Abstract
Background
During the COVID-19 pandemic, efforts to maintain solid-organ transplantation have continued, including the use of SARS-CoV-2–positive heart donors.
Methods
We present our institution's initial experience with SARS-CoV-2–positive heart donors. All donors met our institution's Transplant Center criteria, including a negative bronchoalveolar lavage polymerase chain reaction result. All but 1 patient received postexposure prophylaxis with anti-spike monoclonal antibody therapy, remdesivir, or both.
Results
A total of 6 patients received a heart transplant from a SARS-CoV-2–positive donor. One heart transplant was complicated by catastrophic secondary graft dysfunction requiring venoarterial extracorporeal membrane oxygenation and retransplant. The remaining 5 patients did well postoperatively and were discharged from the hospital. None of the patients had evidence of COVID-19 infection after surgery.
Conclusion
Heart transplants from SARS-CoV-2 polymerase chain reaction–positive donors are feasible and safe with adequate screening and postexposure prophylaxis.
Efforts to maintain solid-organ transplantation during the COVID-19 pandemic include the use of nonlung solid organs from SARS-CoV-2–positive donors, which have been described with good results [1]. However, COVID-19 is known to cause cardiovascular complications such as myocardial injury, myocarditis, myocardial infarction, arrhythmias, cardiomyopathies, and heart failure [2]. We present a case series of heart recipients from SARS-CoV-2–positive donors with non–COVID-19-related deaths at our institution, including 1 case complicated by catastrophic secondary graft dysfunction requiring venoarterial extracorporeal membrane oxygenation (VA-ECMO) and retransplant. This study was approved by Mayo Clinic's institutional review board.
Case Presentations
Of note, all patients underwent orthotopic heart transplantation from SARS-CoV-2–positive donors that met Mayo Clinic's Transplant Center criteria, including a negative bronchoalveolar lavage (BAL) polymerase chain reaction (PCR) result. All patients, except patient 2, received postexposure prophylaxis (PEP) with anti-spike monoclonal antibody therapy, remdesivir, or both.
Patient 1
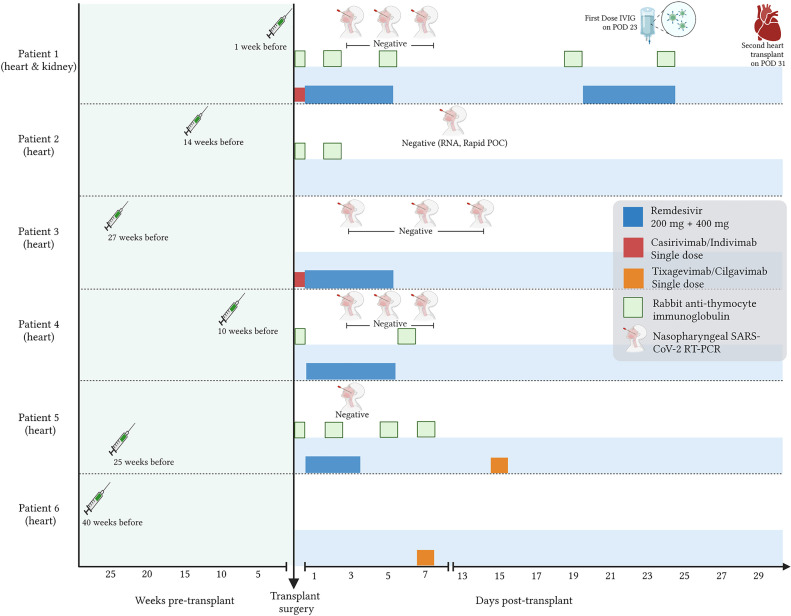
A 65-year-old man presents with end-stage heart failure secondary to ischemic heart disease and dialysis-dependent end-stage renal disease (Table 1 ). His medical history included cardiac arrest requiring an implantable cardioverter-defibrillator (ICD), atrial fibrillation, hypertension, multiple prior ischemic strokes with residual right-sided hemiparesis, obstructive sleep apnea (OSA), and long-standing insulin-requiring type 2 diabetes mellitus (DM2). He received his third dose of the BNT162b2 COVID-19 vaccine 1 week before surgery with a positive antibody titer (spike protein antibody >250 U/mL) pretransplant (Fig 1 ).
Table 1.
Characteristics of SARS-CoV-2–Positive Heart Transplant Recipients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Sex, age, y, ethnicity/race | Male, 65, White | Male, 56, White | Female, 66, White | Male, 41, White | Female, 47, Asian (Indian) | Male, 41, White |
| Organ(s) transplanted (UNOS status) | Heart (4) and kidney | Heart (4) | Heart (3) | Heart (2) | Heart (6) | Heart (4) |
| Previous cardiac surgeries/procedure | CABG | ICD and cardiac resynchronization therapy device | Tricuspid valve repair. 3 ablations for AVNRT and atrial fibrillation |
ICD and intra-aortic balloon pump × 2 | None | ICD |
| Pretransplant organ failure data | ||||||
| Ejection fraction (%) | 18 | 38 | 45 | 19 | 15 | 25 |
| Cardiac index (L/min/m2) | 1.96 | 2.20 | 1.72 | 2.46 | 2.10 | 2.31 |
| Glomerular filtration rate stage | Stage 4 | Stage 2 | Stage 3a | Stage 2 | Stage 3 | Stage 1 |
| COVID-19 data | ||||||
| COVID-19 vaccination status | 3 doses: third dose (BNT162b2) 1 wk prior | 2 doses: second dose (BNT162b2) 14 wk prior |
2 doses: second dose (mRNA-1273) 27 wk prior | 3 doses: third dose (mRNA-1273) 10 wk prior | 3 doses: third dose (BNT162b2) 25 wk prior | 3 doses: third dose (mRNA-1273) 40 wk prior |
| Previous COVID-19 infection | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| SARS-CoV-2 testing posttransplant | Negative on PODs 3.5,7 | Negative on POD 9 | Negative on PODs 3, 7, 14 | Negative on PODs 3, 5, 7 | Negative on POD 3 | Negative on POD 18, 33 |
| Transplant | ||||||
| Cardiopulmonary bypass time (min) | 134 | 164 | 163 | 112 | 236 | 230 |
| Total donor organ ischemic time (min) | 204 | 170 | 157 | 180 | 201 | 97 |
| Immunosuppressive treatment | ||||||
| Induction | rATG | rATG, methylprednisolone | Basiliximab, methylprednisolone | rATG | rATG, methylprednisone | Basiliximab, methylprednisolone |
| Regimen | Mycophenolate, prednisone, tacrolimus | Mycophenolate, prednisone, tacrolimus | Mycophenolate, prednisone, tacrolimus | Mycophenolate, prednisone, tacrolimus | Mycophenolate, prednisone, tacrolimus | Mycophenolate, prednisone, tacrolimus |
| Outcome | Heart retransplant on POD 31. Biopsy of explanted heart showed diffuse myocyte injury and pericarditis. Death on POD 156 | Discharged home on POD 34 | Discharged to an inpatient rehabilitation center on POD 35. Discharged home on POD 48. Third dose of mRNA-1273 3 mo after transplant | Discharged home on POD 11 | Discharged home on POD 21. Fourth dose of BNT162b2 vaccine 2 wk after transplant | Discharged home on POD 9 |
| Post-transplant endomyocardial biopsy* | Total: 3. All negative for rejection |
Total: 11. No. 5 showed moderate ACMR The rest showed mild ACMR |
Total: 10. No. 1, 3, 7, 8, 9 showed mild ACMR. The rest were negative |
Total: 10. No. 5, 6, 7, 8, 9 showed mild ACMR. The rest were negative. |
Total: 6. All negative for rejection |
Total: 4. All showed mild ACMR |
ACMR, acute cell-mediated rejection; AVNRT, atrioventricular nodal reentrant tachycardia; CABG, coronary artery bypass graft; EMB, endomycardial biopsy; ICD, implantable cardioverter-defibrillator; POD, postoperative day; rATG, rabbit antithymocyte immunoglobulin; UNOS, United Network for Organ Sharing.
For patient 1, we consider EMBs obtained after his second heart transplant.
Fig 1.
COVID-19 vaccination, prophylaxis, and testing in heart transplant recipients from SARS-CoV-2–positive donors.
The patient underwent a combined heart-kidney transplant from a SARS-CoV-2-positive donor with a history of nonsevere SARS-CoV-2 infection 2 months previous. The donor tested positive for SARS-CoV-2 via nasopharyngeal PCR 1 day before surgery and had a negative BAL PCR on the day of procurement. Donor vaccination status was unknown (Table 2 ). The heart transplant was performed from 623 nautical miles away with a total donor organ ischemic time of 204 minutes. Initially, moderate primary graft dysfunction was managed with pressors rapidly recovering to a left ventricular ejection fraction (LVEF) of 60% on postoperative day (POD) 5.
Table 2.
Characteristics of SARS-CoV-2–Positive donors
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | Donor 6 | |
|---|---|---|---|---|---|---|
| Sex, age range y, ethnicity/race | Female, 30-40, White | Female, 40-50, White | Male, 20-30, African American | Male, 30-40, White | Female, 40-50, African American | Male, 30-40, White |
| Organ(s) donated | Heart and kidney | Heart | Heart | Heart | Heart | Heart |
| Cause of death | Traumatic | Nontraumatic | Traumatic | Traumatic | Traumatic | Traumatic |
| Medical history | Hypertension Alcohol abuse |
Alcohol abuse Alcoholic cirrhosis Chronic pancreatitis Diabetes mellitus |
None | Chronic hepatitis C Drug abuse Mild coronary artery disease |
Chronic smoker | Chronic smoker Heavy alcohol use |
| Organ data | ||||||
| Cardiac arrest/downtime (min) | Yes (49) | No | No | Yes (50) | No | No |
| CPR administered (min) | Yes (34) | No | No | Yes (20) | No | No |
| LVEF, % | 60 | 61 | 58 | 60 | 60 | 84 |
| Kidney Donor Profile Index, % | 41 | 62 | 45 | 57 | 60 | 9 |
| COVID-19 data | ||||||
| COVID-19 status | NP RT-PCR: positive (1 d prior) BAL RT-PCR: negative (0 d prior) NP RT-PCR: negative (0 d prior) |
NP RT-PCR: negative (5 d prior) BAL RT-PCR: negative (5 d prior) NP RT-PCR: positive (5 d prior) NP RT-PCR: negative (3 d prior) NP RT-PCR: negative (2 d prior) BAL RT-PCR: negative (2 d prior) |
NP RT-PCR: negative (4 d prior) BAL RT-PCR: negative (2 d prior) NP RT-PCR: positive (1 d prior) |
NP antigen: negative (2 d prior) NP RT-PCR: positive (2 d prior) BAL RT-PCR: negative (2 d prior) |
NP RT-PCR: positive (7 d prior) NP RT-PCR: negative (3 d prior) BAL RT-PCR: negative (2 d prior) |
NP RT-PCR: negative (2 d prior) BAL RT-PCR: negative (2 d prior) NP RT-PCR: positive (1 d prior) |
| Previous COVID-19 infection | Yes, 2 mo prior, symptoms not specified | Unknown |
Unknown | Unknown | Unknown | Unknown |
| Vaccination status | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Other infectious risk factors | CMV IgG + EBV IgG + |
EBV IgG + | CMV IgG + EBV IgG + |
Anti-HCV: positive HCV NAAT: negative EBV IgG + |
Anti-CMV: positive EBV IgG + sputum culture: MRSA + |
EBV IgG + EBNA + |
| Laboratories* | ||||||
| WBC (thousand/mcL) | 15.4 | - | 14.2 | 15.1 | 6.0 | 13.7 |
| CPK (µ/L) | 2 | - | - | 253 | 568 | - |
| CKMB (ng/mL) | 8.8 | - | 12.3 | 5.4 | - | 534 |
| Troponin T (ng/mL) | 24 | - | 202 | 138 | 247 (Troponin I) | 0.031 (Troponin I) |
BAL, bronchoalveolar lavage; CMV, cytomegalovirus; CPR, cardiopulmonary resuscitation; EBV, Epstein-Barr virus; EBNA, Ebstein-Barr Nuclear Antigen; HCV, hepatitis C virus; Ig, immunoglobulin; LVEF, left ventricular ejection fraction; MRSA, methicillin-resistant Staphylococcus aureus; NAAT, nucleic acid amplification test; NP, nasopharyngeal; RT-PCR, reverse transcriptase-polymerase chain reaction; WBC, white blood cell count.
Laboratories: most recent value before procurement.
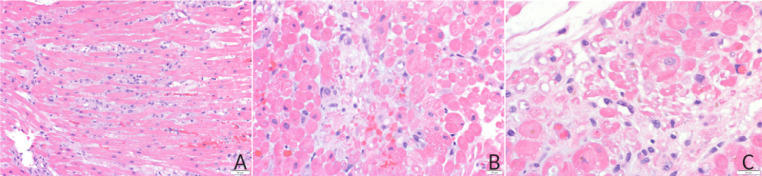
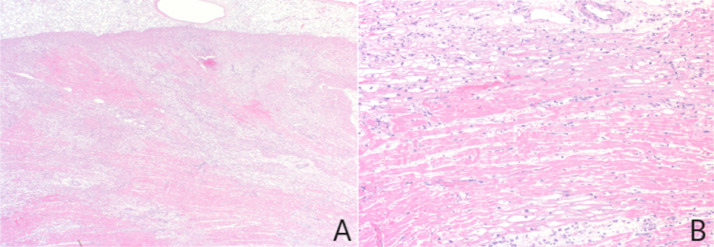
One week later, he developed acute hypotension that persisted despite conservative treatment. A repeat transthoracic echocardiogram demonstrated significant right ventricular (RV) wall thickening and dysfunction and a new decline in LVEF (39%). Given concerns for acute cellular rejection, he received rabbit antithymocyte globulin and 1 plasmapheresis session. During emergent cardiac catheterization, the patient experienced cardiac arrest requiring VA-ECMO and placement of an Impella left ventricular assist device. Endomyocardial biopsy revealed atypical lymphohistiocytic myocarditis with predominant histiocytes and patchy myocyte necrosis without evidence of cellular or antibody-mediated rejection, Quilty lesions, or ischemia (Fig 2 ). Treatment with remdesivir, intravenous immunoglobulin, and high-dose steroids was initiated on the premise that SARS-CoV-2 contributed to the patient's myocarditis and cardiogenic shock of unclear origin. To assess for the presence of SARS-CoV-2, PCR from BAL and institution-based, investigational PCR from whole blood and heart tissue were performed and negative. Given persistent allograft heart dysfunction with marked troponin elevation, the patient underwent repeat heart transplantation 32 days after index surgery. The explant specimen showed diffuse and transmural ischemic injury globally in both ventricles (Fig 3 ). The pattern of injury was most in keeping with global hypoperfusion.
Fig 2.
Transplant endomyocardial biopsy histopathology. (A) Lymphohistiocytic infiltrate diffusely involving the myocardial interstitium. (B) Focal piecemeal myocyte necrosis with (C) myocyte dropout.
Fig 3.
Explant specimen histopathology. (A) Low-power photomicrograph showing diffuse and transmural ischemic changes consisting of patchy sarcoplasmic vacuolization and acute myocardial infarction. (B) High-power photomicrograph showing myocyte vacuolization with areas of contraction band necrosis.
This second transplant was complicated by pulmonary hypertension and RV dysfunction, requiring VA-ECMO for 2 days. Four months after surgery, he had an LVEF of 58% and mild to moderately reduced RV systolic function. The patient remained severely deconditioned with multiple complications, including biopsy-confirmed acute tubular necrosis of the kidney allograft requiring dialysis, myelosuppression, multiple line-related bacteremias, and a sacral pressure ulcer requiring surgical intervention. He was eventually transitioned to comfort care and died on posttransplant day 157.
Patient 2
A 55-year-old man with end-stage heart failure secondary to nonischemic dilated cardiomyopathy underwent a heart transplant and an automatic ICD system explantation. His medical history included atrial fibrillation, ventricular tachycardia (VT) with a cardiac resynchronization therapy device, DM2, mild renal insufficiency, and OSA. He received his second SARS-CoV-2 vaccine 14 weeks before the transplant. His hospitalization was unremarkable, and he was discharged home on POD 34.
Patient 3
A 66-year-old woman with Ebstein anomaly after repair presented with progressive right-sided and systolic heart failure and tricuspid and pulmonary valve regurgitation. Her medical history included nonsustained VT, chronic kidney disease, previous recurrent atrial ablations, and partial pericardiectomy. She received her second SARS-CoV-2 vaccine 6 months before the transplant. One day after surgery, she developed a fever with primary graft dysfunction (ejection fraction 45%) and severe RV dysfunction that improved with inotropic therapy. Hospital stay was prolonged by pericardial and pleural effusions, non–dialysis-requiring acute kidney injury, Clostridioides difficile colitis, and bilateral iliopsoas hematomas. She was discharged from the hospital on POD 48.
Patient 4
A 41-year-old man with end-stage cardiac sarcoidosis with biventricular congestive heart failure on chronic mycophenolate and prednisone presented for a heart transplant. His medical history included liver and pulmonary sarcoidosis, pulmonary hypertension, arrhythmia requiring an ICD, OSA, steroid-induced DM2, and dyslipidemia. The recipient received his third SARS-CoV-2 vaccine 10 weeks before the transplant. He underwent a heart transplant with closure of a patent foramen ovale and de Vega tricuspid annuloplasty of the cardiac allograft. He received 1 week of empirical antibiotics postoperatively for pretransplant fever attributed to occult bacteremia and was discharged from the hospital on POD 11.
Patient 5
A 47-year-old woman with peripartum cardiomyopathy status after dual-chamber ICD implant with progressive decline in LVEF and recent episodes of VT presented for a heart transplant. Her medical history included hepatic steatosis, hyperlipidemia, chronic kidney disease, and gestational diabetes. She received her third SARS-CoV-2 vaccine 25 weeks before the transplant. Her postoperative course was complicated by dialysis-requiring acute kidney injury and retroperitoneal hematoma. She was discharged from the hospital on POD 21.
Patient 6
A 41-year-old man with hypertrophic cardiomyopathy (TNNT-2 mutation) and LV dysfunction (ejection fraction 25%) presented for a heart transplant. His medical history included refractory VT on chronic amiodarone, DM2, hypertension, dyslipidemia, obesity, and OSA. The recipient received his third SARS-CoV-2 vaccine 40 weeks before the transplant. He was admitted to the cardiac intensive care unit 3 weeks before the transplant because of cardiogenic shock requiring milrinone and then underwent a heart transplant with explantation of an automatic ICD. The total donor organ ischemic time included 47 minutes of first cold ischemic time, 268 minutes of Organ Care System Heart perfusion, and 50 minutes of second cold ischemic time. His postoperative course was uneventful, and he was discharged from the hospital on POD 9. On follow-up, he presented with a groin seroma, and his most recent cardiac echocardiogram reported only mild tricuspid valve regurgitation.
Discussion
The Mayo Clinic (Arizona, Florida, Minnesota) has developed institutional guidelines for accepting SARS-CoV-2–positive heart donors. Inclusion criteria include a non–COVID-19 cause of death, a lapse of at least 21 days since symptom onset or positive PCR for donors with previous COVID-19 infection, or asymptomatic status and negative BAL/trachea PCR for donors with an incidental nasopharyngeal PCR-positive test. The heart donor must have normal cardiac function, normal wall thickness, and a nonsignificantly elevated or normal troponin.
Patient 1 experienced posttransplant biventricular failure, elevated troponins, and inflammatory markers. Initially, the differential diagnosis included allograft rejection and infectious myocarditis, which were not supported by pathology specimens. Other causes of graft dysfunction, including acute thrombosis, pulmonary hypertension, and coronary vasculopathy, were also appropriately ruled out [3]. It remains possible that this complication was a sequela of prior COVID-19 infection of the donor heart or recent COVID-19 vaccination [4]. After extensive workup, multisystem inflammatory syndrome in adults (MIS-A) was proposed as a diagnosis of exclusion.
Multisystem inflammatory syndrome is a rare and severe complication that follows SARS-CoV-2 infection in children or, less commonly, in adults [5]. Its diagnostic criteria include the involvement of 1 or more extrapulmonary organs, laboratory evidence of inflammation, and exclusion of other possible causes [5]. Multisystem inflammatory syndrome in adults can present as a cardiogenic shock in patients with prior COVID-19 infection [6]. This condition has recently been associated with COVID-19 vaccination (MIS-V) [7]. Reliable viral identification strategies should be carried out to rule out a concomitant subclinical SARS-CoV-2 infection at the time of vaccination [7]. Vaccination is highly recommended for solid-organ transplant patients. However, because of the possibility of this rare complication, the timing of vaccination may require more study. Prompt identification and immunomodulatory management of MIS-A and MIS-V are key to a favorable outcome [7]. In this case, the treatment also included a second heart transplant from a SARS-CoV-2–negative donor.
In our case series, 6 patients underwent isolated or combined solid-organ transplants from a SARS-CoV-2 PCR-positive donor after a systematic pretransplant screening protocol, PEP, and standard immunosuppressive care. Notable differences between patient 1 and those who preserved heart allograft function are that the former received 5 doses of antithymocyte globulin (to spare calcineurin inhibitor initiation in the setting of allograft kidney injury), had a more recent SARS-CoV-2 vaccination, and a higher total donor organ ischemic time (Fig 1).
The COVID-19 pandemic dramatically affected the thoracic organ transplant rate by disrupting the waitlist process [8]. Because of the growing need for a pool of organ donors, screening protocols were developed in mid-2020 to meet this demand while ensuring organ viability and mitigating the risk of SARS-CoV-2 infection from a potential infected donor. Currently, all potential organ donors are screened with PCR from the respiratory tract specimens, with lower respiratory tract samples in lung donors [9]. Ideally, recipients should have completed a vaccination series against SARS-CoV-2 at least 2 weeks before the transplant [9].
Although donor-derived infections have occurred in lung transplant recipients [10], virus transmission between nonlung organ–infected donors and uninfected recipients has not been reported [1]. The present case series supports this because none of the patients had evidence of acute COVID-19 infection after transplantation. A study that included solid-organ donors with positive lower respiratory samples also reported no transmission [11]. Although promising, it is unclear if this is due to detection protocols or low viral infectivity.
Several reports have been published about SARS-CoV-2–positive heart donors with satisfactory outcomes [1,12]. After the transplant surgery, all recipients who received COVID-19 treatment and maintenance immunosuppression tested negative for SARS-CoV-2 and maintained adequate graft function. Vaccination status was not reported for any recipient.
Conclusions
Nonlung organ transplants from SARS-CoV-2 PCR-positive donors are feasible and safe with adequate screening and PEP. None of the patients had evidence of COVID-19 infection after surgery. Although rare, MIS-A and MIS-V are potentially unexpected and serious complications that raise the question of whether transplant centers should propose recommendations surrounding the timing of donor infection and recipient vaccination in relation to transplant to optimize recipient outcomes.
DISCLOSURES
The Mayo Clinic Foundation provided institutional support for article processing charges.
References
- 1.Neidlinger NA, Smith JA, D'Alessandro AM, et al. Organ recovery from deceased donors with prior COVID-19: a case series. Transpl Infect Dis. 2021;23:e13503. doi: 10.1111/tid.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adu-Amankwaah J, Mprah R, Adekunle AO, Noah MLN, Adzika GK, Machuki JO, et al. The cardiovascular aspect of COVID-19. Ann Med. 2021;53:227–236. doi: 10.1080/07853890.2020.1861644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNamara D, Di Salvo T, Mathier M, Keck S, Semigran M, Dec GW. Left ventricular dysfunction after heart transplantation: incidence and role of enhanced immunosuppression. J Heart Lung Transplant. 1996;15:506–515. [PubMed] [Google Scholar]
- 4.Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am J Cardiol. 2021;157:146–148. doi: 10.1016/j.amjcard.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037–3049. doi: 10.1016/j.vaccine.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coll MD, Yanamandala M, Ferro EG, Nutt CT, Wei EQ, Wang CJ, et al. Early immunosuppression and rapid recovery of cardiogenic shock in multisystem inflammatory syndrome from convalescent COVID-19. JACC Case Rep. 2021;3:1403–1408. doi: 10.1016/j.jaccas.2021.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar KP, Nune A, Ish P, Botchu R, Shashidhara MK, Jain VK. Multisystem inflammatory syndrome after SARS-CoV-2 vaccination (MIS-V), to interpret with caution. Postgrad Med J. 2022;98 doi: 10.1136/postgradmedj-2021-140869. e91. d. [DOI] [PubMed] [Google Scholar]
- 8.Pasrija C, Shah A, Kaczorowski DJ, Lau CL. Cardiac and pulmonary transplant considerations during COVID-19 pandemic. Innovations. 2020;15:314–316. doi: 10.1177/1556984520937007. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health. Special considerations in solid organ transplant, hematopoietic stem cell transplant, and cellular immunotherapy candidates, donors, and recipients, <https://www.covid19treatmentguidelines.nih.gov/special-populations/transplant/>; 2023 [accessed 09.03.23].
- 10.Kaul DR, Valesano AL, Petrie JG, Sagana R, Lyu D, Lin J, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21:2885–2889. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaussen A, Hornby L, Rockl G, O'Brien S, Delage G, Sapir-Pichhadze R, et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105:1405–1422. doi: 10.1097/TP.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 12.Dhand A, Gass A, Nishida S, Kai M, Berger K, Wolf D, et al. Successful transplantation of organs from a deceased donor with early SARS-CoV-2 infection. Am J Transplant. 2021;21:3804–3805. doi: 10.1111/ajt.16706. [DOI] [PMC free article] [PubMed] [Google Scholar]