Abstract
Background
Bacterial and viral infections can occur with SARS‐CoV‐2 infection, but prevalence, risk factors, and associated clinical outcomes are not fully understood.
Methods
We used the Coronavirus Disease 2019‐Associated Hospitalization Surveillance Network (COVID‐NET), a population‐based surveillance system, to investigate the occurrence of bacterial and viral infections among hospitalized adults with laboratory‐confirmed SARS‐CoV‐2 infection between March 2020 and April 2022. Clinician‐driven testing for bacterial pathogens from sputum, deep respiratory, and sterile sites were included. The demographic and clinical features of those with and without bacterial infections were compared. We also describe the prevalence of viral pathogens including respiratory syncytial virus, rhinovirus/enterovirus, influenza, adenovirus, human metapneumovirus, parainfluenza viruses, and non‐SARS‐CoV‐2 endemic coronaviruses.
Results
Among 36 490 hospitalized adults with COVID‐19, 53.3% had bacterial cultures taken within 7 days of admission and 6.0% of these had a clinically relevant bacterial pathogen. After adjustment for demographic factors and co‐morbidities, bacterial infections in patients with COVID‐19 within 7 days of admission were associated with an adjusted relative risk of death 2.3 times that of patients with negative bacterial testing. Staphylococcus aureus and Gram‐negative rods were the most frequently isolated bacterial pathogens. Among hospitalized adults with COVID‐19, 2766 (7.6%) were tested for seven virus groups. A non‐SARS‐CoV‐2 virus was identified in 0.9% of tested patients.
Conclusions
Among patients with clinician‐driven testing, 6.0% of adults hospitalized with COVID‐19 were identified to have bacterial coinfections and 0.9% were identified to have viral coinfections; identification of a bacterial coinfection within 7 days of admission was associated with increased mortality.
Keywords: bacterial coinfection, COVID‐19, COVID‐NET, SARS‐CoV‐2, viral coinfection
1. BACKGROUND
Coinfections, both bacterial and viral, occur with viral respiratory tract infections and can be associated with increased morbidity and mortality 1 , 2 but can also be incidental. 3 Polymicrobial respiratory infections may stem from compromised mucosal lung structure and altered immune responses after an initial infection. 4 , 5 Secondary bacterial infections are known complications of severe influenza infection 6 ; however, studies of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) suggest that bacterial coinfection and secondary infections among patients with COVID‐19 are relatively uncommon. 3 , 7 , 8 Population‐based data are limited on the prevalence of bacterial and viral coinfections in adults with COVID‐19.
We used data collected through Coronavirus Disease 2019‐Associated Hospitalization Surveillance Network (COVID‐NET), a large, geographically diverse US population‐based surveillance platform, to investigate the proportion of viral and bacterial infections among hospitalized adults with laboratory‐confirmed COVID‐19. In this study, our objectives were to (1) compare demographic, radiographic and clinical features, and outcomes among those with bacterial infections, (2) characterize the microbial spectrum of bacterial infections, and (3) describe the prevalence of viral infections. Understanding the epidemiology of bacterial and viral infections in adults hospitalized with COVID‐19 and the association with disease severity can inform testing for coinfections and approach to antimicrobial treatment.
2. METHODS
2.1. Study population
Data were collected through COVID‐NET, 9 a population‐based surveillance system including more than 250 acute‐care hospitals across 99 counties in 14 states (California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah) covering approximately 10% of the US population. COVID‐NET captures laboratory confirmed COVID‐19‐associated hospitalizations, defined as any patient residing in the catchment areas with a positive SARS‐CoV‐2 test during hospitalization or during the 14 days prior to admission. A full case report form is completed on a representative sample of cases stratified by age group and site. For sample selection, random numbers were generated and assigned to each case as previously described. 10 Sampling weights are assigned based on the probability of selection. Trained staff collect demographic information, signs and symptoms, underlying comorbidities, chest imaging, viral and bacterial testing, and outcomes including need for mechanical ventilation, critical care, and death. Individuals with a positive SARS‐CoV‐2 test were included regardless of reason for admission.
2.2. Bacterial pathogens
Adults 18 years or older hospitalized with COVID‐19 during March 2020 and April 2022 and receiving any culture testing within 7 days of admission (including 7 days before or 7 days after) were included in this cross‐sectional analysis of bacterial pathogens. Only pathogens from sterile or respiratory sites were included; cultures from the nasopharynx, urine, or superficial sites (e.g., wound cultures) were excluded. Multiple pathogens were included when present. For individuals with multiple cultures with the same pathogen, results of each bacterial infection and dates of specimen collection were recorded in the case report form. Dates of negative cultures were not recorded. The percent of all sampled cases with any bacterial testing was examined quarterly during the study period.
For individuals with a bacterial infection, the site of culture positivity was classified as sputum, deep respiratory, blood, or other sterile site. Deep respiratory sites included endotracheal aspirate, bronchoalveolar lavage fluid, pleural fluid, and lung tissue. Examples of other sterile sites include cerebrospinal fluid, bone, and peritoneal fluid.
Two physicians trained in infectious diseases reviewed culture information; bacterial infections were defined as clinically relevant organism from a respiratory or sterile site. Individuals categorized as not having a bacterial infection were those who had cultures taken within 7 days of admission (before or after) but tested negative for potentially clinically relevant bacteria. Anaerobes, Bacillus species, Corynebacterium, and coagulase‐negative Staphylococcus (except for Staphylococcus lugdunensis) speciated from blood were excluded unless present in multiple cultures collected on separate days. From sputum cultures, Enterococcus and Streptococcus species (except Streptococcus pneumoniae) were excluded. Coagulase‐negative Staphylococcus were excluded from sputum and deep respiratory cultures as they are usually commensals or contaminants.
2.3. Viral pathogens
Clinician‐directed testing by polymerase chain reaction (PCR) testing results for respiratory syncytial virus (RSV), rhinovirus/enterovirus (RV/EV), influenza (subtypes A, B, or unspecified), adenovirus, human metapneumovirus (HMPV), parainfluenza (serotypes 1–4), and seasonal human coronaviruses (229E, HKU1, NL63, OC43) were also collected from 7 days prior and through 7 days after admission.
2.4. Data analysis
For bacterial infections, we examined characteristics of patients with and without culture testing. Among those with testing, we compared characteristics of those with and without detection of bacterial infections within 7 days before or after hospital admission. We used chi‐square testing for categorical variables and Wilcoxon rank sum tests for continuous variables. We then quantified the most common organisms recovered by site. The same pathogen species and site was only counted once from an individual. We examined whether having a clinically relevant bacterial pathogen with SARS‐CoV‐2 infection was associated with worse outcomes including intensive care unit (ICU) admission, receiving invasive mechanical ventilation (IMV), or death during the hospitalization using multivariable logistic regression analysis with generalized estimating equations (GEE) and controlling for age, sex, race and ethnicity, underlying medical conditions, and time period. Variables significant in bivariate analysis and considered to be relevant to the outcome were included. We present adjusted relative risks (RR) with 95% confidence intervals. A sensitivity analysis on the association of bacterial infection and death was completed excluding individuals with first positive culture on the second day of ICU admission or later. For each respiratory virus, we calculated the proportion of individuals testing positive among all hospitalized adults with COVID‐19 who were tested. Due to low number of viral coinfections, additional multivariable analyses were not performed. Analyses were performed using SAS (version 9.4; SAS Institute). COVID‐NET uses a sampling scheme and collects clinical data on a representative sample of hospitalized adults. 11 All findings are weighted to account for the probability of selection of the sampled patients and adjusted to account for charts with incomplete or missing data. Unweighted counts and weighted percentages are reported except when indicated. The variance estimation was conducted using the Taylor series linearization method.
2.5. Ethical review
This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and conducted consistent with applicable federal law and CDC policy (see e.g., 45 C.F.R. part 46.102(l) (2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.). When required, participating sites obtained approval from respective state and local institutional review boards.
3. RESULTS
Between March 2020 to April 2022, there were a total of 311 292 hospitalizations among adults in COVID‐NET. Of those, a representative sample of 36 490 hospitalized adults had a complete medical chart review. Among 36 490 adults hospitalized in the COVID‐NET catchment area during March 2020 and April 2022, 18 376 (53.3%) had bacterial cultures from sputum, deep respiratory, blood, or other sterile sites (Figure 1A). Of these, 1140, (6.0%) had a bacterial pathogen identified. The percent of all sampled cases with bacterial testing ranged from 46.3% to 70.3% when examined quarterly, and culture testing generally decreased over time (Figure S1). Individuals receiving any bacterial testing were more likely to have underlying conditions (92.1% vs. 81.9%, p < 0.0001), receive ICU level care (30.4% vs. 11.7%, p < 0.0001), and had higher mortality (14.3% vs. 5.1%, p < 0.0001) compared to those without bacterial testing (Table S1). Among those with a bacterial infection, individuals had a median of 1 bacterial infection; the median earliest specimen collection date was on the first day of admission (0.9, interquartile range 0.4–5.0). Among 18 376 patients with bacterial cultures performed (Table 1) within 7 days of admission, sex, age, and race/ethnicity were not significantly different among those with cultures positive for potentially clinically relevant bacteria compared with those with negative cultures. Individuals with bacterial infections were more likely to have underlying medical conditions in the bivariate analysis; 67.3% of those with a bacterial infection had three or more underlying conditions compared with 58.7% of those with bacterial cultures negative for clinically relevant pathogens (p = 0.0017) (Table 1). Those with bacterial infections were more likely to have chronic lung disease, diabetes, cardiovascular disease, obesity, gastrointestinal/liver disease, and renal disease compared with those without bacterial infections within 7 days of admission (Table 1). Cough was less commonly reported in those with a bacterial infection compared with those without a bacterial infection (52.5% vs. 62.9%, p < 0.0001) (Table 1).
FIGURE 1.
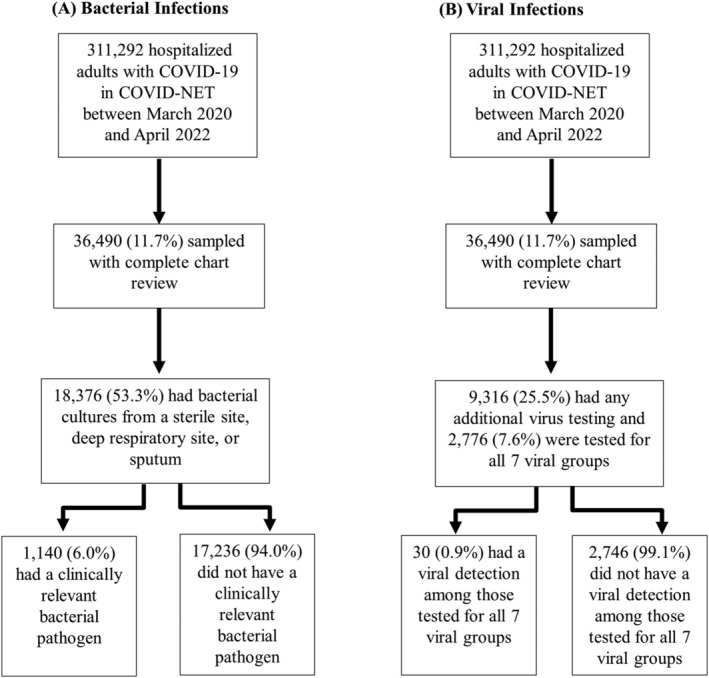
Flowchart of hospitalized adults in COVID‐NET with bacterial and viral infections. Unweighted counts and weighted percentages are reported. The seven viral groups include respiratory syncytial virus, rhinovirus/enterovirus, influenza (subtypes A, B, or unspecified), adenovirus, human metapneumovirus (HMPV), parainfluenza (serotypes 1–4), and common human coronaviruses (229E, HKU1, NL63, OC43).
TABLE 1.
Baseline characteristics of hospitalized adults with COVID‐19 and bacterial testing performed within 7 days of admission, COVID‐NET March 2020–April 2022, stratified by presence of bacterial infections. Unweighted counts and weighted percentages are reported.
| Total sampled hospitalized adults with COVID‐19 tested for a bacterial infection (n = 18 376) | Clinically relevant bacterial infection within ±7 days of admission (n = 1140) | No clinically relevant bacterial infection within first 7 days of admission among those with any bacterial culture performed (n = 17 236) | |||||
|---|---|---|---|---|---|---|---|
| n | Weighted column % with 95% CI | n = 1140 | Weighted column % with 95% CI | n = 17 236 | Weighted column % with 95% CI | p value | |
| Sex | 0.0793 | ||||||
| Male | 10 060 | 54.0 (52.6–55.3) | 697 | 58.2 (53.2–63.1) | 9363 | 53.7 (52.3–55.0) | |
| Female | 8316 | 46.0 (44.7–47.4) | 443 | 41.8 (36.9–46.8) | 7873 | 46.3 (45.0–47.7) | |
| Age category | 0.6917 | ||||||
| 18–34 years | 1865 | 7.3 (6.7–8.0) | 97 | 6.0 (4.1–8.6) | 1768 | 7.4 (6.8–8.1) | |
| 35–54 years | 5185 | 23.3 (22.3–24.4) | 316 | 22.9 (19.2–27.0) | 4869 | 23.4 (22.3–24.4) | |
| 55–74 years | 7317 | 42.1 (40.8–43.4) | 496 | 44.0 (39.1–49.0) | 6821 | 42.0 (40.7–43.3) | |
| ≥75 years | 4009 | 27.2 (26.0–28.5) | 231 | 27.0 (22.6–31.8) | 3778 | 27.2 (25.9–28.6) | |
| Race and ethnicity | 0.2816 | ||||||
| Non‐Hispanic White | 8725 | 49.0 (47.7–50.3) | 548 | 49.2 (44.1–54.3) | 8177 | 49.0 (47.6–50.4) | |
| Non‐Hispanic Black | 3843 | 26.8 (25.6–28.0) | 239 | 29.3 (24.6–34.4) | 3604 | 26.6 (25.3–27.8) | |
| Non‐Hispanic AI/AN | 371 | 1.6 (1.3–1.9) | 38 | 2.1 (1.1–3.5) | 333 | 1.6 (1.3–1.9) | |
| Asian/PI | 1198 | 5.8 (5.1–6.6) | 73 | 4.0 (2.4–6.2) | 1125 | 5.9 (5.2–6.8) | |
| Hispanic | 3547 | 16.8 (15.9–17.8) | 204 | 15.4 (12.1–19.1) | 3343 | 16.9 (16.0–17.9) | |
| Any underlying condition a | 16 696 | 92.1 (91.4–92.8) | 1068 | 96.3 (94.6–97.5) | 15 628 | 91.8 (91.1–92.5) | <0.0001 |
| Major underlying conditions b | 1680 | 7.9 (7.2–8.6) | 72 | 3.7 (2.5–5.4) | 1608 | 8.2 (7.5–8.9) | 0.0017 |
| 0 | 2678 | 12.3 (11.5–13.1) | 74 | 3.8 (2.5–5.6) | 1584 | 8.3 (7.6–9.1) | |
| 1 | 3255 | 16.8 (15.8–17.8) | 151 | 13.2 (9.8–17.2) | 2621 | 13.3 (12.4–14.2) | |
| 2 | 10 763 | 63.0 (61.8–64.3) | 182 | 15.7 (12.2–19.6) | 3419 | 19.7 (18.6–20.8) | |
| 3 or more | 16 696 | 92.1 (91.4–92.8) | 685 | 67.3 (62.4–72.0) | 9126 | 58.7 (57.4–60.1) | |
| Chronic lung disease | 9299 | 54.5 (53.2–55.8) | 369 | 38.0 (32.9–43.3) | 4983 | 30.1 (28.8–31.4) | 0.0018 |
| Diabetes | 6604 | 37.9 (36.6–39.2) | 464 | 42.8 (37.8–47.9) | 5976 | 37.7 (36.3–39.1) | 0.0476 |
| Blood disorders | 669 | 4.1 (3.5–4.7) | 54 | 5.4 (3.2–8.5) | 570 | 3.9 (3.3–4.6) | 0.197 |
| Cardiovascular disease c | 6242 | 38.8 (37.5–40.2) | 477 | 50.4 (45.3–55.5) | 5839 | 40.1 (38.7–41.5) | <0.0001 |
| Neurologic disorders | 3847 | 22.8 (21.6–24.0) | 295 | 25.3 (21.1–29.9) | 3421 | 22.4 (21.2–23.7) | 0.19 |
| Immunocompromising conditions | 2140 | 13.8 (12.8–14.7) | 141 | 15.0 (11.2–19.5) | 1878 | 13.4 (12.5–14.4) | 0.4283 |
| Obesity d | 8715 | 48.5 (47.1–49.8) | 493 | 43.7 (38.6–48.9) | 8029 | 49.2 (47.7–50.6) | 0.0408 |
| Gastrointestinal/liver disease | 1748 | 10.9 (10.1–11.8) | 117 | 9.6 (7.3–12.5) | 1007 | 6.2 (5.5–6.9) | 0.0021 |
| Renal disease | 3102 | 19.5 (18.3–20.6) | 229 | 25.9 (21.4–30.8) | 2752 | 18.8 (17.6–20.1) | 0.0014 |
| Rheumatologic/autoimmune disorder | 1051 | 7.3 (6.6–8.1) | 68 | 7.0 (4.6–10.1) | 917 | 7.2 (6.5–8.1) | 0.8446 |
| Time period e | |||||||
| Pre‐Delta | 14 784 | 63.2 (61.8–64.5) | 846 | 54.5 (49.4–59.5) | 13 938 | 63.7 (62.3–65.1) | 0.0011 |
| Delta | 2442 | 20.6 (19.6–21.6) | 176 | 23.4 (19.4–27.7) | 2266 | 20.4 (19.4–21.5) | |
| Omicron | 1150 | 16.3 (15.0–17.6) | 118 | 22.2 (17.6–27.3) | 1032 | 15.9 (14.6–17.3) | |
| Long‐term care facility residence f | 2601 | 13.0 (12.1–13.9) | 203 | 14.9 (11.8–18.5) | 2339 | 12.8 (11.9–13.8) | 0.2019 |
| Symptoms | |||||||
| Cough | 11 855 | 62.3 (61.0–63.6) | 598 | 52.5 (47.5–57.5) | 11 257 | 62.9 (61.6–64.3) | <0.0001 |
| Shortness of breath | 12 404 | 65.4 (64.1–66.7) | 733 | 58.8 (53.6–63.8) | 11 671 | 65.8 (64.5–67.2) | 0.0054 |
| Congestion | 1855 | 10.6 (9.9–11.4) | 89 | 9.4 (6.9–12.5) | 1766 | 10.7 (9.9–11.5) | 0.3855 |
| Wheezing | 802 | 4.6 (4.1–5.1) | 51 | 4.2 (2.7–6.3) | 751 | 4.6 (4.1–5.2) | 0.6688 |
| Hemoptysis | 271 | 1.2 (0.9–1.4) | 25 | 2.4 (1.0–4.8) | 246 | 1.1 (0.9–1.3) | 0.0283 |
| Chest X‐ray | |||||||
| Abnormal chest X‐ray | 14 735 | 84.5 (83.5–85.5) | 965 | 88.7 (85.5–91.4) | 13 770 | 84.3 (83.2–85.3) | 0.0101 |
| Consolidation | 1401 | 9.1 (8.3–10.0) | 125 | 13.1 (9.9–17.0) | 1276 | 8.8 (8.0–9.7) | 0.006 |
| Lobar infiltrate | 14 735 | 84.5 (83.5–85.5) | 965 | 88.7 (85.5–91.4) | 13 770 | 84.3 (83.2–85.3) | 0.0101 |
| Outcomes | |||||||
| Intensive care required | 6459 | 30.4 (29.2–31.5) | 747 | 60.0 (54.9–64.9) | 5712 | 28.5 (27.3–29.7) | <0.0001 |
| Mechanical ventilation | 3627 | 17.5 (16.6–18.5) | 594 | 47.6 (42.6–52.6) | 3033 | 15.6 (14.7–16.6) | <0.0001 |
| Death | 2535 | 14.3 (13.4–15.3) | 329 | 31.7 (27.2–36.5) | 2206 | 13.2 (12.3–14.1) | <0.0001 |
Note: N in each cell represents unweighted frequency or numerator and N on top row represents denominator. % is prevalence weighted for sampling and non‐response.
Abbreviations: AI/AN, American Indian/Alaska Native; PI, Pacific Islander.
Any underlying conditions include a condition from one of the following major underlying condition categories (see below).
Major underlying conditions include chronic lung disease including asthma; chronic metabolic disease including diabetes; blood disorders/hemoglobinopathies; cardiovascular disease (excluding hypertension); neurologic disorder; immunocompromised condition; renal disease; any obesity; postpartum; gastrointestinal or liver disease; rheumatologic, autoimmune, or inflammatory conditions; other conditions. For definition of major conditions, see Table S4.
Cardiovascular disease excludes hypertension.
Obesity is defined as calculated body mass index (BMI) ≥ 30 kg/m2, and if BMI is missing, by International Classification of Diseases discharge diagnosis codes.
Pre‐Delta: March 2020–June 2021, Delta: July 2021–December 18, 2021, Omicron: December 19, 2021–April 2022.
Long‐term care facility residence includes nursing home/skilled nursing facility, alcohol/drug abuse treatment center, other rehabilitation facility, assisted living/residential care, group/retirement homes, long‐term care facility (LTCF), long‐term acute care hospital (LTACH), or any other psychiatric facility.
Those with bacterial infections were more likely to receive intensive care during hospitalization (60.0% vs. 28.5%, p < 0.0001) and to require mechanical ventilation (47.6% vs. 15.6%, p < 0.0001) compared with those who had bacterial testing performed and were negative for clinically relevant pathogens. In‐hospital death occurred in 329 (31.7%) of those with bacterial infections compared with 13.2% of those without bacterial infections in bivariate analysis (p < 0.0001). Both a respiratory sample culture and blood culture were positive in 9.4% of those with in‐hospital death. After controlling for demographic factors, underlying medical conditions, and time period, adults with COVID‐19 who had bacterial infections within 7 days of admission had 2.28 (95% CI 1.87–2.79) times increased risk for death compared with those with negative bacterial cultures within 7 days of admission (Table 2). After excluding 116 individuals with first positive bacterial culture on the second day of ICU admission or later, those with bacterial infections had 1.81 (95% CI 1.51–2.18) times increased risk for death. Those with a clinically relevant pathogen identified were associated with an increased need for intensive care (RR 2.11 95% CI 1.95–2.23) and mechanical ventilation (RR 3.04 95% CI 2.74–3.37) (Tables S2 and S3).
TABLE 2.
Adjusted relative risk for death among adults hospitalized with COVID‐19 who had bacterial testing performed within 7 days of admission, COVID‐NET March 2020–April 2022.
| Adjusted relative risk for death (95% CI) n = 16 383 | p value | |
|---|---|---|
| Bacterial infection in a respiratory or sterile site within 7 days of hospital admission | 2.28 (1.87, 2.79) | <0.0001 |
| Sex | ||
| Male | 1.19 (0.99, 1.43) | 0.0618 |
| Female | Ref | Ref |
| Age category | ||
| 18–34 years | Ref | Ref |
| 35–54 years | 1.7 (1.16, 2.49) | 0.0069 |
| 55–74 years | 4.01 (2.44, 6.52) | <0.0001 |
| 75 years or more | 5.68 (3.44, 9.36) | <0.0001 |
| Race/ethnicity | ||
| Non‐Hispanic White | Ref | Ref |
| Non‐Hispanic Black | 0.99 (0.88, 1.12) | 0.9123 |
| Non‐Hispanic AI/AN | 1.16 (0.91, 1.49) | 0.2297 |
| Asian/PI | 1.23 (1.07, 1.49) | 0.0031 |
| Hispanic | 1.41 (1.13, 1.76) | 0.0028 |
| Chronic lung disease | 0.95 (0.81, 1.13) | 0.5763 |
| Diabetes | 1.06 (0.94, 1.2) | 0.3423 |
| Cardiovascular disease | 1.25 (1.07, 1.47) | 0.0053 |
| Obesity | 1.09 (0.94, 1.27) | 0.2428 |
| Gastrointestinal/liver disease | 0.83 (0.64, 1.07) | 0.142 |
| Renal disease | 1.29 (1.17, 1.42) | <0.0001 |
| Time period a | ||
| Pre‐Delta | Ref | Ref |
| Delta | 1.39 (1.24, 1.55) | <0.0001 |
| Omicron | 0.70 (0.53, 0.92) | 0.0103 |
Abbreviations: AI/AN, American Indian/Alaska Native; PI, Pacific Islander.
Pre‐Delta: March 2020–June 2021, Delta: July 2021–December 182 021, Omicron: December 19, 2021–April 2022.
Of 1140 patients with a bacterial infection, 35.4% had a positive sputum culture, 44.0% had a positive blood culture, 23.4% had a positive deep respiratory culture, and 3.2% had a positive culture from another sterile site. Among those with positive sputum bacterial cultures, 46.7% were Staphylococcus aureus. Gram‐negative rods were the next most common organisms recovered in sputum, with Pseudomonas aeruginosa (12.8%) and Klebsiella pneumoniae (7.3%) most frequently identified (Figure 2). S. pneumoniae comprised 5.1% of positive sputum cultures. Among 312 deep respiratory cultures, the most common organism reported was S. aureus (43.9%) followed by Gram‐negative rods including P. aeruginosa (10.6%), Escherichia coli (6.1%), and K. pneumoniae (5.8%), (Figure 2). In blood cultures, S. aureus (35.1%) and E. coli (21.9%) were the most common bacteria isolated. Among the 329 (31.7%) who died, 47.5% had a positive blood culture, 36.6% had a positive sputum culture, 27.7% had a positive deep respiratory culture, and 9.3% had both a positive blood and respiratory culture. In the subset of hospitalized adults (n = 329) with COVID‐19 who died, S. aureus (46.3%) and E. coli (17.0%) were the most frequent pathogens in blood, S. aureus (51.7%) and P. aeruginosa (12.4%) in sputum specimens, and S. aureus (40.2%) and P. aeruginosa (17.2%) in deep respiratory specimens.
FIGURE 2.
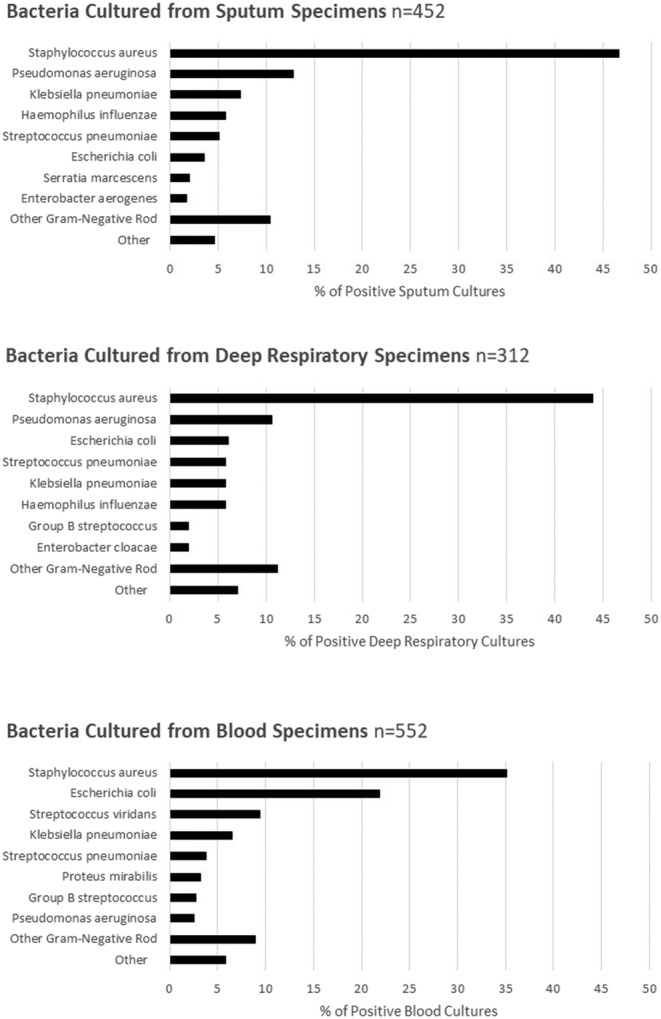
Bacterial cultures from hospitalized sampled adults with COVID‐19 with bacterial pathogens detected in sputum, deep respiratory, or blood cultures within 7 days of admission from Coronavirus Disease 2019‐Associated Hospitalization Surveillance Network (COVID‐NET) from March 2020 to April 2022. This figure includes 1408 bacterial cultures with a clinically relevant organism from 1066 individuals. Deep respiratory sites include endotracheal aspirate, bronchoalveolar lavage fluid, pleural fluid, and lung tissue. Unweighted counts and percentages are reported in this figure.
Among COVID‐19 patients with a complete chart review, 9316 (25.5%) had testing for other respiratory viruses (Figure 1). Of the 9181 individuals tested for influenza (Table 3), 12 (0.1%) were positive; 5639 were tested for RSV, among whom 3 (0.01%) were positive. Of 2766 individuals tested for the seven main viral respiratory groups (parainfluenza, adenovirus, HMPV, RV/EV, RSV, common human coronaviruses, and influenza), another virus was detected in 30 (0.9%). The most commonly detected virus was RV/EV (0.6%) (Table 3). There were two hospitalized adults with COVID‐19, another respiratory virus, and a bacterial pathogen within 7 days.
TABLE 3.
Viral detections by polymerase chain reaction (PCR) in hospitalized adults with COVID‐19 within 7 days of admission from COVID‐NET March 2020–April 2022. Viral groups include polymerase chain reaction (PCR) for influenza (subtypes A, B, or unspecified), rhinovirus/enterovirus, respiratory syncytial virus, adenovirus, human metapneumovirus, parainfluenza (serotypes 1–4), and common human coronaviruses (229E, HKU1, NL63, OC43).
| Viral infection by PCR testing | Detections/tested (%) |
|---|---|
| Rhinovirus/enterovirus | 15/3174 (0.6%) |
| Human metapneumovirus | 6/3213 (0.3%) |
| Parainfluenza | 5/3239 (0.3%) |
| Adenovirus | 4/3237 (0.3%) |
| Common coronavirus type (4 types) | 3/2990 (0.3%) |
| Influenza | 12/9181 (0.1%) |
| Respiratory syncytial virus | 3/5639 (0.01%) |
| Any respiratory co‐detection | 30/2776 (0.9%) |
4. DISCUSSION
Using data from a representative sample of 36 490 cases from 311 292 hospitalized adults with laboratory‐confirmed SARS‐CoV‐2 infection, 6.0% of those with bacterial cultures had evidence of infection with a potentially clinically relevant bacteria within 7 days of admission. Although bacterial infections were identified relatively infrequently during the first week of hospitalizations, among those with bacterial infections identified, nearly one third experienced in‐hospital death. The risk of death was over twofold greater with a detected bacterial infection and COVID‐19 compared with those without evidence of bacterial infections among those subjected to bacterial testing. For clinicians treating patients with COVID‐19 and bacterial infections, understanding that bacterial infections are associated with severe outcomes can inform clinical care.
Our results are consistent with other studies of patients infected with SARS‐CoV‐2 that suggest that bacterial coinfection and secondary infections among patients with COVID‐19 are relatively uncommon 8 when compared with bacterial coinfections observed with influenza and RSV. 7 , 12 , 13 These findings build upon other reports, including meta‐analyses, 7 , 8 which suggest that bacterial infections with COVID‐19 occur in a small subset. Antimicrobial use among hospitalized COVID‐19 early in the pandemic was high, with a meta‐analysis showing 65% prevalence of antibiotic use from studies in the U.S. 14 In settings with minimal empiric antimicrobial use such as that reported from a Japanese hospital, bacterial coinfections with SARS‐CoV‐2 were also uncommon. 15 Similarly, viral coinfections are also relatively infrequent; one US study reported 4.2% of patients of all ages attending inpatient or outpatient care with COVID‐19 had viral codetections. 16
A large population‐based analysis from the United Kingdom concluded that bacterial coinfection were not associated with inpatient death 13 while other studies have reported an association. 17 Our analysis suggests an association between bacterial infection and increased death among hospitalized patients with COVID‐19 who receive bacterial testing. More severe COVID‐19 could lead to increased instrumentation and ports of entry for bacterial infections associated with ICU admission. However, even after excluding those with first positive cultures 2 days or more after ICU admission, an increased risk for death remained. Further studies are needed to untangle these interactions between pathogens and host to determine the role of bacterial infections in the natural history of COVID‐19 and to understand to what extent bacterial infections may drive severe COVID‐19 outcomes.
There are several limitations to this analysis. These findings may not be generalizable to all US adults hospitalized with COVID‐19, and testing practices vary across clinicians and facilities. Additionally, the percent of adults with COVID‐19 receiving bacterial testing changed over time. Assigning clinical significance to specific pathogens is difficult, and without full clinical context, classification of pathogens as clinically relevant may be inaccurate. Detection of viral pathogens by PCR or bacterial pathogens by culture may not indicate active infection or disease. The COVID‐NET platform only collects bacterial pathogens within 7 days of admission, making it difficult to describe the burden of secondary or hospital‐acquired infections over the full course of hospitalization and limits the ability to compare with other studies. The group of patients with negative culture testing did not have dates of culture testing limiting comparison of specific time points. Since the temporal sequence of the presence of multiple pathogens was not available, we are unable to attribute causality of outcomes. The population that had bacterial cultures performed were likely more ill or inherently different from patients with COVID‐19 who did not have bacterial cultures performed which may introduce confounding by indication, limiting the generalizability of the findings to all hospitalized adults with COVID‐19. Incidental COVID‐19 admissions were included in the analysis and may contribute to observed differences.
Although bacterial infections in COVID‐19 patients are relatively infrequent, the presence of bacterial infections is associated with significantly increased disease severity, including increased mortality. As SARS‐CoV‐2 continues to circulate and individuals continue to be hospitalized for COVID‐19, understanding risk factors for bacterial infections and associated outcomes can help guide clinicians in providing optimal care.
AUTHOR CONTRIBUTION
Melisa M. Shah: Conceptualization; investigation; methodology; project administration; visualization; writing‐original draft. Kadam Patel: Conceptualization; validation; data curation; formal analysis; writing‐review and editing. Jennifer Milucky: Conceptualization; methodology; project administration; writing‐review and editing. Christopher A. Taylor: Conceptualization; methodology; project administration; writing‐review and editing. Arthur Reingold: Writing‐review and editing. Isaac Armistead: Writing‐review and editing. James Meek: Writing‐review and editing. Evan J. Anderson: Writing‐review and editing. Andy Weigel: Writing‐review and editing. Libby Reeg: Writing‐review and editing. Kathryn Como‐Sabetti: Writing‐review and editing. Susan L. Ropp: Writing‐review and editing. Alison Muse: Writing‐review and editing. Sophrena Bushey: Writing‐review and editing. Eli Shiltz: Writing‐review and editing. Melissa Sutton: Writing‐review and editing. H. Keipp Talbot: Writing‐review and editing. Ryan Chatelain: Writing‐review and editing. Fiona P. Havers: Conceptualization; investigation; methodology; project administration; visualization; writing‐review and editing.
CONFLICT OF INTEREST STATEMENT
Ms. Leegwater and Ms. Reeg report grants from Michigan Department of Health and Human Services during the conduct of the study. Dr. Anderson reports grants from Pfizer, Merck, PaxVax, Micron, Sanofi‐Pasteur, Janssen, MedImmune, and GSK. Dr. Anderson reports personal fees from Sanofi‐Pasteur, Pfizer, Medscape, Kentucky Bioprocessing, Inc, Sanofi‐Pasteur, Janssen, GSK, WCG and ACI Clinical, and Moderna outside the submitted work. His institution has also received funding from NIH to conduct clinical trials of Moderna and Janssen COVID‐19 vaccines. Mr. Weigel reports grants from CDC/CSTE Cooperative Agreement, during the conduct of the study and grants from CDC/CSTE outside the submitted work. Mr. Teno reports grants from CDC/CSTE Cooperative Agreement during the conduct of the study and grants from CDC/CSTE outside the submitted work. Mrs. Billing reports grants from Council of State and Territorial Epidemiologists (CSTE) during the conduct of the study and grants from Centers for Disease Control and Prevention (CDC) outside the submitted work. Mr. Meek reports grants from CDC during the conduct of the study. Dr. Schaffner reports grants from CDC during the conduct of the study. Dr. Sutton reports grants from CDC Emerging Infections Program during the conduct of the study. Dr. Talbot reports grants from Centers for Disease Control and Prevention, during the conduct of the study. Ms. Yousey‐Hindes reports grants from CDC during the conduct of the study.
Supporting information
Table S1: Baseline characteristics of hospitalized adults with COVID‐19 stratified by presence of bacterial culture testing, COVID‐NET March 2020–April 2022
Table S2: Adjusted relative risk for intensive care among hospitalized adults with COVID‐19 who had bacterial testing performed within 7 days of admission, COVID‐NET March 2020–April 2022
Table S3: Adjusted relative risk for mechanical ventilation among hospitalized adults with COVID‐19 who had bacterial testing performed within 7 days of admission, COVID‐NET March 2020–April 2022
Table S4: Underlying Condition Categories
Figure S1: Percent of sampled cases with bacterial culture testing done quarterly over time, COVID‐NET March 2020–April 2022
ACKNOWLEDGMENTS
The authors would like to thank Sherry Quach, Gretchen Rothrock, Jeremy Roland, Joelle Nadle, Ashley Coates, Monica Napoles, California Emerging Infections Program; Sharon Emmerling, Breanna Kawasaki, Madelyn Lensing, Sarah McLafferty, Jordan Surgnier, Millen Tsegaye, Colorado Department of Public Health and Environment; Ann Basting, Tessa Carter, Maria Correa, Daewi Kim, Carol Lyons, Amber Maslar, Julie Plano, Hazhia Sorosindi, Connecticut Emerging Infections Program, Yale School of Public Health; Emily Fawcett, Annabel Patterson, Taylor Eisenstein: Foundation for Atlanta Veterans Education and Research, Decatur, GA; Atlanta Veterans Affairs Medical Center, Atlanta, GA; Emory University School of Medicine, Atlanta, GA; Katelyn Ward, Jana Manning, Asmith Joseph, Allison Roebling, Chandler Surell, Stephanie Lehman, Suzanne Segler, Grayson Kallas, Marina Bruck, Rayna Ceaser, Sabrina Hendrick, Johanna Hernandez, Hope Wilson; Emory University School of Medicine, Atlanta, GA; Georgia Emerging Infections Program, Georgia Department of Public Health, Atlanta, GA. Atlanta Veterans Affairs Medical Center, Atlanta, GA; Jim Collins, Shannon Johnson, Sue Kim, Libby Reeg, Alexander Kohrman, Lauren Leegwater, Chloe Brown, Alyanna Melicor, Sanchitha Meda, Michigan Department of Health and Human Services; Richard Danila, Grace Hernandez, Kieu My Phi, Melissa McMahon, Jill Reaney, Ruth Lynfield, Minnesota Department of Health; Daniel Sosin, Chad Smelser, Sunshine Martinez, Jasmyn Sanchez, Cory Cline, Melissa Judson, Florent Nkouaga, Mark Montoya, Kelly Plymesser, Adrienne Domen, New Mexico Department of Health; Sarah Lathrop, Kathy M. Angeles, Yadira Salazar‐Sanchez, Sarah A. Khanlian, Nancy Eisenberg, Dominic Rudin, Sarah Shrum Davis, Molly Bleecker, Wickliffe Omondi, Mayvilynne Poblete, Francesca Pacheco, New Mexico Emerging Infections Program; Yassir Talha, Celina Chavez, Jennifer Akpo, Alesia Reed, Murtada Khalifa, CDC Foundation, New Mexico Department of Health; Kerianne Engesser, Grant Barney, Adam Rowe, New York State Department of Health; Virginia Cafferky, Christina Felsen, Maria Gaitán, RaeAnne Kurtz, Christine Long, Thomas Peer, University of Rochester School of Medicine and Dentistry; Sam Hawkins, Public Health Division, Oregon Health Authority; Julie Freshwater, Denise Ingabire‐Smith, Ann Salvator, Rebekah Sutter, Ohio Department of Health; Sam Hawkins, Public Health Division, Oregon Health Authority; Tiffanie Markus, Katie Dyer, Karen Leib, Terri McMinn, Danielle Ndi, Gail Hughett, Emmanuel Sackey, Kathy Billings, Anise Elie, Manideepthi Pemmaraju, Vanderbilt University Medical Center; Amanda Carter, Andrea George, Andrea Price, Andrew Haraghey, Ashley Swain, Keegan McCaffrey, Laine McCullough, Mary Hill, Melanie Crossland, Salt Lake County Health Department.
Shah MM, Patel K, Milucky J, et al. Bacterial and viral infections among adults hospitalized with COVID‐19, COVID‐NET, 14 states, March 2020–April 2022. Influenza Other Respi Viruses. 2023;17(3):e13107. doi: 10.1111/irv.13107
Group Authors: Pam Daily Kirley, California Emerging Infections Program, Oakland, CA; Nisha B. Alden, Colorado Department of Public Health and Environment, Denver, CO; Kimberly Yousey‐Hindes, Connecticut Emerging Infections Program, Yale School of Public Health, New Haven, CT; Kyle P. Openo, Departments of Medicine and Pediatrics, Emory University School of Medicine, Atlanta, GA, Georgia Emerging Infections Program, Georgia Department of Public Health, Atlanta Veterans Affairs Medical Center, Atlanta, GA; Kenzie Teno, Iowa Department of Health, Des Moines, IA; Lauren Leegwater, Michigan Department of Health and Human Services, Lansing, Michigan; Erica Bye, Minnesota Department of Health; Emily B. Hancock, New Mexico Emerging Infections Program, University of New Mexico, Albuquerque, New Mexico; Nancy Spina, New York State Department of Health, Albany, NY; Kevin Popham, University of Rochester School of Medicine and Dentistry, Rochester, NY; Laurie M. Billing, Ohio Department of Health, Columbus, OH; Nasreen Abdullan, Public Health Division, Oregon Health Authority; Portland, Oregon; William Schaffner, Vanderbilt University Medical Center, Nashville, TN; Emily R. Roberts, Salt Lake County Health Department, Salt Lake City, UT
Funding Information This work was supported by the Centers for Disease Control and Prevention through an Emerging Infections Program cooperative agreement (grant CK17‐1701) and through a Council of State and Territorial Epidemiologists cooperative agreement (grant NU38OT000297‐02‐00). The findings and conclusions in this report are those of the authors do not necessarily represent the official position of the United States Department of Health and Human Services, the United States Public Health Service Commissioned Corps, the Centers for Disease Control and Prevention, or the authors' institutions.
Contributor Information
Melisa M. Shah, Email: bgn3@cdc.gov.
Kadam Patel, Email: qbi1@cdc.gov.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community‐acquired pneumonia in adults. Thorax. 2008;63(1):42‐48. doi: 10.1136/thx.2006.075077 [DOI] [PubMed] [Google Scholar]
- 2. Swets MC, Russell CD, Harrison EM, et al. SARS‐CoV‐2 co‐infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet Published online. 2022;399(10334):S014067362200383X. doi: 10.1016/S0140-6736(22)00383-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell K, Fowlkes A, Lynfield R, et al. Viral and bacterial co‐detections in influenza‐positive patients hospitalized with severe acute respiratory illness—Minnesota, 2013–2015. Open Forum Infect Dis. 2016;3(suppl_1):1754. doi: 10.1093/ofid/ofw194.134 [DOI] [Google Scholar]
- 4. Rynda‐Apple A, Robinson KM, Alcorn JF. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83(10):3764‐3770. doi: 10.1128/IAI.00298-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melvin JA, Bomberger JM. Compromised defenses: exploitation of epithelial responses during viral‐bacterial co‐infection of the respiratory tract. PLoS Pathog. 2016;12(9):e1005797. doi: 10.1371/journal.ppat.1005797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah NS, Greenberg JA, McNulty MC, et al. Bacterial and viral co‐infections complicating severe influenza: incidence and impact among 507 U.S. patients, 2013–14. J Clin Virol. 2016;80:12‐19. doi: 10.1016/j.jcv.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lansbury L, Lim B, Baskaran V, Lim WS. Co‐infections in people with COVID‐19: a systematic review and meta‐analysis. J Infect. 2020;81(2):266‐275. doi: 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langford BJ, So M, Raybardhan S, et al. Bacterial co‐infection and secondary infection in patients with COVID‐19: a living rapid review and meta‐analysis. Clin Microbiol Infect. 2020;26(12):1622‐1629. doi: 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CDC . Coronavirus Disease 2019 (COVID‐19)‐Associated Hospitalization Surveillance Network (COVID‐NET): Purpose and Methods. 2021. 12/03/2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
- 10. O'Halloran A, Whitaker M, Patel K, et al. Developing a sampling methodology for timely reporting of population‐based COVID‐19‐associated hospitalization surveillance in the United States, COVID‐NET 2020–2021. Influenza Resp Viruses. 2023;17(1):e13089. doi: 10.1111/irv.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg S, Patel K, Pham H, et al. Clinical trends among U.S. adults hospitalized with COVID‐19, March to December 2020: a cross‐sectional study. Ann Intern Med. 2021;174(10):1409‐1419. doi: 10.7326/M21-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hedberg P, Johansson N, Ternhag A, Abdel‐Halim L, Hedlund J, Nauclér P. Bacterial co‐infections in community‐acquired pneumonia caused by SARS‐CoV‐2, influenza virus and respiratory syncytial virus. BMC Infect Dis. 2022;22(1):108. doi: 10.1186/s12879-022-07089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russell CD, Fairfield CJ, Drake TM, et al. Co‐infections, secondary infections, and antimicrobial use in patients hospitalised with COVID‐19 during the first pandemic wave from the ISARIC WHO CCP‐UK study: a multicentre, prospective cohort study. Lancet Micr. 2021;2(8):e354‐e365. doi: 10.1016/S2666-5247(21)00090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID‐19: rapid review and meta‐analysis. Clin Microbiol Infect. 2021;27(4):520‐531. doi: 10.1016/j.cmi.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komagamine J, Yabuki T, Matsumoto K, Tanaka N. Evaluation of antimicrobial drug use and concurrent infections during hospitalization of patients with COVID‐19 in Japan. JAMA Netw Open. 2022;5(2):e220040. doi: 10.1001/jamanetworkopen.2022.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co‐infection between SARS‐CoV‐2 and other respiratory pathogens. Jama. 2020;323(20):2085‐2086. doi: 10.1001/jama.2020.6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goncalves Mendes Neto A, Lo KB, Wattoo A, et al. Bacterial infections and patterns of antibiotic use in patients with COVID‐19. J Med Virol. 2021;93(3):1489‐1495. doi: 10.1002/jmv.26441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Baseline characteristics of hospitalized adults with COVID‐19 stratified by presence of bacterial culture testing, COVID‐NET March 2020–April 2022
Table S2: Adjusted relative risk for intensive care among hospitalized adults with COVID‐19 who had bacterial testing performed within 7 days of admission, COVID‐NET March 2020–April 2022
Table S3: Adjusted relative risk for mechanical ventilation among hospitalized adults with COVID‐19 who had bacterial testing performed within 7 days of admission, COVID‐NET March 2020–April 2022
Table S4: Underlying Condition Categories
Figure S1: Percent of sampled cases with bacterial culture testing done quarterly over time, COVID‐NET March 2020–April 2022
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


