Abstract
Coronary artery disease (CAD) is an important contributor to the cardiovascular burden in cancer survivors. This review identifies features that could help guide decisions about the benefit of screening to assess the risk or presence of subclinical CAD. Screening may be appropriate in selected survivors based on risk factors and inflammatory burden. In cancer survivors who have undergone genetic testing, polygenic risk scores and clonal hematopoiesis markers may become useful CAD risk prediction tools in the future. The type of cancer (especially breast, hematological, gastrointestinal, and genitourinary) and the nature of treatment (radiotherapy, platinum agents, fluorouracil, hormonal therapy, tyrosine kinase inhibitors, endothelial growth factor inhibitors, and immune checkpoint inhibitors) are also important in determining risk. Therapeutic implications of positive screening include lifestyle and atherosclerosis interventions, and in specific instances, revascularization may be indicated.
Key Words: calcification, coronary artery calcium, coronary artery disease, risk prediction, risk factor, prevention
Abbreviations and Acronyms: ACS, acute coronary syndrome; AYA, adolescent and young adult; CAC, coronary artery calcium; CAD, coronary artery disease; CHIP, clonal hematopoiesis of indeterminate potential; CMR, cardiac magnetic resonance; CTA, computed tomography angiography; CVD, cardiovascular disease; IGF, insulin-like growth factor; LDL, low-density lipoprotein; PCE, pooled cohort equations; PCI, percutaneous coronary intervention; PRS, polygenic risk score; ROS, reactive oxygen species; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor
Central Illustration
Highlights
-
•
CAD results in mortality and morbidity in cancer survivors. This is an area of unmet research need, as little is known about clinical risk evaluation and the best population and modality for screening.
-
•
This risk starts within the first decade of cancer therapy and is associated with the nature of the cancer, treatment, common risk factors, inflammation, and genetic predisposition.
-
•
Quantifying risk and detecting subclinical CAD might enable a means of optimizing the selection of patients for atherosclerotic prevention strategies.
Despite a reported 10 million deaths from cancer,1 there are an estimated 16.9 million cancer survivors in the United States, with the number projected to increase to 22.2 million by 2030.2 Cardiovascular disease (CVD) is the leading non-neoplastic cause of morbidity and mortality in survivors of breast cancer, Hodgkin lymphomas, and childhood cancers like leukemia, treated with radiotherapy and chemotherapy.3 CVD accounts for 27% of all deaths and 56% of noncancer deaths in cancer survivors.4 To date, much interest has focused on reduced left ventricular function and heart failure, especially as a cardiotoxic response to cancer therapy. However, coronary artery disease (CAD) is also a significant cause of mortality and morbidity in survivors.3 For example, in middle-aged women with breast cancer, the 10-year cumulative incidence of hospitalization for CAD was >3%, with 72% of hospitalizations attributed to acute coronary syndromes (ACS).5 The risk of CAD in cancer survivors may be further increased by cancer therapies such as radiation and aromatase inhibitors.6
A process of quantifying CAD risk in cancer survivors might enable a means of optimizing the selection of patients for atherosclerotic prevention strategies. It is important that this screening process is not understood to imply a means of identifying targets for coronary intervention—rather, it is a means of detecting subclinical disease. Limiting the progression of CAD could delay or prevent clinical presentations with myocardial infarction or sudden cardiac death, although it must be acknowledged that early identification of CAD in cancer survivors may not alter the cardiovascular risks, given the interplay of several risk factors. The purpose of this review is to address the knowledge gaps that pose a barrier to adopting a screening process, including understanding the risk of CAD (based on the type of cancer, cancer therapy, and risk factors), mechanistic links between cancer and CAD (including novel risk factors such as clonal hematopoiesis of indeterminate potential [CHIP]), the potential approaches to screening, and the downstream implications of screening, including management of risk factors and CAD (Central illustration).
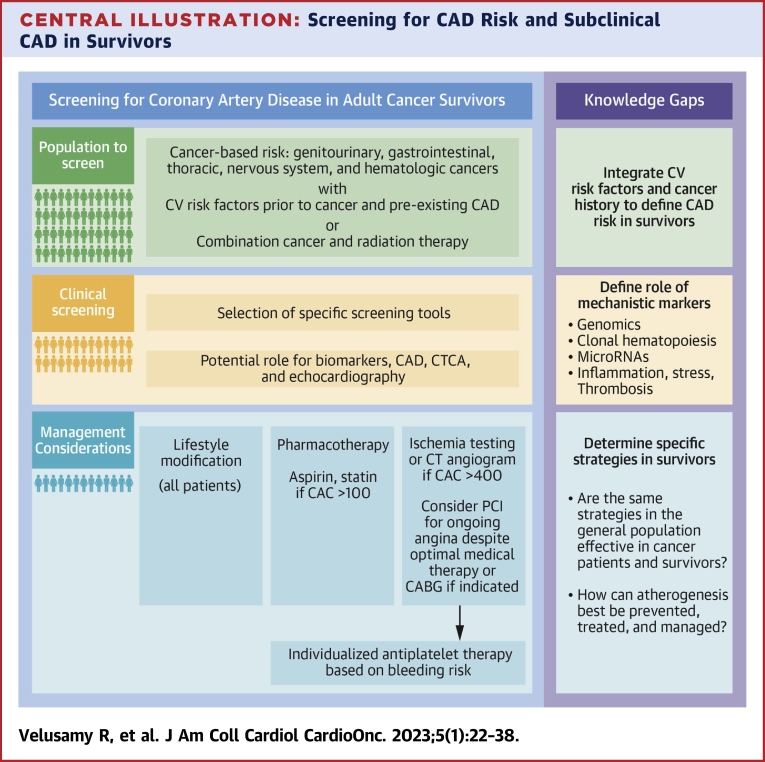
Central Illustration.
Screening for CAD Risk and Subclinical CAD in Survivors
The pillars of a program to identify and reduce risk are based on understanding what population to screen, how to proceed with screening, and the management implications. There are knowledge gaps in each of these aspects and additional research is needed to inform these gaps. CABG = coronary artery bypass grafting; CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; CTCA = computed tomography coronary angiography; CV = cardiovascular; PCI = percutaneous coronary intervention.
Understanding when to screen
A diagnosis of cancer is independently associated with a significantly increased risk for myocardial infarction.7 In cancer survivors over the years 2000 to 2015 in United States, atherosclerosis had a standard mortality ratio of 3.9 over a follow-up of 2 to 11 months and 5.9 over a follow-up of over 120 months.3 This increase in cardiovascular mortality is consistent with data from Canada and Australia.7,8
Cancer survivors have a 1.3- to 3.6-fold increase in developing CAD and a 1.7- to 18.5-fold increase in developing atherosclerotic risk factors compared with the general population.9 The prevalence of pre-existing CAD in cancer survivors ranges from 5.7% (breast) to 20.8% (lung) in the United States.10 However, there are knowledge gaps in understanding the absolute CAD risk for each specific cancer.
The timing of CAD onset is controversial. In patients with newly diagnosed cancer, the 6-month cumulative incidence of myocardial infarction was twice that of matched control patients.11 Although the first decade of survival is reported to have the highest incidence of CAD (44%), falling to 33% between 10 and 19 years, and 23% in those who survive >20 years,12 this is contrary to the usual exponential increment of CAD with age. These findings may reflect several confounders, including ascertainment bias, competing risks, and the impact of the physiologic stresses of cancer treatment unmasking occult coronary disease. Nonetheless, while a life-course survivorship approach to CAD is appropriate, these epidemiologic findings inform the timing of a potential screening strategy; the increase in CAD risk starts within the first decade of cancer therapy. While the increment in CAD mortality starts in the first decade,3 it continues to increase in the second decade of treatment.13
There has been an increase in survival among adolescents and young adults (AYAs) with cancer, causing CVD to become a leading cause of noncancer morbidity and mortality in the long term.14,15 Hodgkin lymphoma is among the most common cancers in AYAs, with an estimated incidence of 3 per 100,000 persons, and a reported 10-year survival rate of >80%.16 The incidence of CAD is close to 20% in AYA survivors who received radiotherapy at 20 years of age, with an estimated cumulative incidence of about 60% 40 years postradiotherapy.16 Childhood Hodgkin lymphoma cancer survivors have an increased risk of developing myocardial infarction when compared with their siblings.15 A 41-fold increase in cardiac deaths was also reported in this population when compared with an age-matched general population. These deaths are observed with mediastinal radiation >42 Gy, indicating that dosage should be a consideration in surveillance. However, the risk of death due to CAD continues to be increased >20 years after therapy, suggesting that the surveillance process might need to be repeated after the first decade of survival in this group. Although most of this risk is caused by cancer therapies, these survivors may also show disproportionately increased risk of traditional, modifiable cardiovascular risk factors. For example, a polygenic risk score (PRS) for blood pressure shows a significantly increased risk for hypertension in long-term survivors of childhood cancer.17
Understanding who to screen
The increased risk of CAD in patients with cancer is attributable to a combination of common mechanisms, shared risk factors, and the impact of cancer therapy. All of these provide insight as to who should best undergo screening.
Mechanisms of the association of cancer and CAD
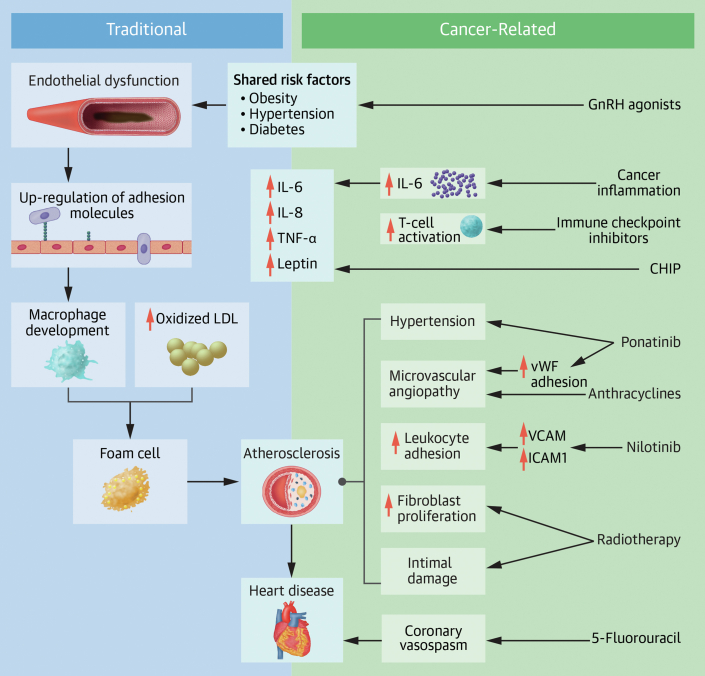
Understanding the mechanisms of CAD in cancer may provide insight into how best to prevent it. Cancer survivors with other cardiovascular risk factors are most prone to CAD,18 with dyslipidemia, tobacco smoking, and obesity documented as key risk factors.19 Most of these risk factors are modifiable by lifestyle changes. In addition to these traditional risk factors, there are a range of novel factors that are particularly related to cancer and its treatment (Figure 1), including drivers of inflammation and coagulation, which also contribute to the development of CAD.20 Emerging research has identified CHIP as a novel driver for hematological malignancy and CVD.21 The association of cancer and CAD is further impacted by the mechanism of atherogenesis in cancer as well as the effect on atherogenesis from cancer therapies.
Figure 1.
Contribution of Traditional and Cancer-Related Risks to Coronary Artery Disease
Cancer potentiates the development of atherosclerosis through factors that contribute to inflammation as well as aspects that potentiate traditional risk factors. CHIP = clonal hematopoiesis of indeterminate potential; GnRH = gonadotropin-releasing hormone; ICAM1 = intercellular adhesion molecule 1; IL = interleukin; LDL = low-density lipoprotein; TNF-α = tumor necrosis factor α; VCAM1 = vascular cell adhesion molecule 1; vWF = von Willebrand factor.
Association of cancer with atherogenic pathways
Atherosclerosis is a lipid-mediated inflammatory disease, and cancer growth is associated with similar pathways.22 The increased risk of atherosclerosis in cancer is due to several mechanisms—mainly inflammation, the prothrombotic state, and oxidative stress. Endothelial dysfunction and injury are important preludes to atherosclerosis.22
Chronic and dysregulated inflammation eventually leads to DNA damage, unregulated cellular proliferation, and tumorigenesis.23 This link is exemplified by the association of ulcerative colitis, pancreatitis, and chronic gastritis with colon,24 pancreatic,25 and gastric cancers,26 respectively. Chronic inflammation is associated with production of reactive oxygen species (ROS) and oxidative stress, which is linked to endothelial dysfunction, inflammation, plaque formation, and smooth muscle growth.27 Atherosclerotic plaque development is further influenced by clonal proliferation of endothelial, smooth muscle cells and macrophages.22 In addition, plaque development is also mediated through angiogenesis, which is the hallmark of cancer, being vital for growth of cancerous cells and metastasis.28 Atherosclerosis is also associated with apoptosis, which drives proliferation and metastasis of cancer cells.29 Apoptosis results in morphological and molecular changes that interfere with plaque stability.30
Cancer and risk factors
In addition to the association of cancer and CAD through chronic inflammatory diseases,20,31 and common risk factors,32 survivors are at an increased risk of developing cardiovascular risk factors (eg, metabolic syndrome) that can further promote CAD.33 Table 1 summarizes the association of cancers with various cardiovascular risk factors.18,19,34, 35, 36 The mechanistic associations summarized in the following section may inform new approaches to CAD diagnosis or prevention in survivors.
Table 1.
| Risk Factor | Common Associated Malignancies |
|---|---|
| Obesity | Esophageal adenocarcinoma, pancreatic, liver, colorectal, breast, endometrial, and kidney |
| Smoking | Lung, larynx, pharynx, upper digestive tract, oral cavity, stomach, and pancreatic |
| Diabetes mellitus | Colorectal, breast, endometrial, hepatic, pancreatic, and bladder |
| Hypertension | Renal cancer (men), breast, and endometrial |
| Alcohol | Oropharyngeal, laryngeal, esophageal, liver, colorectal, and breast |
| Hyperlipidemia | Breast |
| Diet | Colorectal |
| Physical activity | Colorectal, breast, and endometrial |
Obesity is associated with 13 types of cancer,37 and obesity-associated cancers are common (>650,000 occur in the United States each year).38 About 80% of people with CAD have a body mass index >30 kg/m2,39 mediated in part by the association with high levels of circulating low-density lipoprotein (LDL). Obese people also have increased amounts of inflammatory cytokines and hormones produced within the adipose tissue.40 These factors promote angiogenesis, and are key factors in tumorigenesis and tumor spread. For example, interleukin-6 is an inflammatory marker, and its expression is increased in people with obesity compared with lean individuals. Interleukin-6 has been demonstrated in the microenvironment of several cancers such as breast cancer, and is being targeted as potential cancer therapy due to its role in the development, progression, and metastasis of cancers.
In addition to introducing carcinogens,41 smoking reduces the availability of vascular nitric oxide, which is the mediator of endothelial-mediated vasodilation, leading to endothelial dysfunction. This influences the adhesion of monocytes and T lymphocytes to the endothelium and potentiates platelet activation.41 Smoking is also an inflammatory stimulus, evidenced by promoting circulating inflammatory markers such as C-reactive protein, interleukin-6, and tumor necrosis factor. Both inflammatory and vasoactive effects may be measured and targeted.
The risk of cancer appears to be increased in both type 1 and type 2 diabetes mellitus,42 with 8% to 18% of cancer survivors having diabetes mellitus.43 In patients with type 1 diabetes mellitus, the incidence of gastrointestinal, lung, and hematological malignancy is significantly increased for both genders,44,45 whereas the incidence of non-Hodgkin lymphoma and colon cancer is significantly increased for men44 and the incidence of the genitourinary and gastrointestinal cancer is significantly increased for women.44,46,47 With regard to type 2 diabetes mellitus, studies have shown associations between type 2 diabetes mellitus and risks of gastrointestinal, lung, genitourinary, and breast cancer.48
The mechanism underlying the increased prevalence of diabetes mellitus in patients with previous cancer is attributable to common risk factors, inflammation, hyperinsulinemia, and cancer therapies. In addition, the associations of these entities with cancer may be mediated in part through hormonal factors such as insulin and insulin-like growth factor (IGF)-1.49 Insulin binds to insulin receptors A and B, promoting a mitogenic effect that may lead to cancer if the resultant cellular proliferation is not regulated. It also promotes production of IGF-1 by the liver, which further promotes cellular proliferation.49 Hyperglycemia causes nonenzymatic glycosylation of proteins and lipids, which interferes with their molecular structure, hence affecting both their enzymatic activity and their ability to be degraded.49 The interaction between glycosylated proteins and their receptors leads to increased oxidative stress and inflammatory markers, which drive the process of atherosclerosis.
Hypertension is linked to the development of certain cancers—exemplified by a 3-fold increment of risk of renal cancer in African American patients50—as well as cancer-related deaths.51 Angiotensin II stimulates plasma vascular endothelial growth factor, and this may be the link between hypertension and cancer.52 Vascular endothelial growth factor (VEGF) is central to the pathogenesis of tumor growth by stimulating blood vessel formation and is increased in hypertensive subjects.53 Factors for development of hypertension on VEGF inhibitors are largely undefined,54 with neither dose nor treatment duration being shown to influence CAD risk.55 Further research is required to understand this mechanism as well as if treatment of hypertension reduced CAD risk in cancer survivors treated with VEGF inhibitors. In addition, the association of hypertension with CAD is also thought to occur through intimal injury,56 endothelial dysfunction, and plaque instability.56,57
Hyperlipidemia has been cited as a convincing risk factor for breast cancer,58 an association that could be due to the cholesterol metabolite, 27-hydroxycholesterol, being similar in both structure and action to estradiol.59 There is an inverse association between LDL and the risk of other cancers. This could be due to changes in cancer itself, which causes changes in cholesterol metabolism or absorption, as well as cancer cells often expressing receptors that attract cholesterol metabolites necessary to support their growth.60 Hyperlipidemia is the most important risk factor for atherosclerosis, with multiple genes contributing to the etiology of hyperlipidemia and atherosclerosis.61
Cancer diagnoses steadily increase with increasing age, with more than half of the individuals being diagnosed after 65 years of age.62 The mechanism in both aging and cancer is related to time-dependent accumulation of cellular damage. Aging affects vital biological processes in the human body that cause the deterioration of proteins and DNA, leading the involved cells to enter a state of senescence in which they stop growing and dividing. However, senescence mechanisms fail at times, leading to the production of uncontrollable cell growth, similar to pathways that lead to the formation and spread of cancer. Similarly, advanced age predisposes both men and women to high prevalence of CAD. The association of age with inflammation, increased vascular stiffness, endothelial dysfunction, and thrombogenicity20,57 compounds pathways involving oxidative stress, lipid metabolism, genomic stability, and endothelial homeostasis to contribute to vascular aging.57,63
Alcohol use is associated with carcinogenic effects through production of acetaldehyde, which interferes with DNA repair64 and prevents normal cellular turnover. Alcohol also serves as a solvent that makes it easier for other carcinogens to be absorbed.64 Alcohol is associated with increments in circulating estrogen, which may mediate the development of breast cancer in women.65 In addition, it has been reported that CAD follows a dose response with a nonlinear association between alcohol consumption and risk of CAD.66 One possible explanation for this nonlinear association is that consumption of large amounts of alcohol may increase blood pressure and LDL cholesterol, which are risk factors for CAD.66
The role of diet is well established as a risk factor for cancer—with contributors ranging from consumption of carcinogens in food to lifestyle choices impacting obesity, hypertension, and hyperlipidemia that accelerate cancer risk.18 In people with genetic mutations in the folate metabolism pathway, insufficient folate intake increases the risk of both CVD and colorectal cancer.67 The intake of meat has been associated with several cancers, including colorectal cancer.68,69 On the other hand, a diet rich polyphenols, found in fruits, vegetables, and certain plants has been shown to reduce both CVD and cancer by affecting several metabolic pathways, ROS, and IGF-1.70 The same factors are pertinent to CAD risk.
The role of exercise is well established in improving established risk factors such as obesity, hypertension, hyperlipidemia, and diabetes mellitus. Physical activity is shown to reduce risk of colorectal, postmenopausal breast, and endometrial cancers.18,35,36 A randomized controlled study on early breast cancer survivors with elevated body mass index showed that physical activity reduced the 10-year risk of cardiovascular sequelae.71
Standard and novel risk factors are drivers of cancer and CAD. In the heightened risk factor setting of survivorship, these risk factors remain relevant to screening.
Clonal hematopoiesis and atherogenesis
Clonal hematopoiesis, which has been long been associated with hematological malignancies, has now been shown to contribute to CAD by accelerating atherosclerosis.72 Clonal hematopoiesis involves clonal expansion from a single hematopoietic stem arising from somatic mutations that result in a survival and proliferative advantage.73 Somatic mutations are almost ubiquitous in healthy people over 50 years of age, but the clonal outgrowth is extremely low.74 When this mutated clone’s contribution to the peripheral leukocyte pool (known as the variant allele fraction), exceeds 2%, the phenomenon is characterized as CHIP.21 Patients with clonal hematopoiesis have a 1.9 times increment of incident coronary heart disease when compared with control subjects, and patients <50 years of age with clonal hematopoiesis mutations have a 4-fold increment of premature events.72 This is supported by a recent study showing that in patients treated with intensive chemotherapy for acute myeloid leukemia, mutations in CHIP-related genes were associated with the risk of developing cardiovascular events including CAD.21 A higher proportion of clonal burden (>10%) also confers a higher risk of incident coronary heart disease. The increment of risk of CAD associated with CHIP is independent of traditional risk factors.75
The mechanisms linking clonal hematopoiesis and CAD are still being understood. However, a hallmark of CHIP appears to be inflammation, evidenced by increases in levels of various interleukins.72,76,77 Through the use of the UK Biobank it was discovered that individuals with DNMT3A and TET2 CHIP had a lower incidence of CVD if these individuals also had a loss of function mutation in their interleukin-6 receptor.78 In the context of this review, clonal hematopoiesis is also observed in individuals with breast cancer, and these mutated leukocytes have been shown to infiltrate the tumor.79 Whether these mutated leukocytes could also contribute to CAD post-therapy is an interesting question to explore.
Genetics
The presence of a family history can potentially aid in the prediction of CAD risk, as well as guiding clinical management. Part of this association may be due to common environmental risks (such as diet), but much is genetic. The hereditability of death from CAD is 0.57 in men and 0.38 in women, meaning that 38% to 57% of the variance is due to genetic causes.80 Thus, genomic information may have an important role in predicting CAD risk among cancer survivors when combined with other risk factors. PRSs aggregate the effects of many genetic variants across the human genome into a single score and have recently been shown to have predictive value for multiple common diseases including CAD.81 Unlike nongenetic risk factors, these alleles can be detected long before disease presentation,82 and PRSs can be used to identify individuals at risk of CAD and cancer.83,84A PRS for CAD has been used to successfully risk-stratify breast cancer survivors—for example, a retrospective study using a CAD-specific PRS identified breast cancer survivors to have an estimated 33% higher CAD risk independent of established cardiovascular risk factors.83 This potentially suggests an association of PRS and CAD operating through different mechanisms that do not overlap with traditional risk factors. In addition, the assessment of risk with PRS may enable interventions such as statin therapy and lifestyle modification to commence even prior to the onset of CAD. More research is required to make PRSs informative in cancer survivor populations.
CAD associated with cancer therapy
Treatment modalities such as chemotherapy, radiotherapy, and immunotherapy are associated with incident CAD among current cancer patients and survivors. These therapies cause endothelial damage and promote inflammation, which accelerate the process of atherosclerosis. CAD is particularly associated with radiotherapy, compared with other treatment modalities such as chemotherapy and targeted therapies.
Cancer Therapy
Many chemotherapeutic agents affect the endothelium, leading to dysfunction and cell death, and this contributes to the 8% increment in absolute risk of developing CAD over 20 years post-treatment.85,86 The mechanisms of vascular injury from commonly used chemotherapy and the association of chemotherapy-induced endothelial damage with cardiovascular complications are summarized in Table 2.57,87, 88, 89, 90, 91, 92, 93, 94, 95
Table 2.
Association of Chemotherapy-Induced Endothelial Damage With Cardiovascular Complications
| Chemotherapeutics | Cancer87, 88, 89, 90, 91 | Mechanism55,88, 89, 90, 91, 92, 93, 94 |
|---|---|---|
| Anthracyclines (eg, doxorubicin) | Lung, Hodgkin lymphoma, and breast87 | Production of ROS, which promotes breakdown of DNA strands leading to inhibition of DNA synthesis. This cascade causes cell apoptosis, which contributes to accelerated atherosclerosis. |
| Plant alkaloids (eg, paclitaxel and docetaxel) | Breast, colorectal, cervical, endometrial, head and neck, and lung88 | Inhibition of cell proliferation leading to abnormal microtubules. As the tubulin cytoskeleton maintains the integrity of the endothelial layer, plant alkaloids increase the permeability of blood vessels. Endothelial damage promotes the transmigration of LDL and leukocytes into the subintimal space initiating atheroma formation. |
| Alkylating agents (eg, cisplatin) | Testicular, ovarian, bladder and head and neck, gastrointestinal adenocarcinomas, breast, and head and neck tumors | Attachment to DNA, which disrupts DNA repair and eventually leads to apoptosis. Endothelial damage through direct action and production of ROS, which increases oxidative stress and thrombosis via platelet aggregation. Direct cytotoxic effect on the vascular endothelial layer can lead to coronary vasospasm. This class is also associated with elevated lipid profile. |
| Biological therapies/Immunotherapy) (eg, tyrosine kinase inhibitor, immune checkpoint inhibitors) | Colorectal, cervical, lung, and renal | Inhibition of angiogenesis, regression of already formed tumor blood vessels, and altering of blood supply to the tumor cells. Endothelial dysfunction and damage lead to hypertension, cerebral ischemia, and myocardial infarction and are prothrombotic. |
| Antimetabolites (eg, fluorouracil, capecitabine) | Breast, gastrointestinal, and colorectal | Interference with DNA and RNA synthesis by acting as false metabolites, which then prevent DNA synthesis by integration into the DNA strand or blockade of essential enzymes, leading to apoptosis. |
| Hormonal therapies (eg, GnRH agonist) | Breast and prostate | Effect on lipid profile and acceleration of atherosclerosis from hyperlipidemia, particularly increasing LDL and triglycerides, as well as being proinflammatory. |
DNA = deoxyribonucleic acid; GnRH = gonadotropin-releasing hormone; LDL = low-density lipoprotein; RNA = ribonucleic acid; ROS = reactive oxidative species.
Chemotherapy has been associated with anatomic progression of CAD, and influences risk factors to cause metabolic syndrome which in turn increases CAD risk. Platinum-based therapy has been associated with a significantly higher SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) score in lung cancer patients, leading to a higher risk of more severe anatomical CAD.96 The effects of chemotherapy may be ongoing—for example, cisplatin is present in circulating plasma >10 years post-treatment in survivors of testicular cancer.97 Endothelial dysfunction, coupled with the prothrombotic and proinflammatory effects of platinum-based cancer agents,98 may cause continuous stimulation of endothelium, thereby further increasing the risk of atherosclerosis. A significant increase in risk of CAD with an HR of 2.6 was reported in testicular cancer survivors who were treated with cisplatin compared with survivors who had surgery alone.99 In the acute setting, cisplatin may induce acute coronary thrombosis within a week post-treatment.37
Monoclonal antibodies such as VEGF also affect vascular function and thrombosis, increasing the risk of atherosclerosis.100 In addition, VEGF inhibitors have been linked to reduction in coronary reserve with microvascular impairment, takotsubo cardiomyopathy, and coronary vasospasm.101 For example, cancer survivors treated with bevacizumab have a 2.5-fold increased risk of CAD compared with control subjects.102
In addition to cellular dysfunction, fluorouracil agents (including the oral prodrug capecitabine that is commonly used in breast and colorectal cancers) are associated with coronary vasospasm and thrombus formation.103 Fluorouracil contributes to the increase in risk of radiation-induced thrombosis when used concurrently with radiotherapy, effectively acting as a radiosensitizer.104 Myocardial ischemia is reported to range from 1% to 68% of patients treated with fluorouracil (with this variation being attributable to patient characteristics as well as to the presence or absence of caused by sensitizing factors), and 9% with capecitabine.105 The risk of myocardial infarction is increased with fluorouracil and capecitabine, and caution needs to be exercised in cancer survivors with angina.
Tyrosine kinase inhibitors (TKIs) inhibit angiogenesis and mitochondrial function,106 which subsequently causes decreased levels of nitric oxide and increased levels of ROS leading to increased risk of atherosclerosis.107 Ischemic events have been observed in 11 of 38 TKI therapies with no observable dose dependence.108
Hormonal therapies may to affect lipid profile and accelerate atherosclerosis from hyperlipidemia. Gonadotropin-releasing hormone agonists cause hyperlipidemia, particularly increasing LDL and triglycerides, as well as being proinflammatory.95 An observational study on 73,196 men with prostate cancer treated with gonadotropin-releasing hormone agonists showed that 25.3% of men without prior CAD developed CAD.109 On the other hand, aromatase inhibitors have been shown to have varying effects on lipid profile.110 However, hypercholesterolemia is more common in cancer survivors treated with aromatase inhibitors compared with non–aromatase inhibitor therapy, as well as in survivors who have had longer treatment duration of aromatase inhibitor use.111 It has also been shown that cancer survivors treated with an aromatase inhibitor have a 19% higher risk of developing CAD compared with those on tamoxifen therapy.93
Immune checkpoint inhibitors such as programmed death molecule-1 and cytotoxic T cell–associated antigen 4 regulate initiation and proliferation of T cells, macrophages, and platelets.112 The release of inflammatory cytokines after activation of T cells leads to the formation and rupture of unstable atherosclerotic plaques.113 A meta-analysis of cancer survivors who were treated with immune checkpoint inhibitors showed an incidence of myocardial infarction of approximately 1% over a period of 12 to 18 months.114
Radiotherapy
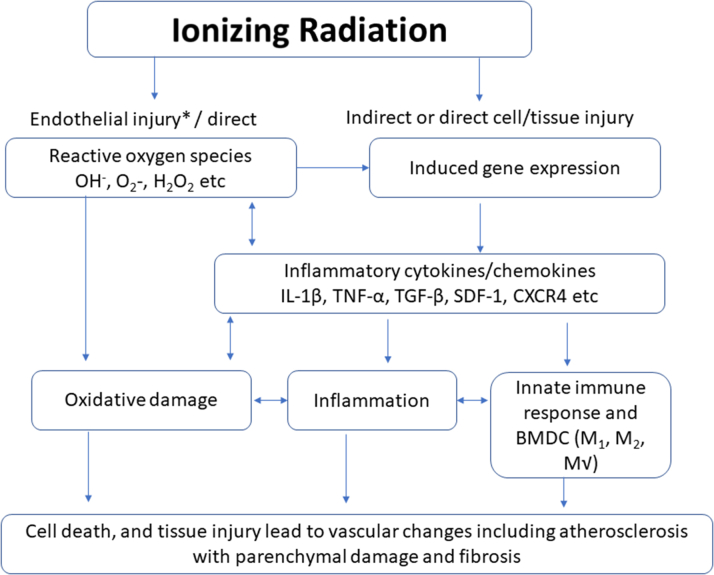
Radiotherapy causes both microvascular and macrovascular changes in coronary arteries. Mechanisms involved in the development of radiotherapy-induced CAD include endothelial damage, generation of ROS, inflammation, and apoptosis (Figure 2).56,67,115,116
Figure 2.
Mechanisms of Radiotherapy-Induced Cardiovascular Disease
The cardiovascular effects of radiotherapy include both endothelial injury and tissue injury.56,57,115,116 BMDC = bone marrow–derived dendritic cell; SDF = stromal cell–derived factor; TGF = transforming growth factor; other abbreviations as in Figure 1.
Hodgkin lymphoma and breast cancer survivors with past mediastinal radiotherapy have a 4- to-6-fold increase risk of CAD compared with nonmediastinal radiotherapy patients.16,93 The single layer of endothelium is susceptible to ionizing radiation,115 and endothelial damage leads to a cascade of inflammatory and thrombotic factors that alter vascular hemostasis, microvascular fibrosis, and myocardial ischemia.117
A study of breast cancer in the United States showed that there was an increase in heart disease mortality in women receiving radiotherapy for left-sided breast cancer in comparison with those undergoing radiotherapy for right-sided breast cancer.86 Over a period of 27.5 years of follow-up, CAD occurred in 5.9% of women who were diagnosed with breast cancer between 25 and 39 years of age and received left-sided radiation therapy, whereas CAD was not reported in women of the same age group but treated with right-sided radiation therapy.118 In women diagnosed with breast cancer between 40 and 54 years of age, CAD was reported in 18.7% in left-sided radiation therapy compared with 6.8% in right-sided radiation therapy.118 One should also note that improvements in radiation technology and delivery have led to significant decreases in radiation doses received by breast cancer patients, leading to an approximately 20% to 70% decrease in CAD.119
Microvascular injury due to radiotherapy is dose dependent,120 and radiation doses >30 Gy are proven to contribute to cardiovascular injury.121 Interestingly, plaque formation secondary to radiation does not completely mimic spontaneous atherosclerosis, as radiation-associated plaque is associated with lower lipid content and intimal hyperplasia,122 and the resulting stenoses are long, smooth, and fibrotic.122 In addition to radiotherapy, prior CAD in cancer survivors was the strongest predictor of coronary events post cancer treatment.123
As seen with cisplatin, there appears to be a temporal delay to the development of CAD after radiotherapy. After a median follow-up of 9 years, 10% of 415 Hodgkin lymphoma patients treated with radiotherapy developed significant CAD.124 Independent of chemotherapy, radiotherapy has been shown to contribute to significantly higher SYNTAX scores in patients with CAD.125
With recent advancements in radiotherapy, it has been noted that improved fractionation and deep inspiration breath-hold show a dose reduction in cardiotoxicity with left-sided breast cancer irradiation.126 Although a reduction in cardiotoxicity has been shown, this has not yet been matched by a reduction in cardiac mortality.126
Screening for atherosclerotic risk and subclinical CAD
Population to screen
Cancer survivors are at increased risk for CAD, but the risk varies by cancer site. Survivors of genitourinary, gastrointestinal, lung, nervous system, and hematologic malignancies are at high risk of CAD.7 Survivors with cardiovascular risk factors prior to cancer therapy initiation, pre-existing CAD, and a combination of both chemotherapy and radiotherapy are also at increased risk, even if they are not at higher risk based on their cancer site.
For screening to be warranted, several criteria must be fulfilled (Table 3). Early detection of plaque formation and initiation of therapies that modify the risk factors for CAD and prevent thrombosis should have the potential to prevent or delay the progression to myocardial infarction. Although screening cancer survivors for CAD is not listed in current guidelines, the topic of CAD risk stratification of cancer survivors is mentioned in consensus statements on cardio-oncology127 and appropriate use.128 The potential role of screening for CAD is only explicit in expert panel guidelines and recommendations and is not explicit in clinical trials. In terms of the management of patients previously exposed to radiotherapy, in addition to annual clinical review (with investigations targeted to symptom status), echocardiography and functional testing are proposed every 5 years in asymptomatic survivors.129
Table 3.
Criteria Established for Screening and Its Application to CAD Assessment in Survivors
| Principle | Application to Survivors177 | Pros and Cons |
|---|---|---|
| Important health problem | CAD is a major cause of death in survivors. | Helps early detection of CAD but can induce anxiety from screening. |
| Accepted treatment | Treatment at the presymptomatic stage changes outcome. Treatment of the undiagnosed developed condition (eg, PCI) may not change outcome. |
Potential reduction in risk of CAD progression and reduces need for coronary angiogram. |
| Accessibility | Imaging and biomarker diagnosis and medical therapy are both readily available. | Minimal pain from blood test, which is routine in care of patients with chronic disease. Radiation risks with coronary CTA, which are minimized by using optimized protocols and potential benefit from screening. |
| Recognizable latent stage | CAC identifies coronary atheroma. | Potential reduction in risk of CAD progression by treatment initiation and reduces need for coronary angiogram. |
| Suitable test | CAC is easy, quick, and sensitive. | Suitable for claustrophobic patients with coronary CTA usually completed in <10 min. |
| Acceptable | CAC provides a low-radiation test, acceptable in the context of CVD risk in survivors. | Radiation risks with coronary CTA, which are minimized by using optimized protocols and potential benefit from screening. |
| Natural history understood | Clear prognostic implications of CAC >0 and CAC >100. Untreated patients develop progressive disease. | CAC score is recognized to represent atherosclerotic burden and confer cardiovascular risk in asymptomatic patients. |
| Agreed policy on who to treat | Adequate control of LDL-C can halt or reverse atherosclerosis, and there are accepted guidelines for starting statins (CAC >100). | Good treatment targets are well established to reduce progression of CAD. |
| Economic justification of case finding | Cost-effectiveness studies needed to show that improvement of health (by avoiding CAD endpoints) justifies the costs. | Reduces progression of CAD and decrease burden on health care overall. |
| Continuing process of case finding | Undefined whether single screening is sufficient. | Need further research for nonradiotherapy patients given that the current guidelines recommend echocardiography for radiotherapy patients at regular intervals. |
CAC = coronary artery calcium; CAD = coronary artery disease.; CTA = computed tomography angiography; CVD = cardiovascular disease; LDL-C = low-density lipoprotein cholesterol.
Clinical risk evaluation
Atypical presentations seem to be more common in cancer patients presenting with ACS than in the general population, with the most common symptoms being dyspnea followed by chest pain.130
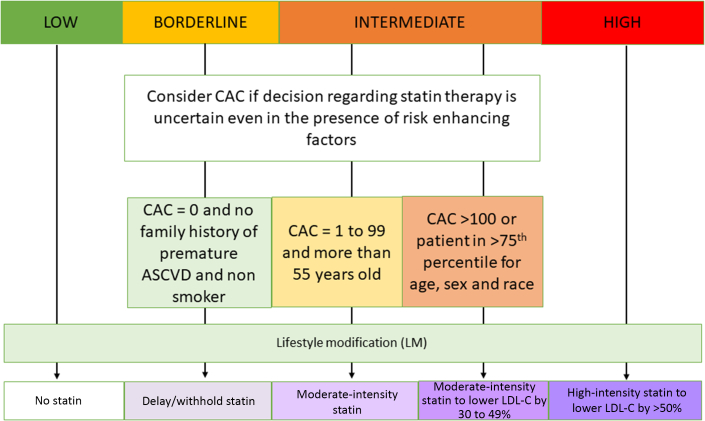
In the general population a variety of risk models are available, using age, sex, race, lipid profile, systolic blood pressure, smoking history, diabetes mellitus, family history, and social determinants.131 Noncancer patients with a 10-year risk of >20% warrant treatment for atherosclerosis without further testing, but the decision to treat is difficult in those at intermediate risk (7.5%-20%) and generally unjustified in those at borderline risk (5%-7.5%).132 Specifically, calcium scoring is of most value to reclassify intermediate risk to either low risk, based on coronary artery calcium (CAC) = 0 (signifying that lifestyle modification is sufficient) or high risk, based on CAC >0 (signifying that subclinical CAD is present and statin therapy is warranted).133,134 Given that the existing primary prevention risk models do not include cancer or cancer therapies (and therefore may underestimate risk in survivors), CAC may be warranted to support clinical decisions in some low-risk patients, but until the value of this is proven, screening models for the general population should be used (Figure 3).
Figure 3.
Treatment Threshold for Atherosclerosis Based on Cardiovascular Disease Risks
The primary determinant of treatment decisions is conventional risk assessment based on noncancer survivors, which may underestimate risk in survivors. For this reason, coronary artery calcium (CAC) scoring may potentially be of even greater value than normal in reclassifying risk and consideration of statin therapy in low- to moderate-risk groups.133,134 ASCVD = atherosclerotic cardiovascular disease; LDL-C = low-density lipoprotein cholesterol.
The European Society of Cardiology in 2022 recommended assessing patients for traditional risk factors prior to commencement of cancer therapy. Despite not including chemotherapy and radiation among cardiovascular risks,135 traditional risk stratification tools such as the Framingham risk score have been found suitable in male cancer patients scheduled to receive hormonal therapy alone.136 Other risk scores to estimate total 5- and 10-year risk of CVD have been developed for breast cancer patients but require external validation.5 Clinical risk scores such as the pooled cohort equations (PCE) can be used to determine the likelihood of an individual dying from a coronary artery event, developing a nonfatal myocardial infarction, or developing a fatal or nonfatal stroke in noncancer populations.132 Using the PCE, a statistically significant reduction was seen in the median 10-year CVD risk in breast cancer survivors who had high CVD risk score following optimization of these cardiovascular risk factors.137 A predictive model of CVD risks from the PCE has also been studied in long-term colorectal cancer survivors and found to have satisfactory accuracy in the prediction of CVD risks.138
In survivors of childhood cancer, the Childhood Cancer Survivor Study risk calculator can be used to screen for CAD. It includes the type of cancer therapy including dosing in addition to the traditional cardiovascular risk factors.139 However, the Childhood Cancer Survivor Study has only been tested in childhood cancer survivors until 50 years of age.
CAC score
Atherosclerosis is an inflammatory process, and CAC is considered a reliable marker of atherosclerosis.140 Several prospective longitudinal studies in the general population have shown the CAC score to represent atherosclerotic burden and confer cardiovascular risk in asymptomatic patients. Computed tomography is a low-risk, noninvasive, and affordable screening tool for many cancers, and coronary artery calcification can be identified on scans done for other reasons, with similar implications to scans done specifically to identify CAC.141 CAC is used to facilitate decision making in those at intermediate risk,142 and patients with a nonzero score >100 should be considered for statin therapy with lifestyle modification, and further coronary evaluation is used in people with severe calcification (>400).133 Subsequent coronary computed tomography angiography (CTA) in symptomatic patients can both identify stenoses and characterize plaque composition.143 A recent guideline has advocated for a greater role of coronary computed tomography in the identification of the CAD burden in survivors.144
Experience with the CAC score to screen for CAD in cancer survivors irrespective of clinical risk status is limited and has only been demonstrated in single-center cohort studies.145 These studies have demonstrated pretreatment CAC being associated with increased cumulative incidence of acute coronary events among breast cancer survivors treated with adjuvant radiotherapy. As a prospective study using CAC has demonstrated CAD in 46% of asymptomatic breast cancer survivors,146 it is possible that decisions informed by CAC may mitigate CAD risk after cancer treatment, but this remains unproven. Similar to the general population, a CAC score of zero indicates a low risk of CAD-related events,128 with the absence of calcification being associated with a favorable 5-year outcome, with patient deaths during this period attributed to malignancy.147 Despite the benefit in using CAC in screening cancer survivors who have received either chemotherapy or radiotherapy, the timing at which this should be undertaken remains unclear.148 It has been recommended that cancer survivors who received chemotherapy with continued vascular toxicity risk (eg, nilotinib, ponatinib, and cisplatin), and/or chest radiotherapy, should be screened every 5 years with coronary CTA or noninvasive stress testing.149 However, the role of CAC has not been well established in cancer survivors, and further research is required.
Although obstructive CAD in symptomatic patients <40 years of age has a low prevalence (3%), the process of coronary atherosclerosis begins decades earlier, and plaques may be present with a CAC score of 0.150 In the absence of supporting evidence for the use of coronary CTA in asymptomatic individuals, other strategies, including PRSs, may be considered for risk assessment in asymptomatic AYA cancer survivors between the ages of 25 and 29 years, who have an increased mortality compared with a noncancer population <40 years of age.3
Other imaging modalities
The joint recommendation from American College of Cardiology, American Society of Echocardiography, and European Association of Cardiovascular Imaging is to monitor radiation-associated heart disease with screening echocardiography 10 years after treatment and then at 5-year intervals in asymptomatic cancer survivors.151 In survivors who are at high risk for radiation-induced heart disease, the recommendation is to screen with echocardiogram at 5 years post-treatment followed by noninvasive stress testing every 5 years thereafter.
If there is ongoing clinical suspicion of CAD with a negative or ambiguous coronary CTA, then other imaging modalities should be considered. Noninvasive stress testing such as stress echocardiography and cardiac magnetic resonance (CMR) are recommended due to their high specificity.129 However, the shortcoming of stress echocardiography is that it is limited to only the identification of flow-limiting coronary stenoses but not the non–flow-limiting coronary plaques, which may rupture and cause an ACS event. Stress CMR has a high sensitivity to detect CAD and quantify the degree of ischemia, and has been proposed to detect myocardial perfusion abnormalities during or after cancer treatment.152 However, global availability of CMR stress testing continues to remain limited. Nuclear stress tests such as cardiac positron emission tomography (PET) may offer accurate assessments of CAD. However, there are no current recommendations use of PET in the cardio-oncology population outside of the usual established methodologies. More research will improve our understanding of the potential use of PET in screening schemas in cardio-oncology patients.153
Biomarkers
Biomarkers can potentially be helpful in the identification and monitoring of CAD in cancer survivors, but no specific CAD markers are available. A prospective study showed that high-sensitivity troponin T is increased in cancer patients and strongly associated with all-cause mortality prior to commencement of any cancer therapy.154 Troponin I can be used as a strong predictor of poor cardiac outcome in patients who had high-dose chemotherapy.155 However, previous research has demonstrated elevated troponin in patients with cancer even prior to their cardiotoxic treatment. This can make it difficult to identify if elevated troponin is associated with advanced cancer rather than CAD.154
Markers such as glycogen phosphorylase isoenzyme BB and heart-type fatty acid binding protein have been suggested for use as risk stratification for CAD in cancer survivors, as they can be found in high concentrations 3 hours after onset of acute myocardial infarction and return to normal levels 12 to 24 hours after an event as well as its independent association with mortality.156 Inflammatory and oxidative stress markers such as C-reactive protein and interleukin have been shown to be significantly elevated during chemotherapy.32 However, these were in small-sample studies, and the elevation could be reflective of the cancer process as well. Other markers such as B-type natriuretic peptide and C-reactive protein do not contribute to CAD detection or progression.157 Further research is required to verify the predictive significance of these cardiac biomarkers in cancer survivors.
There is evolving interest in the use of microRNAs (miRNAs) as biological markers. These are short strands of RNA, usually between 18 and 25 nucleotides, which are predominantly found in the intracellular compartments but can also be found in trace amounts in the extracellular compartments.158 Specific patterns of miRNA have been associated with CAD, including the formation of arteriosclerotic plaque, as well as with ACS.159,160 For example, miR-1, mi-R-21, miR-155, and miR-214 have been associated with CAD in cancer survivors, and these differ from the markers observed in general population for CAD.161 Although miRNAs may be a promising area for early detection, assessment of severity, and prognostication, more research is needed into miRNAs as diagnostic biomarkers.
Management responses to CAD screening
The current selection of survivors for cardioprotective medications follows recommendations to commence therapy for an elevated 10-year CAD risk.162 While discussions about CAD prevention often focus on cardioprotective medication and the selective use of intervention, broader considerations need to be address multiple factors to reduce the risk of CAD in cancer survivors. These include both the individual level—including multidisciplinary management addressing behavioral (tobacco use, diet, and physical activity), biological (blood pressure, lipid profile, and diabetes), and psychosocial (depression, anxiety, acute and chronic life stressors, and lack of social support) factors as well as health systems factors (access to care, screening, diagnosis, and quality of care).163
Pharmacotherapy
Aspirin, angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers, beta-blockers, and statins are cardioprotective in all patients with CAD. However, in an analysis of >35,000 acute myocardial infarction patients, cancer survivors were often undertreated pharmacologically, and this is associated with adverse outcome.164 Long-term aspirin and beta-blocker use has been shown to reduce mortality in cancer survivors with acute myocardial infarction.165 Long-term statin use in cancer survivors is associated with a significant decrease in cancer-related mortality.166 Nonstatin therapies such as ezetimibe or PCSK9 inhibitors use have not been studied in cancer survivors.162 This could be an interesting area of research, as PCSK9 mediates several pathways including lowering LDL cholesterol and is thought to be a novel application to suppress the proliferation and invasion of tumor cells.167
Clinicians need to be mindful of drug-drug interactions between cardioprotective medications and chemotherapeutic agents and their potential clinical significance. Beta-blockers can cause additive bradycardia when combined with TKIs (eg, imatinib, pazopanib, ceritinib, crizotinib), and carvedilol may promote increase chemotherapy drug concentration when used in combination with doxorubicin, paclitaxel, vincristine, and vinblastine due to moderate inhibition of P-glycoprotein.168 Some statin levels (atorvastatin, simvastatin) can increase with TKIs and idelalisib (through inhibition of CYP3A4).168 When these agents are used jointly, rosuvastatin should be used, as it is not influenced by CYP3A4 inhibition.168
Revascularization
The emphasis of the screening process is CAD risk evaluation and detection of subclinical disease, rather than the identification of patients for coronary intervention. Nonetheless, a screening process is likely to identify some patients for potential revascularization. Percutaneous coronary intervention (PCI) or surgical intervention should be considered in cancer patients who are experiencing angina despite optimal medical therapy based on single-center studies. However, prior to consideration of revascularization, several factors such as patient status, status of malignancy, and severity of CAD need to be considered as cancer survivors are prone to bleeding complications. PCI seems to be the preferred invasive revascularization strategy given it is minimally invasive and cancer survivors post PCI were reported to have significantly lower in-hospital mortality rate compared with patients receiving pharmacotherapy only.169 While in patients with a life expectancy <1 year, PCI should still be considered for ACS,170 though further research is required to ascertain the benefit in survivors with ongoing angina.
Thrombocytopenia is common in cancer and is not an absolute contraindication for PCI. Heparin use intraprocedurally and antiplatelet therapy will need to be tailored in the presence of bleeding complications and thrombocytopenia. Platelet function may confer better risk stratification than platelet count, although most studies have only used platelet count.164,171,172 The decision for revascularization should be based on a multidisciplinary team discussion. Cancer survivors with indications for bypass surgery should have the severity of CAD and general health taken into consideration prior to decision making regarding suitability for surgery. Surgery should not be declined, as cancer survivors seem to share similar outcomes when compared with patients without cancer, with the exception of major bleeding.173 For example, in gastric cancer survivors with a prognosis of more than 6 months, bypass surgery can offer symptom relief.174 The use of the internal mammary artery should be avoided after chest-directed radiotherapy due to development of atherosclerotic plaques leading to vessel stenosis,175 although cancer survivors treated with radiotherapy undergoing surgery have similar long-term all-cause mortality as control subjects.176
Conclusions
Cancer survivors have an increased risk of developing CAD compared with the general population. In part, this reflects the common risk factors of CAD and cancer, which also share some novel risk factors such as CHIP. This burden of both mortality and morbidity is increasing as cancer survivorship increases.
Radiotherapy is the most common treatment-related cause of CAD. Survivors of genitourinary, gastrointestinal, lung, nervous system, and hematologic malignancies are at increased risk of CAD. Effective strategies are required to mitigate atherosclerotic CVD in survivors. While the standard control of risk factors is important, the available risk stratification tools have limitations in the cancer population. This is due to exclusion of cancer survivors in clinical trials, and even observational trials of CAD have not collected detailed cancer-related information. A formal CAD screening strategy in survivors would need to be informed by type of cancer and cancer therapy.
In the absence of a formalized guideline, cancer survivors should undertake modification of behavioral, biological, and psychosocial risk factors just as the general population does. More research is required to understand to what extent—and how—CAD screening should be incorporated into cancer survivorship care.
Funding Support and Author Disclosures
Dr Marwick was supported by an Investigator grant from the National Health and Medical Research Council, Canberra, Australia (No. 2008129). Dr Thavendiranathan was supported by the Canadian Institutes of Health Research New Investigator Award (No. 147814), the Ontario Early Research Award, and a Canada Research Chair in Cardio-Oncology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Cancer statistics. https://www.cancer.gov/about-cancer/understanding/statistics
- 3.Sturgeon K.M., Deng L., Bluethmann S.M., et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koczwara B., Meng R., Miller M.D., et al. Late mortality in people with cancer. Med J Aust. 2021;214:318–323. doi: 10.5694/mja2.50879. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Qadir H., Thavendiranathan P., Austin P.C., et al. The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer. J Natl Cancer Inst. 2019;111:854–862. doi: 10.1093/jnci/djy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Qadir H., Austin P.C., Lee D.S., et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2:88. doi: 10.1001/jamacardio.2016.3841. [DOI] [PubMed] [Google Scholar]
- 7.Paterson D.I., Wiebe N., Cheung W.Y., et al. Incident cardiovascular disease among adults with cancer. J Am Coll Cardiol CardioOnc. 2022;4:85–94. doi: 10.1016/j.jaccao.2022.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Y., Otahal P., Marwick T.H., Wills K.E., Neil A.L., Venn A.J. Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015. Cancer. 2019;125:442–452. doi: 10.1002/cncr.31806. [DOI] [PubMed] [Google Scholar]
- 9.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Kindi S.G., Oliveira G.H. Prevalence of preexisting cardiovascular disease in patients with different types of cancer. Mayo Clin Proc. 2016;91:81–83. doi: 10.1016/j.mayocp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Navi B.B., Reiner A.S., Kamel H., et al. Risk of Arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGale P., Darby S.C., Hall P., et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y.J., Nie X.Y., Ji C.C., et al. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong G.T., Chen Y., Yasui Y., et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulrooney D.A., Yeazel M.W., Kawashima T., et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Nimwegen F.A., Schaapveld M., Cutter D.J., et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 17.Sapkota Y., Li N., Pierzynski J., et al. Contribution of polygenic risk to hypertension among long-term survivors of childhood cancer. J Am Coll Cardiol CardioOnc. 2021;3:76–84. doi: 10.1016/j.jaccao.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson C.B., Davis M.K., Law A., Sulpher J. Shared risk factors for cardiovascular disease and cancer. Can J Cardiol. 2016;32:900–907. doi: 10.1016/j.cjca.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Rupprecht S., Finn S., Hoyer D., et al. Association between systemic inflammation, carotid arteriosclerosis, and autonomic dysfunction. Transl Stroke Res. 2020;11:50–59. doi: 10.1007/s12975-019-00706-x. [DOI] [PubMed] [Google Scholar]
- 21.Calvillo-Argüelles O., Schoffel A., Capo-Chichi J.M., et al. Cardiovascular disease among patients with AML and CHIP-related mutations. J Am Coll Cardiol CardioOnc. 2022;4:38–49. doi: 10.1016/j.jaccao.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapia-Vieyra J.V., Delgado-Coello B., Mas-Oliva J. Atherosclerosis and cancer. Arch Med Res. 2017;48:12–26. doi: 10.1016/j.arcmed.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Qu X., Tang Y., Hua S. Immunological approaches towards cancer and inflammation. Front Immunol. 2018;9:563. doi: 10.3389/fimmu.2018.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantini M.C., Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease. Dig Liver Dis. 2021;53:558–565. doi: 10.1016/j.dld.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Cazacu I.M., Farkas N., Garami A., et al. Pancreatitis-associated genes and pancreatic cancer risk. Pancreas. 2018;47:1078–1086. doi: 10.1097/MPA.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu Q., Zhu J., Yu X., et al. Immune response in H. pylori-associated gastritis and gastric cancer. Gastroenterol Res Pract. 2020;2020 doi: 10.1155/2020/9342563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 28.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R.-A., Li Q.-L., Li Z.-S., et al. Apoptosis drives cancer cells proliferate and metastasize. J Cell Mol Med. 2013;17:205–211. doi: 10.1111/j.1582-4934.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan S.-Z., Ooi D.S.-Q., Shen H.-M., Heng C.-K. The atherogenic effects of serum amyloid A are potentially mediated via inflammation and apoptosis. J Atheroscler Thromb. 2014;21:854–867. doi: 10.5551/jat.22665. [DOI] [PubMed] [Google Scholar]
- 31.Hansen P.R. Chronic inflammatory diseases and atherosclerotic cardiovascular disease. Curr Pharm Des. 2018;24:281–290. doi: 10.2174/1381612824666180110102341. [DOI] [PubMed] [Google Scholar]
- 32.Han X.J., Li J.Q., Khannanova Z., Li Y. Optimal management of coronary artery disease in cancer patients. Chronic Dis Transl Med. 2019;5:221–233. doi: 10.1016/j.cdtm.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieli-Conwright C.M., Wong L., Waliany S., Mortimer J.E. Metabolic syndrome and breast cancer survivors: a follow-up analysis after completion of chemotherapy. Diabetol Metab Syndr. 2022;14:36. doi: 10.1186/s13098-022-00807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandini S., Botteri E., Iodice S., et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 35.Schmid D., Behrens G., Keimling M., Jochem C., Ricci C., Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30:397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y., Zhang D., Kang S. Physical activity and risk of breast cancer. Breast Cancer Res Treat. 2013;137:869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 37.Bhaskaran K., Douglas I., Forbes H., dos-Santos-Silva I., Leon D.A., Smeeth L. Body-mass index and risk of 22 specific cancers. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Obesity and cancer. https://www.cdc.gov/cancer/obesity/index.htm
- 39.Alkhawam H., Nguyen J., Sayanlar J., et al. Coronary artery disease in patients with body mass index ≥30 kg/m(2): a retrospective chart analysis. J Community Hosp Intern Med Perspect. 2016;6 doi: 10.3402/jchimp.v6.31483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;4:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii T., Shimada K., Nakai T., Ohbayashi C. MicroRNAs in smoking-related carcinogenesis: biomarkers, functions, and therapy. J Clin Med. 2018;7:98. doi: 10.3390/jcm7050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cignarelli A., Genchi V.A., Caruso I., et al. Diabetes and cancer. Diabetes Res Clin Pract. 2018;143:378–388. doi: 10.1016/j.diabres.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Suh S., Kim K.W. Diabetes and cancer. Diabetes Metab J. 2019;43:733–743. doi: 10.4093/dmj.2019.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carstensen B., Read S.H., Friis S., et al. Cancer incidence in persons with type 1 diabetes. Diabetologia. 2016;59:980–988. doi: 10.1007/s00125-016-3884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sona M.F., Myung S.K., Park K., Jargalsaikhan G. Type 1 diabetes mellitus and risk of cancer. Jpn J Clin Oncol. 2018;48:426–433. doi: 10.1093/jjco/hyy047. [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow A.J., Laing S.P., Qiao Z., et al. Cancer incidence and mortality in patients with insulin-treated diabetes. Br J Cancer. 2005;92:2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friberg E., Orsini N., Mantzoros C.S., Wolk A. Diabetes mellitus and risk of endometrial cancer. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 48.Tsilidis K.K., Kasimis J.C., Lopez D.S., Ntzani E.E., Ioannidis J.P. Type 2 diabetes and cancer. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 49.Kasprzak A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int J Mol Sci. 2021;22:6434. doi: 10.3390/ijms22126434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipworth L., Tarone R.E., McLaughlin J.K. Renal cell cancer among African Americans. BMC Cancer. 2011;11:133. doi: 10.1186/1471-2407-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tini G., Sarocchi M., Tocci G., et al. Arterial hypertension in cancer. Int J Cardiol. 2019;281:133–139. doi: 10.1016/j.ijcard.2019.01.082. [DOI] [PubMed] [Google Scholar]
- 52.Kang Y.S., Park Y.G., Kim B.K., et al. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J Mol Endocrinol. 2006;36:377–388. doi: 10.1677/jme.1.02033. [DOI] [PubMed] [Google Scholar]
- 53.Felmeden D. Endothelial damage and angiogenesis in hypertensive patients. Am J Hypertens. 2003;16:11–20. doi: 10.1016/s0895-7061(02)03149-7. [DOI] [PubMed] [Google Scholar]
- 54.Robinson E.S., Khankin E.V., Karumanchi S.A., Humphreys B.D. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition. Semin Nephrol. 2010;30:591–601. doi: 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khakoo A.Y., Kassiotis C.M., Tannir N., et al. Heart failure associated with sunitinib malate. Cancer. 2008;112:2500–2508. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 56.Rajendran P., Rengarajan T., Thangavel J., et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaoka-Tojo M. Vascular endothelial glycocalyx as a mechanism of vascular endothelial dysfunction and atherosclerosis. World J Cardiovasc Dis. 2020;10:731–749. [Google Scholar]
- 58.Alexopoulos C.G., Blatsios B., Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer. 1987;60:3065–3070. doi: 10.1002/1097-0142(19871215)60:12<3065::aid-cncr2820601234>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 59.Warner M., Gustafsson J.A. On estrogen, cholesterol metabolism, and breast cancer. N Engl J Med. 2014;370:572–573. doi: 10.1056/NEJMcibr1315176. [DOI] [PubMed] [Google Scholar]
- 60.Gabitova L., Gorin A., Astsaturov I. Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res. 2014;20:28–34. doi: 10.1158/1078-0432.CCR-13-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wouters K., Shiri-Sverdlov R., Van Gorp P.J., Van Bilsen M., Hofker M.H. Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice. Clin Chem Lab Med. 2005;43:470–479. doi: 10.1515/CCLM.2005.085. [DOI] [PubMed] [Google Scholar]
- 62.Van Herck Y., Feyaerts A., Alibhai S., et al. Is cancer biology different in older patients? Lancet Healthy Longev. 2021;2:e663–e677. doi: 10.1016/S2666-7568(21)00179-3. [DOI] [PubMed] [Google Scholar]
- 63.Khatana C., Saini N.K., Chakrabarti S., et al. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. oxidative medicine and cellular longevity. 2020;2020 doi: 10.1155/2020/5245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rumgay H., Shield K., Charvat H., et al. Global burden of cancer in 2020 attributable to alcohol consumption. Lancet Oncol. 2021;22:1071–1080. doi: 10.1016/S1470-2045(21)00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Nguyen N., Colditz G.A. Links between alcohol consumption and breast cancer. Womens Health (Lond) 2015;11:65–77. doi: 10.2217/whe.14.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y., Liu D.C., Wang Q.M., et al. Alcohol consumption and risk of coronary artery disease. Nutrition. 2016;32:637–644. doi: 10.1016/j.nut.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Stampfer M., Jahn J.L. Partnerships for promoting prevention. Circulation. 2013;127:1267–1269. doi: 10.1161/CIRCULATIONAHA.113.001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang T., Lee H.G. Advances in research on cis-9, trans-11 conjugated linoleic acid: a major functional conjugated linoleic acid isomer. Crit Rev Food Sci Nutr. 2015;55:720–731. doi: 10.1080/10408398.2012.674071. [DOI] [PubMed] [Google Scholar]
- 69.English D.R., Macinnis R.J., Hodge A.M., et al. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1509–1514. [PubMed] [Google Scholar]
- 70.Baena Ruiz R., Salinas Hernández P. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014;77:202–208. doi: 10.1016/j.maturitas.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Lee K., Tripathy D., Demark-Wahnefried W., et al. Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer. JAMA Oncol. 2019;5:710–714. doi: 10.1001/jamaoncol.2019.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Veiga C.B., Lawrence E.M., Murphy A.J., Herold M.J., Dragoljevic D. Myelodysplasia syndrome, clonal hematopoiesis and cardiovascular disease. Cancers (Basel) 2021;13:1968. doi: 10.3390/cancers13081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young A.L., Challen G.A., Birmann B.M., Druley T.E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7 doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Libby P., Sidlow R., Lin A.E., et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer. J Am Coll Cardiol. 2019;74:567–577. doi: 10.1016/j.jacc.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuster J.J., MacLauchlan S., Zuriaga M.A., et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fidler T.P., Xue C., Yalcinkaya M., et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592:296–301. doi: 10.1038/s41586-021-03341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bick A.G., Pirruccello J.P., Griffin G.K., et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141:124–131. doi: 10.1161/CIRCULATIONAHA.119.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Comen E.A., Bowman R.L., Selenica P., et al. Evaluating clonal hematopoiesis in tumor-infiltrating leukocytes in breast cancer and secondary hematologic malignancies. J Natl Cancer Inst. 2020;112:107–110. doi: 10.1093/jnci/djz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McPherson R., Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res. 2016;118:564–578. doi: 10.1161/CIRCRESAHA.115.306566. [DOI] [PubMed] [Google Scholar]
- 81.Lambert S.A., Abraham G., Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28:R133–R142. doi: 10.1093/hmg/ddz187. [DOI] [PubMed] [Google Scholar]
- 82.Lewis C.M., Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genom Med. 2020;12:44. doi: 10.1186/s13073-020-00742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liou L., Kaptoge S., Dennis J., et al. Genomic risk prediction of coronary artery disease in women with breast cancer: a prospective cohort study. Breast Cancer Res. 2021;23:94. doi: 10.1186/s13058-021-01465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mavaddat N., Michailidou K., Dennis J., et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104:21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baselet B., Rombouts C., Benotmane A.M., Baatout S., Aerts A. Cardiovascular diseases related to ionizing radiation. Int J Mol Med. 2016;38:1623–1641. doi: 10.3892/ijmm.2016.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity. Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 87.McLaughlin M., Florida-James G., Ross M. Breast cancer chemotherapy vascular toxicity. Vasc Biol. 2021;3:R106–R120. doi: 10.1530/VB-21-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao Y., Shang Q., Li W., et al. Antibiotics for cancer treatment. J Cancer. 2020;11:5135–5149. doi: 10.7150/jca.47470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu L., Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. doi: 10.1186/s11658-019-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fung C., Dinh P., Ardeshir-Rouhani-Fard S., et al. Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Adv Urol. 2018;2018 doi: 10.1155/2018/8671832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaulin A.M., Abashina O.E., Duplyakov D.V. Pathophysiological mechanisms of cardiotoxicity in chemotherapeutic agents. Russ Open Med J. 2020;9 [Google Scholar]
- 92.Ferrara N., Adamis A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 93.Hanchate L.P., Sharma S.R., Madyalkar S. Cisplatin induced acute myocardial infarction and dyslipidemia. J Clin Diagn Res. 2017;11:OD05–OD07. doi: 10.7860/JCDR/2017/25546.10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matthews A., Stanway S., Farmer R.E., et al. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ. 2018;363:k3845. doi: 10.1136/bmj.k3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konstantinov S.M., Berger M.R. In: Encyclopedia of Molecular Pharmacology. Offermanns S., Rosenthal W., editors. Springer; 2008. Antimetabolites; pp. 147–152. [Google Scholar]
- 96.Yang Q., Chen Y., Gao H., et al. Chemotherapy-related anatomical coronary-artery disease in lung cancer patients evaluated by coronary-angiography SYNTAX score. Arq Bras Cardiol. 2020;114:1004–1012. doi: 10.36660/abc.20190201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chovanec M., Abu Zaid M., Hanna N., El-Kouri N., Einhorn L.H., Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol. 2017;28:2670–2679. doi: 10.1093/annonc/mdx360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh S., Singh K. Atherosclerosis, ischemia, and anticancer drugs. Heart Views. 2021;22:127–133. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_45_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hjelle L.V., Gundersen P.O., Oldenburg J., et al. Long-term platinum retention after platinum-based chemotherapy in testicular cancer survivors. Anticancer Res. 2015;35:1619–1625. [PubMed] [Google Scholar]
- 100.Ferrara N., Gerber H.-P., Lecouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 101.Kappers M.H.W., Smedts F.M.M., Horn T., et al. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302. doi: 10.1161/HYPERTENSIONAHA.111.173559. [DOI] [PubMed] [Google Scholar]
- 102.Chen X.-L., Lei Y.-H., Liu C.-F., et al. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanduri J., More L.A., Godishala A., Asnani A. Fluoropyrimidine-associated cardiotoxicity. Cardiol Clin. 2019;37:399–405. doi: 10.1016/j.ccl.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Fajardo L.F., Stewart J.R. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244–257. [PubMed] [Google Scholar]
- 105.Tarantini L., Gulizia M.M., Di Lenarda A., et al. ANMCO/AIOM/AICO Consensus Document on clinical and management pathways of cardio-oncology. Eur Heart J Suppl. 2017;19:D370–D379. doi: 10.1093/eurheartj/sux019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodríguez-Hernández M.A., de la Cruz-Ojeda P., López-Grueso M.J., et al. Integrated molecular signaling involving mitochondrial dysfunction and alteration of cell metabolism induced by tyrosine kinase inhibitors in cancer. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown S.-A., Nhola L., Herrmann J. Cardiovascular toxicities of small molecule tyrosine kinase inhibitors: an opportunity for systems-based approaches. Clin Pharmacol Ther. 2017;101(1):65–80. doi: 10.1002/cpt.552. [DOI] [PubMed] [Google Scholar]
- 108.Jin Y., Xu Z., Yan H., et al. A comprehensive review of clinical cardiotoxicity incidence of FDA-approved small-molecule kinase inhibitors. Front Pharmacol. 2020;11:891. doi: 10.3389/fphar.2020.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keating N.L., O'Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 110.Bundred N.J. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005;93:S23–S27. doi: 10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amir E., Seruga B., Niraula S., Carlsson L., Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients. J Natl Cancer Inst. 2011;103:1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 112.Tomita Y., Sueta D., Kakiuchi Y., et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann Oncol. 2017;28:2893–2895. doi: 10.1093/annonc/mdx326. [DOI] [PubMed] [Google Scholar]
- 113.Foks A.C., Kuiper J. Immune checkpoint proteins. Br J Pharmacol. 2017;174:3940–3955. doi: 10.1111/bph.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang-Bo H., Qun Z., Hui-Juan L., et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer. Transl Lung Cancer Res. 2017;6:S8–S20. doi: 10.21037/tlcr.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ministrini S., Carbone F., Montecucco F. Updating concepts on atherosclerotic inflammation. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13467. [DOI] [PubMed] [Google Scholar]
- 116.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Dezorzi C. Radiation-induced coronary artery disease and its treatment: a quick review of current evidence. Cardiol Res Pract. 2018;2018 doi: 10.1155/2018/8367268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carlson L.E., Watt G.P., Tonorezos E.S., et al. Coronary artery disease in young women after radiation therapy for breast cancer. J Am Coll Cardiol CardioOnc. 2021;3:381–392. doi: 10.1016/j.jaccao.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stowe H.B., Andruska N.D., Reynoso F., Thomas M., Bergom C. Heart sparing radiotherapy techniques in breast cancer: a focus on deep inspiration breath hold. Breast Cancer Targets Ther. 2022;14:175–186. doi: 10.2147/BCTT.S282799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 121.Cutter D.J., Schaapveld M., Darby S.C., et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107:djv008. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaworski C., Mariani J.A., Wheeler G., Kaye D.M. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–2328. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 123.Martinou M., Gaya A. Cardiac complications after radical radiotherapy. Semin Oncol. 2013;40:178–185. doi: 10.1053/j.seminoncol.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 124.Hull M.C. Valvular Dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 125.Myrehaug S., Pintilie M., Yun L., et al. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood. 2010;116:2237–2240. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 126.Knöchelmann A.C., Ceylan N., Bremer M. Left-sided breast cancer irradiation with deep inspiration breath-hold: changes in heart and lung dose in two periods. Vivo. 2022;36:314–324. doi: 10.21873/invivo.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 128.Taylor A.J., Cerqueira M., Hodgson J.M., et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/ SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 129.Desai M.Y., Windecker S., Lancellotti P., et al. Prevention, diagnosis, and management of radiation-associated cardiac disease. J Am Coll Cardiol. 2019;74:905–927. doi: 10.1016/j.jacc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 130.Milazzo V., Cosentino N., Campodonico J., et al. Characteristics, management, and outcomes of acute coronary syndrome patients with cancer. J Clin Med. 2020;9:3642. doi: 10.3390/jcm9113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ko D.T., Sivaswamy A., Sud M., et al. Calibration and discrimination of the Framingham risk score and the pooled cohort equations. CMAJ. 2020;192:E442–E449. doi: 10.1503/cmaj.190848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yadlowsky S., Hayward R.A., Sussman J.B., McClelland R.L., Min Y.I., Basu S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20–29. doi: 10.7326/M17-3011. [DOI] [PubMed] [Google Scholar]
- 133.Thomas D.M., Divakaran S., Villines T.C., et al. Management of coronary artery calcium and coronary CTA findings. Curr Cardiovasc Imaging Rep. 2015;8:18. doi: 10.1007/s12410-015-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheong B.Y.C., Wilson J.M., Spann S.J., Pettigrew R.I., Preventza O.A., Muthupillai R. Coronary artery calcium scoring. J Intern Med. 2021;289:309–324. doi: 10.1111/joim.13176. [DOI] [PubMed] [Google Scholar]
- 135.Hess C.N., Roe M.T., Clare R.M., et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lyon A.R., Dent S., Stanway S., et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies. Eur J Heart Fail. 2020;22:1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prior L., Featherstone H., O’Reilly D., et al. Competing mortality risks: predicted cardiovascular disease risk versus predicted risk of breast cancer mortality in patients receiving adjuvant chemotherapy in a single Irish center. Cardiooncology. 2021;7(1):8. doi: 10.1186/s40959-021-00096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jeong S., Lee G., Choi S., et al. Estimating risk of cardiovascular disease among long-term colorectal cancer survivors. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mohammed T., Parekh T., Desai A. Cardiovascular risk management in cancer survivors: Are we doing it right? World J Clin Oncol. 2021;12:144–149. doi: 10.5306/wjco.v12.i3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McClelland R.L., Nasir K., Budoff M., Blumenthal R.S., Kronmal R.A. Arterial age as a function of coronary artery calcium. Am J Cardiol. 2009;103:59–63. doi: 10.1016/j.amjcard.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Detrano R., Guerci A.D., Carr J.J., et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 142.Malguria N., Zimmerman S., Fishman E.K. Coronary artery calcium scoring: current status and review of literature. J Comput Assist Tomogr. 2018;42:887–897. doi: 10.1097/RCT.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 143.Collet C., Conte E., Mushtaq S., et al. Reviewing imaging modalities for the assessment of plaque erosion. Atherosclerosis. 2021;318:52–59. doi: 10.1016/j.atherosclerosis.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 144.Lopez-Mattei J, Yang EH, Baldassarre LA, et al. Cardiac computed tomographic imaging in cardio-oncology. J Cardiovasc Comput Tomogr. Published online September 15, 2022. https://doi.org/10.1016/j.jcct.2022.09.022 [DOI] [PubMed]
- 145.Gal R., Van Velzen S.G.M., Hooning M.J., et al. Identification of risk of cardiovascular disease by automatic quantification of coronary artery calcifications on radiotherapy planning CT scans in patients with breast cancer. JAMA Oncol. 2021;7:1024. doi: 10.1001/jamaoncol.2021.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Puckett L.L., Saba S.G., Henry S., et al. Cardiotoxicity screening of long-term, breast cancer survivors. Cancer Med. 2021;10:5051–5061. doi: 10.1002/cam4.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Whelton S.P., Al Rifai M., Dardari Z., et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality. Eur Heart J Cardiovasc Img. 2019;20:389–395. doi: 10.1093/ehjci/jey176. [DOI] [PubMed] [Google Scholar]
- 148.Roos C.T.G., van den Bogaard V.A.B., Greuter M.J.W., et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother Oncol. 2018;126:170–176. doi: 10.1016/j.radonc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 149.Iliescu C.A., Grines C.L., Herrmann J., et al. SCAI expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2016;87:E202–E223. doi: 10.1002/ccd.26379. [DOI] [PubMed] [Google Scholar]
- 150.Mortensen M.B., Gaur S., Frimmer A., et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7:36–44. doi: 10.1001/jamacardio.2021.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lancellotti P., Nkomo V.T., Badano L.P., et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 152.Windecker S., Kolh P., Alfonso F., et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Rev Esp Cardiol (Engl Ed) 2015;68:144. doi: 10.1016/j.rec.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 153.Biersmith M.A., Tong M.S., Guha A., Simonetti O.P., Addison D. Multimodality cardiac imaging in the era of emerging cancer therapies. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pavo N., Raderer M., Hülsmann M., et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 155.Cardinale D., Colombo A., Sandri M.T., et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 156.O'Donoghue M., de Lemos J.A., Morrow D.A., et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–557. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 157.Endo H., Dohi T., Funamizu T., et al. Long-term predictive value of high-sensitivity C-reactive protein for cancer mortality in patients undergoing percutaneous coronary intervention. Circ J. 2019;83:630–636. doi: 10.1253/circj.CJ-18-0962. [DOI] [PubMed] [Google Scholar]
- 158.Zhang X., Cai H., Zhu M., Qian Y., Lin S., Li X. Circulating microRNAs as biomarkers for severe coronary artery disease. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Melak T., Baynes H.W. Circulating microRNAs as possible biomarkers for coronary artery disease: a narrative review. EJIFCC. 2019;30:179–194. [PMC free article] [PubMed] [Google Scholar]
- 160.Fang Q., Liao Y., Xu Z., Li J., Zhang X., Wang Y. The diagnostic value of circulating microRNAs as biomarkers for coronary artery disease: a meta-analysis. Anatol J Cardiol. 2020;24:290–299. doi: 10.14744/AnatolJCardiol.2020.91582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Siasos G., Bletsa E., Stampouloglou P.K., et al. MicroRNAs in cardiovascular disease. Hellenic J Cardiol. 2020;61:165–173. doi: 10.1016/j.hjc.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 162.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 163.Institute of Medicine (US) In: Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Fuster V., Kelly B.B., editors. National Academies Press; 2010. Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries. [PubMed] [Google Scholar]
- 164.Rohrmann S., Witassek F., Erne P., Rickli H., Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care. 2018;7:639–645. doi: 10.1177/2048872617729636. [DOI] [PubMed] [Google Scholar]
- 165.Sudhakar R. Response to treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35:646. doi: 10.1002/clc.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nielsen S.F., Nordestgaard B.G., Bojesen S.E. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368:576–577. doi: 10.1056/NEJMc1214827. [DOI] [PubMed] [Google Scholar]
- 167.Mahboobnia K., Pirro M., Marini E., et al. PCSK9 and cancer. Biomed Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111758. [DOI] [PubMed] [Google Scholar]
- 168.Zorn S.Z., Thohan V. Drug-drug interactions of common cardiac medications and chemotherapeutic agents. https://www.acc.org/latest-in-cardiology/articles/2018/12/21/09/52/drug-drug-interactions-of-common-cardiac-medications-and-chemotherapeutic-agents
- 169.Guddati A.K., Joy P.S., Kumar G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J Cancer Res Clin Oncol. 2016;142:471–479. doi: 10.1007/s00432-015-2056-5. [DOI] [PubMed] [Google Scholar]
- 170.Vieira R.D., Pereira A.C., Lima E.G., et al. Cancer-related deaths among different treatment options in chronic coronary artery disease. Coron Artery Dis. 2012;23:79–84. doi: 10.1097/MCA.0b013e32834f112a. [DOI] [PubMed] [Google Scholar]
- 171.Banasiak W., Zymliński R., Undas A. Optimal management of cancer patients with acute coronary syndrome. Pol Arch Intern Med. 2018;128:244–253. doi: 10.20452/pamw.4254. [DOI] [PubMed] [Google Scholar]
- 172.Aronson D., Brenner B. Arterial thrombosis and cancer. Thromb Res. 2018;164(suppl 1):S23–S28. doi: 10.1016/j.thromres.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 173.Guha A., Dey A.K., Kalra A., et al. Coronary artery bypass grafting in cancer patients: prevalence and outcomes in the United States. Mayo Clin Proc. 2020;95:1865–1876. doi: 10.1016/j.mayocp.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tsuji Y., Morimoto N., Tanaka H., et al. Surgery for gastric cancer combined with cardiac and aortic surgery. Arch Surg. 2005;140:1109–1114. doi: 10.1001/archsurg.140.11.1109. [DOI] [PubMed] [Google Scholar]
- 175.Durhan G., Erdemir A.G., Yuce Sari S., et al. Does internal mammary node irradiation for breast cancer make a significant difference to the diameter of the internal mammary artery? Correlation with computed tomography. Breast Care (Basel) 2020;15:635–641. doi: 10.1159/000508244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fender E.A., Chandrashekar P., Liang J.J., et al. Coronary artery bypass grafting in patients treated with thoracic radiation: a case–control study. Open Heart. 2018;5 doi: 10.1136/openhrt-2017-000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Principles and practice of screening for disease. J R Coll Gen Pract. 1968;16:318. [Google Scholar]