Abstract
Background
Anaplastic lymphoma kinase (ALK) translocations in metastatic non-small cell lung cancer (3% to 7%) predict for response to ALK-inhibitors (eg, alectinib, first line), resulting in a 5-year survival rate of ∼60% and median progression-free survival of 34.8 months. Although the overall toxicity rate of alectinib is acceptable, unexplained adverse events, including edema and bradycardia, may indicate potential cardiac toxicity.
Objectives
This study’s aim was to investigate the cardiotoxicity profile and exposure–toxicity relationship of alectinib.
Methods
Between April 2020 and September 2021, 53 patients with ALK-positive non-small cell lung cancer treated with alectinib were included. Patients starting with alectinib after April 2020 underwent a cardiac work-up at start, at 6 months and at 1 year at the cardio-oncology outpatients' clinic. Patients already receiving alectinib >6 months underwent 1 cardiac evaluation. Bradycardia, edema, and severe alectinib toxicity (grade ≥3 and grade ≥2 adverse events leading to dose modifications) data were collected. Alectinib steady-state trough concentrations were used for exposure–toxicity analyses.
Results
Left ventricular ejection fraction remained stable in all patients who underwent an on-treatment cardiac evaluation (n = 34; median 62%; IQR: 58%-64%). Twenty-two patients (42%) developed alectinib-related bradycardia (6 symptomatic bradycardia). One patient underwent a pacemaker implantation for severe symptomatic bradycardia. Severe toxicity was significantly associated with a 35% higher alectinib mean Ctrough (728 vs 539 ng/mL, SD = 83 ng/mL; 1-sided P = 0.015).
Conclusions
No patients showed signs of a diminished left ventricular ejection fraction. Alectinib caused more bradycardia than previously reported (42%) with some instances of severe symptomatic bradycardia. Patients with severe toxicity generally had an elevated exposure above the therapeutic threshold.
Key Words: alectinib, anaplastic lymphoma kinase, bradycardia, cardio-oncology, non-small cell lung cancer
Abbreviations and Acronyms: AE, adverse event; ALK, anaplastic lymphoma kinase; CV, cardiovascular; ECG, electrocardiogram; IVC, inferior vena cava; LVEF, left ventricular ejection fraction; MET, mesenchymal epithelial transition; NSCLC, non-small cell lung cancer; OV, outpatient visit; PK, pharmacokinetic; TKI, tyrosine kinase inhibitor
Central Illustration
Personalized treatment for specific molecular-driven subgroups of metastatic non-small cell lung cancer (NSCLC) has significantly improved overall survival for these patients.1,2 One of these aberrations is anaplastic lymphoma kinase (ALK) translocations, which occur in 3% to 7% of the patients with NSCLC, making it an orphan disease.3,4 The ALK-positive subset is younger (median age 58 years), the majority are female, and they have no smoking history compared with all-comer NSCLC.5 Patients with ALK-positive NSCLC can be treated with several lines of ALK inhibitors instead of chemotherapy. Alectinib, a small molecule tyrosine kinase inhibitor (TKI), is the current first-line therapy. An update of the pivotal phase III ALEX study (A Study Comparing Alectinib With Crizotinib in Treatment-Naive Anaplastic Lymphoma Kinase-Positive Advanced Non-Small Cell Lung Cancer Participants) showed a 5-year survival rate of 63% with a median progression-free survival of almost 3 years (34.8 months) for patients with ALK-positive NSCLC treated with alectinib.6 Although alectinib is generally well-tolerated by most patients, long-term toxicities are important to understand, particularly given the impressive increase in life expectancy.6
A common side effect of alectinib is peripheral edema (9% to 17%),5,6 which is also seen in the ALK inhibitor crizotinib. Crizotinib-related peripheral edema is believed to be induced by the mesenchymal epithelial transition (MET) inhibiting effect of crizotinib, which increases capillary permeability through elevated vascular endothelial growth factor (VEGF) signaling, and this effect is also seen in other MET inhibitors.7,8 However, alectinib has no MET inhibiting potential, therefore the cause of alectinib-induced edema remains unknown.9 This shows that insights from other ALK–small molecule TKIs cannot be freely extrapolated to alectinib because they all exhibit different kinase targeting properties and pharmacokinetic and pharmacodynamic profiles10 (Supplemental Table 1). A cardiac cause for edema should be considered because many small molecule TKIs are indeed cardiotoxic,11,12 especially because bradycardia is also a frequent adverse event (AE) of alectinib.13 Limited awareness exists for the potential cardiotoxicity of alectinib, but the durable prognosis of these patients, and consideration of alectinib use in the adjuvant setting (A Study Comparing Adjuvant Alectinib Versus Adjuvant Platinum-Based Chemotherapy in Patients With ALK Positive Non-Small Cell Lung Cancer; NCT03456076, Genetic Testing in Screening Patients With Stage IB-IIIA Non-small Cell Lung Cancer That Has Been or Will Be Removed by Surgery [The ALCHEMIST Screening Trial]; NCT02194738), and use in combination with radiotherapy, which has cardiotoxic properties,14,15 suggest an important need to generate a greater understanding.
The lack of understanding of underlying mechanism of AEs of alectinib, in combination with the infrequency of ALK translocations, makes it difficult for clinicians to make well-informed decisions about interventions, that is, dose reductions, in cases of toxicity. Because the level of alectinib exposure in blood plasma was found to be positively correlated to progression-free survival with a therapeutic target level of 435 ng/mL alectinib,16 a patient might risk a subtherapeutic exposure after dose reduction. An exposure–toxicity relationship has not yet been established.16 Because patients are expected to receive alectinib for several years, it is important to minimize toxicity without undermining its therapeutic effects.
The primary aim of this study was to describe the results of dedicated prospective cardiac follow-up in patients treated with alectinib and to investigate the relationship between alectinib-related edema and cardiac dysfunction. The secondary objective was to describe the frequency, severity, and clinical consequences of cardiac AEs and severe AEs in relation to alectinib exposure.
Materials and Methods
Study design
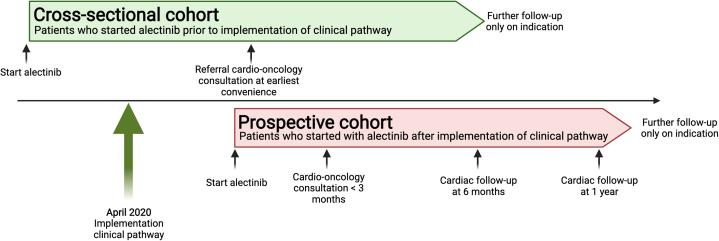
We performed a prospective observational study based on a cardio-oncology clinical pathway at the Erasmus Medical Centre Cancer Institute (CORAL study: MEC 20-0836 approved by the medical ethical committee of the Erasmus MC), which was implemented in April 2020. The patient population consisted of patients with advanced ALK-positive NSCLC treated with alectinib. Depending on the moment of starting alectinib (before or after the implementation of the clinical care pathway), 2 cohorts were developed: a prospective cohort and the cross-sectional cohort (for more details, see the Supplemental Appendix). Patients starting alectinib treatment after implementation of the pathway (prospective cohort) were followed by a cardio-oncologist for the first year of treatment (Figure 1). Patients already on alectinib (cross-sectional cohort) were referred for a 1-time cardio-oncology consultation at their earliest convenience. All patients underwent clinical assessment, electrocardiograms (ECGs), and laboratory values at outpatient visits (OVs) from start of alectinib treatment. All patients provided written consent for data collection, and 47 patients provided additional consent for the collection of pharmacokinetic (PK) samples (START-TKI [Prospective Sampling in Driver Mutation Pulmonary Oncology Patients on Tyrosine Kinase Inhibitors] trial; MEC 16-643, NCT05221372). The PK samples were used to measure alectinib plasma concentrations.
Figure 1.
Schematic Overview of the Clinical Pathway and Patient Cohorts
Patients starting on alectinib from April 2020 onwards were included in the prospective cohort and underwent prospective cardiac follow-up. Patients already on treatment at that moment in time were referred for a 1-time work-up. All patients were included in adverse event collection.
Data collection
Patient characteristics, laboratory values, cardiac investigations, and imaging were collected from medical records from start of treatment until end of treatment with alectinib in a Castor electronic data capture database. Heart rate was extracted from ECGs: at alectinib initiation, 1 week (range 7-14 days), 1 month, and every 3 months after treatment initiation. Cardiac function was measured by echocardiography as part of the clinical pathway. AEs were reported by the treating physician during a dedicated TKI OV. In addition, at every OV, a dedicated oncology nurse specialized in TKI therapy specifically assessed for potential TKI adverse events through a standardized template. The following AEs occurring during treatment with alectinib were collected from the electronic patient records: any grade cardiac-related AEs; bradycardia (defined as a heart rate <60 beats/min), edema, myalgia: and severe toxicity defined as grade ≥3 and/or grade <3 toxicity leading to hospital admission, dose reduction, dose interruption, or discontinuation of treatment. The AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.17 Myalgia was collected as a cardiac-related AE because myalgia might be an indicator for muscle damage that might also affect the cardiomyocytes. Additionally, survival status (dead/alive), date of death, or end of treatment was collected. Patients were included from April 2020 until September 2021, and data cutoff was set at December 1, 2021, to ensure enough follow-up time (>3 months) for the development of AEs.
During the regular multidisciplinary evaluations of the clinical pathway, the exercise ECGs (Results section) that showed chronotropic incompetence were discussed. To gain further unbiased insight in potential chronotropic incompetence, heart rate diaries were introduced starting February 2021. Because a baseline measurement was required, the heart rate diaries were only filled in by new patients. New patients who started on alectinib after this time point were asked to measure their heart rate daily at rest and after a short exercise for 28 days from treatment start using a pulse oximeter provided by the Dutch Society of Pulmonary Physicians (NVALT).
Pharmacokinetic sampling
Patients were prospectively followed, and blood for pharmacokinetic analysis was drawn at every OV until treatment termination. Quantification of alectinib plasma concentrations was performed by a validated liquid-chromatography tandem-mass spectrometric assay.18 The Supplemental Appendix provides more details on the pharmacokinetic analyses.
Statistical analyses
The statistical analyses were performed with SPSS v.28.0.1.0 (IBM SPSS Statistics). Differences between baseline and follow-up echocardiography values were compared using a Wilcoxon signed-rank test (not-normally distributed). Median values are presented with the 25th and 75th percentile (IQR) and P value. Unless stated otherwise, 2-sided P values are reported. The heart rates from ECGs at different time points were analyzed with a mixed effects model with an unstructured covariance type. The unstructured covariance type was the preferred model after testing several models. Time as a factor was added as fixed and random effects. All time points were compared with baseline and 1 year. The Bonferroni correction was used to correct for the multiple comparisons. Model assumptions were evaluated by assessing the residuals. The relationship between bradycardia and other categorical variables, that is, prior small molecule TKI treatment and myalgias, were investigated using Pearson's chi-square test. Similarly, the relation between myalgias and edema was tested using Pearson's chi-square test.
To compare alectinib exposure between patients with and without severe toxicity, mean Ctrough concentrations of alectinib at 600 mg twice daily were analyzed with a U test. If significantly different, a t-test could identify the differences between the average toxic concentration of alectinib in the group with and without severe toxicity. Mean ± SD values are presented and with P values. Similarly, to further investigate the interpatient relationship, the Ctrough of patients experiencing severe toxicity after their dose reduction to 450 mg twice daily was compared with the Ctrough of patients at 600 mg twice daily dose who did not develop severe toxicity. A potential difference in time until first day of severe toxicity was analyzed by Kaplan-Meier analysis. Because the minimal follow-up time was 3 months, and most severe toxicity typically occurs within the first 3 months of treatment, a cutoff time of 6 months was used. The cohort of patients from whom PK data were available was therefore divided in 2 (by median) and divided in 3 tertiles (highest 33% vs the lowest 67%).
Results
Patient characteristics
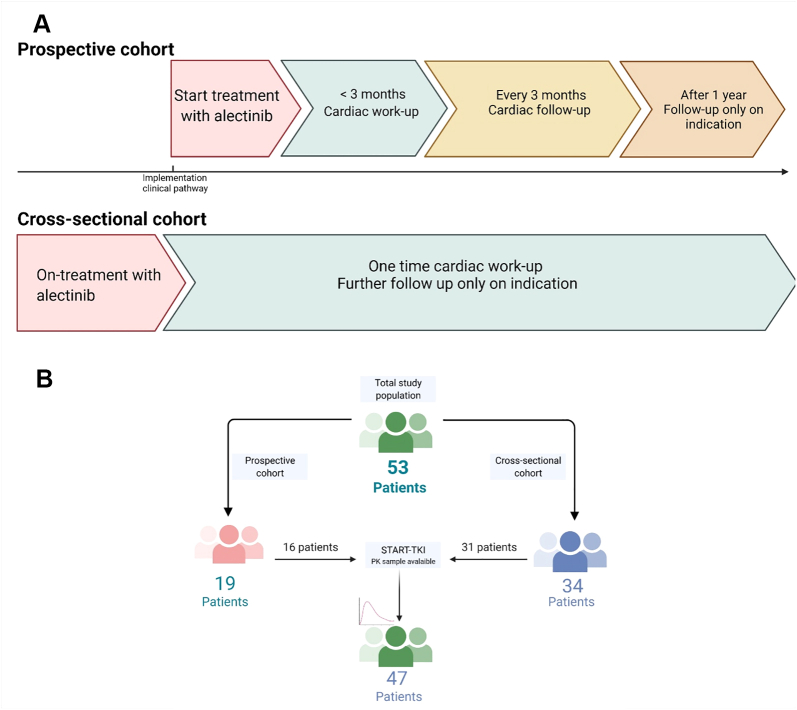
Between April 2020 and September 2021, a total of 53 unique patients were included and were evaluable for assessment of toxicity events. A total of 47 patients were also included in the START-TKI trial and had at least 1 PK sample available (Figure 2). Of the 53 patients, 19 patients were part of the prospective cohort and 34 patients of the cross-sectional cohort (Figure 2). Median age of the total population was 63 years (range 21-82 years), 59% were female, and most had good performance status at the start of treatment (ECOG [Eastern Cooperative Oncology Group] performance score 0 to 1). Seven patients (14%) had more than 1 cardiovascular (CV) risk factor. Patient characteristics at start of alectinib treatment are described in Table 1.
Figure 2.
Flowchart of Patient Inclusion
In total, 53 patients were included, of whom 47 patients were also included in the START-TKI study and had pharmacokinetic samples available. Created by biorender.com.
Table 1.
Baseline Patient Characteristics (N = 53)
| Age, y | 63 (21-82) |
| Female | 31 (59) |
| Male | 22 (41) |
| ECOG performance status | |
| 0-1 | 43 (81) |
| ≥2 | 9 (17) |
| Unknown | 1 (2) |
| Smoker status | |
| Never | 36 (68) |
| Former/active | 15 (29) |
| Unknown | 2 (4) |
| Prior therapy | 30 (57) |
| Small molecule TKI | 14 (26) |
| Chemo | 17 (32) |
| CoRTX | 4 (8) |
| Known brain or LM metastasis, n = 50 | 14 (26) |
| >1 CV risk factor, n = 51 | 7 (14) |
| Follow-up time, mo | 21 (1-49) |
Values are median (range) or n (%).
Chemotherapy received was platinum with pemetrexed, except for 1 patient (carboplatin + paclitaxel). Cardiovascular (CV) risk factors included hypertension, diabetes mellitus, dyslipidemia, familial history, and prior CV events.
CoRTX = concurrent chemoradiation; ECOG = Eastern Cooperative Oncology Group; LM = leptomeningeal; TKI = small molecule tyrosine kinase inhibitor.
Cardiac function and edema
Nineteen patients underwent an echocardiogram at baseline, and all had a normal systolic left ventricular ejection fraction (LVEF) of >50% (median 65%; IQR: 61%-67%) (Supplemental Table 2). Thirteen of 19 patients had at least 1 follow-up echocardiography after 6 months. Cancer progression or death (n = 2), withdrawn consent for echocardiography (n = 1), logistical issues (n = 1), and data cutoff (n = 3) were major reasons for echocardiograms not being performed at 6 months. No significant differences were seen between LVEF at baseline, at 6 months (n = 11; median 61%; IQR: 60%-63%; P = 0.15) and at 9 to 12 months (n = 12; median 60%; IQR: 57%-64%; P = 0.14). LVEF did not decline to <50% in any of the patients, nor were there signs of an increased of pressure in the inferior vena cava (IVC); median IVC diameter at baseline 17 mm; IQR: 14-18 mm, median at 9 to12 months 16 mm; IQR: 13-20 mm; P = 0.21.
Of the 34 patients included in the cross-sectional cohort, 22 underwent an echocardiogram. The median time on alectinib treatment at time of echocardiography was 24 months (range 7-38 months). Patients’ decision (n = 4) or cessation of treatment with alectinib (n = 8) were the major reasons for lack of echocardiography. All patients had a normal systolic LVEF of >50% (median 63%; IQR: 59%-64%), except for 1 patient with pre-existing heart failure. In addition, no patients showed signs of increased pressure in the IVC based on IVC collapse and IVC diameter (median 17 mm; IQR: 14-18 mm).
Alectinib-related edema was reported in 7 patients (13%); grade 1 in 4 patients, and grade 2 in 3 patients. In 2 patients, edema was the reason for a dose reduction. Four patients underwent an echocardiogram, but showed no signs of LVEF dysfunction. One patient had more than 1 CV risk factor. No patients with an albumin measurement at time of edema had evidence for hypoalbuminemia (n = 6). In the patients for whom N-terminal pro–B-type natriuretic peptide (NT-proBNP) was available around the time of edema (n = 6), no age-adjusted abnormal values were seen (Supplemental Table 3).
Alectinib-related bradycardia
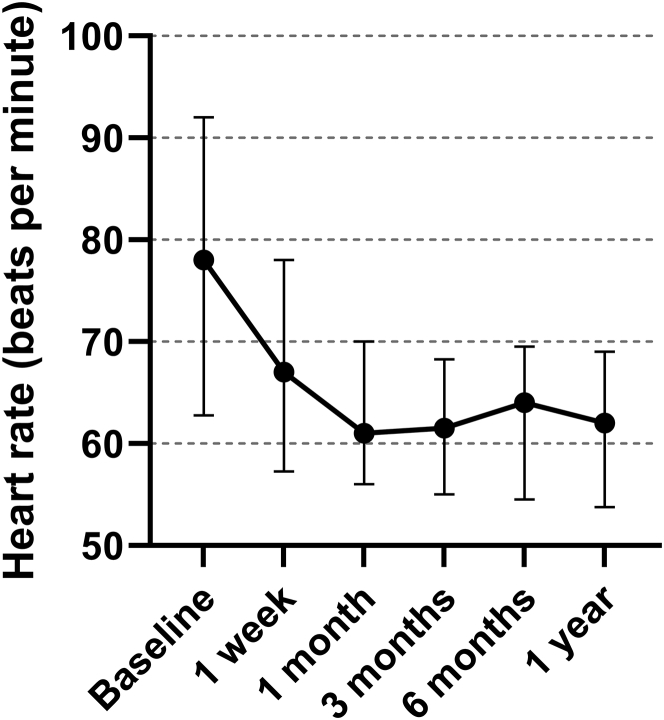
During treatment with alectinib, there was a significant decrease in heart rate (P < 0.001 for all time points compared with baseline). Besides the baseline heart rate, the heart rate at the other time points was not significantly different from the heart rate at 1 year (all P values >0.50), suggesting stability of the heart rate after an initial decrease. Median heart rates are depicted in Figure 3. No prolongation of QTc interval or other conduction changes were observed. Detailed ECG data can be found in Supplemental Table 2.
Figure 3.
Median Heart Rate of Consecutive Electrocardiograms
This graph shows the course of the median heart rate by electrocardiograms at different time points and associated 25th and 75th percentile range.
During treatment with alectinib, 31 patients experienced bradycardia (both related and unrelated), including 8 patients with bradycardia at baseline (grade 1). Twenty-two patients (42%) were assessed as related to alectinib; 16 patients were grade 1, 5 patients grade 2, and 1 patient grade 3 (Table 2). Median time to bradycardia was 3 weeks (range 1-126 weeks), and 91% (20/22) developed bradycardia within 3 months. In 9 patients (17%), bradycardia led to a dose reduction. One-half of these patients (5/9) had no improvement of the bradycardia (Figure 3), and 3 patients required an additional dose reduction. The patient with grade 3 bradycardia had refractory symptoms of bradycardia (frequent spells of dizziness, without syncope) after the second dose reduction, for which a pacemaker implantation was necessary. After the pacemaker implantation, the patient was safely re-escalated to 600 mg twice daily.
Table 2.
Summary of Adverse Events Related to Alectinib
| Any Grade (N = 53) | Grade ≥3 (n = 26) | |
|---|---|---|
| Preferred term adverse event | ||
| Bradycardia | 22 (42) | |
| Myalgia | 16 (30) | |
| Edema | 7 (13) | |
| Preferred term for severe adverse events | ||
| CK elevation | 9 (17) | 3 (6) |
| AST/ALT increase | 5 (9) | 3 (6) |
| Bradycardia | 6 (11) | 1 (2) |
| Myalgia | 6 (11) | — |
| Creatinine increase | 3 (6) | — |
| Blood bilirubin increase | 2 (4) | 2 (4) |
| Skin rash | 2 (4) | 2 (4) |
| Fatigue | 1 (2) | — |
| Edema | 2 (4) | — |
| Pneumonitis | 1 (2) | — |
| Severe toxicity consequence | ||
| Hospital admission | 1 (2) | |
| Dose reduction | 25 (47) | |
| Treatment interruption | 17 (32) | |
| Treatment termination | 2 (4) |
Values are n (%).
AST = aspartate aminotransferase; ALT = alanine aminotransferase; CK = creatine kinase.
No relationships between bradycardia and prior ALK–small molecule TKI treatment (P = 0.76), nor between bradycardia and more than 1 CV risk factor (P = 0.69), and bradycardia and beta-blockade therapy was found (P > 0.99).
Three patients with bradycardia underwent an exercise ECG as advised by the cardiologist based on the clinical presentation of the bradycardia. None of the patients reached the target heart rate (77%, 70%, and 54%) that indicated chronotropic incompetence (local cutoff <80% of target heart rate19). However, all patients had a normal or more than normal exercise capacity. The chronotropic incompetence was not clinically significant.
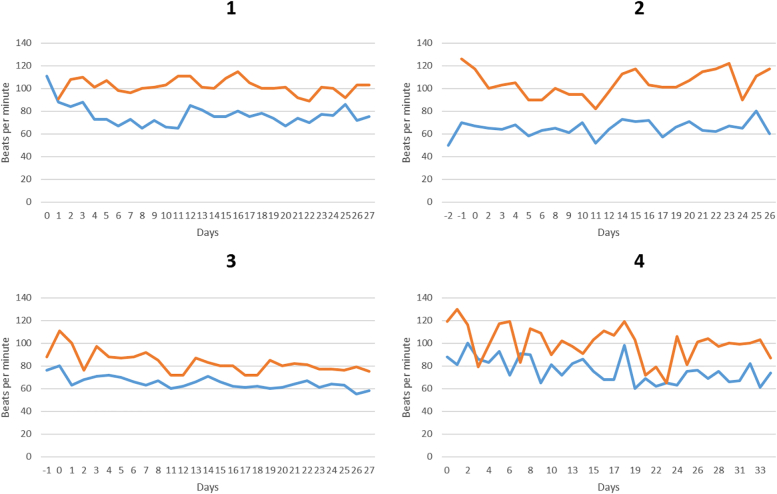
At termination of the clinical pathway, 4 patients had completed a heart rate diary (Figure 4). Two of them developed alectinib-related bradycardia (Patients 3 and 4) and showed a decrease in heart rate during exercise, potentially indicating chronotropic incompetence. One patient (Patient 2) had bradycardia at baseline, which did not worsen and was considered as unrelated to alectinib.
Figure 4.
Overview of the Heart Rate Diaries
The blue line depicts heart rate at rest, and the orange line depicts heart rate after exercise. Day 0 is the day of start alectinib. Patients 2 and 3 started recording their heart rate before starting alectinib indicated by days −1 and −2. Days with incomplete data were not included. Patients 3 and 4 developed alectinib-related bradycardia.
Myalgias
Myalgias related to alectinib were reported in 16 patients (30%) (grade 1 n = 12, and grade 2 n = 4) and led to a dose reduction in 6 patients. The majority (13/16) had a creatine kinase increase (grade 1 n = 7; grade 2 n = 4; grade 3 n = 1; grade 4 n = 1). Myalgias improved in 5 patients, and 1 patient required a second dose reduction. There was a significant association between myalgia and edema (P = 0.002). Six patients with edema also had myalgia, 5 of which occurred simultaneously. No association could be found between the presence of myalgia and bradycardia (P = 1.00).
Exposure-toxicity relationship
From 47 patients, 270 PK samples were drawn during the study period. Fifty-nine samples had to be excluded because Tmax was <5 hours. From 36 patients, Ctrough at 600 mg twice daily was calculated. Median Ctrough at 600 mg twice daily was 569 ng/mL, with an IQR of 450 to 780 ng/mL. Twenty-seven patients had Ctrough at 600 mg twice daily of more than 435 ng/mL (therapeutic threshold).
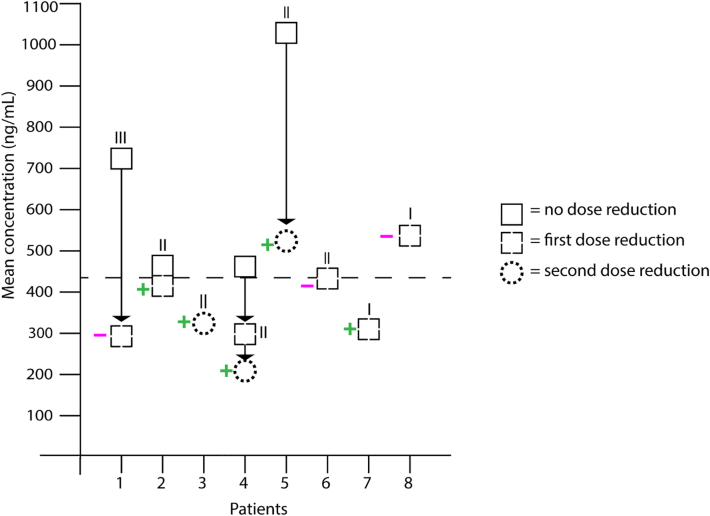
From 8 patients who received a dose reduction for bradycardia (n = 9), PK samples were available either before or after dose reduction, or at both time points. The PK data showed that patients could develop bradycardia at low, but still therapeutic, alectinib concentrations (Figure 5). And after a dose reduction, many patients (5/8) dropped below the therapeutic exposure (based on the threshold of 435 ng/mL).
Figure 5.
Exposure–Toxicity Association
Mean plasma concentrations of alectinib before and after dose reduction due to bradycardia. The Roman numerals indicate the grade of severity of bradycardia. Plus means the bradycardia improved, minus means the bradycardia remained unchanged or worsened. A sample before dose reduction was not available for all patients. Patient 4 developed grade 2 bradycardia after a first dose reduction for a grade 3 rash.
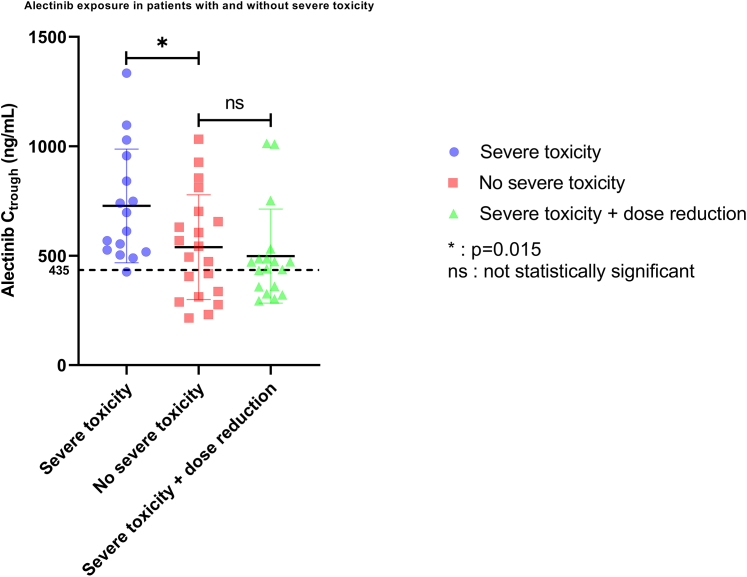
In total, 26 patients (49%) experienced severe alectinib-related toxicity (Table 2). The most frequent severe toxicities were creatine kinase increase, aspartate aminotransferase/alanine aminotransferase increase, bradycardia, and myalgia. In the group for whom a Ctrough at 600 mg twice daily was available, at least 1 event of severe toxicity occurred in 16 patients (44%). The occurrence of severe toxicity was significantly associated with a higher alectinib Ctrough (Figure 6) (P = 0.039). Average Ctrough was 35% higher in patients who experienced severe toxicity (728 ± 260 ng/mL vs 539 ± 239 ng/mL; 1-sided P = 0.015). One patient with severe toxicity had a subtherapeutic alectinib concentration (<435 ng/mL), compared with 40% of the patients without severe toxicity (Figure 6).
Figure 6.
Plasma Exposure per Toxicity Group
This figure shows the difference in alectinib minimal plasma concentration (Ctrough) between patients with (blue dots) and without severe toxicity (red squares) when treated with alectinib 600 mg twice daily. The green triangles are Ctrough concentrations from patients with severe toxicity who were reduced in alectinib dose to 450 mg twice daily because of severe toxicity. Data shown as mean ± SD. ∗P < 0.05; ns = not statistically significant. The dotted line at 435 ng/mL shows the therapeutic threshold of alectinib.
When the mean Ctrough of 18 patients with severe toxicity after dose reduction to 450 mg twice daily was compared with the mean Ctrough of 20 patients without severe toxicity (who continued on 600 mg twice daily), no difference in exposure was found (Figure 6) (P = 0.80). Mean Ctrough in the latter group was <10% higher (539 ± 239 ng/mL vs 498 ± 215 ng/mL; P = 0.57).
Median time to severe toxicity was 2.7 weeks (IQR: 1.9-4.0 weeks). Dividing the cohort in 2 based on median Ctrough (<569 ng/mL vs >5,693 ng/mL) did not show a significant difference in time to onset of severe toxicity: median 2.26 months vs not reached; P = 0.17. In the one-third of patients with the higher Ctrough (>700 ng/mL), however, it was clear that severe toxicity occurred significantly earlier compared with the two-thirds of patients with a lower Ctrough (median 2.3 [95% CI: 0.0 to 8.6] months vs not reached; P = 0.030).
Discussion
To our knowledge, this is the first study performing prospective cardiac follow-up, reporting on the real-world (cardio)toxicity of alectinib, and demonstrating a safety-exposure relationship in treatment with alectinib. In this study, intensive prospective cardiac follow-up showed that alectinib-related edema was not associated with decreased cardiac function (Central Illustration). Our data furthermore showed alectinib-related bradycardia to be far more common than previously reported. Dose reductions due to bradycardia left many patients exposed to subtherapeutic levels of alectinib. However, in general, patients with severe toxicity related to alectinib did have a relatively high exposure to alectinib.
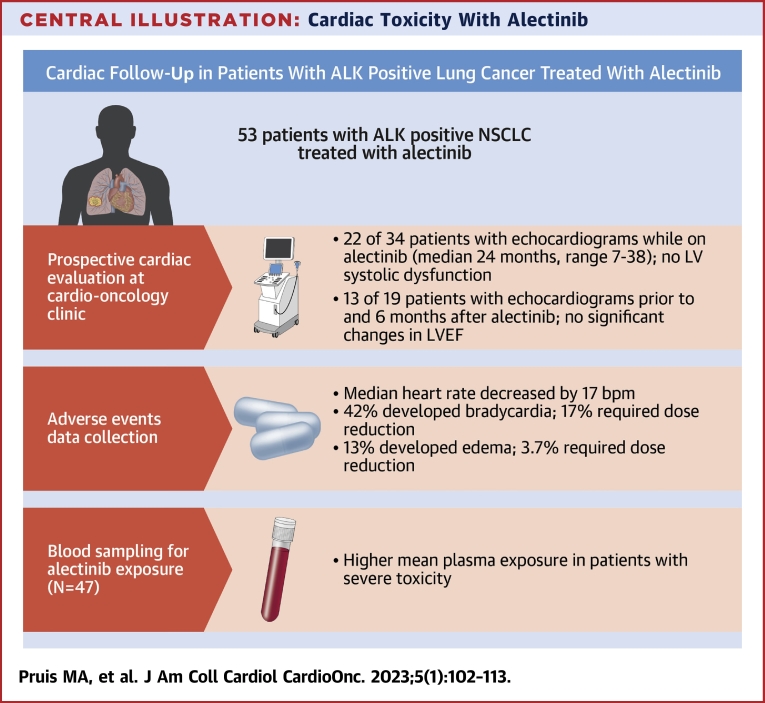
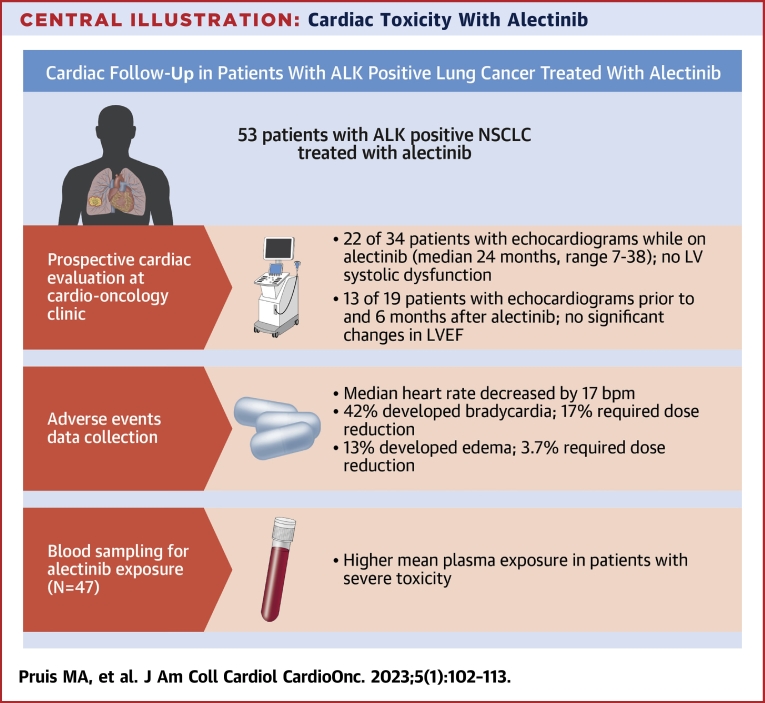
Central Illustration.
Cardiac Toxicity With Alectinib
Main results of prospective cardiac follow-up, adverse events collection, and pharmacokinetic sampling in patients with anaplastic lymphoma kinase (ALK)-positive lung cancer treated with alectinib. AE = adverse event; bpm = beats/min; LV = left ventricular; LVEF = left ventricular ejection fraction; NSCLC = non-small cell lung cancer; PFS = progression-free survival; PMI = pacemaker implantation; TDM = therapeutic drug monitoring.
We prospectively confirmed earlier retrospective studies that alectinib does not seem to induce heart failure.20 Systolic dysfunction as a cause for edema could also be excluded, as well as hypoalbuminemia. Although our sample size was small, we do not believe our results suggest a need for routine screen for systolic dysfunction. Our reported frequency of edema (13%) was comparable to the large clinical trials (9%-17%).5,6 By contrast, we reported a higher prevalence of bradycardia (42%) compared with phase II and phase III trials (0.01%-30.0%), which included all bradycardia regardless of relatedness.6,21, 22, 23 In this study, bradycardia was the most common reason for a dose reduction, even if patients were asymptomatic. Based on these results, we formulated our site-specific general recommendations for management of bradycardia and severe toxicity (Figure 7). These recommendations clearly need to be validated in a larger prospective study. Because most patients in this population were active and some even regularly performed strenuous exercise, further prospective research should be done into the clinical relevance of potential chronotropic incompetence to better guide these patients. In cases of persistent symptomatic bradycardia, pacemaker implantation should be considered, which could allow for dose escalation. Although these patients have advanced lung cancer, their expected life expectancy typically surpasses the required expectancy for a pacemaker implantation of 1 year.24 Currently, the European Medicines Agency does not recommend routine heart rate measurements during alectinib treatment.13 However, with the high prevalence and early occurrence of bradycardia, this might be indicated for the first 6 months to identify patients at risk for symptomatic bradycardia. Increased heart rate monitoring should be accompanied with clear instructions for dose reductions to avoid unnecessary dose reductions.
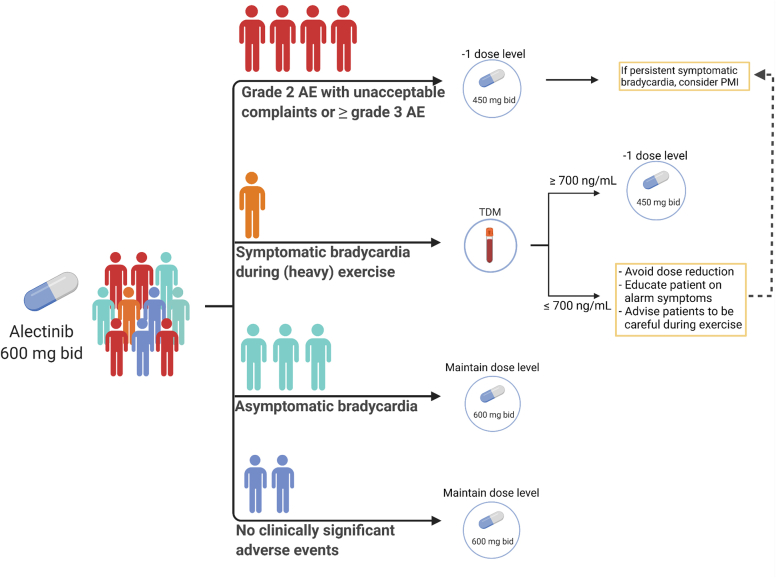
Figure 7.
Our Institutional Recommendations for Management of Alectinib-Related Toxicity
The person icons reflect the frequency in an alectinib population. These recommendations are based upon our cohort findings and clinical experience and need to be further validated. AE = adverse events; bid = twice a day; PMI = pacemaker implantation; TDM = therapeutic drug monitoring. Created by biorender.com.
The mechanism of alectinib-induced bradycardia is poorly understood.25 Bradycardia is also described with other ALK–small molecule TKIs, but the frequency varies between trials, and limited real-world data are available.26,27 One proposed mechanism might be a direct inhibition of cardiac pacemaker cells in the sinus atrial node, but this has only been researched with crizotinib in mouse models.25 Other anti-cancer therapies rarely cause bradycardia, and no clear pattern of mode of action could be found.28, 29, 30, 31 Because lorlatinib, another highly selective ALK inhibitor, does not seem to cause bradycardia, the bradycardia could be caused by off-target inhibition of other pathways of alectinib.32,33
In clinical practice, we observed that dose reductions and severe toxicity due to alectinib-related toxicity were more prevalent than in clinical trials.34 This may be due to our definition of severe toxicity, which not only included grade 3 events, but also milder adverse events that were relevant to the patient. Adverse events that are not compatible with long-term dosing should be taken into account in defining a toxic threshold. Quantification of a Ctrough after 1 or 2 weeks could identify patients at risk of severe toxicity. Based on our findings, we expect the toxic plasma concentration limit to be >700 ng/mL, but this should be further validated. Defining a therapeutic window with a therapeutic threshold and a toxic limit would support clinicians in making decisions about dose modifications. Personalized dosing with alectinib is especially interesting, because it shows high interpatient variability, and suffers from a large positive food effect.35 Currently, a randomized controlled trial is ongoing to further investigate the therapeutic drug monitoring–guided alectinib dosing (NCT05525338).
Study limitations
Because rapid initiation with alectinib in these patients had the highest priority, it was not possible to consistently obtain echocardiography before start of treatment. Because a decrease in LVEF would be expected after a longer period,36, 37, 38 we believe it was justified to perform an echocardiography within 3 months after starting treatment, although we cannot exclude earlier declines. Diastolic function was also not systematically assessed; as a result, we cannot exclude abnormal diastolic function as the cause of edema. The relatively small sample size and missing data should be taken into account when interpreting these results. Nevertheless, we provide new insights that motivate additional clinical research into alectinib. In our mixed model, the residuals showed a slight deviation from normality. However, because the differences between heart rate at baseline and during treatment were very clear, we do not expect this slight deviation to have an impact on the final conclusion.
Conclusions
Involvement of cardiologists, both by cardio-oncology outpatient service and in research, is pivotal to better understand cardiotoxicity of novel anticancer drugs.39 Further clinical and translational research into alectinib-related bradycardia, potential chronotropic incompetence, edema, and the exposure–toxicity relationship is highly recommended. Because ALK translocations are uncommon, sharing clinical observations from experience is pivotal to ensure that patients worldwide treated with alectinib receive up-to-date and consistent management of toxicity to ensure long-term safe dosing.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients treated with alectinib, bradycardia is a more common side effect than previously reported (40% of the patients) and can result in clinically significant symptoms. Regular heart rate measurements in the first period after treatment initiation may be useful. Alectinib appears to have an exposure–toxicity association, and therapeutic drug monitoring might prevent severe toxicity.
TRANSLATIONAL OUTLOOK: Investigations into the etiology of the alectinib-induced bradycardia are needed to increase our understanding of alectinib’s inhibiting properties. Further research needs to be performed into the pharmacokinetics of alectinib with the aim of further defining and validating a toxic threshold for accurate therapeutic drug monitoring.
Funding Support and Author Disclosures
Dr Veerman has received honoraria from Eli Lily. Dr Paats has received honoraria from Bayer, Eli Lily, Novartis, Pfizer, and Roche; and has received expert testimonial fees from AstraZeneca, Eli Lily, Merck, and Takeda. Dr Mathijssen has received grants or contracts from Astellas, Bayer, Boehringer Ingelheim, Cristal Therapeutics, Pamgene, Pfizer, Novartis, Roche, and Servier. Dr Manintveld has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novartis. Dr Dingemans has received honoraria from Eli Lily, AstraZeneca, Jansen, Chiesi, Pfizer, and Takeda; has received grants from Amgen; has participated on a data safety monitoring board or advisory board for Boehringer Ingelheim, Amgen, Bayer, Pharmamar, and Sanofi; and has had a leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, for Roche. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Figures 1 and 5 were created with BioRender. Dutch Society of Pulmonary Physicians (NVALT) provided oximeters. The authors thank Sara Baart, Msc, PhD, for her statistical expertise.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Surveillance, Epidemiology, and End Results (SEER) National Cancer Institute; Bethesda, MD: 2022. Cancer Stat Facts: Lung and Bronchus Cancer. [Google Scholar]
- 2.Imyanitov E.N., Iyevleva A.G., Levchenko E.V. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103194. [DOI] [PubMed] [Google Scholar]
- 3.Chia P.L., Mitchell P., Dobrovic A., et al. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423–432. doi: 10.2147/CLEP.S69718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pikor L.A., Ramnarine V.R., Lam S., et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82:179–189. doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated alk-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 6.Camidge D.R., Dziadziuszko R., Peters S., et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14:1233–1243. doi: 10.1016/j.jtho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Largeau B., Cracowski J.-L., Lengellé C., et al. Drug-induced peripheral oedema: An aetiology-based review. Br J Clin Pharmacol. 2021;87:3043–3055. doi: 10.1111/bcp.14752. [DOI] [PubMed] [Google Scholar]
- 8.Dagogo-Jack I., Moonsamy P., Gainor J.F., et al. A phase 2 study of capmatinib in patients with MET-altered lung cancer previously treated with a MET inhibitor. J Thorac Oncol. 2021;16:850–859. doi: 10.1016/j.jtho.2021.01.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakamoto H., Tsukaguchi T., Hiroshima S., et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Costa R.B., Costa R.L.B., Talamantes S.M., et al. Systematic review and meta-analysis of selected toxicities of approved ALK inhibitors in metastatic non-small cell lung cancer. Oncotarget. 2018;9:22137–22146. doi: 10.18632/oncotarget.25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng H., Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106:21–34. doi: 10.1161/CIRCRESAHA.109.206920. [DOI] [PubMed] [Google Scholar]
- 12.Kartik A., Joe E., Barry T., Eric H.B. Osimertinib-induced cardiotoxicity. J Am Coll Cardiol CardioOnc. 2019;1:172–178. [Google Scholar]
- 13.European Medicines Agency (EMA) European Medicines Agency; Amsterdam, the Netherlands: 2021. Alecensa: EPAR - Product Information. [Google Scholar]
- 14.Wang K., Eblan M.J., Deal A.M., et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banfill K., Giuliani M., Aznar M., et al. Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol. 2021;16:216–227. doi: 10.1016/j.jtho.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenland S.L., Geel D.R., Janssen J.M., et al. Exposure-response analyses of anaplastic lymphoma kinase inhibitors crizotinib and alectinib in non-small cell lung cancer patients. Clin Pharmacol Ther. 2021;109:394–402. doi: 10.1002/cpt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Cancer Therapy Evaluation Program (CTEP) National Institutes of Health; Bethesda, MD: 2021. Common Terminology Criteria for Adverse Events (CTCAE) [Google Scholar]
- 18.Veerman G.D.M., Lam M.H., Mathijssen, et al. Quantification of afatinib, alectinib, crizotinib and osimertinib in human plasma by liquid chromatography/triple-quadrupole mass spectrometry; focusing on the stability of osimertinib. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1113:37–44. doi: 10.1016/j.jchromb.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Peter H.B., Dalane W.K. Chronotropic incompetence. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waliany S., Zhu H., Wakelee H., et al. Pharmacovigilance analysis of cardiac toxicities associated with targeted therapies for metastatic NSCLC. J Thorac Oncol. 2021;16(12):2029–2039. doi: 10.1016/j.jtho.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Hida T., Nokihara H., Kondo M., et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 22.Morcos P.N., Bogman K., Hubeaux S., et al. Effect of alectinib on cardiac electrophysiology: results from intensive electrocardiogram monitoring from the pivotal phase II NP28761 and NP28673 studies. Cancer Chemother Pharmacol. 2017;79:559–568. doi: 10.1007/s00280-017-3253-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C., Kim S.W., Reungwetwattana T., et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437–446. doi: 10.1016/S2213-2600(19)30053-0. [DOI] [PubMed] [Google Scholar]
- 24.Tracy C.M., Epstein A.E., Darbar D., et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z., Huang T.-Q., Nepliouev I., et al. Crizotinib inhibits hyperpolarization-activated cyclic nucleotide-gated channel 4 activity. Cardiooncology. 2017;3:1. doi: 10.1186/s40959-017-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirne F., Zhou S., Kappel C., et al. ALK inhibitor-induced bradycardia: a systematic-review and meta-analysis. Lung Cancer. 2021;161:9–17. doi: 10.1016/j.lungcan.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Bedas A., Peled N., Maimon Rabinovich N., et al. Efficacy and safety of ALK tyrosine kinase inhibitors in elderly patients with advanced ALK-positive non-small cell lung cancer: findings from the real-life cohort. Oncol Res Treat. 2019;42:275–282. doi: 10.1159/000499086. [DOI] [PubMed] [Google Scholar]
- 28.Seto T., Esaki T., Hirai F., et al. Phase I, dose-escalation study of AZD7762 alone and in combination with gemcitabine in Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol. 2013;72:619–627. doi: 10.1007/s00280-013-2234-6. [DOI] [PubMed] [Google Scholar]
- 29.Goldman J.W., Laux I., Chai F., et al. Phase 1 dose-escalation trial evaluating the combination of the selective MET (mesenchymal-epithelial transition factor) inhibitor tivantinib (ARQ 197) plus erlotinib. Cancer. 2012;118:5903–5911. doi: 10.1002/cncr.27575. [DOI] [PubMed] [Google Scholar]
- 30.Maroun J.A., Stewart D.J. Phase I study of tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide, NSC 286193) Invest New Drugs. 1990;8(suppl 1):S33–S39. doi: 10.1007/BF00171982. [DOI] [PubMed] [Google Scholar]
- 31.Schlitt A., Jordan K., Vordermark D., et al. Cardiotoxicity and oncological treatments. Dtsch Arztebl Int. 2014;111:161–168. doi: 10.3238/arztebl.2014.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw A.T., Bauer T.M., de Marinis F., et al. first-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 33.Zhou S., Cirne F., Kappel C., El-Kadi A., et al. Bradycardia associated with ALK inhibitors in the treatment of non-small cell lung cancers: a systematic review and meta-analysis. Eur Heart J. 2021;42 doi: 10.1093/eurheartj/ehab724.2848. [DOI] [PubMed] [Google Scholar]
- 34.Yang J.C., Ou S.I., De Petris L., et al. Pooled systemic efficacy and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small cell lung cancer. J Thorac Oncol. 2017;12:1552–1560. doi: 10.1016/j.jtho.2017.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veerman G.D.M., Hussaarts K., Jansman F.G.A., et al. Clinical implications of food-drug interactions with small-molecule kinase inhibitors. Lancet Oncol. 2020;21:e265–e279. doi: 10.1016/S1470-2045(20)30069-3. [DOI] [PubMed] [Google Scholar]
- 36.Lidbrink E., Chmielowska E., Otremba B., et al. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat. 2019;174:187–196. doi: 10.1007/s10549-018-5058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valentina G., Daniel J.L., Vicente V., et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 38.Kamphuis J.A.M., Linschoten M., Cramer M.J., et al. J. Early- and late anthracycline-induced cardiac dysfunction: echocardiographic characterization and response to heart failure therapy. Cardio-Oncology. 2020;6:23. doi: 10.1186/s40959-020-00079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pareek N., Cevallos J., Moliner P., et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur J Heart Fail. 2018;20:1721–1731. doi: 10.1002/ejhf.1292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.