of the GTPases was precipitated as described in Materials and Methods. (A) Rac1-GTP precipitated with GST-PAK1 and total Rac1 present in the lysates were analyzed by immunoblotting with an anti-Rac1 antibody. The histogram represents the relative proportions of GTP-loaded Rac1, with the 0-h time point being arbitrarily set as 100% loading. (B) Cdc42 was analyzed as described in panel A with an anti-Cdc42 antibody. (C) Ras-GTP loading was assayed as described in panels A and B using GST-RalGDSRBD to bind the GTPase. (D) At the indicated times, cells were collected, and their viability was assayed by trypan blue exclusion. The results represent means and SDs from three experiments.
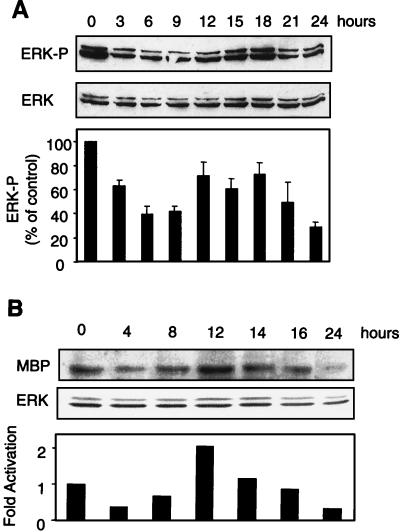
It was previously shown that inhibition of the Erk cascade inhibits apoptosis of cells deprived of anchorage (30). Consequently, we studied Erk activation following the loss of adherence (Fig. 2). The activated form of Erk was detected by immunoblotting with an antibody directed specifically against the phosphorylated forms of Erk1 and Erk2. Placing MEFs in suspension in medium containing 10% FCS led to an initial decrease in the level of phospho-Erk followed by a transient peak of the active form of Erk, whose onset varied between 12 and 15 h in different experiments (Fig. 2A). This increase in the level of phospho-Erk reflected increased kinase activity (Fig. 2B). While the activation was modest relative to the basal activity observed in adherent cells, it was highly reproducible. It is presumably physiologically significant, since PD98059, a pharmacological inhibitor of MEK1, the Erk-activating kinase, promotes the survival of anchorage-deprived cells (30; data not shown).
FIG. 2.
Erk phosphorylation status in anchorage-deprived MEFs. Cells were kept in suspension over a layer of 1% agar in culture medium supplemented with 10% FCS. (A) At the indicated times, cells were collected and lysed, and proteins were analyzed by immunoblotting. Activated Erk was detected by its phosphorylation status using an antibody specific for Erk1 and Erk2 doubly phosphorylated on Thr 202 and Tyr 204 (ERK-P). (B) At the indicated times, cells were collected, lysed, and electrophoresed in MBP-containing SDS-polyacrylamide gels. After renaturation, kinase activity was revealed by MBP phosphorylation in situ. Band intensities were quantified by scanning with a PhosphorImager and are represented as the ratio of activated Erk to total Erk, with the ratio found in exponentially grown adherent cells set as 100%. For the Western analysis, the results are presented as means and SDs from three experiments.
Nuclear translocation of activated Erk is essential for apoptotic signaling by dominant-negative forms of Cdc42 and Rac1.
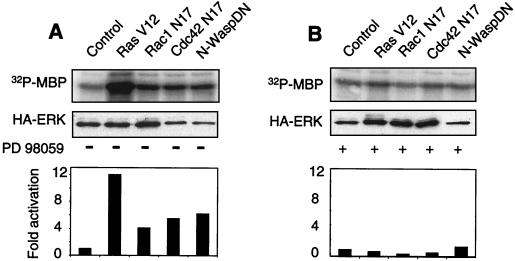
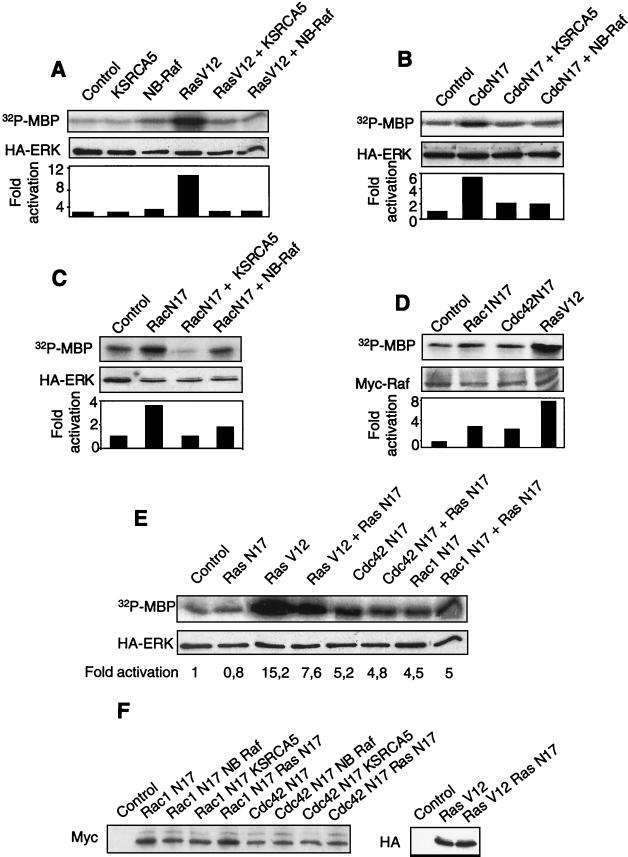
Inhibition of Rac1 or Cdc42 signaling in adherent cells mimics the effects of loss of adherence to the ECM (30). We therefore determined whether the extinction of these GTPases in monolayer cell cultures also led to the activation of Erk (Fig. 3A). Adherent MEFs were cotransfected with HA-tagged Erk2 and one of the following constructs: a dominant-negative mutant of Rac1 (Rac1N17); a dominant-negative mutant of Cdc42 (Cdc42N17); a Cdc42-interacting domain of N-WASP, which acts as an inhibitor of endogenous Cdc42 (N-WASP DN) (30); a constitutively activated form of H-Ras (RasV12), used as a positive control; or an empty pcDNA3 vector, used as a negative control. Erk2 activity was assayed 24 h later by in vitro phosphorylation of MBP following Erk immunoprecipitation with an anti-HA antibody. As expected, RasV12 strongly activated Erk, as did the inhibition of either Rac1 or Cdc42; however, the latter effect was never as strong as that obtained with RasV12. In all instances, the MEK1 inhibitor PD98509 blocked Erk activation (Fig. 3B). It is noteworthy that both inhibitors of Cdc42 signaling that we have used, which have totally different modes of action, led to Erk activation. It was previously shown that they are indistinguishable in inducing apoptosis in primary mouse fibroblasts (30).
FIG. 3.
Inhibition of Rac1 or Cdc42 signaling in adherent MEFs activates Erk. (A) Exponentially growing cells were cotransfected with vectors coding for an HA-tagged Erk2 construct and one of the following: dominant-negative form of Rac1 (Rac1N17), dominant-negative form of Cdc42 (Cdc42N17), N-WASP DN, constitutively active Ras (RasV12), or pcDNA3 as a negative control. At 24 h after transfection, cells were serum starved by culturing in medium supplemented with 1% FCS for 16 h and lysed, and HA-Erk2 was immunoprecipitated with anti-HA monoclonal antibody 12CA5. Kinase activity was assayed by in vitro phosphorylation of GST-MBP (upper panel). Erk2 activation was normalized (lower panel) relative to the total amount of transfected HA-Erk2, as quantitated by immunoblotting with antibody 12CA5 (middle panel). (B) Analysis like that in panel A, except that the MEK1 inhibitor PD98059 (20 μM) was included in the culture medium.
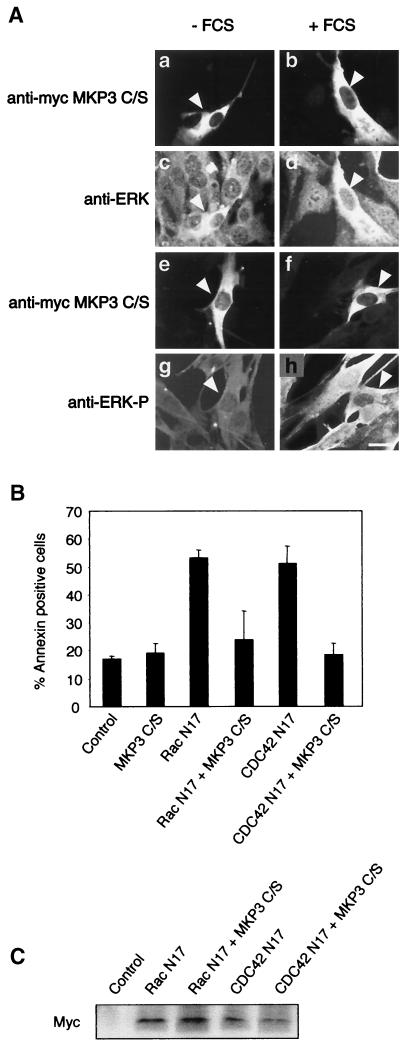
We wished to determine if proapoptotic Erk signaling required its nuclear translocation. We therefore assayed the effects of the retention of Erk in the cytoplasm by a mutant form of MAPK phosphatase 3, MKP3C/S. MKP3C/S localizes to the cytoplasm and, despite its lack of phosphatase activity, retains the ability to bind activated Erk. As a consequence, it anchors active Erk in the cytoplasm, preventing phosphorylation of the nuclear substrates of Erk but allowing phosphorylation of its cytoplasmic targets (4). We confirmed that, for control cells kept in low levels of serum, Erk is detected in the cytoplasm and there is little phospho-Erk labeling (Fig. 4A, panels c and g). Upon stimulation with 20% FCS, phospho-Erk accumulates in the nucleus within 10 min of treatment, while the maximal accumulation of total Erk in the nucleus occurs 2 h after stimulation (Fig. 4A, panels d and h). Under the same conditions, there is no detectable Erk translocation in cells transfected with MKP3C/S (Fig. 4A, panels b and d), but Erk activation takes place, as judged by cytoplasmic phospho-Erk-specific immunofluorescence (Fig. 4A, panels f and h). Notably, transfection of MKP3C/S protected cells from the apoptotic effects of dominant-negative forms of Rac1 or Cdc42 (Fig. 4B and C). These data confirm the requirement for Erk signaling in apoptosis induction in our experimental system (30). Furthermore, they strongly suggest that the nuclear substrates of Erk are involved in proapoptotic signaling.
FIG. 4.
Cytoplasmic retention of activated Erk inhibits RacN17- and Cdc42N17-induced apoptosis. (A) Adherent MEFs were transfected with myc epitope-tagged MKP3C/S. After 24 h, the cells were starved by culturing in medium containing 1% FCS for 24 h (panels a, c, e, and g) and then were stimulated with 20% serum for 10 min (panels f and h) or 2 h (panels b and d). Cells were stained with anti-ERK antibody (panels c and d) or anti-phospho-ERK antibody (panels g and h). The transfected cells (arrowheads) were identified by immunofluorescence detection of myc-MKP3C/S (panels a, b, e, and f). Bar, 10 μm. The results shown are representative of four independent experiments. (B) Adherent cells were cotransfected with the indicated constructs and truncated rat CD2 as a marker of transfection. Apoptosis in the transfected-cell population was assayed by the Annexin V binding assay. The results represent the means and SDs from two independent experiments performed in triplicate. (C) Western blot analysis of the levels of expression of the myc epitope-tagged GTPases with the different cotransfection combinations.
Dominant-negative mutants of Cdc42 and Rac1 activate Erk via Raf.
MEK activation by the Raf family of MAPK kinase kinases is sensitive to overexpression of the kinase domain of KSR, which forms a stable complex with MEK, thereby preventing its phosphorylation by Raf proteins (12). Similarly, in our system, transfection of the vector expressing only the kinase domain of mKsr-1 (CA5) strongly inhibited Erk activation by RasV12 in primary fibroblasts (Fig. 5A). Notably, KsrCA5 also inhibited Erk activation due to the extinction of Cdc42 (Fig. 5B) or Rac1 (Fig. 5C) signaling. These results suggested that a Raf protein was implicated in the signal emanating from dominant-negative mutants of Rac1 and Cdc42 and leading to Erk activation. To confirm this interpretation, we overexpressed a dominant-negative mutant of Raf, NB-Raf, which lacks the catalytic domain of the kinase. As expected (Fig. 5A), NB-Raf strongly inhibited Erk activation by RasV12. Likewise, this dominant-negative mutant of Raf inhibited Erk activation due to the extinction of Cdc42 (Fig. 5B) or Rac1 (Fig. 5C) signaling. To further confirm that the inhibition of Rho GTPases could result in Raf activation in primary fibroblasts, we tested the effects of dominant-negative mutants of Cdc42 and Rac1 on the kinase activity of a myc epitope-tagged B-Raf protein. The results (Fig. 5D) indicate that both mutants led to the activation of Raf, as measured by an in vitro kinase assay. Thus, the signal transduction pathway leading from the extinction of Cdc42 and Rac1 GTPases to Erk involves both Raf and MEK.
FIG. 5.
Inhibition of Rac1 or Cdc42 signaling in adherent MEFs activates Erk via the MAPK kinase kinase Raf. (A to C and E) Exponentially growing cells were cotransfected with a vector coding for an HA-tagged Erk2 construct and vector pcDNA3 (control) or a plasmid encoding either a constitutively active form of Ras (RasV12) (A) or a dominant-negative form of Cdc42 (CdcN17) (B), Rac1 (RacN17) (C), or Ras (RasN17) (E). Dominant-negative mutants of mKsr-1 (CA5) and B-Raf (NB-Raf), which prevent MEK activation by Raf proteins, were included as indicated. At 24 h after transfection, the cells were serum starved by culturing in medium containing 1% FCS for 16 h, lysed, and immunoprecipitated with anti-HA monoclonal antibody 12CA5. Erk2 kinase activity was assayed by in vitro phosphorylation of GST-MBP and normalized relative to the total amount of transfected HA-Erk, quantitated by Western blotting with antibody 12CA5. (D) Cells were transfected with vectors encoding the indicated GTPases or empty vector pcDNA3 as a negative control and a construct coding for myc epitope-tagged B-Raf. After 24 h, cells were starved for serum for 16 h, lysed, and incubated with anti-myc monoclonal antibody 9E10. Raf activity in the immunoprecipitate was measured by an indirect kinase assay based on serial additions of purified GST-MEK1, GST-Erk2, and GST-MBP. The total amount of myc epitope-tagged B-Raf protein was estimated by immunoblotting with antibody 9E10 and was used to normalize the kinase activity. (F) Western blot analysis indicating the level of expression of the GTPases in the different cotransfections. Rac1N17 and Cdc42N17 are tagged with a myc epitope, RasV12 is tagged with HA, and RasN17 is not tagged.
We next examined whether Ras, which acts upstream of Raf in growth factor and integrin signaling, was also responsible for Raf activation in the pathway linking GDP-bound Rho GTPases to Erk. To this end, we transiently coexpressed the dominant-negative form of Ras (RasN17) with the dominant-negative form of Cdc42 or Rac1 (Fig. 5E). The presence of RasN17 alone had no detectable effect on basal Erk activity. The construct had biological activity, since its coexpression with constitutively active Ras (RasV12) gave rise to 50% inhibition of Erk activation. However, the presence of RasN17 had no effect on Erk activation by the dominant-negative forms of Cdc42 and Rac1. We verified that there were no significant variations in the levels of expression of the transfected GTPases in the different cotransfections (Fig. 5F). Our data suggest, but do not formally prove (34), that Ras does not participate in the signal transduction pathway leading from dominant-negative forms of Cdc42 and Rac1 to the Raf-MEK-Erk cascade.
Akt is involved in survival signaling by Rho GTPases.
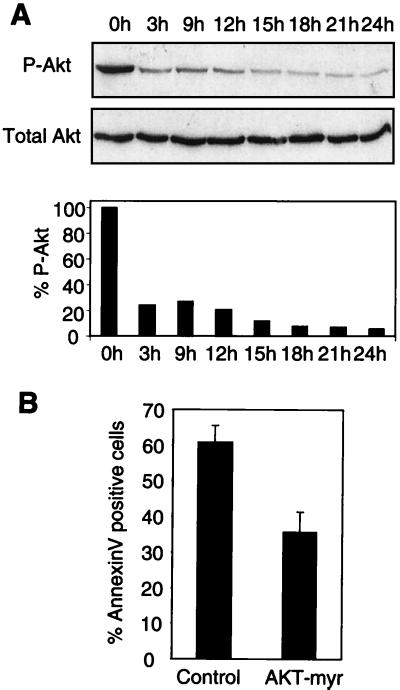
The serine/threonine kinase Akt is implicated in survival signaling due to adhesion of cells to the ECM (27). This implication led us to examine the effect of anchorage loss on the level of activation of endogenous Akt in MEFs (Fig. 6A), as judged by the relative amount of the enzyme phosphorylated on serine 473. The loss of attachment led to a rapid decrease in the level of this phosphorylated form of Akt in primary fibroblasts. In particular, the level of active Akt dropped to 10 to 15% of that present in exponentially growing adherent cells after 12 h of culturing in suspension. The loss of Akt activity compromised cell viability, since forced expression of the membrane-targeted, myristoylated form of the kinase significantly suppressed apoptosis in suspended MEFs (Fig. 6B).
FIG. 6.
Akt activity is regulated by anchorage. (A) Exponentially growing MEFs were deprived of anchorage by culturing in complete medium over a layer of 1% agar. At the indicated time points, cells were collected, lysed, and analyzed by immunoblotting with polyclonal antibodies directed against Akt phosphorylated on serine 473 (P-Akt) or Akt (total Akt). A Western blot and a histogram from a representative experiment are shown. (B) Exponentially growing MEFs were cotransfected with an expression vector encoding a myristoylated form of Akt (Akt-myr) or an empty vector as a control. After 24 h, the cells were trypsinized and placed in suspension over a layer of 1% agar for 18 h. Cell viability was assayed by Annexin V-fluorescein isothiocyanate labeling in the transfected population identified by anti-CD2 staining. Results represent the mean and range for two independent experiments.
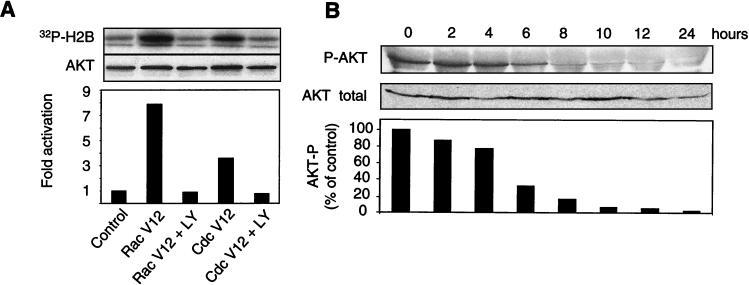
To determine if Rac1 and Cdc42 GTPases had any effect on the kinase activity of Akt, we coexpressed constitutively active forms of the GTPases (Rac1V12 and Cdc42V12) together with HA-tagged wild-type Akt in adherent MEFs (Fig. 7A). At 24 h after transfection, Akt was immunoprecipitated with the anti-HA antibody, and its activity was assayed in vitro. Both GTPases activated Akt in a manner dependent on PI(3)K, since the PI(3)K pharmacological inhibitor LY294002 totally abolished Akt activation by Rac1 and Cdc42.
FIG. 7.
Akt acts downstream of Rac1 and Cdc42. (A) HA-tagged Akt was expressed in exponentially growing MEFs together with a constitutively active form of Rac1 or Cdc42 or empty vector pcDNA3 as a control. Where indicated (+ LY), LY294002 (50 μM), a pharmacological inhibitor of PI(3)K, was added to the culture medium. After 24 h, HA-Akt was immunoprecipitated, and its activity was assayed in vitro by phosphorylation of histone H2B. (B) Adherent MEFs were cultured in complete medium containing 0.1 ng of C. difficile toxin B/ml. At the indicated times, the cells were lysed and analyzed by immunoblotting for the presence of Akt phosphorylated on serine 473 (P-Akt) and total Akt. The blots were scanned, and the relative level of phosphorlyated Akt was plotted as a percentage of the control (no toxin B treatment), arbitrarily set as 100%.
Next we examined whether the inhibition of Rho GTPases leads to diminished Akt activity. Clostridium difficile toxin B is a specific inhibitor of Rac1, Cdc42, and RhoA GTPases (24). Culturing of MEFs in medium containing 0.1 ng of toxin B/ml leads to detachment of cells within 24 h, followed by death (data not shown). The data presented in Fig. 7B show that this treatment also leads to a strong inhibition of Akt kinase activity, visible within 2 h of treatment and highly significant from 6 h onward. Although Rac1 and Cdc42 are not the only substrates of C. difficile toxin B, these results are consistent with the model in which the inhibition of these GTPases leads to a decrease in the activity of Akt. Taken together, these data show that PI(3)K and Akt act downstream of Rac1 and Cdc42 in the control of cellular survival following the loss of ECM contact.
Inhibition of Akt activates Erk.
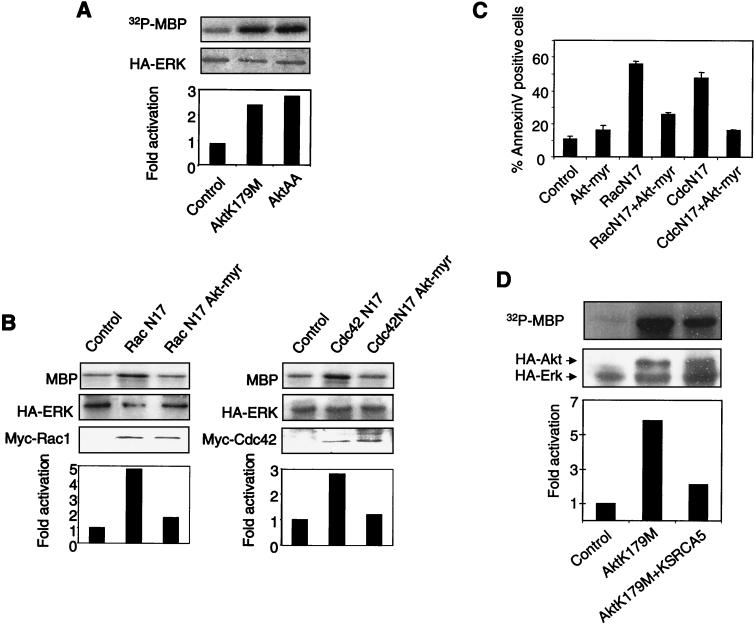
It has recently been reported that Akt can modulate the Erk cascade via inhibitory phosphorylation of both Raf-1 and B-Raf (20, 46, 59). It seemed possible that decreased Akt activity, resulting from the inhibition of Rho GTPases, could account for the Erk activation that we observed. To test this idea, we used two different dominant-negative Akt mutants: kinase-dead Akt K179M and Akt T308AS473A (AktAA), in which alanine residues replace the residues that are found to be phosphorylated in the active form of the enzyme and that are critical for the maintenance of Akt activity. The dominant-negative effect of these mutants was verified by their ability to inhibit wild-type Akt activity (data not shown). The two Akt mutants were expressed in adherent MEFs together with the HA-tagged Erk2 construct. At 24 h after transfection, Erk2 was immunoprecipitated, and its activity was assayed in vitro. As shown in Fig. 8A, both Akt mutants gave rise to increased Erk2 activity.
FIG. 8.
Dominant-negative Akt mutants lead to Erk activation. (A) MEFs were cotransfected with either of two Akt dominant-negative mutants, Akt K179M or Akt T308AS473A (AktAA), or an empty vector as a negative control. The cells were processed as described in the legend to Fig. 3A. (B) Cells were transfected with an HA-tagged Erk2 construct, a dominant-negative mutant of either Rac1 or Cdc42, or a myristoylated form of Akt (Akt-myr). At 24 h after transfection, cells were lysed, HA-Erk was immunoprecipitated, and its kinase activity was measured. (C) MEFs were cotransfected with a truncated rat CD2 antigen and either pcDNA3 as a negative control or a dominant-negative form of Rac1 or Cdc42 (RacN17 or CdcN17). A myristoylated form of Akt (Akt-myr) was also included where indicated. Cell survival was quantitated by Annexin V labeling of the transfected subpopulation identified by anti-CD2 staining. Results represent means and SDs for three experiments. (D) Cells were transfected with HA-tagged Erk2 and the HA-tagged Akt K179M mutant with or without m-Ksr1 (CA5). Erk2 kinase activity was assayed as described in the legend to Fig. 3A.
If inhibition of Akt leads to Erk activation and to apoptosis in the context of the lack of Rac1 or Cdc42 signaling, we reasoned that myristoylated form of Akt should prevent Erk activation and apoptosis induction by dominant-negative forms of these GTPases. To test this prediction, we coexpressed the dominant-negative form of either Cdc42 or Rac1 together with myristoylated Akt in MEFs. The constitutively active Akt indeed inhibited Erk activation (Fig. 8B) and promoted the survival (Fig. 8C) of cells expressing RacN17 or Cdc42N17 GTPase.
Since we have shown that inhibitory forms of Cdc42 and Rac1 control Akt activity but also contribute to the Erk kinase cascade on the level of Raf, it was important to test whether the Raf/MEK pathway was involved in the release of Erk inhibition by inactive Akt. We found that Erk activation by an inhibitory form of Akt was strongly diminished by mKsr-1 (CA5) (Fig. 8D). It would thus appear that attenuation of Rac1 or Cdc42 signaling, either by loss of anchorage or by overexpression of the dominant-negative forms of the GTPases, signals to Raf via Akt inhibition and thereby culminates in transient activation of Erk.
Inhibition of Akt, together with activation of the p53 tumor suppressor, is sufficient for the induction of apoptosis in primary fibroblasts.
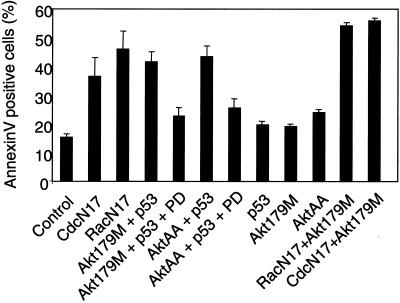
It was previously shown that the extinction of Rac1 or Cdc42 signaling led to the activation of Erk and of the p53 tumor suppressor and that both were necessary for the ensuing apoptosis (30). Here, we show that Erk activation is mediated by inhibition of Akt. In order to determine whether simultaneous activation of p53 and inhibition of Akt were sufficient to elicit the apoptotic response, we cotransfected MEFs with dominant-negative mutants of Akt and wild-type p53. Apoptosis in the transfected subpopulation of cells was quantified; it was identified by the expression of the cotransfected CD2 marker. As shown in Fig. 9, inhibition of Akt signaling together with p53 overexpression (Akt179M + p53 or AktAA + p53) led to an apoptotic response indistinguishable from that induced by inhibition of Cdc42 or Rac1, while neither p53 nor Akt mutants were significantly toxic on their own. The Erk cascade appears to be an effector of this apoptotic response, since death was alleviated by the concomitant presence of PD98509 (Fig. 9). Furthermore, Rho GTPases and Akt seem to lie on the same pathway of apoptosis signaling, since the simultaneous inhibition of both does not significantly increase the apoptotic response (Fig. 9, CdcN17 + Akt179M and RacN17 + Akt179M). Thus, simultaneous loss of Akt activity and p53 activation appear to be both necessary and sufficient for apoptosis induction by dominant-negative forms of Cdc42 and Rac1.
FIG. 9.
Inhibition of Akt, in conjunction with p53 activation, mimics apoptosis induction by dominant-negative forms of Rac1 and Cdc42. Exponentially growing MEFs were cotransfected with truncated rat CD2 and a combination of the following constructs, as indicated below the bars: a dominant-negative Cdc42 mutant (CdcN17), a dominant-negative Rac1 mutant (RacN17), wild-type p53 (p53), a kinase-dead Akt mutant (Akt179M), and an inactive Akt mutant (AktAA). Empty vector pcDNA3 was used as a control. Where indicated, the MEK inhibitor PD98059 (20 μM) was included in the culture medium. At 48 h after transfection, apoptotic cells in the transfected subpopulations were scored by Annexin V labeling. The data represent the means and SDs from three experiments.
Apoptotic response as a function of the intensity of Erk signaling.
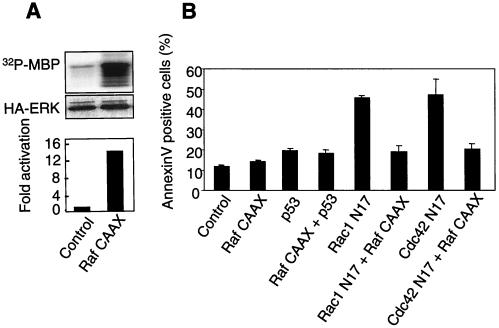
It has been shown that relatively modest Erk activation, which results from the inhibition of Rac1 and Cdc42 or Akt signaling, is required for the apoptotic response of primary mouse fibroblasts (30; this work). On the other hand, numerous reports in the literature show that Erk activation plays a protective role, including in the context of anoikis. A possible explanation for this discrepancy would be different cellular responses to modest and strong Erk cascade activation. We used a membrane-targeted form of Raf (Raf-CAAX) to test this hypothesis. As expected, expression of this construct in MEFs gives rise to strong constitutive Erk activation (Fig. 10A) without any apparent toxicity, either by itself or in conjunction with wild-type p53 expression. Furthermore, Raf-CAAX expression protects cells from apoptosis induced by inhibitory forms of Rac1 or Cdc42 GTPases (Fig. 10B). It would thus appear that while modest Erk activation is required for apoptosis in our experimental system, the more substantial intensity of Erk signaling is indeed protective, in agreement with data reported by others (31, 48).
FIG. 10.
High-intensity Erk signaling protects cells from apoptosis induced by Rac1 or Cdc42 inhibition. (A) Exponentially growing MEFs were cotransfected with Raf-CAAX- and HA-tagged Erk2-encoding vectors. After 24 h, cells were collected, and Erk activity was measured by an in vitro kinase assay after immunoprecipitation with an anti-HA antibody. (B) Cells were transfected with the indicated constructs. After 48 h, apoptosis was measured by Annexin V labeling of the transfected subpopulation. Data represent means and SDs of six measurements from two independent experiments.
DISCUSSION
Loss of anchorage leads to inactivation of multiple signal transduction pathways (recently reviewed in reference 2). Our results show that Rac1 and Cdc42 are key elements in controlling the ensuing apoptosis. Interestingly, there is no hierarchical organization of apoptosis signaling through these two pathways; rather, they appear to act in parallel (results not shown). We have used two different inhibitors of Cdc42 signaling: Cdc42N17 and N-WASP DN. Their modes of action are expected to be totally different: inhibition of the upstream exchange factors for Cdc42N17 and sequestration of the GTP-bound form of the GTPase for N-WASP DN. The fact that they give identical results both in assays of the induction of apoptosis (30) and in the activation of downstream signaling pathways (this work) strongly argues that their effects are physiologically significant.
We have identified two events required downstream of these GTPases, which together are necessary and sufficient for anoikis, namely, activation of the p53 tumor suppressor and inactivation of the serine/threonine kinase Akt. Both events are known to sensitize cells to apoptosis, but neither one alone is sufficient to kill primary cells. The mechanism of p53 activation during anoikis is currently under investigation. It is independent of the Erk cascade, since it is not altered in the presence of pharmacological inhibitors of MEK, PD98509 and UO126 (results not shown). In the present work, we dissected the signal transduction pathway leading from loss of the GTP-bound and, consequently, accumulation of the GDP-bound forms of the Rho GTPases to Erk via Akt. We have not investigated whether other Akt effectors known to participate in apoptosis control (reviewed in reference 11) are also instrumental in our experimental system. Rather, we have concentrated on the Erk pathway, whose role in the control of apoptosis remains controversial. Indeed, it is generally considered that Erk activation is associated with cellular survival, while the stress-activated jun N-terminal kinase cascade often correlates with induction of apoptosis (recently reviewed in reference 9).
The signaling pathways described in the present work have been elucidated largely, but not exclusively, in adherent cells by the use of appropriate dominant-negative mutants. Although all signaling cascades affected by placing cells in suspension are clearly not involved in cellular responses to the inhibition of Rho GTPases in a monolayer cell culture, we have identified a subset of these pathways which are similarly affected under both experimental conditions. These include the loss of GTP loading of Rho GTPases, activation of p53, inhibition of Akt signaling, and moderate Erk activation. Importantly, these signaling pathways are sufficient for the induction of apoptosis in adherent cells. It thus appears legitimate to use a simpler experimental system, based on a monolayer cell culture, to study some of the signaling pathways involved in the control of anoikis.
The role of p38, another stress-activated MAPK positively regulated by active Rac1 and Cdc42 GTPases, seems to depend on the experimental system and the isoform of p38 that is examined; however, some evidence suggests an antiapoptotic function for this signaling pathway, probably via modulation of NF-κB activity (23, 52). It has been reported that p38 activation correlates with cell survival of anchorage-deprived fibroblasts (30), in agreement with its reported role in promoting cell cycle progression in the same experimental system (43). However, apoptosis (either triggered by loss of attachment or inhibition of Rac1 or Cdc42 signaling) is not simply a consequence of p38 inhibition, since a pharmacological inhibitor of this kinase is not toxic to cells (30) (data not shown).
In our experimental system, Erk activation and nuclear translocation are clearly required for apoptosis. This situation is unusual but not without precedent (25, 57), suggesting that the cellular response to Erk activation depends strongly on the physiological context of the cell. For example, apoptosis caused by the loss of anchorage in the fibroblastic cell line CCL39 is inhibited by exogenous Erk activation via an estrogen-inducible chimeric protein, Raf-1–ER (31). Interestingly, Raf-1–ER signaling rescues epithelial MDCK cells from a variety of apoptotic stimuli, despite the fact that it also promotes apoptotic signaling via transforming growth factor β secretion (32). These data link Erk to cellular survival in this particular context of robust and sustained Erk activation. In contrast, it has been demonstrated (30) that modest Erk activation is essential for apoptosis in response to the loss of anchorage in primary mouse fibroblasts. This discrepancy is likely due to differences in cell type and physiology. One important element is the p53 tumor suppressor, which is essential for apoptosis resulting from the loss of anchorage of both immortalized and primary mouse fibroblasts. Accordingly, CCL39 cells appear to express a mutant form of this tumor suppressor (unpublished data). An additional difference between the two experimental systems is the intensity of Erk activation, and there is ample precedent for both the strength and the duration of Erk signaling determining cellular destiny (26, 44, 53, 58). Strong Erk activation, which rescues suspension-grown CCL39 and MDCK cells from anoikis (31, 32), also signals survival in adherent fibroblasts deprived of Rac1 or Cdc42 signaling. On the other hand, Erk activation in anchorage-deprived MEFs, which is associated with apoptosis, is modest, typically not exceeding a factor of 2 compared to the level observed in cells grown under normal, anchorage-dependent conditions.
The intensity of signaling through a kinase cascade is related in part to the origin of the signal. Our data clearly demonstrate that, in the case of Erk activation by the extinction of Rac1 or Cdc42 signaling, the signal contributes to the MAPK pathway at the level of Raf by a mechanism not inhibited by RasN17. We interpret the lack of Erk activation observed in the presence of the NB-Raf construct as being due to a direct inhibition of endogenous Raf, rather than to titration of Ras. Indeed, independent of its ability to bind Ras-GTP, the N terminus of Raf can also inhibit endogenous Raf through a direct interaction with the catalytic domain of the kinase, thereby mimicking intramolecular inhibition of the C-terminal catalytic domain by the N-terminal regulatory domain (10). Therefore, we assume that the mutant used in this and other studies (6, 17) is able to inhibit endogenous Raf, whatever the status of Ras GTP loading. Consequently, the results obtained with the NB-Raf mutant are not in contradiction with those obtained with the RasN17 mutant and are in agreement with interference of Akt with the Erk pathway at the level of Raf rather than Ras.
Most often, extracellular signals activate the Erk pathway via Ras activation, which recruits Raf to the membrane, where it is subsequently activated by phosphorylation (reviewed in reference 37). However, alternative mechanisms for Raf activation exist. For example, all three isotypes of protein kinase C (conventional, novel, and atypical) have been described to activate the Erk pathway either independently of Ras (50) or via Ras but through a mechanism insensitive to inhibition by the dominant-negative form of this GTPase (36).
Another mechanism of Ras-independent, Raf-dependent Erk activation involves signaling through PI(3)K (8, 28, 55). PI(3)K has been reported to act both upstream and downstream of Rho GTPases (reviewed in reference 3). It controls the activation of Rac1 (45), which in turn can activate the PAK family of serine/threonine kinases (33). Some members of this family can phosphorylate Raf-1, leading to the activation of the Erk cascade (28, 55). Certain steps in this pathway are not clear, however, since Rac1 has never been shown to activate Erk. On the other hand, PAK has recently been reported to play a major role in relaying anchorage-dependent protein kinase A signaling to Erk (22). Furthermore, PI(3)K has been reported to attenuate Raf activity through its downstream effector, Akt (20, 46, 59). Akt can physically interact with and phosphorylate both Raf-1 and B-Raf, on serine 259 for the former and on multiple residues for the latter, resulting in inhibition of their activity in a cell type- and differentiation stage-specific manner. In our experimental system, the phosphorylation of Raf1 on serine 259 by activated Akt can be demonstrated with 293 cells but not with MEFs (data not shown). This could be due to a technical problem of assay sensitivity, since MEFs express significantly lower levels of Raf than 293 cells. Alternatively, serine 259 phosphorylation might not be involved in the modulation of Raf activity by Akt in our experimental system. Our results strongly support the notion of a functional relationship between Akt and Raf in anoikis, without addressing the mechanism of this regulation. We show that, for mouse primary fibroblasts, the constitutively active forms of either Rac1 or Cdc42 activate Akt in a manner dependent on PI(3)K activity, confirming that the previously described link between these Rho GTPases and Akt (18, 38) also operates in primary cells. Furthermore, the loss of Akt activity in anchorage-deprived cells as well as in adherent cells upon inhibition of Cdc42 or Rac1 signaling, is instrumental in inducing apoptosis, since cell death is inhibited by forced expression of activated Akt. One downstream target of Akt signaling is the Erk cascade, more precisely, the MAPK kinase kinase Raf. Our results demonstrate that, at least for primary fibroblasts, activation of Erk following Akt downregulation is essential in apoptosis induced by the loss of Rho GTPase signaling.
Several questions are raised by the concomitant existence of both positive and negative signals leading from PI(3)K to Erk. It has been argued that such a mechanism might be important for the fine-tuning of the activation signal (49), implying that the signal intensity is a major determinant of the cellular response. Alternatively, a cell might not use all the transduction mechanisms available. In support of the latter idea, PI(3)K in adipocytes is stimulated similarly by exposure of cells to both insulin and platelet-derived growth factor (39), but only insulin leads to a substantial stimulation of Akt. Such specificity of signaling has been correlated with the distribution of PI(3)K lipid substrates, but it is likely to also depend on the sequestration of signaling molecules to scaffolding complexes (recently reviewed in reference 5).
Yet another consequence of the intricacies of the cross talk between several signaling pathways is exemplified by our results. The current paradigm for the Ras superfamily GTPases is their oscillation between the active, GTP-bound state and the inactive, GDP-bound form. However, the discovery of negatively regulated effectors, including the Erk cascade, sheds new light on the underlying mechanisms of signal transduction by these GTPases. Indeed, the loss of the GTP-loaded form leads to an alleviation of inhibitory signaling that ultimately results in the initiation of a positive signal. This argument does not necessarily imply interactions of the GDP-bound form with distinct molecular partners. Interestingly, an activity specific for the GDP-bound form of Ras has recently been described (54). An even more clear-cut situation exists in yeast, where GTP-bound and GDP-bound forms of the Ras-related small GTPase Bud1 bind to distinct partners and form different multiprotein complexes, both essential for bud site selection (42). Although our data do not support such a model in the regulation of anoikis, the results reported here delineate a novel signaling pathway, which initiates at Rho family GTPases and controls apoptosis via the PI(3)K-Akt and Erk cascades and the p53 tumor suppressor.
ACKNOWLEDGMENTS
We are grateful to A. Brunet, P. Chavrier, A. Hall, P. Lenormand, C. Marshall, C. Norbury, S. Roche, M. Vandromme, M. White, and E. Yonish-Rouach for various plasmids used in this work and to P. Boquet for the generous gift of C. difficile toxin B. We thank Damien Gregoire for help with the anoikis experiments, Pierre Travo for help with immunofluorescence microscopy, and Bob Hipskind for invaluable comments on the manuscript.
This work was supported by INSERM, CNRS, and Association pour la Recherche contre le Cancer (support given to U.H.). T.F.F. is the recipient of Career Development Award DAMD17-00-1-0214 and O.Z. is the recipient of a fellowship from Association pour la Recherche contre le Cancer.
REFERENCES
- 1.Alessi D R, Cohen P, Ashworth A, Cowley S, Leevers S J, Marshall C J. Assay and expression of mitogen-activated protein kinase, MAP kinase kinase, and Raf. Methods Enzymol. 1995;255:279–290. doi: 10.1016/s0076-6879(95)55031-3. [DOI] [PubMed] [Google Scholar]
- 2.Aplin A E, Howe A K, Juliano R L. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burack W R, Shaw A S. Signal transduction: hanging on a scaffold. Curr Opin Cell Biol. 2000;12:211–216. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 6.Busca R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne J P, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao T S, Byron K L, Lee K M, Villereal M, Rosner M R. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992;267:19876–19883. [PubMed] [Google Scholar]
- 8.Chaudhary A, King W G, Mattaliano M D, Frost J A, Diaz B, Morrison D K, Cobb M H, Marshall M S, Brugge J S. Phosphatidylinositol 3-kinase regulates raf1 through Pak phosphorylation of serine 338. Curr Biol. 2000;10:551–554. doi: 10.1016/s0960-9822(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 9.Cross T G, Scheel-Toellner D, Henriquez N V, Deacon E, Salmon M, Lord J M. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 10.Cutler R E, Jr, Stephens R M, Saracino M R, Morrison D K. Autoregulation of the Raf-1 serine/threonine kinase. Proc Natl Acad Sci USA. 1998;95:9214–9219. doi: 10.1073/pnas.95.16.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 12.Denouel-Galy A, Douville E M, Warne P H, Papin C, Laugier D, Calothy G, Downward J, Eychene A. Murine Ksr interacts with MEK and inhibits Ras-induced transformation. Curr Biol. 1998;8:46–55. doi: 10.1016/s0960-9822(98)70019-3. [DOI] [PubMed] [Google Scholar]
- 13.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 14.Franke T F, Cantley L C. Apoptosis. A Bad kinase makes good [news] Nature. 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- 15.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 16.Frisch S M, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia J, de Gunzburg J, Eychene A, Gisselbrecht S, Porteu F. Thrombopoietin-mediated sustained activation of extracellular signal-regulated kinase in UT7-Mpl cells requires both Ras-Raf-1- and Rap1-B-Raf-dependent pathways. Mol Cell Biol. 2001;21:2659–2670. doi: 10.1128/MCB.21.8.2659-2670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genot E M, Arrieumerlou C, Ku G, Burgering B M, Weiss A, Kramer I M. The T-cell receptor regulates Akt (protein kinase B) via a pathway involving Rac1 and phosphatidylinositide 3-kinase. Mol Cell Biol. 2000;20:5469–5478. doi: 10.1128/mcb.20.15.5469-5478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giancotti F G. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 20.Guan K L, Figueroa C, Brtva T R, Zhu T, Taylor J, Barber T D, Vojtek A B. Negative Regulation of the Serine/Threonine Kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- 21.Howe A, Aplin A E, Alahari S K, Juliano R L. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 22.Howe A K, Juliano R L. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol. 2000;2:593–600. doi: 10.1038/35023536. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov V N, Ronai Z. p38 protects human melanoma cells from UV-induced apoptosis through down-regulation of NF-kappaB activity and Fas expression. Oncogene. 2000;19:3003–3012. doi: 10.1038/sj.onc.1203602. [DOI] [PubMed] [Google Scholar]
- 24.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 25.Kauffman-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G I. Suppression of c-myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 26.Kerkhoff E, Rapp U R. High-intensity Raf signals convert mitotic cell cycling into cellular growth. Cancer Res. 1998;58:1636–1640. [PubMed] [Google Scholar]
- 27.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King A J, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall M S. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 29.Lassus P, Hibner U. Detection and quantification of apoptosis in transiently transfected adherent cells. Nucleic Acids Res. 1998;26:5233–5234. doi: 10.1093/nar/26.22.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassus P, Roux P, Zugasti O, Philips A, Fort P, Hibner U. Extinction of Rac1 and Cdc42Hs signaling defines a novel p53 dependent apoptotic pathway. Oncogene. 2000;19:2377–2385. doi: 10.1038/sj.onc.1203553. [DOI] [PubMed] [Google Scholar]
- 31.Le Gall M, Chambard J C, Breittmayer J P, Grall D, Pouyssegur J, Van Obberghen-Schilling E. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann K, Janda E, Pierreux C E, Rytomaa M, Schulze A, McMahon M, Hill C S, Beug H, Downward J. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 2000;14:2610–2622. doi: 10.1101/gad.181700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manser E, Leung T, Salihuddin H, Zhao Z S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 34.Marais R, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 35.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marais R, Light Y, Paterson H F, Mason C S, Marshall C J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 37.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 38.Nishida K, Kaziro Y, Satoh T. Anti-apoptotic function of Rac in hematopoietic cells. Oncogene. 1999;18:407–415. doi: 10.1038/sj.onc.1202301. [DOI] [PubMed] [Google Scholar]
- 39.Oatey P B, Venkateswarlu K, Williams A G, Fletcher L M, Foulstone E J, Cullen P J, Tavare J M. Confocal imaging of the subcellular distribution of phosphatidylinositol 3,4,5-trisphosphate in insulin- and PDGF-stimulated 3T3–L1 adipocytes. Biochem J. 1999;344 Pt 2:511–518. [PMC free article] [PubMed] [Google Scholar]
- 40.Ory S, Munari-Silem Y, Fort P, Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113:1177–1188. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- 41.Papin C, Denouel-Galy A, Laugier D, Calothy G, Eychene A. Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J Biol Chem. 1998;273:24939–24947. doi: 10.1074/jbc.273.38.24939. [DOI] [PubMed] [Google Scholar]
- 42.Park H O, Bi E, Pringle J R, Herskowitz I. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc Natl Acad Sci USA. 1997;94:4463–4468. doi: 10.1073/pnas.94.9.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philips A, Roux P, Coulon V, Bellanger J M, Vie A, Vignais M L, Blanchard J M. Differential effect of Rac and Cdc42 on p38 kinase activity and cell cycle progression of nonadherent primary mouse fibroblasts. J Biol Chem. 2000;275:5911–5917. doi: 10.1074/jbc.275.8.5911. [DOI] [PubMed] [Google Scholar]
- 44.Qui M S, Green S H. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 45.Ren X D, Schwartz M A. Regulation of inositol lipid kinases by Rho and Rac. Curr Opin Genet Dev. 1998;8:63–67. doi: 10.1016/s0959-437x(98)80063-4. [DOI] [PubMed] [Google Scholar]
- 46.Rommel C, Clarke B A, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos G D, Glass D J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 47.Roux P, Gauthier-Rouviere C, Doucet-Brutin S, Fort P. The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Curr Biol. 1997;7:629–637. doi: 10.1016/s0960-9822(06)00289-2. [DOI] [PubMed] [Google Scholar]
- 48.Rytömaa M, Lehmann K, Downward J. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene. 2000;19:4461–4468. doi: 10.1038/sj.onc.1203805. [DOI] [PubMed] [Google Scholar]
- 49.Scheid M P, Woodgett J R. Protein kinases: six degrees of separation? Curr Biol. 2000;10:R191–R194. doi: 10.1016/s0960-9822(00)00349-3. [DOI] [PubMed] [Google Scholar]
- 50.Schonwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz M A, Baron V. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr Opin Cell Biol. 1999;11:197–202. doi: 10.1016/s0955-0674(99)80026-x. [DOI] [PubMed] [Google Scholar]
- 52.Schwenger P, Alpert D, Skolnik E Y, Vilcek J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation. Mol Cell Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart S, Guan K L. The dominant negative Ras mutant, N17Ras, can inhibit signaling independently of blocking Ras activation. J Biol Chem. 2000;275:8854–8862. doi: 10.1074/jbc.275.12.8854. [DOI] [PubMed] [Google Scholar]
- 55.Sun H, King A J, Diaz H B, Marshall M S. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr Biol. 2000;10:281–284. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- 56.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 57.van den Brink M R, Kapeller R, Pratt J C, Chang J H, Burakoff S J. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J Biol Chem. 1999;274:11178–11185. doi: 10.1074/jbc.274.16.11178. [DOI] [PubMed] [Google Scholar]
- 58.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]