Abstract
Background
Smoking is well known to be associated with a higher prevalence and incidence of liver diseases such as advanced fibrosis. However, the impact of smoking on developing nonalcoholic fatty liver disease remains controversial, and clinical data on this is limited. Therefore, this study aimed to investigate the association between smoking history and nonalcoholic fatty liver disease (NAFLD).
Methods
Data from the Korea National Health and Nutrition Examination Survey 2019-2020 were used for the analysis. NAFLD was diagnosed according to an NAFLD liver fat score of >-0.640. Smoking status was classified as into nonsmokers, ex-smokers, and current smokers. Multiple logistic regression analysis was conducted to examine the association between smoking history and NAFLD in the South Korean population.
Results
In total, 9,603 participants were enrolled in this study. The odds ratio (OR) for having NAFLD in ex-smokers and current smokers in males was 1.12 (95% confidence interval [CI]: 0.90–1.41) and 1.38 (95% CI: 1.08–1.76) compared to that in nonsmokers, respectively. The OR increased in magnitude with smoking status. Ex-smokers who ceased smoking for <10 years (OR: 1.33, 95% CI: 1.00–1.77) were more likely to have a strong correlation with NAFLD. Furthermore, NAFLD had a dose-dependent positive effect on pack-years, which was 10 to 20 (OR: 1.39, 95% CI: 1.04–1.86) and over 20 (OR: 1.51, 95% CI: 1.14–2.00).
Conclusion
This study found that smoking may contribute to NAFLD. Our study suggests cessation of smoking may help management of NAFLD.
Keywords: smoking, smoking behavior, smoking history, smoking cessation, tobacco, pack-years, nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease. It is a condition in which neutral fat accumulates excessively in the liver (1, 2). Although there are some differences in its frequency from country to country, it has been reported that 6.3 to 33% and an average of approximately 20% of patients worldwide have been affected by the disease (3). The prevalence of NAFLD is rapidly increasing in Asian countries due to the increase in Westernized eating habits, obesity, and the diabetic population (4, 5). In addition, between 10 and 29% of patients with nonalcoholic fatty hepatitis develop cirrhosis within 10 years and between 4 and 27% of patients develop liver cancer (6, 7). Furthermore, patients with NAFLD have a higher mortality rate than healthy controls, and the mortality rate related to liver disease is also high (8–11). Therefore, NAFLD must be managed immediately due to its expected serious public health burden and significant social costs (12, 13).
Tobacco smoke contains more than 7,000 chemicals, of which at least 250 are known to be harmful, such as ammonia and hydrogen cyanide (14, 15). Smoking is closely related to chronic diseases, such as cardiovascular diseases, cancer, and type 2 diabetes (16–19), which are also related to NAFLD (20–22). Previous studies have suggested smoking is associated with increased prevalence and incidence of liver diseases (23, 24). In particular, it has been reported to be an independent risk factor for the progression of advanced fibrosis in patients with primary biliary cirrhosis (23) and chronic hepatitis C (24).
A positive association between smoking and NAFLD has been continuously reported (25–27). An experimental study suggested cigarettes accelerated the progression of NAFLD in obese mice-fed diets (25). Furthermore, a study conducted in mice without apolipoprotein E, a condition wherein fatty liver is easily occurs, found that nicotine in electronic cigarettes (e-cigarettes) causes genetic mutations and promotes NAFLD outbreaks (26). Other studies have shown that the activation of sterol regulatory element-binding proteins (SREBPs), which stimulate the synthesis of fatty acids in the liver, is associated with NAFLD (27). These studies provided evidence of the mechanism of the relationship between smoking and the prevalence of NAFLD. However, most studies are experimental studies conducted on animals, and there are not many studies conducted on humans.
Therefore, this study aimed to examine the association between smoking history and NAFLD in a representative population and to explain whether smoking behavior plays a potential role in developing NAFLD.
Materials and methods
Data
The study used cross-sectional data from the 2019–2020 National Health and Nutrition Examination Survey (KNHANES), conducted by the Korea Centers for Disease Control and Prevention Agency (KDCA). The KNAHENS is a self-report survey using a stratified, multistage, cluster sampling design conducted annually for South Koreans of all ages to evaluate the health and nutritional status. The survey provides data for the evaluation and development of health policies and programs and does not require ethical approval from the ethics review board, as the KNHANES conforms to the Declaration of Helsinki.
Study population
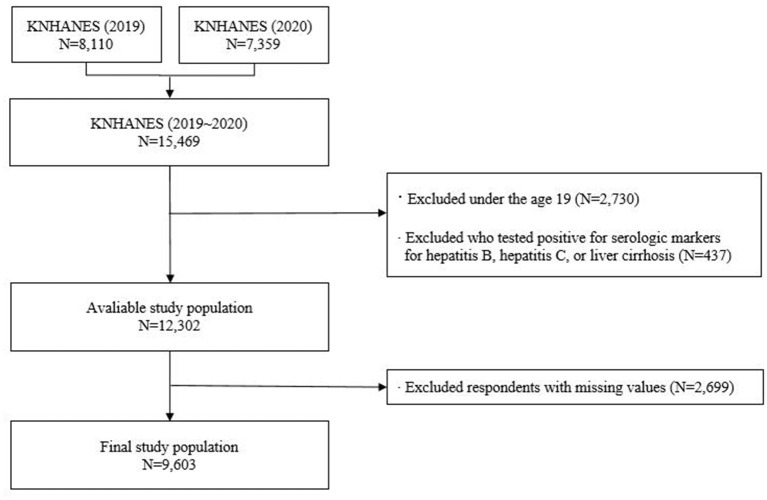
Of the 15,469 survey participants, we excluded those under 19 years of age and those who did not participate in a KNHANES smoking questionnaire survey (n = 2,730). Furthermore, participants who tested positive for serologic markers for liver diseases (hepatitis B, hepatitis C, and liver cirrhosis) were excluded (n = 437). Participants with missing data were also excluded (n = 2,699). Consequently, a final sample of 9,603 participants was analyzed in this study (Figure 1). As a study that examined the effects of smoking on NAFLD, participants with alcohol-related fatty liver disease were also excluded based on their biochemical and clinical profiles.
Figure 1.
Flowchart of the study participants showing the inclusion and exclusion.
Variables
The main dependent variable was the prevalence of NAFLD. NAFLD was diagnosed according to the NAFLD liver fat score developed by the Department of Medicine and the Minerva Medical Research Institute at Helsinki University (28). The NAFLD liver fat score formula was derived using a multivariate logistic regression model using metabolic syndrome, type 2 diabetes, fasting insulin (fS), serum aspartate aminotransferase (AST) ratio, and AST to serum alanine aminotransferase (ALT) ratio (28): NAFLD liver fat score = −0.89 + 1.18 × metabolic syndrome (yes = 1 / no = 0) + 0.45 × diabetes (yes = 2 / no = 0) + 0.15 × fS-insulin (mu/L) + 0.04 × fS-AST (U/L) – 0.94 × AST/ALT. Participants were considered to have NAFLD if their liver fat score of NAFLD was >−0.640 as the optimal cutoff point (28).
The primary independent variable was the smoking status of the participants, which was divided into three groups: (1) nonsmokers, (2) ex-smokers, and (3) current smokers. This was defined based on the questions: 'Do you currently smoke conventional cigarettes?'; “Do you currently smoke e-cigarettes?”. This classification was the same as that of a previous study that used the same research tool to investigate smoking behavior (29).
The covariates included demographic factors (sex, age, marital status, and educational level), socioeconomic factors (household income, region, and occupational categories), behavioral health patterns (current drinking status, physical activity), and health-related factors (body mass index (BMI), diagnosis of hypertension, and diagnosis of diabetes).
Statistical analysis
All estimates were calculated using sample weight procedures to improve representativeness and generalize the data. Clusters and strata were assigned to the study population. The general characteristics of the study group, represented by frequencies and percentages for categorical variables, means and standard deviations for continuous variables, were based on descriptive analysis. After adjusting for covariates, a multiple logistic regression analysis was performed to assess the relationship between smoking and NAFLD. Subgroup analyzes were also performed according to age, current drinking status, physical activity, BMI, and diagnosis of hypertension and diabetes. Furthermore, we also performed a subgroup analysis for a more complete analysis of smoking behavior, including smoking cessation status (SCS) and pack years. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Table 1 shows the characteristics of the study population. Of the 9,603 participants, 4,063 were men (42.3%) and 5,540 were women (57.7%). Among males, 1,249 (30.7%) were current smokers, 1,674 (41.2%) were ex-smokers, and 1,140 (28.1%) were nonsmokers. Among the females, 259 (4.7%) were current smokers, 312 (5.6%) were ex-smokers, and 4,969 (89.7%) were nonsmokers. In total, 1,433 (35.3%) men and 1,278 (23.1%) women reported NAFLD.
Table 1.
General characteristics of the study population.
| Variables | Nonalcoholic fatty liver disease (NAFLD) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||||||||
| Total | Yes | No | P- value | Total | Yes | No | P- value | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Total ( N =9,603) | 4,063 | 100.0 | 1,433 | 35.3 | 2,630 | 64.7 | 5,540 | 100.0 | 1,278 | 23.1 | 4,262 | 76.9 | |||
| Smoking Behavior | 0.0014 | 0.5628 | |||||||||||||
| Nonsmoker | 1,140 | 28.1 | 354 | 31.1 | 786 | 68.9 | 4,969 | 89.7 | 1,156 | 23.3 | 3,813 | 76.7 | |||
| Ex-smoker | 1,674 | 41.2 | 629 | 37.6 | 1,045 | 62.4 | 312 | 5.6 | 65 | 20.8 | 247 | 79.2 | |||
| Current smoker | 1,249 | 30.7 | 450 | 36.0 | 799 | 64.0 | 259 | 4.7 | 57 | 22.0 | 202 | 78.0 | |||
| Age (Mean, SD) | 51.6 | 17.2 | 52.2 | 15.9 | 51.3 | 17.9 | < 0.0001 | 51.8 | 16.4 | 49.8 | 16.3 | 58.6 | 14.9 | < 0.0001 | |
| Marital status | 0.0185 | 0.1441 | |||||||||||||
| Married | 2,884 | 71.0 | 1,043 | 36.2 | 1,841 | 63.8 | 3,664 | 66.1 | 835 | 22.8 | 2,829 | 77.2 | |||
| Divorced, Separated | 166 | 4.1 | 67 | 40.4 | 99 | 59.6 | 343 | 6.2 | 94 | 27.4 | 249 | 72.6 | |||
| Single, widow | 1,013 | 24.9 | 323 | 31.9 | 690 | 68.1 | 1,533 | 27.7 | 349 | 22.8 | 1,184 | 77.2 | |||
| Educational level | 0.8249 | < 0.0001 | |||||||||||||
| Middle school or below | 878 | 21.6 | 317 | 36.1 | 561 | 63.9 | 1,715 | 31.0 | 621 | 36.2 | 1,094 | 63.8 | |||
| High school | 1,472 | 36.2 | 513 | 34.9 | 959 | 65.1 | 1,787 | 32.3 | 381 | 21.3 | 1,406 | 78.7 | |||
| College or over | 1,713 | 42.2 | 603 | 35.2 | 1,110 | 64.8 | 2,038 | 36.8 | 276 | 13.5 | 1,762 | 86.5 | |||
| Household income | 0.6334 | < 0.0001 | |||||||||||||
| Low | 651 | 16.0 | 224 | 34.4 | 427 | 65.6 | 1,046 | 18.9 | 353 | 33.7 | 693 | 66.3 | |||
| Mid-low | 985 | 24.2 | 364 | 37.0 | 621 | 63.0 | 1,360 | 24.5 | 334 | 24.6 | 1,026 | 75.4 | |||
| Mid-high | 1,129 | 27.8 | 396 | 35.1 | 733 | 64.9 | 1,488 | 26.9 | 297 | 20.0 | 1,191 | 80.0 | |||
| High | 1,298 | 31.9 | 449 | 34.6 | 849 | 65.4 | 1,646 | 29.7 | 294 | 17.9 | 1,352 | 82.1 | |||
| Region | 0.2872 | < 0.0001 | |||||||||||||
| Metropolitan | 1,720 | 42.3 | 584 | 34.0 | 1,136 | 66.0 | 2,466 | 44.5 | 502 | 20.4 | 1,964 | 79.6 | |||
| Urban | 1,505 | 37.0 | 540 | 35.9 | 965 | 64.1 | 2,034 | 36.7 | 459 | 22.6 | 1,575 | 77.4 | |||
| Rural | 838 | 20.6 | 309 | 36.9 | 529 | 63.1 | 1,040 | 18.8 | 317 | 30.5 | 723 | 69.5 | |||
| Occupational categories | 0.6403 | < 0.0001 | |||||||||||||
| White | 1,155 | 28.4 | 422 | 36.5 | 733 | 63.5 | 1,263 | 22.8 | 174 | 13.8 | 1,089 | 86.2 | |||
| Pink | 404 | 9.9 | 137 | 33.9 | 267 | 66.1 | 835 | 15.1 | 191 | 22.9 | 644 | 77.1 | |||
| Blue | 1,308 | 32.2 | 449 | 34.3 | 859 | 65.7 | 822 | 14.8 | 217 | 26.4 | 605 | 73.6 | |||
| Inoccupation | 1,196 | 29.4 | 425 | 35.5 | 771 | 64.5 | 2,620 | 47.3 | 696 | 26.6 | 1,924 | 73.4 | |||
| Current drinking status | 0.0315 | < 0.0001 | |||||||||||||
| Never or occasionally | 1,273 | 31.3 | 435 | 34.2 | 838 | 65.8 | 3,310 | 59.7 | 892 | 26.9 | 2,418 | 73.1 | |||
| 2–4 times/month | 1,478 | 36.4 | 498 | 33.7 | 980 | 66.3 | 1,633 | 29.5 | 297 | 18.2 | 1,336 | 81.8 | |||
| 2–4 times/week | 1,312 | 32.3 | 500 | 38.1 | 812 | 61.9 | 597 | 10.8 | 89 | 14.9 | 508 | 85.1 | |||
| Physical activity | < 0.0001 | < 0.0001 | |||||||||||||
| Adequate | 1,906 | 46.9 | 613 | 32.2 | 1,293 | 67.8 | 2,216 | 40.0 | 425 | 19.2 | 1,791 | 80.8 | |||
| Inadequate | 2,157 | 53.1 | 820 | 38.0 | 1,337 | 62.0 | 3,324 | 60.0 | 853 | 25.7 | 2,471 | 74.3 | |||
| BMI | < 0.0001 | < 0.0001 | |||||||||||||
| Normal | 1,155 | 28.4 | 132 | 11.4 | 1,023 | 88.6 | 2,494 | 45.0 | 175 | 7.0 | 2,319 | 93.0 | |||
| Underweight | 92 | 2.3 | 3 | 3.3 | 89 | 96.7 | 272 | 4.9 | 6 | 2.2 | 266 | 97.8 | |||
| Overweight | 1,069 | 26.3 | 274 | 25.6 | 795 | 74.4 | 1,116 | 20.1 | 268 | 24.0 | 848 | 76.0 | |||
| Obesity of stage 1 | 1,472 | 36.2 | 796 | 54.1 | 676 | 45.9 | 1,351 | 24.4 | 597 | 44.2 | 754 | 55.8 | |||
| Obesity of stages 2&3 | 275 | 6.8 | 228 | 82.9 | 47 | 17.1 | 307 | 5.5 | 232 | 75.6 | 75 | 24.4 | |||
| Diagnosis of hypertension | < 0.0001 | < 0.0001 | |||||||||||||
| Yes | 1,105 | 27.2 | 530 | 48.0 | 575 | 52.0 | 1,290 | 23.3 | 577 | 44.7 | 713 | 55.3 | |||
| No | 2,958 | 72.8 | 903 | 30.5 | 2,055 | 69.5 | 4,250 | 76.7 | 701 | 16.5 | 3,549 | 83.5 | |||
| Diagnosis of diabetes | < 0.0001 | < 0.0001 | |||||||||||||
| Yes | 466 | 11.5 | 299 | 64.2 | 167 | 35.8 | 500 | 9.0 | 339 | 67.8 | 161 | 32.2 | |||
| No | 3,597 | 88.5 | 1,134 | 31.5 | 2,463 | 68.5 | 5,040 | 91.0 | 939 | 18.6 | 4,101 | 81.4 | |||
| Year | 0.0018 | 0.1354 | |||||||||||||
| 2019 | 2,088 | 51.4 | 689 | 33.0 | 1,399 | 67.0 | 2,932 | 52.9 | 653 | 22.3 | 1,983 | 77.7 | |||
| 2020 | 1,975 | 48.6 | 744 | 37.7 | 1,231 | 62.3 | 2,608 | 47.1 | 625 | 24.0 | 2,279 | 76.0 | |||
Table 2 presents the results of the multiple regression analysis for the relationship between smoking and NAFLD stratified by sex after adjusting for all covariates. Among male participants, the odds ratios (OR) for NAFLD among ex-smokers and current smokers were 1.12 (95% confidence interval [CI]: 0.90–1.41) and 1.38 (95% CI: 1.08–1.76), respectively. In women, the OR for NAFLD among ex-smokers and current smokers were 1.32 (95% CI: 0.86–2.01) and 1.18 (95% CI: 0.76–1.83), respectively. Ex-smokers and current smokers exhibited an increasing trend of OR for NAFLD compared to that in nonsmokers, although there were statistically significant associations only in current smokers among males.
Table 2.
Results of factors associated between smoking and nonalcoholic fatty liver disease.
| Variables | Nonalcoholic fatty liver disease (NAFLD) | ||||
|---|---|---|---|---|---|
| Male | Female | ||||
| OR | 95% CI | OR | 95% CI | ||
| Smoking Behavior | |||||
| Nonsmoker | 1.00 | 1.00 | |||
| Ex-smoker | 1.12 | (0.90 – 1.41) | 1.32 | (0.86 – 2.01) | |
| Current smoker | 1.38 | (1.08 – 1.76) | 1.18 | (0.76 – 1.83) | |
|
Age
Marital status |
1.00 | (1.00 – 1.01) | 1.01 | (1.00 – 1.83) | |
| Married | 1.00 | 1.00 | |||
| Divorced, Separated | 1.29 | (0.80 – 2.07) | 1.31 | (0.93 – 1.84) | |
| Single, widow | 0.84 | (0.64 – 1.10) | 0.91 | (0.72 – 1.13) | |
| Educational level | |||||
| Middle school or below | 1.00 | 1.00 | |||
| High school | 1.07 | (0.79 – 1.44) | 1.03 | (0.78 – 1.36) | |
| College or over | 1.06 | (0.77 – 1.45) | 0.73 | (0.53 – 1.01) | |
| Household income | |||||
| Low | 1.04 | (0.74 – 1.44) | 0.83 | (0.60 – 1.16) | |
| Mid-low | 1.17 | (0.92 – 1.49) | 0.73 | (0.55 – 0.95) | |
| Mid-high | 0.97 | (0.78 – 1.21) | 0.68 | (0.51 – 0.89) | |
| High | 1.00 | 1.00 | |||
| Region | |||||
| Metropolitan | 1.00 | 1.00 | |||
| Urban | 1.06 | (0.87 – 1.30) | 1.12 | (0.91 – 1.38) | |
| Rural | 1.08 | (0.85 – 1.38) | 1.34 | (1.01 – 1.77) | |
| Occupational categories | |||||
| White | 0.88 | (0.65 – 1.19) | 0.81 | (0.61 – 1.07) | |
| Pink | 0.78 | (0.55 – 1.09) | 0.94 | (0.72 – 1.23) | |
| Blue | 0.71 | (0.55 – 0.92) | 0.63 | (0.48 – 0.82) | |
| Inoccupation | 1.00 | 1.00 | |||
| Current drinking status | |||||
| Never or occasionally | 1.00 | 1.00 | |||
| 2–4 times/month | 0.89 | (0.72 – 1.09) | 0.85 | (0.70 – 1.04) | |
| 2–4 times/week | 1.06 | (0.86 – 1.31) | 0.60 | (0.43 – 0.84) | |
| Physical activity | |||||
| Adequate | 1.00 | 1.00 | |||
| Inadequate | 1.44 | (1.20 – 1.71) | 1.33 | (1.11 – 1.59) | |
| BMI | |||||
| Normal | 1.00 | 1.00 | |||
| Underweight | 0.50 | (0.14 – 1.78) | 0.44 | (0.17 – 1.15) | |
| Overweight | 2.84 | (2.09 – 3.86) | 4.21 | (3.28 – 5.41) | |
| Obesity of stage 1 | 10.44 | (7.94 – 13.72) | 11.48 | (8.92 – 14.78) | |
| Obesity of stages 2&3 | 50.57 | (32.58 – 78.48) | 62.42 | (41.29 – 94.35) | |
| No | 1.00 | 1.00 | |||
| Yes | 1.42 | (1.14 – 1.77) | 1.96 | (1.55 – 2.48) | |
| Diagnosis of diabetes | |||||
| No | 1.00 | 1.00 | |||
| Yes | 4.36 | (3.28 – 5.80) | 6.06 | (4.41 – 8.31) | |
| Year | |||||
| 2019 | 1.00 | 1.00 | |||
| 2020 | 1.06 | (0.88 – 1.27) | 0.95 | (0.79 – 1.15) | |
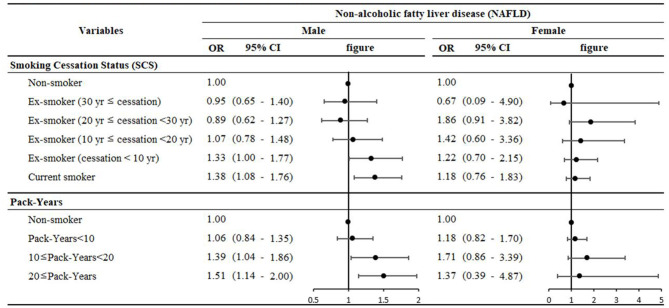
Figure 2 presents the results of the stratified subgroup analysis of the association between SCS and pack years, indicating the effect of the number of cigarettes and the smoking period on NAFLD according to smoking behavior. In general, with nonsmokers as the reference category, the OR for NAFLD increased linearly as smoking cessation decreased and pack years increased in males. Specifically, an ex-smoker with smoking cessation for < 10 years (OR: 1.33, 95% CI: 1.00–1.77) and a current smoker (OR: 1.38, 95% CI: 1.08–1.76) had the strongest statistically significant association compared to a nonsmoker, as classified based on the smoking cessation period. Furthermore, an ex-smoker and current smoker with 10 to 20 pack years (OR: 1.39, 95% CI: 1.04–1.86) and over 20 pack years (OR: 1.51, 95% CI: 1.14–2.00), respectively, was more likely to have a strong relationship with NAFLD compared to a nonsmoker.
Figure 2.
Results of the subgroup analysis stratified by smoking cessation and pack-years.
Table 3 shows the results of the independent variable subgroup analysis, representing the ORs for NAFLD stratified by the smoking status. Among current male smokers, cases of never or occasional drinking (OR: 1.78, 95% CI: 1.14–2.78), adequate physical activity (OR: 1.55, 95% CI: 1.09–2.21), BMI indicating overweight (OR: 2.31, 95% CI: 1.40–3.83), no diagnosis of hypertension (OR: 1.42, 95% CI: 1.07–1.87), and no diagnosis of diabetes (OR: 1.39, 95% CI: 1.08–1.79) showed the strongest associations with NAFLD compared to male nonsmokers. In women, drinking 2 to 4 times per month (current smokers: OR: 1.39, 95% CI: 1.08–1.79), normal BMI (ex-smokers: OR: 2.74, 95% CI: 1.28–5.88), and BMI indicating stage 2 and 3 obesity (ex-smokers: OR: 4.36, 95% CI: 1.14–16.71) showed the strongest associations with NAFLD compared to those in nonsmokers.
Table 3.
Results of subgroup analysis stratified by independent variables.
| Nonalcoholic fatty liver disease (NAFLD) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||||
| Non | Ex-smoker | Current smoker | Non | Ex-smoker | Current smoker | |||||||
| OR | OR | 95% CI | OR | 95% CI | OR | OR | 95% CI | OR | 95% CI | |||
| Age | ||||||||||||
| 20–29 | 1.00 | 0.53 | (0.21 – 1.37) | 1.06 | (0.54 – 2.09) | 1.00 | 0.82 | (0.18 – 3.63) | 1.12 | (0.32 – 3.92) | ||
| 30–39 | 1.00 | 0.71 | (0.33 – 1.51) | 1.18 | (0.65 – 2.16) | 1.00 | 1.68 | (0.41 – 6.84) | 0.62 | (0.15 – 2.51) | ||
| 40–49 | 1.00 | 1.35 | (0.71 – 2.58) | 1.47 | (0.76 – 2.86) | 1.00 | 1.40 | (0.61 – 3.22) | 0.74 | (0.26 – 2.13) | ||
| 50–59 | 1.00 | 1.14 | (0.62 – 2.08) | 1.38 | (0.72 – 2.62) | 1.00 | 2.67 | (1.06 – 6.73) | 3.16 | (1.12 – 8.86) | ||
| 60–69 | 1.00 | 1.58 | (0.94 – 2.67) | 1.51 | (0.83 – 2.76) | 1.00 | 0.78 | (0.33 – 1.86) | 1.07 | (0.44 – 2.63) | ||
| ≥70 | 1.00 | 0.98 | (0.58 – 1.66) | 0.99 | (0.43 – 2.27) | 1.00 | 0.71 | (0.18 – 2.85) | 0.38 | (0.10 – 1.39) | ||
| Current drinking status | ||||||||||||
| Never or occasionally | 1.00 | 0.97 | (0.64 – 1.46) | 1.78 | (1.14 – 2.78) | 1.00 | 1.12 | (0.60 – 2.06) | 0.61 | (0.33 – 1.13) | ||
| 2–4 times/month | 1.00 | 1.05 | (0.74 – 1.50) | 1.21 | (0.84 – 1.76) | 1.00 | 1.30 | (0.59 – 2.87) | 2.28 | (1.14 – 4.56) | ||
| 2–4 times/week | 1.00 | 1.52 | (0.95 – 2.43) | 1.56 | (1.01 – 2.42) | 1.00 | 2.56 | (0.95 – 6.95) | 0.91 | (0.36 – 2.31) | ||
| Physical activity | ||||||||||||
| Adequate | 1.00 | 1.07 | (0.77 – 1.50) | 1.55 | (1.09 – 2.21) | 1.00 | 1.28 | (0.67 – 2.44) | 0.99 | (0.48 – 2.03) | ||
| Inadequate | 1.00 | 1.15 | (0.84 – 1.58) | 1.28 | (0.90 – 1.83) | 1.00 | 1.33 | (0.78 – 2.28) | 1.27 | (0.74 – 2.19) | ||
| BMI | ||||||||||||
| Underweight | 1.00 | – | – | – | – | – | 1.00 | – | – | – | – | |
| Normal | 1.00 | 1.32 | (0.74 – 2.34) | 1.14 | (0.57 – 2.25) | 1.00 | 2.74 | (1.28 – 5.88) | 2.16 | (0.96 – 4.85) | ||
| Overweight | 1.00 | 1.63 | (0.96 – 2.75) | 2.31 | (1.40 – 3.83) | 1.00 | 0.79 | (0.25 – 2.49) | 1.04 | (0.41 – 2.65) | ||
| Obesity of stage 1 | 1.00 | 0.97 | (0.71 – 1.33) | 1.14 | (0.82 – 1.60) | 1.00 | 0.77 | (0.38 – 1.57) | 1.01 | (0.52 – 1.96) | ||
| Obesity of stages 2&3 | 1.00 | 0.86 | (0.28 – 2.60) | 1.73 | (0.63 – 4.77) | 1.00 | 4.36 | (1.14 – 16.71) | 1.21 | (0.25 – 5.81) | ||
| Diagnosis of hypertension | ||||||||||||
| No | 1.00 | 1.07 | (0.82 – 1.41) | 1.42 | (1.07 – 1.87) | 1.00 | 1.33 | (0.81 – 2.19) | 1.28 | (0.79 – 2.07) | ||
| Yes | 1.00 | 1.16 | (0.74 – 1.82) | 1.05 | (0.62 – 1.78) | 1.00 | 1.35 | (0.61 – 2.99) | 0.90 | (0.34 – 2.37) | ||
| Diagnosis of diabetes | ||||||||||||
| No | 1.00 | 1.06 | (0.83 – 1.35) | 1.39 | (1.08 – 1.79) | 1.00 | 1.29 | (0.83 – 2.01) | 1.22 | (0.76 – 1.95) | ||
| Yes | 1.00 | 1.79 | (0.85 – 3.80) | 0.91 | (0.39 – 2.13) | 1.00 | 2.14 | (0.24 – 19.16) | 1.19 | (0.30 – 4.67) | ||
Discussion
The general findings were that there is an association between smoking and NAFLD, and the risk of having NAFLD has a dose-dependent negative association with the duration of smoking cessation and a positive association with pack years. Given these results, our study suggests that ex-smokers with an SCS of fewer than 10 years had associations similar to those seen in current smokers, while ex-smokers whose SCS was more than 20 years had no association. Furthermore, we found a strong linear association between the duration of smoking and the number of cigarettes smoked per day. These findings are consistent with the results of a previous study (30) and may provide supporting evidence for an association between smoking history and NAFLD. Smoking cessation reduces the incidence of NAFLD. However, due to the low number of female smokers in Korea, we could not find a relationship between smoking and NAFLD among females. However, although not statistically significant, the OR of former smokers and current smokers was higher than that of nonsmokers. This reflects the recall bias of self-reported data due to the poor perception of female smokers in Korea (31).
Smoking has been identified, as an adjunct to obesity, as a causative factor for NAFLD in animal and clinical studies (25, 32). This study found no association between smoking behavior and NAFLD in men with stages 1, 2, and 3 obesity; however, in overweight men and normal women, smoking behavior was a significant risk factor associated with NAFLD compared to nonsmoking. This supports the results of a previous study (33) suggesting that while severe obesity directly affects NAFLD in BMI groups, smoking may have an independent relationship in normal or overweight groups. A mechanism that explains the independent role of BMI in the association between smoking and NAFLD is that the antiestrogenic effect of cigarette smoking leads to a change in body fat distribution (34–36). Therefore, normal and overweight smokers who may not be evaluated for NAFLD should receive more attention to prevent NAFLD.
According to the multiple parallel hits hypothesis theory, the pathophysiological mechanisms of NAFLD indicate the causes of insulin resistance, genetic and epigenetic factors, mitochondrial dysfunction, endoplasmic reticulum stress, microbiota, chromatic low-grade injury, and dysfunction of adipose tissue (37, 38). In insulin-resistant patients, liver fat production can be further induced by activation of transcription factors such as SREBP-1 (38, 39). Many studies have shown that tobacco increases lipid accumulation in liver cells by regulating the activity of 5′-AMP-activated protein kinase (AMPK) and SREBP-1, two important molecules involved in lipid synthesis (27, 40–42). It is considered a mechanism between smoking and NAFLD, especially based on previous studies that show a decisive role in liver fat accumulation in SREBP-1, when tobacco smoke is exposed to mice and cultured hepatocytes (27).
However, the effects of smoking on NAFLD remain controversial, with inconsistent results (43). One study reported that active smoking was associated with fibrosis in patients with NAFLD (25), but another study showed a lack of significant relationship between active smoking and NAFLD (44). Several experimental studies in mice have shown that nicotine, a dangerous substance in cigarettes, promotes the development of NAFLD or accelerates its progression (25–27). A systematic review and meta-analysis of 20 observational studies showed that smoking was significantly associated with NAFLD (43). Furthermore, second-hand smoking increases the risk of NAFLD around 1.38 times (43). Based on these mechanisms, experimental studies and cohort studies that consider additional confounders are needed.
This study had several limitations. First, it was a cross-sectional study. It may not establish temporal relations and may have found an inverse causal relationship. Therefore, caution is warranted when interpreting the results. More research is needed to clarify the association between smoking and NAFLD. Second, KNHANES data were collected through self-report surveys. Hence, data on health-related status, socioeconomic variables, and smoking status may not be reliable and accurate. In particular, this can lead to recall bias and is likely to be underestimated in the case of smoking. Third, although the liver fat score for NAFLD was demonstrated for the ROC curves for detecting NAFLD (sensitivity of 86% and specificity of 71%), there were still tiny errors of false-positive or false-negative results. In addition, due to the characteristics of the KNHANES called secondary data, the diagnosis of NAFLD was not measured by the instrument investigation, so steatosis could not be confirmed by methods such as CAP, ultrasound, and liver biopsy. Therefore, we calculated and considered the NAFLD liver fat score instead. Fourth, it could not differentiate among the various smoking types, such as conventional cigarette use, electronic cigarette use, or both. Besides, we could not calculate the pack years for e-cigarettes because the KNHANES did not include this information. Finally, we cannot exclude the possibility of unrecognized confounders, although we adjusted for known confounders in the relationship between smoking and NAFLD.
Despite these limitations, our study had several notable strengths. First, the study was based on the KNHANES data, a nationally representative dataset collected by the KDCA. This is useful for health-related research because it is updated annually to reflect the changes in the actual health situation of Koreans. In addition, it is a statistic that can generalize the study results to the general population because the survey is performed by reliable and representative random cluster sampling. Second, we calculated the SCS and pack years for ex-smokers and current smokers. The study showed a significant association between current smoking behavior in men and smoking status considering SCS and pack-years. Therefore, our results suggest that smoking status has the opportunity to be considered as a measure of intervention to reduce the risk of NAFLD when SCS and pack years are taken into account.
Conclusion
In conclusion, this study found that current smoking was associated with NAFLD in men in the South Korean population. In particular, we suggest an association between NAFLD and ex-smoker and current smoker status with a short smoking cessation period or many pack years. Given these results, smoking has a potential effect on NAFLD, and smoking cessation should be considered in the prevention and management of NAFLD. It is best to stop smoking considering health status and behavior to avoid serious diseases. More prospective studies and clinical trials are required to clarify the relationship between smoking history and NAFLD.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.kdca.go.kr/index.es?sid=a2.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YSJ designed the study, collected data, performed statistical analysis, and drafted the manuscript. YSJ, HJJ, YSP, E-CP, and S-IJ contributed to the discussion. S-IJ is the guarantor of this work, has full access to all the study data, and assumes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed and edited the drafts of the manuscript, approved the final version, and approved the final manuscript.
Acknowledgments
The authors express sincere appreciation to our colleagues and professor from the Department of Public Health, Graduate School of Yonsei University, for their advice on this manuscript. We thank KDCA, which provided the KNAHNES.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2022R1F1A1062794).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. (2011) 332:1519–23. 10.1126/science.1204265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. (2012) 56:234–40. 10.1016/j.jhep.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. (2012) 55:2005–23. 10.1002/hep.25762 [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. (2013) 10:686–90. 10.1038/nrgastro.2013.171 [DOI] [PubMed] [Google Scholar]
- 5.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. (2008) 12:733–46. 10.1016/j.cld.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. (2009) 13:511–31. 10.1016/j.cld.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 7.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. (2010) 51:1820–32. 10.1002/hep.23594 [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. (2005) 129:113–21. 10.1053/j.gastro.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. (2006) 44:865–73. 10.1002/hep.21327 [DOI] [PubMed] [Google Scholar]
- 10.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. (2009) 7:234–8. 10.1016/j.cgh.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 11.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. (2010) 59:1410–5. 10.1136/gut.2010.213553 [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. (2016) 63:776–86. 10.1002/hep.28376 [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services . The Health Consequences of Smoking −50 Years of Progress: A Report of the Surgeon General. Reports of the Surgeon General; (2014). [Google Scholar]
- 15.Centers for Disease Control and Prevention (US) National Center for Chronic Disease Prevention and Health Promotion (US) and and Office on Smoking and Health . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Publications and Reports of the Surgeon General; (2010). [PubMed] [Google Scholar]
- 16.Jatoi NA, Jerrard-Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension. (2007) 49:981–5. 10.1161/hypertensionaha.107.087338 [DOI] [PubMed] [Google Scholar]
- 17.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. (2008) 300:2765–78. 10.1001/jama.2008.839 [DOI] [PubMed] [Google Scholar]
- 18.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. (2007) 298:2654–64. 10.1001/jama.298.22.2654 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . WHO Report on the Global Tobacco Epidemic, 2021: Addressing new and emerging products. (2021). [Google Scholar]
- 20.Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. (2021) 110:921–37. 10.1007/s00392-020-01709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. (2020) 2020:3920196. 10.1155/2020/3920196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. (2016) 67:103–17. 10.1146/annurev-med-090514-013832 [DOI] [PubMed] [Google Scholar]
- 23.Zein CO, Beatty K, Post AB, Logan L, Debanne S, McCullough AJ. Smoking and increased severity of hepatic fibrosis in primary biliary cirrhosis: a cross validated retrospective assessment. Hepatology. (2006) 44:1564–71. 10.1002/hep.21423 [DOI] [PubMed] [Google Scholar]
- 24.Pessione F, Ramond MJ, Njapoum C, Duchatelle V, Degott C, Erlinger S, et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. (2001) 34:121–5. 10.1053/jhep.2001.25385 [DOI] [PubMed] [Google Scholar]
- 25.Azzalini L, Ferrer E, Ramalho LN, Moreno M, Domínguez M, Colmenero J, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology. (2010) 51:1567–76. 10.1002/hep.23516 [DOI] [PubMed] [Google Scholar]
- 26.Hasan KM, Friedman TC, Shao X, Parveen M, Sims C, Lee DL, et al. E-cigarettes and western diet: important metabolic risk factors for hepatic diseases. Hepatology. (2019) 69:2442–54. 10.1002/hep.30512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan H, Shyy JY, Martins-Green M. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. (2009) 51:535–47. 10.1016/j.jhep.2009.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. (2009) 137:865–72. 10.1053/j.gastro.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 29.Yoon YJ, Lee MS, Jang KW, Ahn JB, Hurh K, Park EC. Association between smoking cessation and obstructive spirometry pattern among Korean adults aged 40-79 years. Sci Rep. (2021) 11:18667. 10.1038/s41598-021-98156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takenaka H, Fujita T, Masuda A, Yano Y, Watanabe A, Kodama Y. Non-alcoholic fatty liver disease is strongly associated with smoking status and is improved by smoking cessation in japanese males: a retrospective study. Kobe J Med Sci. (2020) 66:E102–e12. [PMC free article] [PubMed] [Google Scholar]
- 31.Kang HG, Kwon KH, Lee IW, Jung B, Park EC, Jang SI. Biochemically-verified smoking rate trends and factors associated with inaccurate self-reporting of smoking habits in Korean women. Asian Pac J Cancer Prev. (2013) 14:6807–12. 10.7314/apjcp.2013.14.11.6807 [DOI] [PubMed] [Google Scholar]
- 32.Mallat A, Lotersztajn S. Cigarette smoke exposure: a novel cofactor of NAFLD progression? J Hepatol. (2009) 51:430–2. 10.1016/j.jhep.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Dai M, Bi Y, Xu M, Xu Y, Li M, et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. (2013) 23:115–21. 10.2188/jea.je20120067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. Iii Effects of cigarette smoking. JAMA. (1989) 261:1169–73. [PubMed] [Google Scholar]
- 35.Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause. (2004) 11:104–9. 10.1097/01.Gme.0000079740.18541.Db [DOI] [PubMed] [Google Scholar]
- 36.Windham GC, Mitchell P, Anderson M, Lasley BL. Cigarette smoking and effects on hormone function in premenopausal women. Environ Health Perspect. (2005) 113:1285–90. 10.1289/ehp.7899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acierno C, Caturano A, Pafundi P, Nevola R, Adinolfi L, Sasso F. Nonalcoholic fatty liver disease and type 2 diabetes: pathophysiological mechanisms shared between the two faces of the same coin. Explor Med. (2020) 1:287–306. [Google Scholar]
- 38.Caturano A, Acierno C, Nevola R, Pafundi PC, Galiero R, Rinaldi L, et al. Non-alcoholic fatty liver disease: From pathogenesis to clinical impact. Processes. (2021) 9:135. 10.3390/pr9010135 [DOI] [Google Scholar]
- 39.George J, Liddle C. Nonalcoholic fatty liver disease: pathogenesis and potential for nuclear receptors as therapeutic targets. Mol Pharm. (2008) 5:49–59. 10.1021/mp700110z [DOI] [PubMed] [Google Scholar]
- 40.Jung EJ, Kwon SW, Jung BH, Oh SH, Lee BH. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J Lipid Res. (2011) 52:1617–25. 10.1194/jlr.M015263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SP, Nam SH, Friedman M. Mechanism of the antiadipogenic-antiobesity effects of a rice hull smoke extract in 3T3-L1 preadipocyte cells and in mice on a high-fat diet. Food Funct. (2015) 6:2939–48. 10.1039/c5fo00469a [DOI] [PubMed] [Google Scholar]
- 42.Ponciano-Rodríguez G, Méndez-Sánchez N. Cigarette smoking and fatty liver. Ann Hepatol. (2010) 9:215–8. [PubMed] [Google Scholar]
- 43.Akhavan Rezayat A, Dadgar Moghadam M, Ghasemi Nour M, Shirazinia M, Ghodsi H, Rouhbakhsh Zahmatkesh MR, et al. Association between smoking and non-alcoholic fatty liver disease: a systematic review and meta-analysis. SAGE Open Med. (2018) 6:2050312117745223. 10.1177/2050312117745223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavez-Tapia NC, Lizardi-Cervera J, Perez-Bautista O, Ramos-Ostos MH, Uribe M. Smoking is not associated with nonalcoholic fatty liver disease. World J Gastroenterol. (2006) 12:5196–200. 10.3748/wjg.v12.i32.5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.kdca.go.kr/index.es?sid=a2.