Abstract
Background
Evidence indicates that chronic stress promotes progression of colorectal liver metastases (CLM). Mangiferin is the active chemical constituent of the rhizomes of Anemarrhena asphodeloides Bunge. Mangiferin (MGF) exerts anti-inflammatory, anti-proliferative, anti-angiogenic, anti-fibrotic and antioxidant effects in a variety of cancers. Its mechanism in chronic stress and tumor growth is still poorly understood.
Methods
To investigate the effects of MGF on the CLM and tumor-associated depression, activated hepatic stellate cells (a-HSCs), HT-29 CRC cells, were used in chronic unpredictable mild stress (CUMS) of tumor-bearing models. Potential antidepressant activity was determined by FST, TST, SIT and serum cytokine (IL-6, IL-18 and TNF-α) examination. Downstream signaling molecules were detected by Western blot, immunohistochemistry and fluorescence microscopy.
Results
CUMS induced depression behavior and depression-related cytokines and promoted tumor growth in CLM. MGF-treated mice significantly improved chronic stress behaviors by reducing depression-related cytokines. In addition, MGF treatment inhibits WAVE2 signaling pathway, leading to TGF-β1 induced HSC inhibition, thereby reducing depressive behavior and tumor growth in CLM.
Conclusion
MGF can alleviate CUMS induced tumor growth and the treatment of CLM patients with MGF may be beneficial.
Keywords: Chronic stress, Colorectal liver metastases, Mangiferin
1. Introduction
Colorectal cancer (CRC) is a major global health problem, with 2020 statistics showing that 576,858 colorectal cancer patients died [1], approximately 50% of CRC patients develop liver metastases, and that the 5-year overall survival rate after surgery is approximately 40%.
Due to informed diagnosis, lack of social support, clinical symptoms during chemotherapy [2], depression is a common and easily overlooked concomitant disorder during cancer treatment [3]. The prevalence of both depression and anxiety in CRC patients has been shown to be high [4] and epidemiological data show that 54.37% of Chinese colon cancer patients have depressive symptoms [5].57.9% of colon cancer patients are diagnosed with depression after surgery [6] and previous studies have demonstrated that tumor-related depression leads to increased mortality, poor prognosis [7]. Patients' depression and overall survival may be affected by the availability of any medications and psychological coping strategies.
New evidence indicates that antidepressants aim at the serotonergic system may play a key role in tumorigenesis and progression, growth inhibition in CRC in the mouse model [8]. Potential effect of serotonin has been proved to the contribution to tumorigenesis, metastasis and poor prognosis in CRC patients [9,10]. However, due to the heterogeneity in tissue distribution of serotonin receptors, different antidepressants are not effective in treating depressed patients with CRC [11]. Therefore, it is important to explore strategies to reduce depression in CRC patients.
In Chinese traditional medicine, Anemarrhena asphodeloides has been commonly used with curative effects on febrile diseases, such as fever, bronchitis and diarrhea. It exerted neuroprotective activities in depression. Meanwhile, it also showed biological activities of inflammation improvement and anticancer effect [12]. Mangiferin (Formula:C19H18O11, also named as 1,3,6,7-Tetrahydroxyxanthone C2-β-D-glucoside) is the active chemical components isolated from the rhizomes of Anemarrhena asphodeloides Bunge, which has been identified as a xanthanoid with anti-proliferative, anti-angiogenic and antioxidant effects in a variety of cancers, including colorectal colitis and cancer [13,14]. Emerging evidence suggests that MGF has antidepressant effects in mouse chronic stress model [15]. Recent research has shown that MGF inhibits Rac1/WAVE2 signaling in by suppressing invasive motility in breast cancer cells [16]. Our previous studies found that WAVE2 was involved in the CLM tumor growth and could possibly serve as a therapeutic target for CLM [17]. With regard to MGF's above-mentioned pharmacological properties, mouse CLM model was applied to validate the novel role of MGF in chronic stress-induced tumor growth in CLM.
2. Materials and methods
2.1. Ethical approval
The project was ethically approved by Ethics Committee of XiangYa Hospital, Central South University, Permit Number: N02019030913. Animal care and procedures were conducted according to the ministry of science and technology's guiding opinions on treating experimental animals.
2.2. Primary HSC cultures
Mice were liver perfused through the inferior vena cava with pronase (Nordmark, Cat# S17465022) and collagenase D (Roche) after anesthesia with ketamine and xyaline. To remove the hepatocytes, cell suspension was centrifuged at 50 g for 1 min, supernatant was collected and centrifuged at 800 g for 7 min. After removing supernatant, HSCs were then separated by 18% Nycodenz gradient by density gradient centrifuge for 20 min. HSCs were cultured in media containing 10% fetal bovine serum (FBS).
2.3. Conditioned media culture and CRC cell lines
The human CRC HT-29 cell line was purchased from the Typical Culture Collection (Manassas, VA, USA) and cultured in and 100 U/mL Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS with penicillin and streptomycin. A serum-free DMEM medium was diluted with fresh media (1:1) to make HT-29-conditioned medium (HT-29-CM).
2.4. Immunohistochemistry (IHC) and fluorescence microscopy detection
Protocols for this study have been described previously [18]. 6-μm thick paraffin-embedded mice liver sections were stained with a rabbit polyclonal to CD31 (1:200; Abcam, ab32457).
Fluorescence staining was performed as follow. TGF-β1 treated HSCs were fixed in 4% paraformaldehyde, permeabilized in 0.02% Triton X-100/PBS, serum blocked, and incubated with polyclonal antibody α-smooth muscle actin (α-SMA) (1:1000, Abcam, Cat. # ab5694). HSCs were serum blocked before incubation with secondary fluorochrome-conjugated antibodies. DAPI was used as a nuclear marker.
2.5. WB assay
Proteins were first extracted using a radioimmunoprecipitation assay lysis buffer containing phenylmethylsulfonyl fluoride, Na2VO3, NaF and protease inhibitor (Roche, Cat. #11873580001). Proteins (50 μg) were then denatured on 10–14% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and transferred to nitrocellulose membranes as described previously.
2.6. Reagents
Mangiferin and terbutaline were obtained from Sigma-Aldrich (Cat. #4773-96-0 and 139508-58-0). The stock solution was used to prepare different concentrations in the test media. including β2-AR polyclonal antibody (Cat. #8513, CST), WAVE2 polyclonal antibody (Cat. # YT4898, ImmunoWay), VEGF polyclonal antibody (Cat. # sc-7269, Santa Cruz Biotechnology), α-smooth muscle actin (α-SMA) (Abcam, Cat. # ab5694), a polyclonal antibody against p-SMAD2 (Cat. #3104, CST) at a 1:1000 and GAPDH mouse monoclonal antibody (Cat. # ab8245, Abcam) at 1:5000 dilution.
2.7. In vivo orthotopic implantation assay
Male BALB/c mice with weight between 18 and 22 g were used at 6–8 weeks. They were supplied with lab mice diet and drinking water and housed under controlled temperature and lighting conditions. HT29-mixed HSC cells (7 × 106) were injected subcutaneously into athymic BALB/c mice [17]. Explanted tumor xenografts were minced into three (1 mm) pieces, then orthotopically implanted into the livers of BALB/c nude mice. The chronic unpredictable mild stress (CUMS) procedure was conducted as previously described [19]. Mice were treated with different doses of MGF. As controls, CLM mice were given only saline. Mice were categorized by different doses of MGF as follows: Group I, sterile saline solution (0,9% NaCl) as the negative control; Group II, MGF 10 mg/kg; Group III, MGF 50 mg/kg; Group IV, MGF 100 mg/kg. MGF and sterile saline solution were administered intragastric intubation twice per week [14]. Each experimental condition involved six mice. At the end of the experiment, mice were subjected to behavioral tests and serum cytokine measurement to assess depression levels.
2.8. Behavioral tests
Depressive behaviors were assessed by the tail suspension test (TST), forced swim test (FST) and the sucrose intake test (SIT). The protocol procedures of behavioral tests were referred to previous works [20]. Briefly, TST was performed as the tails of mice were wrapped with tape from the base to the end and placed upside down on a hook using tape. The time when the mice last did not actively try to escape was quantified using an automated device (BioSeb, Chaville, France). FST was performed 24 h after the TST as described previously. In brief, each mouse was placed individually in a transparent cylinder (height: 25 cm; diameter: 15 cm) filled with water. An analysis of immobility and activity time in the last 4 min was conducted using SMART v3.0 software (Panlab SL, Barcelona, Spain) after each mouse had swum for 6 min. SIT measured 1% sucrose intake for 1 h between 19:00 and 20:00 every Monday. Prior to the SIT, all mice were denied water and food. After the test, the mice were allowed to eat and drink freely.
2.9. ELISA assay
For the collection of murine serum, fresh blood was collected, deposited at 4 °C and then centrifuged. Murine serum norepinephrine was measured by Norepinephrine ELISA Kit (Abbexa, abx150363) and corticosterone was measured by Corticosterone (17-Deoxycortisol) ELISA Kit (Abbexa, abx052182) according to the assay protocol. Murine cytokine serum were measured by IL-6 ELISA Kits (Abcam, ab242772), IL-18 ELISA Kits (Abcam, ab218808) and TNF-1α ELISA Kits (Abcam, ab208348). At 450 nm, the microplate reader measured absorbance.
2.10. Masson's trichrome staining
Masson's trichrome staining was performed according to the manufacturer's instruction (Solarbio, China). The sections were then incubated with Bouin's Fluid for 2 h, followed by 10 min of hematoxylin and 2 min of eosin. Fibrosis assessment analysis completed using Image J software [21].
2.11. Statistical analysis
Data were expressed as mean ± standard deviation (SD) of the mean, and then analyzed using GraphPad Prism 9.4.1 software (GraphPad Software, Inc., La Jolla, CA). Two-tailed Student's t-test was used to compare groups. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. CUMS induced depression behavior and depression-related cytokines in CLM
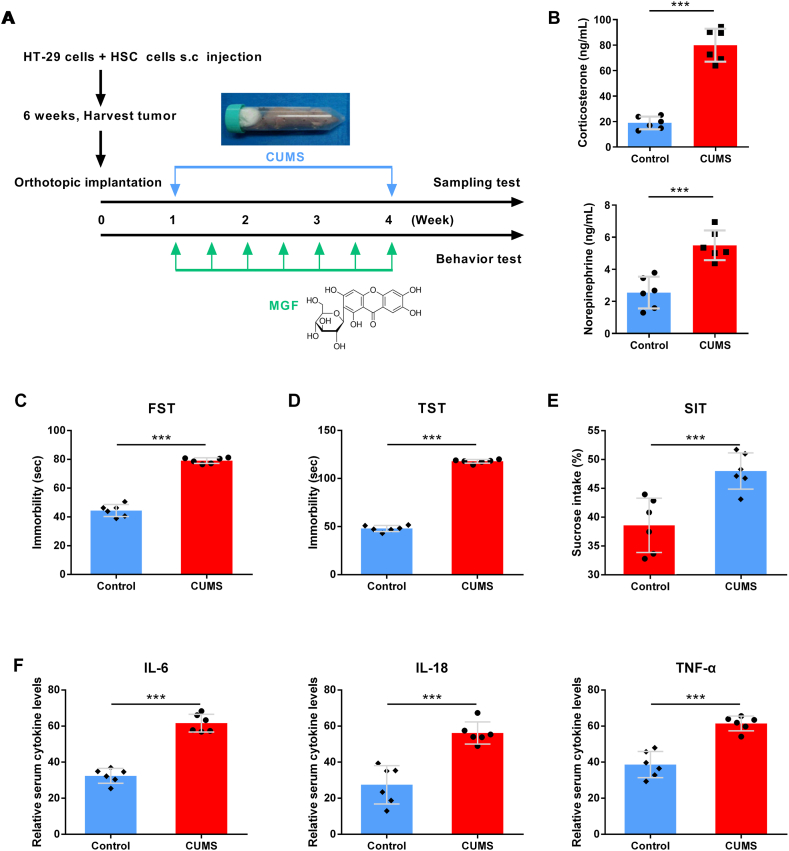
We performed a murine CLM model to examine the effects of chronic unpredictable mild stress (CUMS) on tumor growth, and evaluated the role of MGF in chronic stress. Depression behavior and depression-related cytokines was analyzed after daily CUMS during the four weeks (Fig. 1A). The stress-related hormones norepinephrine and corticosterone were both markedly higher in the serum of stressed mice than in controls (Fig. 1B). As shown in Fig. 1C, D and 1E, the CUMS group reported significantly more immobility behavior and more sugar intake than the control group in FST, TST, SIT. Significantly, serum levels of depression-related cytokine IL-6, IL-18 and TNF-α in CUMS group were higher than control (Fig. 1F).
Fig. 1.
Chronic stress induces depression behavior and depression-related cytokines in CLM mouse model. (A) Chemical structure of mangiferin and an illustration of our experiment design. (B) Serum norepinephrine and corticosterone concentrations in control and CUMS group. (C) Depressive behavior by FST, (D) Depressive behavior by TST, (E) Depressive behavior by SIT, (F) Serum IL-6 level, (E) Serum IL-18 level, (F) Serum TNF-α level. Data are presented as the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001.
3.2. CUMS promoted tumor growth of CLM
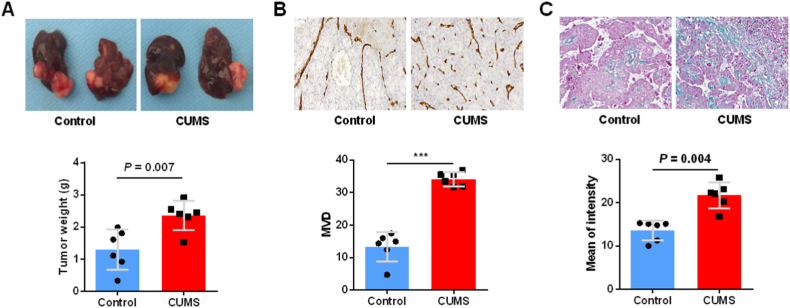
In stressed mice bearing HT-29 and HSC cells, the tumor weight was significantly higher than in control mice (Fig. 2A). HT29+HSC-CUMS group had significantly higher microvascular density (MVD) than the HT29+HSC-control group, according to IHC staining (Fig. 2B). Additionally, we measured tumor extracellular matrix in both stressor-free and stressor-loaded CLM models, and chronic stress facilitated the remodel of extracellular matrix (Fig. 2C). Based on these data, daily CUMS promotes CLM growth.
Fig. 2.
CUMS induces marked tumor growth in CLM. (A) HT29+HSC-CUMS and HT29+HSC-control tumor size was compared. (B) MVD in HT29+HSC-CUMS and HT29+HSC-control group. (C) Masson's trichrome staining of xenograft. Data are presented as the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001.
3.3. CUMS induced HSC activation in CLM via β2-AR and WAVE2 signaling
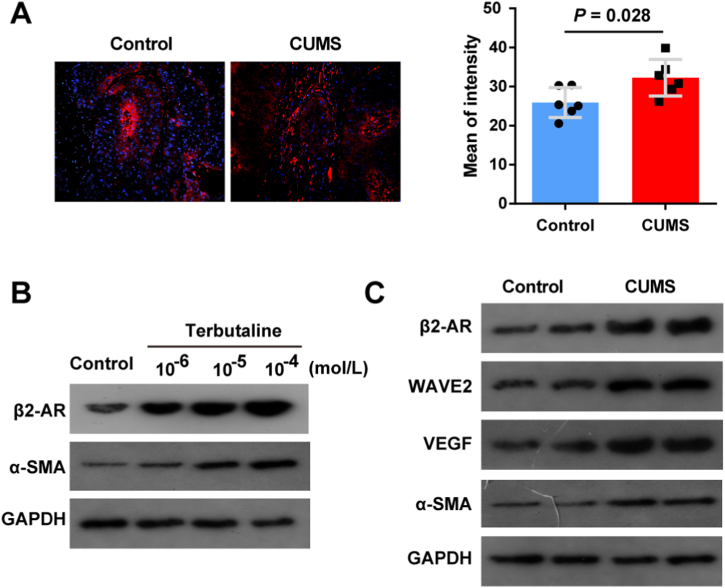
Hepatic stellate cells (HSCs) are a key promoter in CLM microenvironment, which transdifferentiate into pro-tumor myofibroblasts through a TGF-β-dependent mechanism [22]. α-SMA could be used to quantify the activation of HSCs since it is an activation marker for HSCs. α-SMA IF staining showed that chronic stress enhanced HT-29 tumor growth in mice (Fig. 3A). Due to the role of β2-AR in regulating the properties of HSCs [23], Terbutaline, a selective agonist of β2-AR, was used to facilitate HSC activation. To further prove the role of β2-AR in HSCs, HSC cells were treated with terbutaline at the concentrations of 10−6-10−4 mol L−1. Expression of β2-AR and α-SMA was downregulated in HSC cells stimulated with terbutaline increasing concentrations (Fig. 3B). Based on above results, expression of HSC activation and MVD markers in HT29+HSC-CUMS were evaluated by Western blot. β2-AR, WAVE2, α-SMA and VEGF were significant overexpressed in HT29+HSC-CUMS group, retrospectively (Fig. 3C).
Fig. 3.
CUMS induces HSC activation of CLM in mice. (A) IF staining for α-SMA detection in HT29+HSC-CUMS and HT29+HSC-control group. (B) Expression of β2-AR and α-SMA was measured in HSC cells stimulated with terbutaline. (C) HT29+HSC-CUMS and HT29+HSC-control groups were compared for HSC activation and MVD markers. Data are presented as the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. MGF inhibits depressive behavior and related cytokines
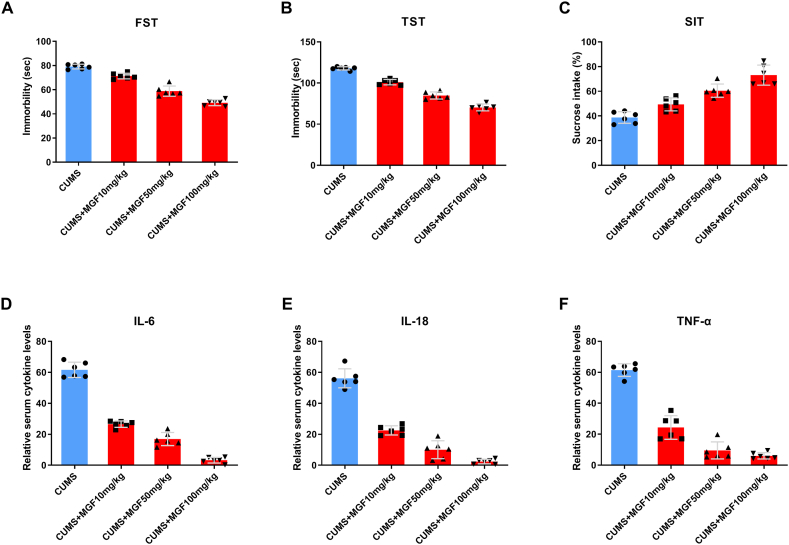
Compared to CUMS control mice, we found that MGF dose-dependently reduced immobility time in the FST (Fig. 4A) and TST (Fig. 4B). We also found that MGF dose-dependently increased sugar intake in SIT compared to CUMS control mice (Fig. 4C), suggesting that MGF may improve depressive status in CLM mice. To understand the mechanism of MGF-induced depression remission in CLM mice, we analyzed serum levels of IL-6, IL-18 and TNF-α. We found that MGF dose-dependently reduced serum depression-related cytokines compared to control group (Fig. 4D–F). These data suggest that the antidepressant effect of MGF may be due to reduced depressive behavior and related cytokines in CLM mice.
Fig. 4.
MGF inhibits depressive behavior and related cytokines. (A) FST, (B) TST, (C) SIT, (D) IL-6, (E) IL-18, (F) TNF-α.
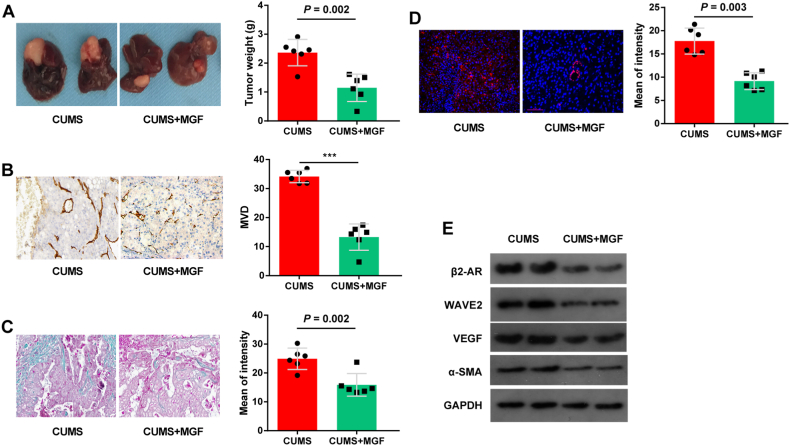
3.5. MGF inhibits tumor growth of CLM in vivo
The tumor weights of the HT29+HSC-MGF mice were lower than those of the HT29+HSC-Control mice (Fig. 5A). Moreover, MVD in HT29+HSC-Control group was significantly higher compared to HT29+HSC-MGF (Fig. 5B), whereas Masson's trichrome staining indicated that the HT29+HSC-Control had significantly higher ECM (Fig. 5C), than those in the HT29+HSC-MGF group. Besides, in the HT29+HSC-MGF group, IF staining revealed higher levels of a-HSCs than in the control group (Fig. 5D). Compare to control group, β2-AR, WAVE2, VEGF and α-SMA levels were decreased significantly in HT29+HSC-MGF group (Fig. 5E). In light of these findings, MGF reduced activation of the TGF-β1 signaling pathway via WAVE2 on HSCs in CLM. These evidences suggest that MGF reduced the activation of WAVE2-dependent TGF-β1 signaling pathway in CLM.
Fig. 5.
MGF reduces promoting effects of HSCs on tumor growth in mice. (A) HT-29 cells (0.5 × 106) mixed with 0.5 × 106 a-HSCs expressing tumors were dissected from nude mice, and tumor size was compared between the HT29+HSC-Control and HT29+HSC-MGF groups (left). (B) MVD in HT29+HSC-CUMS and HT29+HSC- MGF group. (C) Masson's trichrome staining of xenograft. (D) IF staining for α-SMA detection shows that MGF inhibits HT-29 tumor cell growth in mice. (E)Western blot showed that expression of β2-AR, WAVE2, VEGF and α-SMA decreased significantly in HT29+HSC-MGF group. Data are presented as the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001.
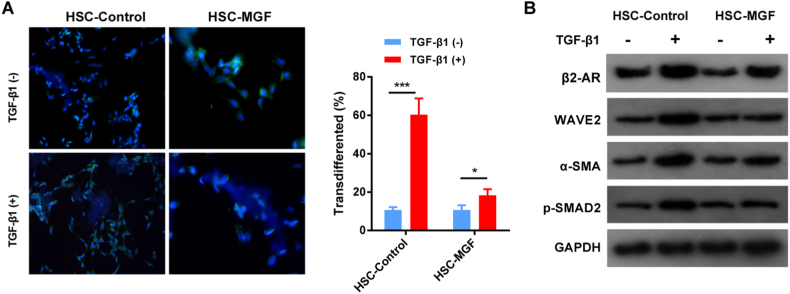
3.6. MGF inhibits activation of HSC in tumor growth via the WAVE2 signaling pathway
Previous studies have shown that MGF inhibits cellular Rac1/WAVE2 signaling protein expression, thereby suppressing invasive breast cancer cell motility [16]. WAVE2 can regulate myofibroblast activation in HSC through the TGF-β1 signaling pathway [17]. Therefore, we investigated whether MGF could inhibit the TGF-β1 signaling pathway to regulate myofibroblast activation in activated HSC. Immunofluorescence staining of α-SMA quantification showed that 24 h TGF-β1 stimulation in control cells induced the development of α-SMA-positive stress fibers in more than 60% of the HSC, whereas only 18% of the HSC myofibroblast activation when MGF treated. On the other hand, HSC myofibroblast activation in control group was similar to MGF treated when TGF-β1 absent. (Fig. 6A). Compared to control cells, protein blots showed significantly reduced α-SMA and p-SMAD2 activation in MGF-treated HSC (Fig. 6B). Western-blots showed activation of β2-AR and WAVE2 was enhanced in HSC when TGF-β1 treated in control group. MGF significantly reduced activation of both β2-AR and WAVE2 in HSC, which inhibition processes was TGF-β1 dependent (Fig. 6B).
Fig. 6.
MGF inhibits activation of HSCs via the WAVE2 signaling pathway. (A) Transduced HSCs treated with TGF-1 (2.5 ng/mL) and IF for SMA show that MGF consistently suppresses TGF-1-induced myofibroblast differentiation. (B) Following 24 h of serum starvation and treatment with TGF-1, control and MGF-treated HSCs are shown. Western blot analysis for detection of HSC activation markers, α-SMA and p-SMAD2, and activator β2-AR and TGF-β1. Data are presented as the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
It is well known that depression itself may be a facilitator of tumor progression [24]. Depression and tumor growth may be related to innate immune activation and inflammation [25]. Pro-inflammatory cytokines are known to regulate key neurobiological correlates of depression [26]. Psychological stress is a common cause of CRC patients and can be a potential indicator of poor prognosis and clinical staging of advanced cancer.
There is evidence that depression is positively associated with the development of CRC. However, the potential mechanisms underlying the effects of psychological stress on CLM have not been fully and systematically explored. We hypothesize that chronic stress may affect the CRC tumor microenvironment and the regulation of WAVE2. We used the CUMS procedure to induce depression in mouse models and to establish murine models. The results showed that CLM deterioration was significantly accelerated in the CUMS + tumor group. In addition, IL-6, IL-18 and TNF-α expression was increased in the tumor microenvironment, accompanied by an increase in TGF-β1 levels and activation of HSCs. Several studies have revealed that TGF-β1 activated p-Akt and p-STAT3 in response to IL-6 stimulation [27]. Depending on context-dependent pro- and anti-cell proliferation functions of TGF-β1 [28], our results suggest a significant increase in α-SMA and p-SMAD2 after CUMS + tumor treatment. Thus, we demonstrate that chronic stress induced activation of HSCs by TGF-β1 signaling of transcriptionally active SMAD complexes is critical for depression-promoted CRC migration [29].
Subsequently, we investigated pharmacological effects of MGF in CLM-CUMS model. According to the literature, MGF attenuated lipid peroxidation, and neuronal damage and shown neuroprotective effects in vitro and in vivo. MGF further increases endogenous antioxidant levels, thereby protecting against oxidative stress within neurons [30]. Antioxidant and immunomodulation attenuated the depression-like behavior and cognitive impairment seen in a mammary tumor mouse model [31]. In line with previous reports, we found that mice treated with MGF significantly improved depression-related behaviors, by reducing depression-related cytokines, IL-6, IL-18 and TNF-α [19,32]. These results support a model in which MGF plays an anti-depression and anti-CLM role [15].
Previously, our team had proved that WAVE2 signaling participated in CLM tumor microenvironment by activating HSCs through TGF-β1, thus potentially being a latent therapeutic target [17]. Our results further demonstrated that this process is effectively blocked by MGF. In prostate cancer cells, MGF inhibits cell invasion by suppressing MMP9 expression and NF-κB activation [33]. MGF can inhibit tumor growth and metastasis by fatty acid metabolism and NF-κB signaling pathways in vitro in human HT29 colon cells [14]. Overall, these evidences support the view that MGF has been shown to exert antitumor, antiangiogenic and antimetastatic effects in cancer. From another perspective, we observed that MGF treatment inhibits WAVE2 signaling pathway, leading to TGF-β1 induced HSC inhibition, thereby reducing depressive behavior and tumor growth in CLM. The findings reported here shed new light on a potent mechanism of chronic stress-induced tumor growth in colorectal liver metastases. Moreover, our work contributes to existing knowledge of MGF by providing a novel mechanism of suppressing chronic stress-induced tumor growth.
5. Conclusion
In conclusion, our results suggest that MGF alleviate CUMS induced tumor growth effects in a CLM model, possibly through TGF-β1 deactivation via WAVE2 signaling. Our findings in this study provide a new understanding of the relationship of chronic unpredictable mild stress and colorectal liver metastases. Another important practical implication is that MGF exhibited a novel role in anti-chronic stress-induced colorectal liver metastases. A greater focus on MGF could produce interesting findings that account more for its pharmacological effects in CLM. Further research could also be conducted to determine the effectiveness of MGF in other cancers and psychosomatic diseases.
Author contribution statement
Yiming Tao: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xuefei Tian: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Jia Luo: Performed the experiments; Wrote the paper.
Hongyi Zhu; Yi Chu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Lei Pei: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
Dr. Lei Pei was supported by National Natural Science Foundation of China [81502545].
Yiming Tao was supported by National Natural Science Foundation of China [81372630; U20A20408; 82074450].
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13753.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y.N., Huang M.L., Kao C.H. Prevalence of depression and anxiety in colorectal cancer patients: a literature review. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta R.D., Roth A.J. Psychiatric considerations in the oncology setting. Ca - Cancer J. Clin. 2015;65:300–314. doi: 10.3322/caac.21285. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Saenz de Tejada M., Bilbao A., Baré M., Briones E., Sarasqueta C., Quintana J.M., Escobar A. Association between social support, functional status, and change in health-related quality of life and changes in anxiety and depression in colorectal cancer patients. Psycho Oncol. 2017;26:1263–1269. doi: 10.1002/pon.4303. [DOI] [PubMed] [Google Scholar]
- 5.Hong J.S., Tian J. Prevalence of anxiety and depression and their risk factors in Chinese cancer patients. Support. Care Cancer. 2014;22:453–459. doi: 10.1007/s00520-013-1997-y. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L., Sun H. The longitudinal changes of anxiety and depression, their related risk factors and prognostic value in colorectal cancer survivors: a 36-month follow-up study. Clin. Res. Hepatol. Gastroenterol. 2021;45 doi: 10.1016/j.clinre.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Pitman A., Suleman S., Hyde N., Hodgkiss A. Depression and anxiety in patients with cancer. Bmj. 2018;361:k1415. doi: 10.1136/bmj.k1415. [DOI] [PubMed] [Google Scholar]
- 8.Schneider M.A., Heeb L., Beffinger M.M., Pantelyushin S., Linecker M., Roth L., Lehmann K., et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abc8188. [DOI] [PubMed] [Google Scholar]
- 9.Sakita J.Y., Bader M., Santos E.S., Garcia S.B., Minto S.B., Alenina N., Brunaldi M.O., et al. Serotonin synthesis protects the mouse colonic crypt from DNA damage and colorectal tumorigenesis. J. Pathol. 2019;249:102–113. doi: 10.1002/path.5285. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y., Wang D., Zhang N., Wang Z., Pang L. Plasma serotonin level is a predictor for recurrence and poor prognosis in colorectal cancer patients. J. Clin. Lab. Anal. 2018;32 doi: 10.1002/jcla.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannen V., Bader M., Sakita J.Y., Uyemura S.A., Squire J.A. The dual role of serotonin in colorectal cancer. Trends Endocrinol. Metabol. 2020;31:611–625. doi: 10.1016/j.tem.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Dan Y., Yang D., Hu Y., Zhang L., Zhang C., Zhu H., et al. The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2014;153:42–60. doi: 10.1016/j.jep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Dou W., Zhang J., Ren G., Ding L., Sun A., Deng C., Wu X., et al. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int. Immunopharm. 2014;23:170–178. doi: 10.1016/j.intimp.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Gonzalez J.C., Hernández-Balmaseda I., Declerck K., Pérez-Novo C., Logie E., Theys C., Jakubek P., et al. Antiproliferative, antiangiogenic, and antimetastatic therapy response by mangiferin in a syngeneic immunocompetent colorectal cancer mouse model involves changes in mitochondrial energy metabolism. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.670167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao C., Su M., Zhou F. Mangiferin inhibits hippocampal NLRP3 inflammasome and exerts antidepressant effects in a chronic mild stress mice model. Behav. Pharmacol. 2017;28:356–364. doi: 10.1097/fbp.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 16.Deng Q., Tian Y.X., Liang J. Mangiferin inhibits cell migration and invasion through Rac1/WAVE2 signalling in breast cancer. Cytotechnology. 2018;70:593–601. doi: 10.1007/s10616-017-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan F., He D., Hu K., Wang D., Zhang S., Li J., Wang Z., et al. WAVE2 enhanced hepatic stellate cells activity in colorectal liver metastases. Cancer Manag. Res. 2020;12:7671–7680. doi: 10.2147/cmar.S259125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan F., Zhu H., Tao Y., Yu N., Pei Q., Liu H., Zhou Y., et al. Neuron navigator 2 overexpression indicates poor prognosis of colorectal cancer and promotes invasion through the SSH1L/cofilin-1 pathway. J. Exp. Clin. Cancer Res. 2015;34:117. doi: 10.1186/s13046-015-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q., Ding W., Qian Z., Jiang G., Sun C., Xu K. Chronic unpredictable mild stress accelerates the growth of bladder cancer in a xenograft mouse model. Psychol. Res. Behav. Manag. 2020;13:1289–1297. doi: 10.2147/prbm.S288983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Chen Y., Dai C., Shang Y., Xie J. Ginsenoside Rh2 alleviates tumor-associated depression in a mouse model of colorectal carcinoma. Am J Transl Res. 2016;8:2189–2195. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C., Yao X., Xu Y., Zhang Q., Wang H., Zhao L., Wen G., et al. Dahuang Zhechong Pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J. Ethnopharmacol. 2019;238 doi: 10.1016/j.jep.2019.111878. [DOI] [PubMed] [Google Scholar]
- 22.Tan H.X., Gong W.Z., Zhou K., Xiao Z.G., Hou F.T., Huang T., Zhang L., et al. CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol. Ther. 2020;21:258–268. doi: 10.1080/15384047.2019.1685157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.Q., Peng W.T., Shan S., Wu J.J., Li N., Du J.J., Sun J.C., et al. β-arrestin2 regulating β2-adrenergic receptor signaling in hepatic stellate cells contributes to hepatocellular carcinoma progression. J. Cancer. 2021;12:7287–7299. doi: 10.7150/jca.59291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greer J.A., Jacobs J.M., El-Jawahri A., Nipp R.D., Gallagher E.R., Pirl W.F., Park E.R., et al. Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J. Clin. Oncol. 2018;36:53–60. doi: 10.1200/jco.2017.73.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W., Zheng L., Xu L., Tu J., Gu X. Pinocembrin mitigates depressive-like behaviors induced by chronic unpredictable mild stress through ameliorating neuroinflammation and apoptosis. Mol. Med. 2020;26:53. doi: 10.1186/s10020-020-00179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currier M.B., Nemeroff C.B. Depression as a risk factor for cancer: from pathophysiological advances to treatment implications. Annu. Rev. Med. 2014;65:203–221. doi: 10.1146/annurev-med-061212-171507. [DOI] [PubMed] [Google Scholar]
- 27.Fan H., Jiang C., Zhong B., Sheng J., Chen T., Chen Q., Li J., et al. Matrine ameliorates colorectal cancer in rats via inhibition of HMGB1 signaling and downregulation of IL-6, TNF-α, and HMGB1. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/5408324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamprecht S., Sigal-Batikoff I., Shany S., Abu-Freha N., Ling E., Delinasios G.J., Moyal-Atias K., et al. Teaming up for trouble: cancer cells, transforming growth factor-β1 signaling and the epigenetic corruption of stromal naïve fibroblasts. Cancers. 2018;10 doi: 10.3390/cancers10030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui S., Lin H., Cui Y., Wen W., Cui X., Shen C., Mo H., et al. Depression promotes lung carcinoma progression by regulating the tumor microenvironment in tumor-bearing models of C57BL/6J mice. Neurosci. Lett. 2021;754 doi: 10.1016/j.neulet.2021.135851. [DOI] [PubMed] [Google Scholar]
- 30.Walia V., Chaudhary S.K., Kumar Sethiya N. Therapeutic potential of mangiferin in the treatment of various neuropsychiatric and neurodegenerative disorders. Neurochem. Int. 2021;143 doi: 10.1016/j.neuint.2020.104939. [DOI] [PubMed] [Google Scholar]
- 31.Casaril A.M., Domingues M., Bampi S.R., Lourenço D.A., Smaniotto T., Segatto N., Vieira B., et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain Behav. Immun. 2020;84:229–241. doi: 10.1016/j.bbi.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Qi H., Ma J., Liu Y.M., Yang L., Peng L., Wang H., Chen H.Z. Allostatic tumor-burden induces depression-associated changes in hepatoma-bearing mice. J. Neuro Oncol. 2009;94:367–372. doi: 10.1007/s11060-009-9887-3. [DOI] [PubMed] [Google Scholar]
- 33.Dilshara M.G., Kang C.H., Choi Y.H., Kim G.Y. Mangiferin inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-κB activity. BMB Rep. 2015;48:559–564. doi: 10.5483/bmbrep.2015.48.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.