Abstract
Background
Osteosarcoma and Ewing sarcoma patients face a significant risk of cardiotoxicity as defined by left ventricular dysfunction and heart failure (HF).
Objectives
This study sought to evaluate the association between age at sarcoma diagnosis and incident HF.
Methods
A retrospective cohort study was performed at the largest sarcoma center in the Netherlands among patients with an osteosarcoma or Ewing sarcoma. All patients were diagnosed and treated over a 36-year period (1982-2018) and followed until August 2021. Incident HF was adjudicated through the universal definition of heart failure. Determinants including age at diagnosis, doxorubicin dose, and cardiovascular risk factors were entered as fixed or time-dependent covariates into a cause-specific Cox model to assess their impact on incident HF.
Results
The study population consisted of 528 patients with a median age at diagnosis of 19 years (Q1-Q3: 15-30 years). Over a median follow-up time of 13.2 years (Q1-Q3: 12.5-14.9 years), 18 patients developed HF with an estimated cumulative incidence of 5.9% (95% CI: 2.8%-9.1%). In a multivariable model, age at diagnosis (HR: 1.23; 95% CI: 1.06-1.43) per 5-year increase, doxorubicin dose per 10-mg/m2 increase (HR: 1.13; 95% CI: 1.03-1.24), and female sex (HR: 3.17; 95% CI: 1.11-9.10) were associated with HF.
Conclusions
In a large cohort of sarcoma patients, we found that patients diagnosed at an older age are more prone to develop HF.
Key Words: age, anthracyclines, cancer, cardio-oncology, heart failure, sarcoma
Abbreviations and Acronyms: AYA, adolescent and young adult; CTX, cardiotoxicity; HF, heart failure
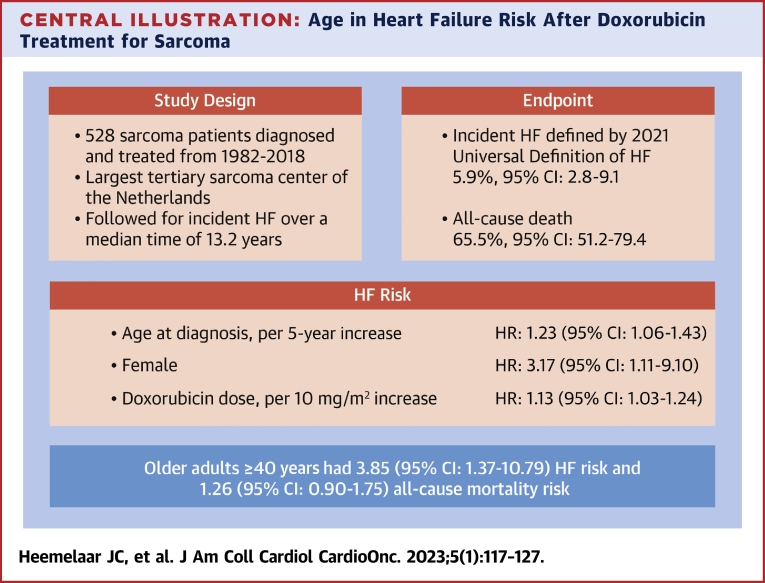
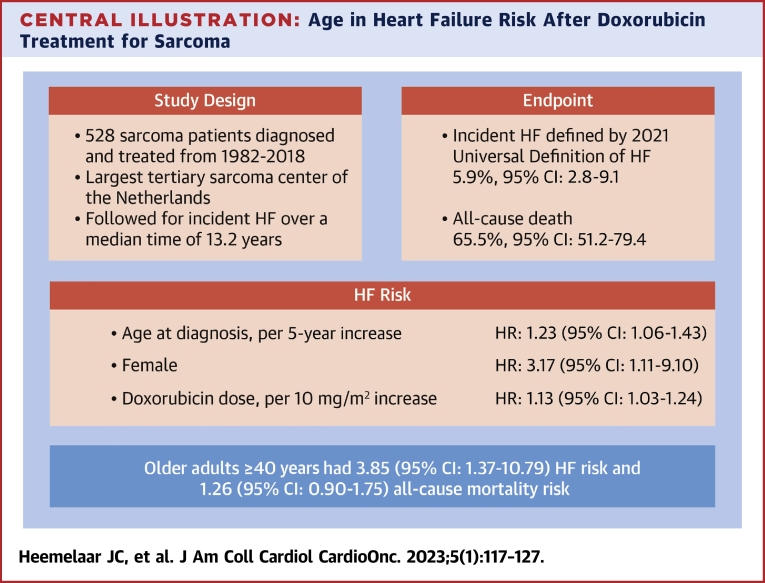
Central Illustration
Although bone sarcoma is rare in the general population, it is the second most common malignancy among children and young adults. The incidence of the most common type of malignant bone sarcoma—osteosarcoma—is estimated at 0.8 to 1.1 of 100,000 patient-years based on the EUROCARE database.1 Incidence peaks around puberty and after 40 years of age.2, 3, 4 Ewing sarcoma is less common with an incidence of 0.3 of 100,000 patient-years and is mostly diagnosed in adolescents and young adults (AYAs). The first-line treatment of bone sarcoma is surgical treatment in combination with neo(adjuvant) systemic treatment containing high-dosage anthracyclines, most frequently doxorubicin.5,6 Yet, it is well-known that anthracyclines carry a lifelong increased risk of cardiotoxicity (CTX) in the form of cardiomyopathy and heart failure (HF). The occurrence of CTX is a significant limiting factor in sarcoma treatment.7, 8, 9, 10 Therefore, it is important to identify patients at increased risk for CTX and detect CTX at an early stage to promptly start cardioprotective treatment. Several algorithms that use clinical parameters including age, pre-existing cardiovascular disease, and anthracycline dosage are used in CTX screening.11, 12, 13
However, the majority of sarcoma patients are younger than 30 years of age, and this impairs risk stratification algorithms for CTX based on clinical parameters because traditional cardiovascular risk factors are rarely present in young patients. Little is known about the incidence compared with sarcoma patients diagnosed at childhood. Fortunately, childhood sarcoma survivors usually are enrolled in lifetime care and research programs for the surveillance of late effects of cancer treatment.14 Although older patients are less likely to have long-term surveillance, they have a much larger burden of cardiovascular risk factors. More data are also needed to understand the CTX risk in the older population.
Therefore, the aim of this study was to determine the incidence of HF in a large cohort of osteosarcoma and Ewing sarcoma patients across diverse age groups treated with doxorubicin and to evaluate the effect of age at sarcoma diagnosis on HF risk. Furthermore, the competing risk of death was studied because the 5-year overall survival rate after diagnosis is approximately 60% depending on the age group.2
Methods
Design and setting
This single-center retrospective analysis of CTX of sarcoma treatment was performed at the largest tertiary sarcoma center in the Netherlands (Leiden University Medical Center) among patients with an osteosarcoma or Ewing sarcoma. All patients were diagnosed and treated over a 36-year period (1982-2018) at Leiden University Medical Center and followed until August 2021. Data collection on exposure to cardiotoxic chemotherapy, traditional cardiovascular risk factors, medications, and HF at follow-up was performed between March and August 2021. This study was conducted in collaboration between the departments of cardiology, medical oncology, and orthopedic surgery. The study was approved by the regional Institutional Review Board (Medical Ethics Committee Leiden The Hague Delft [METC-LDD], approval code: G21.041), and the requirement for informed consent was waived. The study complies with the Declaration of Helsinki.
Patient selection
All patients from the institutional osteosarcoma and Ewing sarcoma registries were considered for this study. The main inclusion criterion was exposure to doxorubicin-containing chemotherapy regimens. The study population was derived from 2 separate cohorts: 1) skeletal nonfacial high-grade osteosarcoma patients treated with surgery and (neo)adjuvant chemotherapy who had been reported on in a previous study;2 and 2) all treated Ewing sarcoma patients identified through hospital billing codes. All diagnoses were histology proven. Patients were excluded if they were treated with palliative intent, if the cumulative doxorubicin dosage was missing, or if follow-up data were unavailable. Follow-up was performed through a review of electronic health records and the institutional oncology registry (OncDoc).
Measurement of study endpoints and exposures
The primary study endpoint was defined as incident HF according to the 2021 universal definition of heart failure15 as summarized in Table 1. The secondary study endpoints were urgent HF-related hospitalizations and non–HF-related cardiac hospitalizations. Endpoints were adjudicated by 2 independent reviewers; in case of disagreement, a third reviewer was requested, and the decision was made by the majority.
Table 1.
Universal Definition of Heart Failure15
|
|
In patients hospitalized for HF, sepsis is an important differential diagnosis. To assess for this alternative explanation for hospitalization, we have added the systemic inflammatory response syndrome + documented infection criteria to the adjudication process.16
Information regarding demographics, cancer diagnosis, location, relapse, chemotherapy regimen, the use of radiotherapy, and/or surgery was extracted from the sarcoma registries. Relapse of disease was defined as local recurrence, progression of metastasis, or new metastasis. The cumulative chemotherapy dose was extracted from pharmacy records. This was based on the chemotherapy protocol, admitted cycles, treatment alterations, and/or discontinuations. Prevalent cardiovascular history, traditional cardiovascular risk factors, smoking status, and medications at the time of diagnosis were acquired through chart review of medical records. Hypercholesterolemia was defined as total cholesterol >5.0 mmol/L, a low-density lipoprotein cholesterol >2.5 mmol/L, the use of lipid-lowering drugs, or a diagnosis of hypercholesterolemia. Diabetes was diagnosed as a random glucose measurement ≥11.1 mmol/L, the use of diabetes medication, or a history of diabetes mellitus. Arterial hypertension was defined as the use of antihypertensive medication or having a history of hypertension.
Statistical methods
Patients were categorized based on sarcoma type and age group by a cutoff ≥40 years (children/AYAs vs older adults at diagnosis). This cutoff was chosen based on the Evenhuis et al2 cutoff for “older adults” opposed to children and AYAs. Baseline characteristics were stratified by sarcoma type and compared using the Student’s t-test or Fisher exact test as appropriate. Overall and according to age group, cumulative incidence curves of HF were constructed and compared using Fine and Gray’s method.
Cause-specific Cox regression models were used to evaluate the effect of covariates on the occurrence of HF in the presence of competing risks (death) as well as all-cause mortality.17 Subjects were followed until the event of interest occurred, the competing risk occurred (HF model), the last known follow-up, or August 1, 2021, whichever occurred first. In this way, the association of each predictor with incident HF could be assessed together with the impact on overall survival. All variables in Table 4 were considered for the multivariable all-cause death model, whereas only the top 4 variables with P < 0.10 in the univariable analysis were considered for the multivariable HF model because of a low number of events. Because of the limited number of events and multicollinearity, we chose to include the “any cardiovascular risk factor as a summary measure in our adjusted analysis instead of the individual covariates hypercholesterolemia and hypertension. Both unadjusted and adjusted HRs with 95% CIs are presented. Age at diagnosis was analyzed both as a continuous variable and, for illustrative purposes, stratified by age group. To account for a change in the cardiovascular risk profile and cancer stage over follow-up, arterial hypertension, hypercholesterolemia, diabetes mellitus, and relapse of sarcoma were analyzed as time-dependent covariates. Sex, cumulative doxorubicin dose, radiotherapy use, and smoking status at baseline were treated as fixed covariates. The proportional hazard assumption was evaluated using the Schoenfeld residuals method. As a sensitivity analysis, Fine and Gray’s competing risk regression was performed to estimate the subdistribution HR (sHR) of several covariates on incident HF. Traditional cardiovascular risk factors could only be entered in the model as time-fixed covariates because of the risk of bias when using Fine and Gray’s method and time-varying covariates.18 Kaplan-Meier methods were used to estimate the median follow-up time and the median overall survival and to construct survival curves. The log-rank test was used to compare survival curves.
Table 2.
Baseline Characteristics of the Study Population
| Total(N = 528) | Osteosarcoma(n = 365) | Ewing Sarcoma(n = 163) | P Value | |
|---|---|---|---|---|
| Age at diagnosis, y | 19 (15-30) | 19 (16-33) | 19 (14-28) | 0.046 |
| Female | 208 (39.4) | 156 (42.7) | 52 (31.9) | 0.019 |
| Cumulative doxorubicin dose, mg/m2 | 396 ± 83 | 414 ± 93 | 357 ± 29 | <0.001 |
| >300 | 479 (90.7) | 324 (88.8) | 155 (95.1) | 0.021 |
| >400 | 294 (55.7) | 294 (80.5) | 0 (0.0) | <0.001 |
| Neo(adjuvant) radiotherapy | 134 (25.7) | 45 (12.3) | 89 (56.7) | <0.001 |
| Radiotherapy protocol (n = 522) | <0.001 | |||
| Definitive radiotherapy | 35 (6.6) | 1 (0.3) | 34 (20.9) | |
| Preoperative radiotherapy | 16 (3.0) | 2 (0.5) | 14 (8.6) | |
| Postoperative radiotherapy | 80 (15.2) | 42 (11.5) | 38 (23.3) | |
| Other | 3 (0.6) | 0 (0.0) | 3 (1.8) | |
| Surgery as part of treatment | 484 (91.7) | 365 (100.0) | 119 (73.0) | <0.001 |
| Prior cardiovascular disease at time of sarcoma diagnosis | 3 (0.6) | 2 (0.5) | 1 (0.6) | 0.93 |
| Coronary artery disease | 1 (0.2) | 0 (0.0) | 1 (0.6) | |
| Supraventricular arrythmia | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Renal disease | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Traditional cardiovascular risk factor at time of diagnosisa | 20 (3.8) | 16 (4.4) | 4 (2.5) | 0.28 |
| Arterial hypertension | 9 (1.7) | 8 (2.2) | 1 (0.6) | 0.20 |
| Hypercholesterolemia | 8 (1.5) | 5 (1.4) | 3 (1.8) | 0.68 |
| Diabetes mellitus | 4 (0.8) | 4 (1.1) | 0 (0.0) | 0.18 |
| Active smoking | 52 (9.8) | 37 (10.1) | 15 (9.2) | 0.74 |
| Use of cardiovascular medication | 5 (0.9) | 4 (1.1) | 1 (0.6) | 0.60 |
Values are median (Q1-Q3), n (%), or mean ± SD. Data per variable are complete unless noted in parentheses after variable name.
Excluding active smoking.
Interaction terms were constructed for sex and sarcoma types to assess for effect modification. Because of prior reports on conflicting findings regarding anthracycline-induced CTX, menopausal status, and age at diagnosis,19 a table with observed crude risk per age group (children/AYA vs older adults at diagnosis) and sex was constructed. Continuous variables are presented as mean ± SD or median with 25th and 75th percentiles (Q1-Q3), and categoric data are presented as frequencies and percentages. Normality of variable distribution was evaluated graphically. The Wilcoxon rank sum test was used to compare non-normally distributed continuous data. Available case analysis was used to handle missing data.
P < 0.05 was considered statistically significant. Stata version 16.1 (Stata Corp) was used for all analyses.
Results
Study population
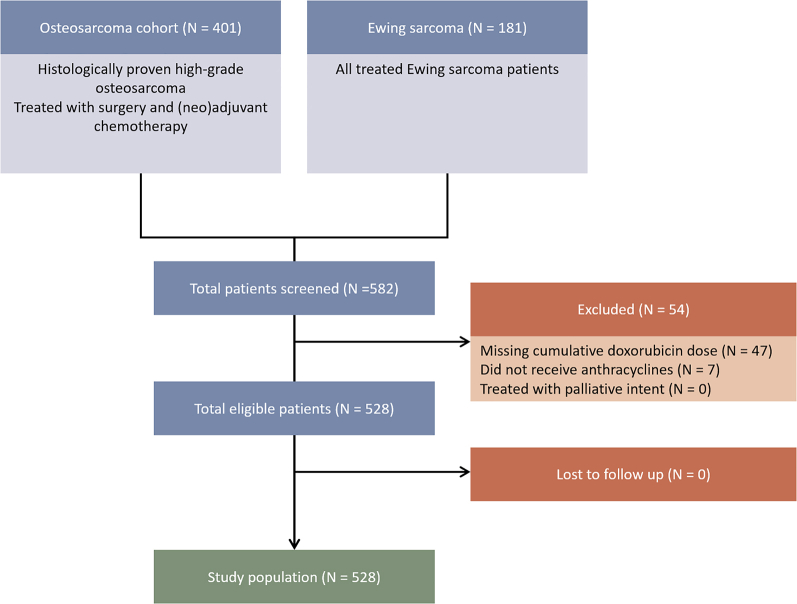
A total of 582 subjects (401 osteosarcoma patients and 181 Ewing sarcoma patients) were considered for this study. After the exclusion of 54 patients, the study population was composed of 528 patients (Figure 1). The baseline characteristics are provided in Table 2. On average, the study population was young (median age = 19 [Q1-Q3: 15-30; range: 1-70] years at diagnosis), the majority were male (60.6%), and the baseline cardiovascular disease or cardiovascular risk factors were infrequently observed (0.6% and 3.8%, respectively, excluding active smoking). None of the patients had prevalent HF at sarcoma diagnosis. The cumulative doxorubicin dose was high in the study population with a mean administered dose of 396 ± 83 mg/m2 (range 75-750 mg/m2). Compared with osteosarcoma patients (n = 365), Ewing sarcoma patients (n = 163) received a lower cumulative dose of anthracyclines (414 ± 93 vs 357 ± 29; P < 0.001). Twenty-four- to 72-hour infusion protocols were used in line with the EURAMOS-1 (European and American Osteosarcoma Study-1) and EuroEwing-99 protocol.5,20,21 Dexrazoxane was not used at our institution. Furthermore, Ewing sarcoma patients were more likely to undergo concurrent radiotherapy (56.7% vs 12.3%). Although all osteosarcoma patients underwent surgery, this was the case for 73.0% of Ewing sarcoma patients. There were no significant differences observed in baseline cardiovascular burden between sarcoma types.
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Diagram of Patient Selection
All patients of the institutional Ewing sarcoma and osteosarcoma registries were considered for study participation. Patients are enrolled in these registries based on International Classification of Diseases-10th Revision diagnoses and have histologically confirmed disease. The study population was composed of 528 patients who were exposed to doxorubicin-containing chemotherapy regimens and were treated with curative intent.
Follow-up
A summary of follow-up and study endpoints is provided in Table 3. In total, 18 patients developed HF (crude rate 3.4%) in a median observation time of 5.7 (Q1-Q3: 2.1-13.1) years. The estimated cumulative incidence of HF was 5.9% (95% CI: 2.8%-9.1%). Eleven patients were hospitalized for HF during follow-up (2.1%). An overview of the results of the adjudication process can be found in Supplemental Table 1. Systemic inflammatory response syndrome criteria could be assessed in 10 of 11 hospitalized HF patients and met the criteria for a sepsis diagnosis in none of the cases. In 9 of 18 patients who developed HF, HF admission was the first manifestation of HF. Furthermore, during follow-up, 14 urgent non-HF, cardiac-related hospitalizations occurred. The most common indications were coronary artery disease (n = 5) and supraventricular tachycardia/atrial fibrillation (n = 4).
Table 3.
Crude Primary and Secondary Event Rates During Follow-Up (N = 528)
| Median follow-up time,a y (95% CI) | 13.2 (12.5-14.9) |
| Died during follow-up,a % (95% CI) | 65.5 (51.2-79.4) |
| Primary endpoint: incident heart failure | 18 (3.4) |
| Secondary endpoints | |
| Relapse | 280 (54.7) |
| Heart failure admission | 11 (2.1) |
| Reasons for hospitalization | |
| Coronary artery disease | 5 (0.9) |
| Supraventricular tachycardia including atrial fibrillation | 4 (0.8) |
| Valvular heart disease | 1 (0.2) |
| VA/OHCA not relating to decreased LVEF | 1 (0.2) |
| Conduction abnormalities requiring pacemaker implantation | 1 (0.2) |
| Pericardial disease excluding pericarditis carcinomatosis | 1 (0.2) |
Values are n (%) unless otherwise indicated.
LVEF = left ventricular ejection fraction; VA/OHCA = ventricular arrhythmia/out-of-hospital cardiac arrest.
Estimated by the Kaplan-Meier method.
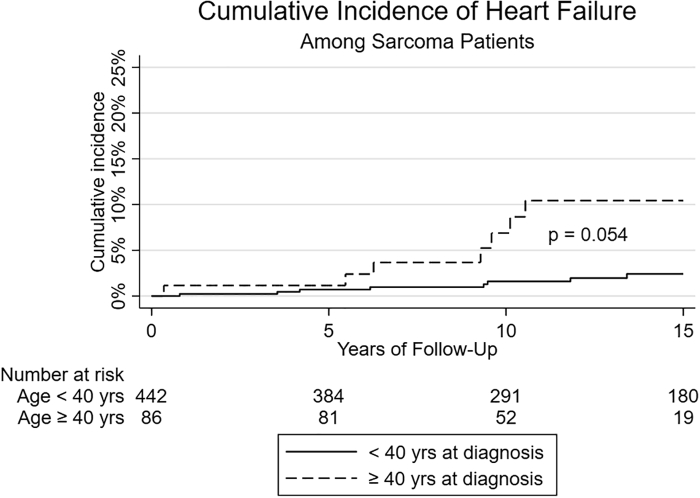
At the end of the follow-up, 65.5% (95% CI: 51.2%-79.4%) of patients had died. The overall median survival after sarcoma diagnosis in the study population was estimated at 12.4 years (Q1-Q3: 2.3-not available [NA] years), but Ewing sarcoma patients had a significantly worse prognosis (5.7 years [Q1-Q3: 2.5-NA years] vs 22.8 years [Q1-Q3: 2.5-NA years] in osteosarcoma patients; log-rank test: P = 0.014). The vast majority of patients died of malignancy-related causes (86.3%) (Supplemental Table 2). The cumulative incidence curves of HF for children/AYAs and older adults are presented in Figure 2. There was a borderline significant difference in the cumulative incidence function (P = 0.054).
Figure 2.
Cumulative Incidence of HF at Follow-Up
The estimated cumulative incidence of heart failure (HF) was 5.9%, but older adults (aged ≥40 years, dashed line) at diagnosis showed a higher cumulative incidence of HF compared with patients diagnosed at childhood or adolescents and young adults (solid line). Cumulative incidence curves were constructed and compared using Fine and Gray’s method.
Association between cardiovascular and oncologic predictors and CTX
Table 4 presents the regression estimates. Age at diagnosis showed a significant association with incident HF with an increase in risk of 23% per 5-year increase in age (HR = 1.23; 95% CI: 1.06-1.43; P = 0.007). A nearly 4-fold increased risk of HF was observed in older adult sarcoma patients (age ≥40 years vs age < 40 years) when adjusted for sex, the cumulative doxorubicin dose, and ≥1 (time-dependent) cardiovascular risk factor (HR: 3.85; 95% CI: 1.37-10.79; P = 0.010). Furthermore, the cumulative doxorubicin dose (per 10-mg/m2 increase) and female sex were both significantly associated with incident HF (HR: 1.13; 95% CI: 1.03-1.24; P = 0.008 and HR: 3.17; 95% CI: 1.11-9.10; P = 0.032, respectively).
Table 4.
Cause-Specific Cox Proportional Hazard Regression With the Competing Risk of Death
| Determinant (No. of Subjects With Exposure) | Heart Failure Modela |
All-Cause Death Model |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| HR (95% CI) | P Value | HR | P Value | HR (95% CI) | P Value | HR | P Value | |
| Age at diagnosis, per 5-y increase | 1.27 (1.11-1.45) | <0.001 | 1.23 (1.06-1.43) | 0.007 | 1.07 (1.03-1.11) | 0.001 | 1.04 (1.00-1.09) | 0.074 |
| Female sex (n = 208) | 3.29 (1.17-9.24) | 0.024 | 3.17 (1.11-9.10) | 0.032 | 0.74 (0.57-0.96) | 0.021 | 0.93 (0.71-1.20) | 0.57 |
| Doxorubicin dose, per-10 mg/m2 increase | 1.10 (1.02-1.18) | 0.011 | 1.13 (1.03-1.24) | 0.008 | 0.97 (0.96-0.98) | <0.001 | 0.99 (0.97-1.00) | 0.038 |
| Any cardiovascular risk factorb (n = 50) | 3.83 (1.25-11.71) | 0.019 | 2.10 (0.57-7.72) | 0.264 | 0.77 (0.42-1.42) | 0.41 | 0.73 (0.38-1.39) | 0.34 |
| Received radiotherapy (n = 140) | 0.61 (0.14-2.68) | 0.52 | 2.25 (1.74-2.90) | <0.001 | 1.65 (1.24-2.18) | 0.001 | ||
| Ewing sarcomac (n = 163) | 0.18 (0.02-1.36) | 0.097 | 1.37 (1.06-1.79) | 0.007 | 1.18 (0.87-1.58) | 0.29 | ||
| Relapseb (n = 280) | 1.07 (0.35-3.26) | 0.91 | 20.50 (14.05-29.91) | 0.015 | 19.24 (13.14 -28.18) | <0.001 | ||
Only the top 4 univariable predictors were chosen for the multivariable heart failure model because of the limited number of events.
Analyzed as a time-varying covariate.
Reference category = osteosarcoma. In separate multivariable models, the HR for age ≥ 40 years at diagnosis adjusted for female sex, doxorubicin dose, and cardiovascular risk factors was 3.85 (95% CI: 1.37-10.79; P = 0.010) and 1.26 (95% CI: 0.90-1.75; P = 0.18) for the heart failure and all-cause death models, respectively.
In a sensitivity analysis, when Fine and Gray’s competing risk regression was used instead of cause-specific Cox regression, similar adjusted results were observed for age per 5-year increase (sHR: 1.18; 95% CI: 1.01-1.39; P = 0.042) and age ≥40 years at diagnosis (sHR: 3.55; 95% CI: 1.22-10.31; P = 0.020). The full estimates table is provided in Supplemental Table 3.
After adjusting for several cancer-related factors and cardiovascular risk factors, age at diagnosis was not significantly associated with the competing risk of all-cause death. Conversely, there was a very strong association between sarcoma relapse and all-cause death (HR: 19.24; 95% CI: 13.14-28.18; P < 0.001).
Furthermore, the interaction between older adults and female sex was assessed. Although the interaction term was nonsignificant (P = 0.193), based on the observed absolute risk per age group and sex, women diagnosed as older adults had the highest risk of HF after treatment (11.1% vs 0.7% in child/AYA males and 4.9% in child/AYA females) (Table 5).
Table 5.
Observed Crude Rate of HF by Age Group and Sex
| Age Group/Sex Category | Observed Crude Rate of HF |
|---|---|
| Male children/AYA at diagnosis | 0.7 (2/279) |
| Female children/AYA at diagnosis | 4.9 (8/163) |
| Male older adults at diagnosis | 7.3 (3/41) |
| Female older adults at diagnosis | 11.1 (5/45) |
Values are % (n/N).
AYA = adolescents and young adults (<40 years of age); HF = heart failure.
Discussion
In this 36-year experience of CTX in a tertiary sarcoma center, the estimated cumulative incidence of HF was 5.9% (95% CI: 2.8%-9.1%). A sarcoma diagnosed later in life was associated with a nearly 4-fold increased risk of HF during follow-up. In this paper, we present a large, comprehensive study of CTX in sarcoma patients that used a standardized definition of HF, included a broad range of patients, followed patients for an extended period of time, and considered the competing risk of death (Central Illustration).
Central Illustration.
Age in Heart Failure Risk After Doxorubicin Treatment for Sarcoma
In a large cohort of osteosarcoma and Ewing sarcoma patients, covering all age groups, we performed a retrospective study to assess the impact of age at sarcoma diagnosis on the risk of heart failure (HF) adjudicated according to the universal definition of HF. When adjusting for the cumulative doxorubicin dose, female sex, and the presence of traditional cardiovascular risk factors, older adults at diagnosis had a near 4-fold increased risk of HF compared with patients diagnosed at childhood or adolescent/young adult age. Age at diagnosis was not a significant predictor of all-cause mortality. The competing risk of death was strongly determined by cancer-related factors such as relapse.
Anthracycline-related CTX in sarcoma
Over the past few decades, many studies have focused on CTX of anthracyclines, but only a few have focused on sarcoma patients. This is remarkable because in sarcoma treatment, although it is a rare malignancy, the cumulative dosage of anthracyclines used is higher than in many other cancers; therefore, the risk of CTX is substantial.10,22, 23, 24 Furthermore, the available studies on CTX in sarcoma patients primarily focus on childhood and adolescent patients, and data regarding patients diagnosed at an older age are lacking. The largest study on CTX in sarcoma patients (n = 883 osteosarcoma, n = 543 Ewing sarcoma) by Longhi et al25 found in a child and adolescent population an incidence of 2.0% and 1.3% CTX for osteosarcoma and Ewing sarcoma, respectively. Paulides et al22 also reported on CTX in pediatric sarcoma patients enrolled in the prospective Late Effects Surveillance System. Among 265 patients, they found that 7.5% of patients developed CTX, but only 1.5% developed clinically relevant CTX.
The available studies that included patients diagnosed at an older age were either of small sample size or limited follow-up. Our study is the first study to report the incidence of a standardized HF definition with a sufficiently long follow-up for older sarcoma patients. A report from the prospective Israel Cardio-Oncology Registry showed a 14% incidence of CTX (defined as a reduction in echocardiography-derived left ventricular ejection fraction ≤53%) among 43 patients with a mean age of 58 years.23 About one-third had traditional cardiovascular risk factors at baseline; however, the investigators did not find a significant association between baseline cardiovascular risk factor burden and CTX during follow-up, similar to our study after adjusting for age at diagnosis and cumulative doxorubicin dosage. In a post hoc analysis of CTX in the ANNOUNCE (A Study of Doxorubicin Plus Olaratumab [LY3012207] in Participants With Advanced or Metastatic Soft Tissue Sarcoma) phase III randomized trial among 504 soft tissue sarcoma patients with a median age of 57 years, symptomatic cardiac events were reported through the Common Terminology Criteria for Adverse Events 4.0.26 Among all doxorubicin dose ranges, they found a low incidence of ≥ grade 3 events (1.1%-2%), but this study was limited by a 28-week follow-up for CTX adverse events.
A noteworthy finding is that there was a suggestion of effect modification, although nonsignificant, of female sex on the effect of older age at diagnosis; the highest absolute risk of HF was observed among women diagnosed as older adults followed by older adult men and child/AYA females. These findings are roughly in line with conclusions of a review of the literature regarding this topic by Wilcox et al19 that showed that menopausal state, sex, and age at diagnosis are key determinants of anthracycline CTX. In childhood cancer survivors, prepubertal females were at an increased risk for CTX, whereas older age women and men had a similar risk. However, there is considerable heterogeneity in the definitions of CTX, treatment regimens, malignancies, and follow-up times, which limits the ability to make solid conclusions.
Another interesting finding was that a higher cumulative doxorubicin dose was not only associated with higher rates of HF but also with slightly lower HR of all-cause death (HR: 0.99; 95% CI: 0.97-1.00). A higher on-target cumulative dose of doxorubicin is likely the result of complete doxorubicin administration. Conversely, a lower cumulative doxorubicin dose is likely caused by interrupted or discontinued treatment because of cancer treatment–related complications or cancer progression, which is associated with a poor prognosis.
Age and CTX risk
Although a very young age at diagnosis has been described previously as a risk factor for anthracycline-induced CTX,27 to our knowledge this is 1 of the few studies that evaluated all age groups. In a meta-analysis of 18 studies with 49,017 patients with all malignancy types, the authors found an increased risk of CTX with increasing age at diagnosis and a higher cumulative doxorubicin dose, which is similar to our findings. However, unlike our study, they also found that prevalent arterial hypertension and diabetes was associated with CTX.28 Furthermore, in a heterogeneous cohort of cancer patients over 65 years of age, the incidence of CTX was approximately 10%.8 For this reason, older age is mentioned as a risk factor in the 2016 European Society of Cardiology consensus document on cardiovascular toxicity of cancer treatment.29 The prevalence of arterial hypertension and diabetes in our study was too low at baseline to make conclusions on their impact on incident HF. In any case, previous studies in cancer patients have shown the importance of optimizing cardiovascular risk factors in cancer patients on mitigating the risk of CTX.30,31
One can speculate that at older age the accumulated burden of subclinical cardiovascular disease and the increasing prevalence of left ventricular (diastolic) dysfunction reduce the resilience of the myocardium to cardiotoxic exposure and make these patients more prone to deterioration of left ventricular function after cardiotoxic chemotherapy. Another explanation was suggested by Robert et al32 in 1983. They observed that with increasing age the clearance of doxorubicin declined, which may add to increased CTX risk.
However, we did not observe the association between a very young age at diagnosis and an increased risk of HF. Lipshultz et al33 included 33 osteosarcoma patients (median age at diagnosis = 14 years, range 2.8-28.9 years) in an echocardiographic study with a minimum of 2 years of follow-up and found that younger age was associated with higher rates of cardiac abnormalities. However, in our study, 35 patients were diagnosed below 10 years of age, and none of these patients developed HF. The competing risk of death by malignancy was not likely to play a role because 30 of 35 patients were alive at the end of the follow-up.
Clinical implications
The implications of our findings are 3-fold: 1) periodic monitoring for CTX in patients diagnosed with sarcoma at an older age should be considered; 2) this older age group may benefit from specific preventive strategies, including dexrazoxane34; and 3) prompt initiation of HF treatment may be useful to prevent clinical deterioration.
Our study shows that sarcoma patients diagnosed at an older age may need additional monitoring surveillance for 2 reasons. First, patients diagnosed at a younger age are frequently enrolled in long-term, late effect of cancer treatment surveillance protocols that include periodic echocardiograms, whereas patients diagnosed at an older age may not be included in such programs. Second, as demonstrated earlier, our study shows that patients diagnosed at an older age have a higher risk of acquiring HF.
Although for the past several decades anthracycline-induced cardiomyopathy was considered irreversible, this has been debated in recent years.35 In our population, we observed that only 1 of 5 patients in whom cardioprotective medications were started because of a decrease in left ventricular ejection fraction in the outpatient clinic were subsequently hospitalized for HF in follow-up. This suggests that with timely detection and treatment of CTX on monitoring visits through echocardiography or cardiac magnetic resonance, clinical deterioration may be averted. Our work may help to further provide data to support screening guidelines.
Sarcoma is a cancer diagnosis with a poor prognosis; the 5-year overall survival rate is estimated at 59% depending on the age group and sarcoma type. Long-term survivors still face a myriad of long-term sequelae that include both CTX (∼5%) and second malignant neoplasms (∼5%).25
Study limitations
We present a large cohort study across a broad age range in a rare patient population within the limits of a single-center study. There is a risk of misclassification of the endpoint because cardiac biomarkers (1 of 2 minor criteria in the universal definition of HF) were not routinely measured at our institution in the 1980s and 1990s in sarcoma patients. This makes the probability of meeting the endpoint lower for patients followed in this time period or delays the HF diagnosis until the occurrence of clinical symptoms of HF. However, there was no association between age group and missing biomarker assessment. Therefore, these measurements are considered missing at random for both age groups. Also, there is a risk of differential follow-up for childhood cancer survivors enrolled in intensive and lifelong late effect monitoring programs compared with patients diagnosed as older adults. However, the follow-up interval and length were similar for both groups; the median follow-up time in children and AYAs was 5.8 years (Q1-Q3: 2.0-13.3) vs 5.5 years (Q1-Q3: 2.2-11.0) in older adults. Therefore, we assume that the effect of differential follow-up is limited. Lastly, the cause of death could not be recovered in ∼10% of patients. In most cases, this was because of treatment discontinuation after cancer progression, after which follow-up was suspended.
Conclusions
In a large cohort of osteosarcoma and Ewing sarcoma patients across a broad age range, we found that patients diagnosed at an older age are more prone to develop HF. Age at diagnosis was not associated with the competing risk of all-cause death after correcting for cancer-related factors such as relapse of disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients treated for sarcoma with doxorubicin, the cumulative incidence of HF was 5.9% (95% CI: 2.8%-9.1%). When patients were diagnosed at >40 years of age, they had nearly a 4-fold increased risk of developing CTX compared with sarcoma patients diagnosed at childhood or AYA age.
TRANSLATIONAL OUTLOOK: Sarcoma patients diagnosed at an older age may have the most benefit from long-term CTX monitoring and preventive measures during treatment. Future studies should evaluate the effect of these measures in this subgroup of sarcoma patients.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Stiller C.A., Craft A.W., Corazziari I. Survival of children with bone sarcoma in Europe since 1978: results from the EUROCARE study. Eur J Cancer. 2001;37:760–766. doi: 10.1016/s0959-8049(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 2.Evenhuis R.E., Acem I., Rueten-Budde A.J., et al. Survival analysis of 3 different age groups and prognostic factors among 402 patients with skeletal high-grade osteosarcoma. Real world data from a single tertiary sarcoma center. Cancers (Basel) 2021;13:486. doi: 10.3390/cancers13030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss S.J., Frezza A.M., Abecassis N., et al. Bone sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:1520–1536. doi: 10.1016/j.annonc.2021.08.1995. [DOI] [PubMed] [Google Scholar]
- 4.Kumar N., Gupta B. Global incidence of primary malignant bone tumors. Curr Orthop Pract. 2016;27:530–534. [Google Scholar]
- 5.Whelan J.S., Bielack S.S., Marina N., et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderton J., Moroz V., Marec-Bérard P., et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours – EURO EWING 2012 protocol. Trials. 2020;21:96. doi: 10.1186/s13063-019-4026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith L.A., Cornelius V.R., Plummer C.J., et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 9.Kremer L.C.M., van Dalen E.C., Offringa M., Voûte P.A. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002;13:503–512. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 10.van Dalen E.C., van der Pal H.J., Kok W.E., Caron H.N., Kremer L.C. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Čelutkienė J., Pudil R., López-Fernández T., et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC) Eur J Heart Fail. 2020;22:1504–1524. doi: 10.1002/ejhf.1957. [DOI] [PubMed] [Google Scholar]
- 12.Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 13.Curigliano G., Lenihan D., Fradley M., et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leerink J.M., de Baat E.C., Feijen E.A.M., et al. Cardiac disease in childhood cancer survivors: risk prediction, prevention, and surveillance. J Am Coll Cardiol CardioOnc. 2020;2:363–378. doi: 10.1016/j.jaccao.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozkurt B., Coats A.J.S., Tsutsui H., et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 16.Bone R.C., Balk R.A., Cerra F.B., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 17.Schuster N.A., Hoogendijk E.O., Kok A.A.L., Twisk J.W.R., Heymans M.W. Ignoring competing events in the analysis of survival data may lead to biased results: a nonmathematical illustration of competing risk analysis. J Clin Epidemiol. 2020;122:42–48. doi: 10.1016/j.jclinepi.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Austin P.C., Latouche A., Fine J.P. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med. 2020;39:103–113. doi: 10.1002/sim.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox N.S., Rotz S.J., Mullen M., et al. Sex-specific cardiovascular risks of cancer and its therapies. Circ Res. 2022;130:632–651. doi: 10.1161/CIRCRESAHA.121.319901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan B., Kirton L., Marec-Berard P., et al. Comparison of two chemotherapy regimens in Ewing sarcoma (ES): overall and subgroup results of the Euro Ewing 2012 randomized trial (EE2012) J Clin Oncol. 2020;38 11500-11500. [Google Scholar]
- 21.Juergens C., Weston C., Lewis I., et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47:22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 22.Paulides M., Kremers A., Stöhr W., et al. Prospective longitudinal evaluation of doxorubicin-induced cardiomyopathy in sarcoma patients: a report of the late effects surveillance system (LESS) Pediatr Blood Cancer. 2006;46:489–495. doi: 10.1002/pbc.20492. [DOI] [PubMed] [Google Scholar]
- 23.Shamai S., Rozenbaum Z., Medinsky O., et al. Cardio-toxicity among patients with sarcoma: a cardio-oncology registry. BMC Cancer. 2020;20:609. doi: 10.1186/s12885-020-07104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen B.V., Skovsgaard T., Nielsen S.L. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 25.Longhi A., Ferrari S., Tamburini A., et al. Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients. Cancer. 2012;118:5050–5059. doi: 10.1002/cncr.27493. [DOI] [PubMed] [Google Scholar]
- 26.Jones R.L., Wagner A.J., Kawai A., et al. Prospective evaluation of doxorubicin cardiotoxicity in patients with advanced soft-tissue sarcoma treated in the ANNOUNCE phase III randomized trial. Clin Cancer Res. 2021;27:3861–3866. doi: 10.1158/1078-0432.CCR-20-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt C.B., Ransom J.L., Evans W.E. Age-related adriamycin cardiotoxicity in children. Cancer Treat Rep. 1978;62:1381–1385. [PubMed] [Google Scholar]
- 28.Lotrionte M., Biondi-Zoccai G., Abbate A., et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 30.Murray J., Bennett H., Bezak E., Perry R. The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: systematic review. Eur J Prev Cardiol. 2021;29:463–472. doi: 10.1093/eurjpc/zwab006. [DOI] [PubMed] [Google Scholar]
- 31.Michos E.D., Marshall C.H. Healthy lifestyle benefits both cancer and cardiovascular disease. J Am Coll Cardiol CardioOnc. 2021;3:675–677. doi: 10.1016/j.jaccao.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert J., Hoerni B. Age dependence of the early-phase pharmacokinetics of doxorubicin. Cancer Res. 1983;43:4467–4469. [PubMed] [Google Scholar]
- 33.Lipshultz S.E., Lipsitz S.R., Mone S.M., et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 34.Li J., Chang H.-M., Banchs J., et al. Detection of subclinical cardiotoxicity in sarcoma patients receiving continuous doxorubicin infusion or pre-treatment with dexrazoxane before bolus doxorubicin. Cardiooncology. 2020;6:1. doi: 10.1186/s40959-019-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardinale D., Iacopo F., Cipolla C.M. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. 2020;7:26. doi: 10.3389/fcvm.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.