Abstract
Soybean meal (SBM) is a cost-effective alternative protein source to replace costly fish meal in aquaculture. This present study determined to measure the effects of replacing fish meal (FM) protein with SBM on growth, feed utilization, and health condition of stinging catfish, Heteropneustes fossilis. Four isonitrogenous (35 %) diets were applied in four treatment groups designed as SBM0, SBM25, SBM50, and SBM75, where 0 %, 25 %, 50 %, and 75 % of FM protein were substituted by SBM, respectively. Significantly higher mean final weight (g), weight gain (g), percent weight gain (%), specific growth rate (% day−1), and protein efficiency ratio (PER) were recorded in SBM0, SBM25, and SBM50 groups than SBM75 group. Consequently, significantly lower feed conversion ratio (FCR) was found in SBM0, SBM25, and SBM50 groups than SBM75 group. Moreover, protein content of whole-body carcass was significantly higher in SBM25 and lower in SBM0 group however, lipid content was significantly higher in SBM0 and SBM75 than in other groups. Hemoglobin, red blood cells, and white blood cells were significantly higher in SBM0, SBM25, and SBM50 groups compared to SBM75. However, the higher the substitution of FM protein by SBM in diets higher the values of glucose. Morphological analysis of the intestine including villi length (μm), width (μm), and area (mm2); crypt depth (μm); wall thickness (μm); abundance of goblet cell (GB); and muscle thickness (μm) showed an increasing trend in fish fed diet containing upto 50 % replacement of FM protein by SBM. Therefore, the results suggest that SBM could replace upto 50 % FM protein in diets of H. fossilis without compromising growth, feed efficiency, and health status.
Keywords: Diet replacement, Fish meal, Soybean meal, Stinging catfish
1. Introduction
Heteropneustes fossilis (stinging catfish), is high-priced and widespread among the air-sac catfishes because of its suitable content of quality protein and iron (Bhatt, 1968, Anon, 1982). It can survive in less oxygen content water therefore suitable for commercial aquaculture (Haniffa and Sridhar, 2002). The production cost is low with high market demand because of its superior quality flesh having high nutritional and therapeutic value (Alam et al., 2009). As well, H. fossilis are highly fecund and adaptable to artificial diets at high temperatures and salinity fluctuation (Radhakrishnan and Sugumaran, 2010; Jhingran, 1991, Thakur, 1991). Due to these promising characteristics related to production, H. fossilis attains considerable attention for culture in Southeast Asia over the past few years.
Feed cost broadly represents around 70 % of the total operational cost because proteins are the high-priced dietary source of semi-intensive or intensive grow-out farming operations (Hossain et al., 2020a, Hossain et al., 2020b). One of the biggest targets of successful aquaculture is to attain highest growth by investing lowest inputs at lowest price. Fish meal (FM), a high-priced feed ingredient, is generally considered one of the major sources of protein for aquafeed production because of its higher protein along with stable amino acids, higher digestible energy, and micronutrients (Tacon and Jackson, 1985). The higher prices and irregular supply of FM demand the search for alternatives with lower prices and highly available plant feedstuffs such as soybean, rapeseed meal, moong, guar, sorghum, etc. (Robinson and Li, 2007, Uddin et al., 2007).
Among all plant protein sources, soybean meal (SBM) represents the high protein content, most secured amino acid profile, stable source, and realistic cost (Meng et al., 2020, Pervin et al., 2020). Several studies reported SBM to replace FM in diets for several fish species owing to the source of quality protein of soybean throughout the world (Nyirenda et al., 2000, Kalla et al., 2003). Nonetheless, the effects of replacing FM protein with SBM on the growth and physiological status of fish are species specific along with their feeding mechanisms (Zhou et al., 2018). In general, SBM is better utilized by herbivorous and omnivorous fish than carnivorous fish species. Moreover, the complete or partial substitution of FM with SBM do not affect the growth and physiology of various herbivorous and omnivorous species of fish (Liu et al., 2021). Siddique et al. (2014) suggested that 15 % replacement of FM protein with SBM did not have any significant difference in the growth of H. fossilis when compared with 100 % FM-containing diet. Therefore, there had been a possibility of replacing FM protein with SBM in diets of H. fossilis fry more than 15 %. Pervin et al. (2020) reported that 75 % substitution of FM by SBM showed no substantial changes in the growth and physiology of Oreochromis niloticus. Moreover, Mohammadinafchi et al. (2014) reported no considerable variations in Mesopotamichthys sharpeyi when the replacement level was 100 %. However, the effects of the replacement of FM protein with SBM on growth performance and physiology of stinging catfish have not been well-documented. Moreover, due to the presence of lower content of methionine and higher content of anti-nutrients, there is a limitation to using SBM as fish feed (Ollie et al., 1994). Besides, SBM may be a cost-effective and highly available alternative protein source to replace costly fish meal in aquaculture. Therefore, the present study was projected to investigate the effects of replacing FM protein with SBM at different substitution levels on growth, feed efficiency, and health condition of stinging catfish.
2. Materials and methods
2.1. Preparation of diets
Available fresh feed ingredients were used to formulate four isonitrogenous (35 % crude protein) diets where 0 %, 25 %, 50 %, and 75 % of FM protein were substituted by SBM in SBM0, SBM25, SBM50, and SBM75 groups, respectively. The formulation and proximate composition of different diets are presented in Table 1. Dry feedstuffs were first ground with a crusher machine for diet preparation. After sieving ground ingredients, all ingredients were thoroughly mixed and added distilled water at 30 % level (El-Saidy and Gaber, 1997). Pellets were made for all experimental diets with a pellet machine. After that processed pellets (1.0 mm size) were oven dried at 55 °C and refrigerated at 4 °C until further use (Yang et al., 2004).
Table 1.
Ingredients and nutrient composition of experimental diets fed to H. fossilis.
| Ingredients (g 100 g−1) | Diets |
|||
|---|---|---|---|---|
| SBM0 | SBM25 | SBM50 | SBM75 | |
| aFish meal | 46.23 | 34.68 | 23.11 | 11.57 |
| bSoybean meal | 0.00 | 17.85 | 35.68 | 53.53 |
| Mustard oil cake | 15.34 | 19.72 | 24.49 | 28.77 |
| Rice bran | 15.17 | 9.14 | 7.24 | 0.60 |
| Wheat bran | 18.29 | 13.63 | 4.52 | 0.58 |
| *Premix | 1.00 | 1.00 | 1.00 | 1.00 |
| Molasses | 4.00 | 4.00 | 4.00 | 4.00 |
| Nutrient composition (%) | ||||
| Moisture | 12.85 | 12.82 | 13.64 | 14.36 |
| Crude protein | 35.57 | 35.54 | 35.04 | 35.23 |
| Crude lipid | 7.87 | 7.34 | 7.56 | 7.33 |
| Ash | 13.38 | 13.04 | 10.42 | 8.54 |
| Crude fibre | 4.20 | 4.36 | 5.13 | 5.34 |
| Nitrogen free extract | 26.13 | 26.90 | 28.21 | 29.20 |
| Energy (kcal/g) | 3.81 | 3.80 | 3.84 | 3.87 |
*Premix supplied the following vitamins and minerals (mg or g or IU/2.5 kg of diet): A, 12,500 IU; B1, 2.5 g; B2, 5 g; B6, 4 g; B12, 12 mg; D3, 2,500,000 IU; E, 20 g; K3, 4 g; Nicotinic acid, 12.5 g; Folic acid, 800 mg; Biotin, 100 mg; Cobalt, 400 mg; Copper, 10 g; Iron, 60 g; Iodine, 400 mg; Manganese, 60 g; Zinc, 50 g; Selenium, 150 g.
Fish meal (56.46 % crude protein), supplied by Blueline Foods (India) Pvt. ltd.
Soybean meal (36.58 % crude protein), supplied by Fresh, Meghna Group of Industries (Narayanganj, Bangladesh).
2.2. Feeding trials
The experimental fish (H. fossilis) was gathered from a renowned hatchery in the Jashore division of Bangladesh named “Maa Fatima Hatchery”. Six hundred fingerlings of H. fossilis (average size 1.55 ± 0.00 g fish−1) were stocked in twelve plastic tanks (150 L capacity each) under four groups with three replications (50 fingerlings in each tank) for 14 weeks in a temporary shed set up on the premises of the Faculty of Fisheries, Patuakhali Science and Technology University, Dumki, Patuakhali, Bangladesh. Detailed procedures of tank preparation were followed as per Billah et al. (2022). Before starting the feeding trial, all the fish were fed for one week with a basal diet to adjust to the experimental systems and conditions. During the trial, the feed was given at the rate of 3 % body weight to all fish daily (2 times at 8:00 and 18:00 h) (Yang et al., 2011). Based on the recorded data of the total weight of fish, the ration was regulated for each treatment at fortnightly sampling. Siphoning was performed to remove uneaten feeds daily. Water exchange was completed from tap water every three days intervals. To observe water quality parameters, respective monitoring systems were applied throughout the experimental periods. The observed parameters were within a suitable range for the culture of fish. The recorded values of temperature, dissolved oxygen, pH, ammonia–nitrogen, and nitrite-nitrogen were 26.6–31.6 °C, 4.5–6.1 mg l−1, 6.7–8.2, 0.1–0.5 mg l−1, and 0.02–0.05 mg l−1, respectively.
2.3. Analysis of growth, feed utilization, and survival rate
After 14 weeks, data were recorded for the quantity and total weight of fish in each tank. Individual length (cm) and weight (g) were measured by randomly collecting fifteen (15) fishes. The growth parameters such as weight gain (g), percent weight gain (%), and specific growth rate (% day−1), feed utilization parameters such as feed conversion ratio and protein efficiency ratio, and survival (%) of H. fossilis were measured with the noted data are as follows.
-
•
Weight gain (g) = Mean final weight – Mean initial weight
-
•
Percent weight gain (%) = × 100
-
•
Specific growth rate (% day−1) = × 100
-
•
Feed conversion ratio (FCR) =
-
•
Protein efficiency ratio (PER) =
-
•
Survival (%) = × 100
2.4. Proximate composition analysis
The proximate composition of feed ingredients, feed, and whole-body carcass from each treatment was analyzed following methods described by AOAC (2000) with little modifications. Triplicate samples were used to analyze the moisture, protein, lipid, ash, and fibre on percent basis (%). Carbohydrate was estimated by deducting the total percentages of analyzed compositions from 100 (Castell and Tiews, 1980). The values of crude protein, fat, and carbohydrate were used to calculate the calorific value of the feeds (Jauncey and Ross, 1982).
2.5. Hemato-biochemical analysis
The value of hemoglobin (Hb), red blood cells (RBCs), white blood cells (WBCs), and blood glucose (Glu) of stinging catfish were determined as g/dL, ×106 /mm3, ×103 /mm3, and mg/dL, respectively as per the methods described by Billah et al. (2022).
2.6. Histological examination
After 14 weeks, two randomly selected H. fossilis were used for each treatment to perform morphological analysis of the intestine as per the procedures followed by Billah et al. (2022). The following morphological parameters as villus length (μm), villus width (μm), villus area (mm2), crypt depth (μm), thickness of the intestinal wall (μm), abundance of goblet cell (GB), and thickness of muscle (μm) were determined for this experiment.
2.7. Statistical analysis
A statistical comparison of data obtained from the proposed study was made with SPSS software. The normality and homogeneity of variances of data were confirmed before any statistical analysis. ANOVA (one-way analysis of variance) was applied to observe significant variations (P < 0.05) among diets. Presentation of data was done as mean ± SE (standard error). Moreover, the Tukey test was selected for detecting variations between diets.
3. Results
3.1. Growth performance, feed utilization and survival
After 14-week feeding trials, the performance of growth, utilization of feed, and survival of H. fossilis were calculated (Table 2). Significantly (P < 0.05) higher mean final weight (g), weight gain (g), percent weight gain (%), specific growth rate (% day−1), and protein efficiency ratio (PER) was recorded in SBM0, SBM25, and SBM50 groups than SBM75 group. The FCR values were significantly (P < 0.05) lower in SBM0, SBM25, and SBM50 groups compared to SBM75 group. Moreover, there were no significant variations (P > 0.05) in the survival rate of H. fossilis among different feeding groups (Table 2).
Table 2.
Growth and feed utilization of H. fossilis fed different experimental diets (mean ± SE).
| Parameters | Diets |
|||
|---|---|---|---|---|
| SBM0 | SBM25 | SBM50 | SBM75 | |
| Initial weight (g) | 1.55 ± 0.00 | 1.55 ± 0.00 | 1.55 ± 0.00 | 1.55 ± 0.00 |
| Final weight (g) | 8.91 ± 0.64a | 8.33 ± 0.28a | 8.28 ± 0.05a | 6.10 ± 0.25b |
| Weight gain (g) | 7.36 ± 0.64a | 6.78 ± 0.28a | 6.73 ± 0.05a | 4.55 ± 0.25b |
| Percent weight gain (%) | 475.05 ± 41.63a | 437.38 ± 18.41a | 433.91 ± 3.59a | 293.55 ± 16.13b |
| Specific growth rate (% day−1) | 1.74 ± 0.07a | 1.67 ± 0.03a | 1.66 ± 0.01a | 1.36 ± 0.04b |
| Feed conversion ratio (FCR) | 3.15 ± 0.7b | 3.29 ± 0.08b | 3.03 ± 0.07b | 3.87 ± 0.06a |
| Protein efficiency ratio (PER) | 0.77 ± 0.01a | 0.76 ± 0.02a | 0.76 ± 0.01a | 0.58 ± 0.01b |
| Survival (%) | 97.63 ± 0.63 | 94.50 ± 3.50 | 96.80 ± 0.58 | 95.61 ± 2.64 |
The values in the same row that are followed by the different superscript letters are significantly different (P < 0.05).
3.2. Proximate composition of experimental fish
The proximate composition of the whole-body carcass is presented in Table 3. The significantly higher moisture content (%) was found in SBM25 and SBM50 groups and it was significantly lower in SBM0 and SBM75 groups (P < 0.05). However, a reverse condition was observed in case of lipid content (%). The protein content (%) was significantly higher in SBM25 group compared to other groups (P < 0.05) and significantly lower protein content (P < 0.05) was found in SBM0 group (Table 3). While significantly higher content of ash (%) was found in the SBM50 group and lower in the SBM25 group (P < 0.05) (Table 3). No significant difference was observed in crude fibre (P > 0.05) content (%) of whole-body carcass among different groups (Table 3). Nitrogen-free extract was found significantly higher in SBM0, SBM25, and SBM75 groups and significantly lower in SBM50 group (P < 0.05).
Table 3.
Proximate composition of H. fossilis fed different experimental diets (mean ± SE).
| Proximate composition (%) | Diets |
|||
|---|---|---|---|---|
| SBM0 | SBM25 | SBM50 | SBM75 | |
| Moisture | 76.11 ± 0.12b | 76.60 ± 0.02a | 76.70 ± 0.05a | 76.00 ± 0.01b |
| Crude protein | 14.44 ± 0.01c | 14.91 ± 0.10a | 14.61 ± 0.01bc | 14.81 ± 0.02ab |
| Crude lipid | 2.13 ± 0.02a | 1.98 ± 0.03b | 1.88 ± 0.01b | 2.15 ± 0.02a |
| Ash | 2.69 ± 0.06b | 2.23 ± 0.02d | 3.15 ± 0.01a | 2.47 ± 0.03c |
| Crude fibre | 0.90 ± 0.20 | 1.12 ± 0.01 | 1.13 ± 0.01 | 0.96 ± 0.17 |
| Nitrogen free extract | 3.73 ± 0.06a | 3.16 ± 0.19a | 2.53 ± 0.01b | 3.61 ± 0.02a |
The values in the same row that are followed by the different superscript letters are significantly different (P < 0.05).
3.3. Hemato-biochemical study
Hemato-biochemical analysis of stinging catfish for instance hemoglobin (Hb), red blood cells (RBCs), white blood cells (WBCs), and blood glucose (Glu) were calculated for all the fish groups (Table 4). The SBM0, SBM25, and SBM50 groups showed significantly higher content of Hb, RBCs, and WBCs, however, the SBM75 group detected significantly lower values of these blood parameters (P < 0.05). Moreover, glucose showed significantly higher and lower (P < 0.05) values in the SBM75 and SBM0 groups, respectively than in other groups (Table 4).
Table 4.
Hemato-biochemical parameters of H. fossilis fed different experimental diets (mean ± SE).
| Blood parameters | Diets |
|||
|---|---|---|---|---|
| SBM0 | SBM25 | SBM50 | SBM75 | |
| Hb (g/dL) | 12.33 ± 0.03a | 12.25 ± 0.01a | 12.23 ± 0.03a | 10.38 ± 0.03b |
| RBC (×106 / mm3) | 1.33 ± 0.02a | 1.31 ± 0.04a | 1.32 ± 0.03a | 1.09 ± 0.01b |
| WBC (×103 / mm3) | 1.60 ± 0.02a | 1.59 ± 0.07a | 1.62 ± 0.01a | 1.25 ± 0.02b |
| Glucose (mg/ dL) | 52.22 ± 0.04d | 54.00 ± 0.04c | 57.5 ± 0.07b | 72.00 ± 0.03a |
The values in the same row that are followed by the different superscript letters are significantly different (P < 0.05).
3.4. Histological study
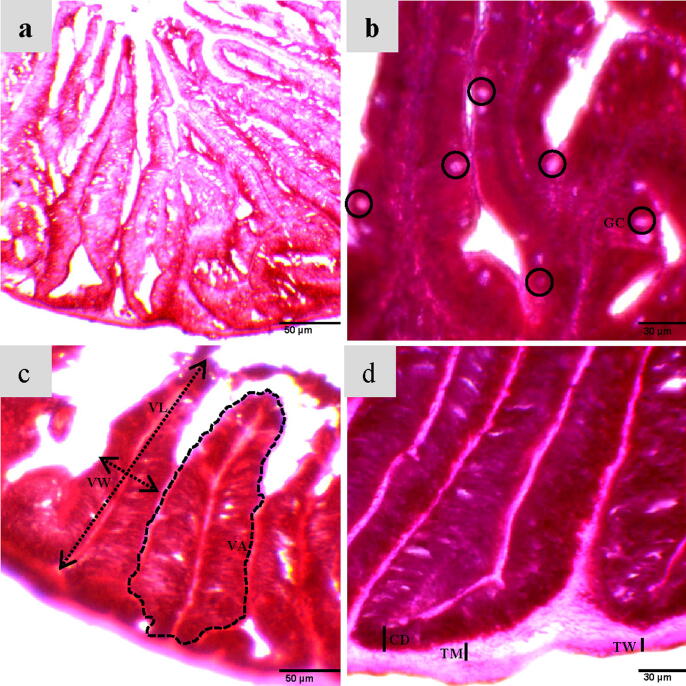
The intestinal histo-morphological measurements of H. fossilis such as length (μm), width (μm), and area of the villi (mm2), crypt depth (μm), thickness of wall (μm), abundance of goblet cell (GB), and thickness of muscle (μm) are presented in Table 5 and Fig. 1. The values of these parameters varied significantly (P < 0.05) among the SBM0, SBM25, SBM50, and SBM75 groups. The increasing trend of histological parameters was observed with increasing dietary soybean meal and it continued upto 50 % replacement level and then declined to 75 %. The examined values of histo-morphological parameters were significantly (P < 0.05) higher in the SBM50 group. An importantly similar tendency was detected also in the case of the number of goblet cells (immune response variable).
Table 5.
Changes in intestinal morphology of H. fossilis fed different experimental diets (mean ± SE).
| Parameters | Diets |
|||
|---|---|---|---|---|
| SBM0 | SBM25 | SBM50 | SBM75 | |
| Villus length (μm) | 97.0 ± 6.24b | 101.02 ± 4.06b | 147.30 ± 6.40a | 91.55 ± 3.10b |
| Villus width (μm) | 62.67 ± 3.90b | 73.33 ± 5.07ab | 96.67 ± 4.53a | 64.36 ± 2.20b |
| Villus area (mm2) | 7.75 ± 0.39b | 10.87 ± 1.26ab | 14.68 ± 1.57a | 8.02 ± 0.41b |
| Crypt depth (μm) | 22.67 ± 3.73ab | 23.33 ± 1.33ab | 34.67 ± 1.32a | 21.38 ± 0.74b |
| Thickness of wall (μm) | 9.00 ± 0.84b | 10.33 ± 1.13b | 18.00 ± 1.85a | 9.09 ± 0.73b |
| Abundance of goblet cell (GB) | 71.30 ± 3.25b | 90.00 ± 6.70ab | 112.00 ± 7.00a | 78.09 ± 1.78b |
| Thickness of muscle (μm) | 5.09 ± 0.08c | 8.10 ± 0.03b | 13.86 ± 0.85a | 7.00 ± 0.30bc |
The values in the same row that are followed by the different superscript letters are significantly different (P < 0.05).
Fig. 1.
Histological changes of the intestine of H. fossilis fed different experimental diets for 14 weeks; (a) control (SBM0) diet, (b) GC = Goblet cell, (c) VA = Villus area, VL = Villus length, VW = Villus width, (d) CD = Crypt depth, TW = Thickness of wall, TM = Thickness of muscle.
4. Discussion
The growth performance of stinging catfish, H. fossilis was significantly higher in SBM0, SBM25, and SBM50 groups compared to SBM75. Moreover, efficient feed utilization was also found in SBM0, SBM25, and SBM50 groups than SBM75 group. This means that replacement of FM protein with SBM upto 50 % did not show any significant difference in growth and feed utilization. Liu et al. (2021) reported that SBM can replace upto 50 % of fish meal in juvenile Liza haematocheila based on weight gain and feed efficiency. Wang et al. (2015) reported substitution of FM protein with SBM upto 40 % in the diet of Pseudobagras ussuriensis had no negative effects on growth, while best feed utilization was obtained upto 50 % substitution level. Conversely, in this present study, upto 50 % replacement level showed better growth performance and this replacement level was somewhat lower than other studies for instance blue catfish (Webster et al., 1995) and rainbow trout (Yang et al., 2011), where 60 % of FM protein can be substituted with SBM without compromising growth. These differences vary from species to species, size, types of ingredients and process of formulation, inclusion and value of SBM, and different culture systems (Wang et al., 2015). Shukla et al. (2018) suggested that 100 % substitution of FM protein with SBM improved growth performance in H. fossilis when the protein content of the FM (23.76 %) was less than SBM (37.83 %), which means that the deteriorative quality of the fish meal. In this present study, the protein content of FM was higher (56.46 %) than SBM (36.58 %).
At large, FM can be partly substituted by SBM without hampering development in some aquatic animals. In this study, 75 % replacement of FM protein with SBM decreased the growth of stinging catfish. Liu et al. (2021) showed that SBM above 75 % in the diet of Liza haematocheila reduced the growth and feed efficiency. Similar reports were also suggested by Yang et al. (2011), where 80 % dietary inclusion level of SBM significantly reduced the growth of Oncorhynchus mykiss. Diet containing SBM protein reduce growth and feed efficiency because of the existence of anti-nutrients, bitter taste, lower digestibility of protein, scarcity of essential amino acid (Francis et al., 2001, Wang et al., 2006, Yang et al., 2011). In the present study, the reduced growth rate of stinging catfish found from high soybean meal-containing diet (SBM75) could be due to their morphological and physiological variations for diets high in animal protein. Moreover, the FCR values of this present study were slightly higher because of the culture systems and removal of uneaten feeds. At times, leftover feeds were not possible to collect because of suspension in water, and this situation leads to excess feeding. While, in the nutritional study, the supply of feed should be smooth enough, therefore overfeeding is better than underfeeding (Tacon and Cowey, 1985). Billah et al. (2022) reported the FCR values of stinging catfish 3.55 to 4.35 when FM protein was totally replaced by SBM. In this study, the survival rate found around 94 to 98 % is similar to the results of 92 to 100 % reported by Liu et al. (2021), where FM protein was replaced with various percentages of soybean meal protein.
Substitution of FM protein with SBM in diets of H. fossilis affected the values of whole-body carcass composition such as moisture, crude protein, crude lipid, ash, crude fibre, and nitrogen-free extract. Comparable results were also reported in Ussuri catfish (Wang et al., 2015), Nile tilapia (Ajani et al., 2016), and European seabass (Kaushik et al., 2004). While some other studies showed that the replacement of FM protein with SBM in diets did not affect the proximate composition of rainbow trout (Yang et al., 2011) and European sea bass (Tibaldi et al., 2006). In this study, significantly higher and lower whole-body carcass protein was found in the SBM25 and SBM0 groups, respectively. However, lipid content was significantly higher in SBM0 and SBM75 groups than in other groups. Moreover, moisture and lipid content also showed reverse conditions.
Hemato-biochemical studies are commonly used for the evaluation of different physical conditions of fish (Pradhan et al., 2012, Sharmin et al., 2016, Jahan et al., 2019, Billah et al., 2022). In this present experiment, there was no significant variation observed in Hb, RBCs, and WBCs upto 50 % replacement of FM protein by soybean meal in diets and then there was a decreasing trend of these values at 75 % replacement level. Dawood et al. (2015) obtained Hb and Glu levels of 10.7 to 12.5 g/dL and 68 to 97.3 mg/dL, respectively in Amberjack juveniles fed 0 to 45 % soybean meal-containing diets. The increasing level of Hb in fish blood may have had better oxygen transport in tissues leading to improved growth (Esmaeili, 2021, Hossain et al., 2022). Hosseini and Khajepour (2013) reported that a higher level of replacement of FM protein by soybean meal lowers the value of Hb, RBC, and WBC in beluga. In this study, the blood glucose level was reversed with blood hemoglobin. Moreover, in this study, the higher the replacement of FM protein by soybean meal in diets higher the values of glucose in stinging catfish. Parallel reports were also stated by Liu et al. (2021) in juvenile redlip mullet and Hosseini and Khajepour (2013) in beluga. Previous reports suggested that the hematology of fish varies on fish species, size, physical and environmental conditions, feed ingredients and formulation, source of quality protein, vitamins, and probiotics (Osuigwe et al., 2005).
The morphological changes in the intestine have been observed in some fish species owing to the inclusion of SBM in the diets (Shiu et al., 2015, Garcia-Ortega et al., 2016). The present study showed that the dietary replacement of FM protein with SBM upto 50 % increased intestinal shape with increasing the histo-morphological factors of the intestine and then decline to 75 % replacement level. Increased intestinal shape (villus length, width, area, and thickness) designates the evaginations of the intestinal mucosa, which increases the absorption of intestinal nutrients and improves fish growth performance and feed consumption (Ferguson et al., 2010, Pirarat et al., 2011, Khojasteh et al., 2012, Jahan et al., 2021, Islam et al., 2021). In general, plant feedstuff contains cellulose (plant fiber), which is digested by cellulase produced by the gut bacteria of many fish species such as chitinase in crustaceans. Moreover, herbivorous and some omnivorous fishes are more able to digest cellulose than carnivorous fishes. For this reason, in this present study, the shape of the intestine was increased by replacing FM protein with SBM upto 50 % in the diet. Further replacing of SBM in the diet reduced the intestinal shape of stinging catfish. Comparable effects were also described in orange-spotted grouper (Wang et al., 2017) and Japanese seabass (Zhang et al., 2018). In this study, the abundance of goblet cells increased by substituting FM protein with SBM upto 50 % level, which is responsible for the production of mucus that provides gel coating over the surface layer of epithelium and defends from bacterial invasion (Johansson et al., 2008).
Based on the results it was suggested that SBM could replace upto 50 % FM protein in diets of H. fossilis without compromising growth, feed efficiency, and health status. More studies should be carried out in production trials for a longer duration on H. fossilis by using 50 % replacement of FM protein with SBM in the diet.
Author contributions
Sumon Howlader experimented, collected, and tabulated the data; Kanij Rukshana Sumi planned the study, designed, supervised, analyzed data, and wrote the manuscript; Subroto Sarkar and Sheikh Masum Billah assisted Sumon Howlader in conducting the experiments and data collection; Mohammad Lokman Ali assisted Sumon Howlader to conduct the experiments and revised the manuscript; Md. Shahjahan facilitated the laboratory to analyze histology and hematology and edited the manuscript; Jewel Howlader helped in data analysis and revised the manuscript; All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research work was supported by the Research and Training Centre (Grant no. 5921, Fish-1 and 4829, Fish-90), Patuakhali Science and Technology University.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sumon Howlader, Email: PG05803@pgs.pstu.ac.bd.
Kanij Rukshana Sumi, Email: krsumi@pstu.ac.bd.
Subroto Sarkar, Email: PG05764@pgs.pstu.ac.bd.
Sheikh Masum Billah, Email: PG05790@pgs.pstu.ac.bd.
Mohammad Lokman Ali, Email: lokman@pstu.ac.bd.
Jewel Howlader, Email: jewelhrt@pstu.ac.bd.
Md Shahjahan, Email: mdshahjahan@bau.edu.bd.
References
- Ajani E.K., Orisasona O., Omitoyin B.O., Osho E.F. Total replacement of fishmeal by soybean meal with or without methionine fortification in the diets of Nile tilapia, Oreochromis niloticus. J. Fish. Aquat. Sci. 2016;11(3):238–243. [Google Scholar]
- Alam M.S., Jahan M., Hossain M.M., Islam M.S. Population genetic structure of three major river populations of rohu, Labeo rohita (Cyprinidae; Cypriniformes) using icrosatellite DNA markers. Genes Genom. 2009;31:43–51. [Google Scholar]
- Anon, 1982. Pakistan National Report. In: Spagnesi, M. (ed.), Proc. Conference on Conservation of Wetlands of International Importance especially as Waterfowl Habitat, cagliari, Italy, 24–29 November 1980. Suppl. Richerche di Biologia della Selvaggina, VIII 1, 893–905
- AOAC, 2000. Official methods of Analysis, 17th ed. Association of Official Analytical Chemist, Washington DC, pp. 2200
- Bhatt V.S. Studies on the biology of some fresh water fishes. part -Vll. H. fossilis (Bloch) Indian J. Fish. 1968;15:99–115. [Google Scholar]
- Billah S.M., Sumi K.R., Howlader S., Sarkar S., Ferdous Z., Islam S.M., Shahjahan M. Effects of supplemental L-methionine for total replacement of fish meal by soybean meal on growth, feed utilisation and health status of stinging catfish, Heteropneustes fossilis fry. Aquacult. Fish Fish. 2022:1–9. [Google Scholar]
- Castell, J.D., K. Tiews (eds.), 1980. Report of the EIFAC, IUNS and ICES working group on standardization of methodology in fish nutrition research. EIFAC Technical Paper 36, Hamburg, Federal Republic of Germany, 21-23 March, 1979. 24 pp.
- Dawood M.A., Koshio S., Ishikawa M., Yokoyama S. Seriola dumerili juveniles; BioMed Res. Int.: 2015. Effects of partial substitution of fish meal by soybean meal with or without heat-killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack; p. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saidy D.M.S., Gaber M.M.A. Total replacement of fish meal by soybean meal, with various percentages of supplemental L-methionine, in diets for Nile tilapia, Oreochromis niloticus fry. Ann. Agric. Sci. Moshtohor. 1997;35:1223–1238. [Google Scholar]
- Esmaeili M. Blood performance: a new formula for fish growth and health. Biology (Basel) 2021;10:1–17. doi: 10.3390/biology10121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R.M.W., Merrifield D.L., Harper G.M., Rawling M.D., Mustafa S., Picchietti S., Balcázar J.L., Davies S.J. The effect of Pediococcus acidilactici on the gut microbiota and immune status of on-growing red tilapia (Oreochromis niloticus) J. Appl. Microbiol. 2010;109:851–862. doi: 10.1111/j.1365-2672.2010.04713.x. [DOI] [PubMed] [Google Scholar]
- Francis G., Makkar H.P.S., Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199:197–227. [Google Scholar]
- Garcia-Ortega A., Kissinger K.R., Trushenski J.T. Evaluation of fish meal and fish oil replacement by soybean protein andalgal meal from schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus. Aquaculture. 2016;452:1–8. [Google Scholar]
- Haniffa M.A., Sridhar S. Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis) using human chorionic gonadotropin and synthetic hormone (ovaprim) Vet. Arhiv. 2002;72:51–56. [Google Scholar]
- Hossain M.M., Ali M.L., Khan S., Haque M.M., Shahjahan M. Use of Asian watergrass as feed of grass carp. Aquacult. Rep. 2020;18 [Google Scholar]
- Hossain M.K., Hossain M.M., Mim Z.T., Khatun H., Hossain M.T., Shahjahan M. Multi-species probiotics improve growth, intestinal microbiota and morphology of Indian major carp mrigal Cirrhinus cirrhosus. Saudi J. Biol. Sci. 2022;9 doi: 10.1016/j.sjbs.2022.103399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.M., Rahman M.H., Ali M.L., Khan S., Haque M.M., Shahjahan M. Development of a low-cost polyculture system utilizing Hygroryza aristata floating grass in the coastal wetlands of Bangladesh. Aquaculture. 2020;527 [Google Scholar]
- Hosseini S.A., Khajepour F. Effect of partial replacement of dietary fish meal with soybean meal on some hematological and serum biochemical parameters of juvenile beluga, Huso huso. Iran. J. Fish. Sci. 2013;12(2):348–356. [Google Scholar]
- Islam S.M.M., Rohani M.F., Shahjahan M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac. Reports. 2021;21 [Google Scholar]
- Jahan N., Islam S.M.M., Rohani M.F., Hossain M.T., Shahjahan M. Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture. 2021;545 [Google Scholar]
- Jahan A., Nipa T.T., Islam S.M., Uddin M.H., Islam M.S., Shahjahan M. Striped catfish (Pangasianodon hypophthalmus) could be suitable for coastal aquaculture. J. Appl. Ichthyol. 2019;35(4):994–1003. [Google Scholar]
- Jauncey K., Ross B. University of Stirling, Stirling; Institute of Aquaculture: 1982. A guide to tilapia feeds and feeding. [Google Scholar]
- Jhingran V.G. In: Fish and Fisheries of India. Jhingran V.G., editor. Hindustan published corporation; Delhi, India: 1991. Nutrition of cultivated fishes; pp. 572–576. [Google Scholar]
- Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U.S.A. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla A., Garg S.K., Kaushik C.P., Arasu A.R.T., Dinodia G.S. Effect of replacement of fishmeal with processed soybean on growth, digestibility and nutrient retention in Mugil cephalus (Linn.) fry. Indian J. Fish. 2003;50(4):509–518. [Google Scholar]
- Kaushik, S.J., Cove‘s, D., Dutto, G., Blanc, D., 2004. Almost total replacement of fishmeal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 230, 391–404.
- Khojasteh S.M.B., Mahdi S., Khojasteh B. The morphology of the post-gastric alimentary canal in teleost fishes: a brief review. Int. J. Aquat. Sci. 2012;3:2008–8019. [Google Scholar]
- Liu T., Han T., Wang J., Liu T., Bian P., Wang Y., Cai X. Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile redlip mullet Liza haematocheila. Aquac. Rep. 2021;20 [Google Scholar]
- Meng F., Li B., Xie Y., Li M., Wang R.X. Substituting fishmeal with extruded soybean meal in diets did not affect the growth performance, hepatic enzyme activities, but hypoxia tolerance of Dolly Varden (Salvelinus malma) juveniles. Aquac. Res. 2020;51(1):379–388. [Google Scholar]
- Mohammadinafchi F., Mohammadiazarm H., Yavari V. Evaluation effect of soybean meal and baker’syeast on resistance to anoxia stress and blood biochemical parameters of fingerlings (Mesopotamichthys sharpeyi Günther, 1874) Int. J. Biosci. 2014;5(8):215–222. [Google Scholar]
- Nyirenda, J., Mwabumba, M., Kaunda, E., Sales, J., 2000. Effect of substituting animal protein sources with soybean meal in diets of Oncorhynchus karongae (Trewavas 1941). Naga, The ICLARM Quarterly, Volume 23, No. 4, October-December.
- Ollie J.J., Krogdahl A., Hjelmeland K. Soybean trypsin inhibitors in diets for Atlantic salmon (Slmo salar) effects on nutrient digestibilities and trypsin in pyloric caeca homogenate and intestinal content. Comp. Biochem. Physiol. A. 1994;109:923–928. doi: 10.1016/0300-9629(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Osuigwe D.I., Obiekezie A.I., Onuoha G.C. Some haematological changes in hybrid catfish (Heterobranchus longifilis × Clarias gariepinus) fed different dietary levels of raw and boiled jackbean (Canavalia ensiformis) seed meal. Afr. J. Biotechnol. 2005;4:1017–1021. [Google Scholar]
- Pervin M.A., Jahan H., Akter R., Omri A., Hossain Z. Appraisal of different levels of soybean meal in diets on growth, digestive enzyme activity, antioxidation, and gut histology of tilapia (Oreochromis niloticus) Fish Physiol. Biochem. 2020;46(4):1397–1407. doi: 10.1007/s10695-020-00798-5. [DOI] [PubMed] [Google Scholar]
- Pirarat N., Pinpimai K., Endo M., Katagiri T., Ponpornpisit A., Chansue N., Maita M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011;91:e92–e97. doi: 10.1016/j.rvsc.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Pradhan S.C., Patra A.K., Sarkar B., Pal A. Seasonal changes in hematological parameters of Catla catla (Hamilton 1822) Comp. Clin. Path. 2012;21(6):1473–1481. [Google Scholar]
- Radhakrishnan M.V., Sugumaran E. Effect of sugarcane bagasse and supplemental feed on certain reproductive characteristics of the catfish Heteropneutes fossilis (Bloch) J. Fish. Int. 2010;5(4):58–60. [Google Scholar]
- Robinson E.H., Li M.H. office of Agricultural Communications. Mississippi State University, USA; 2007. Catfish Protein Nutrition (Revised). Bulletin 1153. [Google Scholar]
- Sharmin S., Salam M.A., Haque F., Islam M.S., Shahjahan M. Changes in hematological parameters and gill morphology in common carp exposed to sub-lethal concentrations of malathion. Asian J. Med. Biol. Res. 2016;2(3):370–378. [Google Scholar]
- Shiu Y.L., Hsieh S.L., Guei W.C., Tsai Y.T., Chiu C.H., Liu C.H. Using bacillus subtilis e20-fermented soybean meal as replacement for fish meal in the diet of orange-spotted grouper (Epinephelus coioides, Hamilton) Aquacult. Res. 2015;46(6):1403–1416. [Google Scholar]
- Shukla A., Kaur V.I., Kumar P., Ansal M.D., Dhawan A., Mishra V. Utilization of dietary soybean meal and groundnut meal as fish meal replacement in Heteropnuestes fossilis (Bloch.) Int. J. Curr. Microbiol. App. Sci. 2018;7(6):734–746. [Google Scholar]
- Tacon, A.G.J., Jackson, A.J., 1985. Utilization of conventional and unconventional protein sources in practical fish feeds, in: Cowey, C.B., Mackie, A.M., Bell, J.G. (Eds.), Nutrition and Feeding of Fish. Academic Press, London, UK, pp. 119–145.
- Tacon A.G., Cowey C.B. In: Fish Energetics-New Perspectives. Tytler P., Calow P., editors. Croom Helm; London: 1985. Protein and amino acid requirements; pp. 155–183. [Google Scholar]
- Thakur N.K. Possibilities and problem of catfish culture in India. J. Inland Fish. Soc. India. 1991;23:80–90. [Google Scholar]
- Tibaldi E., Hakim Y., Uni Z., Tulli F., de Francesco M., Luzzana U., Harpaz S. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax) Aquaculture. 2006;261:182–193. [Google Scholar]
- Uddin, M.N., Rahman, Shahjahan, M., 2007. Effects of duckweed (Lemna minor) as supplementary feed on monoculture of gift strain of tilapia (Oreochromis niloticus). Progress. Agric. 18, 183–188
- Wang Y., Kong L.J., Li C., Bureau D.P. Effect of replacing fish meal with soybean meal on growth, feed utilization and carcass composition of cuneate drum (Nibea miichthioides) Aquaculture. 2006;261:1307–1313. [Google Scholar]
- Wang Y.R., Wang L., Zhang C.X., Song K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides) Aquac, Rep. 2017;5:52–57. [Google Scholar]
- Wang Y., Yu S., Wang Y., Che J., Zhao L., Bu X., Yang Y. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion of juvenile Pseudobagrus ussuriensis. Aquac. Res. 2015;47(10):3145–3155. [Google Scholar]
- Webster C.D., Goodgame-Tiu L.S., Tidwell J.H. Total replacement fish meal by soybean meal, with various percentages of supplemental L-methionine, in fish diets for blue catfish, Ictalurus furcatus (Leseur) Aquac. Res. 1995;26:299–306. [Google Scholar]
- Yang Y.H., Wang Y.Y., Lu Y., Li Q.Z. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion on rainbow trout (Oncorhynchus mykiss) Aquac. Int. 2011;19:405–419. [Google Scholar]
- Yang Y., Xie S., Cui Y., Lei W., Zhu X., Yang Y., Yu Y. Effect of replacement of dietary fish meal by meat and bone meal and poultry by-product meal on growth and feed utilization of gibel carp. Carassius auratus gibelio. Aquac. Nutr. 2004;10(5):289–294. [Google Scholar]
- Zhang C., Rahimnejad S., Wang Y.R., Lu K., Song K., Wang L., Mai K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture. 2018;483:173–182. [Google Scholar]
- Zhou Z., Ringø E., Olsen R.E., Song S.K. Dietary effects of soybean products on gut microbiota and immunity of aquatic animals: a review. Aquac. Nutr. 2018;24:644–665. [Google Scholar]