Abstract
Objective
To compare the prevalence of contraception in breast cancer (BC) patients at risk of unintentional pregnancy (i.e. not currently pregnant or trying to get pregnant) and matched controls.
Study design
The FEERIC study (Fertility, Pregnancy, Contraception after BC in France) is a prospective, multicenter case-control study, including localized BC patients aged 18–43 years, matched for age and parity to cancer-free volunteer controls in a 1:2 ratio. Data were collected through online questionnaires completed on the Seintinelles research platform.
Results
In a population of 1278 women at risk of unintentional pregnancy, the prevalence of contraception at study inclusion did not differ significantly between cases (340/431, 78.9%) and controls (666/847, 78.6%, p = 0.97). Contrarily, the contraceptive methods used were significantly different, with a higher proportion of copper IUD use in BC survivors (59.5% versus 25.0% in controls p < 0.001). For patients at risk of unintentional pregnancy, receiving information about chemotherapy-induced ovary damage at BC diagnosis (OR = 2.47 95%CI [ 1.39–4.37] and anti-HER2 treatment (OR = 2.46, 95% CI [ 1.14–6.16]) were significantly associated with the use of a contraception in multivariate analysis.
Conclusion
In this large French study, BC survivors had a prevalence of contraception use similar to that for matched controls, though almost one in five women at risk of unintentional pregnancy did not use contraception. Dedicated consultations at cancer care centers could further improve access to information and contraception counseling.
Keywords: contraception, Breast cancer, Survivorship, Contraceptive counseling, Emergency contraception
1. Introduction
Breast cancer (BC) is the most common cancer in women. Approximately 11 000 women under the age of 45 years are diagnosed with BC annually in France [1]. Over the last decade, physicians have begun to pay more attention to the possibility of pregnancy following BC. Concerns have been raised over decades about the impact on recurrence of breast cancer after a pregnancy and BC patients have long been advised against conception in the future, due to fears that pregnancy could adversely affect their breast cancer outcome. However, many data have since emerged to indicate that pregnancy does not have a detrimental effect on survival [2], regardless of ER status [3,4], and the presence or absence of a germline BRCA mutation [5].
Contraception after BC has been little studied. However, it is a particularly important issue because pregnancy planning in these patients is crucial from a medical point of view, as highlighted in a previous study [6]. Patients who do not wish to become pregnant should actively avoid pregnancy, particularly during tamoxifen treatment, as this drug is known to have potential teratogenic effects [7]. Moreover, chemotherapy-induced amenorrhea might be associated with an unpredictable resumption of menses, potentially resulting in an unwanted pregnancy. Effective, safe, well-tolerated contraception is therefore of considerable importance for this population.
Female hormonal contraception has been available for over 50 years and is used by more than 300 million women worldwide [8]. Hormonal contraceptives are classically contraindicated in BC patients, and women are generally advised to stop hormonal contraceptive use at the time of BC diagnosis. Classic options for alternative contraceptive methods, according to guidelines [9], include intrauterine device (IUD), or barrier methods. Thus, breast cancer survivors have few contraceptive options, mostly non-hormonal methods. Previous studies have shown that sexually active cancer survivors have lower rates of use of World Health Organization tier I-II contraceptive methods [[10], [11], [12]], and are considered at high risk of unintended pregnancy [13]. In the FIRST cohort [10], breast cancer patients were found to be three times less likely to use emergency contraception than other cancer survivors.
The FEERIC study was designed to compare fertility, pregnancy and contraception outcomes in young BC survivors and matched cancer-free women. The objective of the study described here was to analyze contraception use during follow-up and to compare contraceptive use between BC survivors and matched controls.
2. Materials and methods
2.1. Study design and data collection
The design of The FEERIC (Fertility, Pregnancy, Contraception after BC in France) study has been described elsewhere [14]. Briefly, the FEERIC study is a prospective case-control study assessing the impact of BC treatment on fertility, pregnancy, and contraception in young BC survivors. Women were recruited from March 13, 2018 to June 27, 2019. The study was launched by the Seintinelles research network. The scientific board of the Seintinelles approved the FEERIC project in December 2015, and the ethics board of Sud Ouest Outre Mer II approved the project on October 5, 2017. Seintinelles is a collaborative social network created in 2012 to accelerate the recruitment of French volunteers for cancer research studies, by connecting researchers with men and women of various ages, social and medical backgrounds with or without a history of cancer. In September 2022, the network included more than 37 000 French citizens willing to participate in research studies. Cases and controls were recruited by both (i) the Seintinelles network through the sending of a newsletter to the pool of volunteers to invite them to participate to the survey; and by (ii) nine breast care/oncofertility and gynecology centers (See appendix material and methods).
Volunteers matching the inclusion criteria (11 questions) were sent a link to the survey and were asked to complete a baseline form at inclusion and follow-up forms every six months (a total of six forms). Data were collected via self-administered online questionnaires released through the Seintinelles research platform. The current study concerns baseline characteristics and attitudes to contraception at study inclusion.
2.2. Study population
The inclusion criteria for cases were: female patients aged from 18 to 43 years with a previous diagnosis of localized, relapse-free BC (invasive or in situ) and who had completed treatment (surgery, chemotherapy, and radiotherapy) at the time of enrollment. The exclusion criteria were previous hysterectomy and/or bilateral oophorectomy and/or bilateral salpingectomy. The controls were women aged from 18 to 43 years, free from BC or other cancers, who had not undergone hysterectomy, bilateral oophorectomy or bilateral salpingectomy (Fig. S1).
We initially planned to match each BC patient (case) for age (±2 years) and parity with two volunteers (controls) recruited prospectively within the Seintinelles network and one control from the patient's close circle of friends and relatives. However, too few controls of this second type were recruited. We therefore pooled these controls with the volunteers recruited through the Seintinelles network, and each case was matched to two controls based on age and previous parity.
We excluded the women who were attempting to conceive or pregnant at inclusion (cohort 1 on the study flow chart), to define a subpopulation of women at risk of unintentional pregnancy (cohort 2).
2.3. Contraception
2.3.1. Prevalence of contraception
The prevalence of contraception was defined as the percentage of women currently using, or whose sexual partner was currently using, at least one method of contraception, regardless of the method used.
2.3.2. Classification of contraceptive methods
Each contraceptive method was classified according to three classifications: (i) the WHO contraceptive effectiveness tier classification [15,16] (Tier 1/Tier 2/Tier 3 & 4); (ii) The hormonal nature of the contraception method (hormonal versus non-hormonal); The reversibility of the contraception method (definitive versus reversible) (See appendix material and methods).
2.4. Emergency contraception
Emergency contraception was defined as methods of contraception (oral or IUD) used to prevent pregnancy after sexual intercourse. These contraceptive methods were not included in the previous classification of contraceptive methods, and data were collected in a dedicated section of the form.
2.5. Study endpoint
The primary outcome measure was a comparison of the prevalence of contraception at study inclusion between cases and controls, for women at risk of unintentional pregnancy. Secondary endpoints included the comparison between the type of contraceptive methods use between cases and controls, the analysis of factor associated with contraceptive use, and the description of the use of emergency contraception.
2.6. Statistical analysis
The study population is described in terms of frequencies for qualitative variables, or medians and associated ranges for quantitative variables. Associations between continuous and categorical variables were assessed using Student's t-tests or Wilcoxon-Mann-Whitney tests where indicated. Associations between categorical variables were assessed with Chi-squared tests, or with Fisher's exact test if at least one category included fewer than three patients. A value of p ≤ 0.05 was considered statistically significant. For identification of the factors predictive of contraceptive use in BC patients at risk unintentional pregnancy, variables were introduced into a univariate logistic regression model. A multivariate logistic model was then generated with a forward stepwise selection procedure, with all covariates included having a likelihood ratio test p-value ≤0.05. Data were processed and statistical analyses were performed with R software version 3.1.2 (www.cran.r-project.org, (R Foundation for Statistical Computing, 2009)).
3. Results
3.1. Prevalence of contraception and contraceptive methods
3.1.1. Population matching
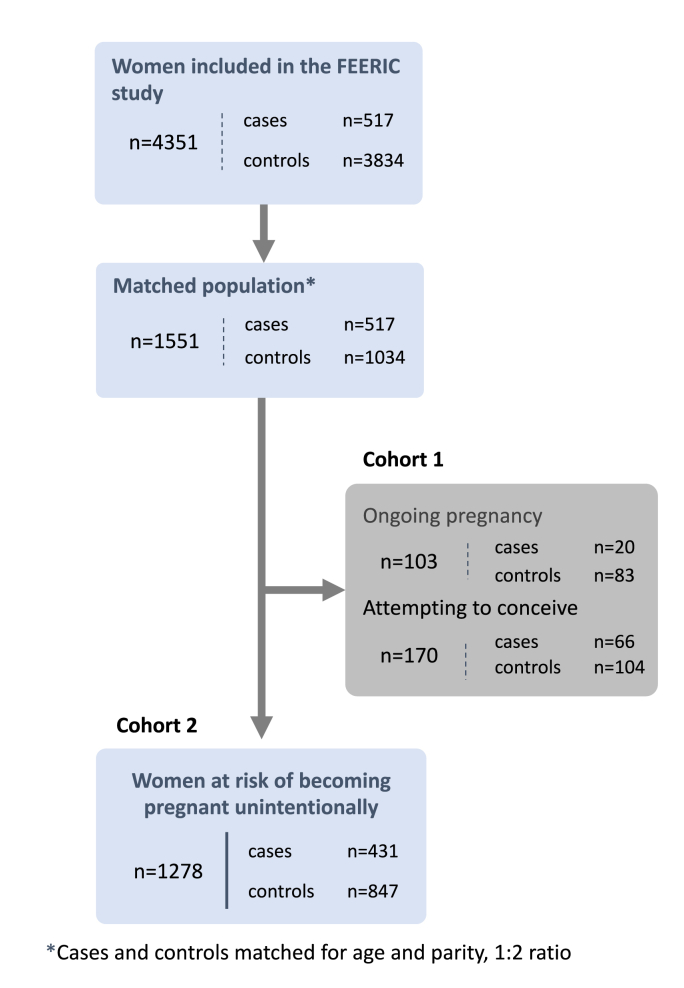
We matched the 517 BCE patients with a control population of 3834 cancer-free volunteers included in the study on the basis of matching for age ( ± 2 years old) and parity in a 1:2 ratio, resulting in an overall population of 1551 women (cases n = 517, controls n = 1034). After exclusion of the patients who were pregnant (n = 103) or trying to conceive (n = 170), 1278 women remained for the analyses (case n = 431, controls n = 847) (Fig. 1). Median age at inclusion in this study was 37.1 years. Median time between BC diagnosis and inclusion in the study was 30.8 months. The controls were significantly more obese or overweight and were more likely to be current smokers and to have concomitant comorbidity than the cases (Table 1).
Fig. 1.
Flow chart for the study cohort.
Table 1.
Characteristics of the women (cases and controls) in the population at risk of unintentional pregnancy.
| Variable | Class | All | Case | Control | p |
|---|---|---|---|---|---|
| n = | 1278 | 431 | 847 | ||
| Age at study inclusion | 37.1 (4.2) | 37.18 (4.28) | 37.00 (4.22) | 0,494 | |
| Age at study inclusion | <30 | 98 (7.7) | 36 (8.4) | 62 (7.3) | 0,424 |
| [30–35 [ | 344 (26.9) | 104 (24.1) | 240 (28.3) | ||
| [35–40 [ | 514 (40.2) | 177 (41.1) | 337 (39.8) | ||
| ≥40 | 322 (25.2) | 114 (26.5) | 208 (24.6) | ||
| Study level | Bachelor or lower | 137 (10.7) | 50 (11.6) | 87 (10.3) | 0,528 |
| University | 1141 (89.3) | 381 (88.4) | 760 (89.7) | ||
| Profession (class) | Intermediate | 547 (42.8) | 189 (43.9) | 358 (42.3) | 0,847 |
| Low CSP or unemployed | 124 (9.7) | 42 (9.7) | 82 (9.7) | ||
| Superior | 607 (47.5) | 200 (46.4) | 407 (48.1) | ||
| Marital status (current) | Single | 258 (20.2) | 77 (17.9) | 181 (21.4) | 0,161 |
| Coupled up | 1020 (79.8) | 354 (82.1) | 666 (78.6) | ||
| BMI (4 classes) | Underweight | 61 (4.8) | 23 (5.3) | 38 (4.5) | 0,007 |
| Normal weight | 828 (64.8) | 303 (70.3) | 525 (62.0) | ||
| Pre-obesity | 260 (20.3) | 74 (17.2) | 186 (22.0) | ||
| Obesity | 129 (10.1) | 31 (7.2) | 98 (11.6) | ||
| Smoking status | Current | 205 (16.0) | 54 (12.5) | 151 (17.8) | 0,017 |
| Former | 463 (36.2) | 174 (40.4) | 289 (34.1) | ||
| Never | 610 (47.7) | 203 (47.1) | 407 (48.1) | ||
| Comorbidities | No | 839 (65.6) | 305 (70.8) | 534 (63.0) | 0,007 |
| Yes | 439 (34.4) | 126 (29.2) | 313 (37.0) | ||
| Stroke | 11 | 3 | 8 | ||
| VTE | 32 | 11 | 21 | ||
| Hypertension | 26 | 7 | 19 | ||
| Diabetes | 6 | 2 | 4 | ||
| Dyslipidemia | 37 | 7 | 30 | ||
| Thyroid disease | 81 | 25 | 56 | ||
| Renal failure | 3 | 0 | 3 | ||
| Depression | 166 | 48 | 118 | ||
| Other | 189 | 50 | 139 | ||
| Comedications | No | 981 (76.8) | 321 (74.5) | 660 (77.9) | 0,191 |
| Yes | 297 (23.2) | 110 (25.5) | 187 (22.1) | ||
| Previous pregnancy (at BC diagnosis) | No | 353 (27.6) | 118 (27.4) | 235 (27.7) | 0,942 |
| Yes | 925 (72.4) | 313 (72.6) | 612 (72.3) | ||
| Familial history of BC | No | 624 (48.8) | 196 (45.5) | 428 (50.5) | 0,153 |
| At least 1 first degree relative | 255 (20.0) | 97 (22.5) | 158 (18.7) | ||
| At least 1 s degree relative | 399 (31.2) | 138 (32.0) | 261 (30.8) | ||
| Gynecological follow-up | No | 228 (17.8) | 72 (16.7) | 156 (18.4) | 0,497 |
| Yes | 1050 (82.2) | 359 (83.3) | 691 (81.6) |
Each BC patient (case) was matched for age and parity to two volunteers (controls). We excluded 103 women currently pregnant and 170 women attempting to conceive from the analyses.
Abbreviations: breast cancer (BC); body mass index (BMI); socioprofessional category (SPC).
“n” denotes the number of patients. Categorical variables are expressed as absolute numbers (percentages in brackets). Continuous variables are expressed as mean values, with the standard deviation in brackets. There were no missing data.
Regarding partnered status, the p-value for the association between partnered status and contraceptive use (single: 76.3% versus coupled up: 85.8%) did not reach statistical significance, though we found a trend in such association (p = 0.07).
3.1.2. Prevalence of contraception and contraceptive methods
Overall, the prevalence of contraception did not differ between cases (340/431, 78.9%) and controls (666/847, 78.6%, p = 0.97). There were no association between contraceptive use at study inclusion and the prior use of contraception before BC diagnosis after univariate analysis (p = 0.198). Within the population of BC patients at risk of unintentional pregnancy, the factors associated with the use of contraception were younger age, the information about chemotherapy-induced ovary damage received at BC diagnosis, the information about contraception received at BC diagnosis, and anti-HER2 treatment but were not associated with having visited a gynecologist in the year before breast cancer diagnosis (Table 2).
Table 2.
Factors associated with the use of contraception in patients with contraceptive needs.
| Univariate |
Multivariate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Class | All | With contraception | OR | OR (95%) | p | ||||
| Age at study inclusion (continuous) | 0,91 | [0.85–0.98] | 0,011 | |||||||
| Age at study inclusion | <30 | 34 | 30 | 88,20% | 1 | |||||
| [30–35 [ | 97 | 89 | 91,80% | 1,48 | [0.37–5.07] | 0,543 | ||||
| [35–40 [ | 163 | 134 | 82,20% | 0,62 | [0.17–1.71] | 0,396 | ||||
| ≥40 | 109 | 87 | 79,80% | 0,53 | [0.15–1.52] | 0,273 | ||||
| Study level | Bachelor or lower | 44 | 37 | 84,10% | 1 | |||||
| University | 359 | 303 | 84,40% | 1,02 | [0.4–2.28] | 0,957 | ||||
| Profession (class) | Intermediate | 176 | 153 | 86,90% | 1 | |||||
| Low CSP or unemployed | 40 | 34 | 85% | 0,85 | [0.34–2.45] | 0,747 | ||||
| Superior | 187 | 153 | 81,80% | 0,68 | [0.38–1.2] | 0,182 | ||||
| Marital status (current) | Single | 59 | 45 | 76,30% | 1 | |||||
| Coupled up | 344 | 295 | 85,80% | 1,87 | [0.93–3.6] | 0,067 | ||||
| BMI (4 classes) | Underweight | 21 | 16 | 76,20% | 1 | |||||
| Normal weight | 287 | 242 | 84,30% | 1,68 | [0.53–4.54] | 0,334 | ||||
| Pre-obesity | 69 | 61 | 88,40% | 2,38 | [0.65–8.19] | 0,172 | ||||
| Obesity | 26 | 21 | 80,80% | 1,31 | [0.32–5.49] | 0,703 | ||||
| Smoking status | Current | 53 | 41 | 77,40% | 1 | |||||
| Former | 164 | 138 | 84,10% | 1,55 | [0.7–3.3] | 0,261 | ||||
| Never | 186 | 161 | 86,60% | 1,88 | [0.85–4.01] | 0,106 | ||||
| Comorbidities | No | 289 | 248 | 85,80% | 1 | |||||
| Yes | 114 | 92 | 80,70% | 0,69 | [0.39–1.24] | 0,205 | ||||
| Comedications | No | 299 | 253 | 84,60% | 1 | |||||
| Yes | 104 | 87 | 83,70% | 0,93 | [0.52–1.75] | 0,816 | ||||
| Prior use of contraception (before BC diagnosis) | No | 90 | 72 | 80,00% | 1 | |||||
| Yes | 313 | 268 | 85,60% | 1,49 | [0.8–2.69] | 0,198 | ||||
| Previous pregnancy (at BC diagnosis) | No | 103 | 85 | 82,50% | 1 | |||||
| Yes | 300 | 255 | 85% | 1,2 | [0.65–2.15] | 0,551 | ||||
| Previous children (at BC diagnosis) | No | 135 | 114 | 84,40% | 1 | |||||
| Yes | 268 | 226 | 84,30% | 0,99 | [0.55–1.74] | 0,976 | ||||
| Number of children (3 classes) | 0 | 135 | 114 | 84,40% | 1 | |||||
| 1 | 91 | 78 | 85,70% | 1,11 | [0.53–2.39] | 0,793 | ||||
| More than 1 | 177 | 148 | 83,60% | 0,94 | [0.5–1.73] | 0,843 | ||||
| Pregnancy desire (at BC diagnosis) | Attempted pregnancy | 39 | 31 | 79,50% | 1 | |||||
| Future pregnancy desire | 156 | 136 | 87,20% | 1,75 | [0.67–4.24] | 0,225 | ||||
| No | 188 | 155 | 82,40% | 1,21 | [0.48–2.77] | 0,662 | ||||
| Pregnant at diagnosis | 20 | 18 | 90% | 2,32 | [0.51–16.52] | 0,318 | ||||
| Gynecological follow-up | No | 64 | 52 | 81,20% | 1 | |||||
| Yes | 339 | 288 | 85% | 1,3 | [0.63–2.54] | 0,455 | ||||
| Fertility counseling (at BC diagnosis) | No | 108 | 81 | 75% | 1 | 1 | ||||
| Yes | 284 | 251 | 88,40% | 2,54 | [1.43–4.47] | 0,001 | 2,47 | [ 1.39–4.37 ] | 0,002 | |
| Contraception counseling | No | 136 | 107 | 78,70% | 1 | |||||
| Yes | 267 | 233 | 87,30% | 1,86 | [1.07–3.2] | 0,026 | ||||
| Quality of contraception counseling | Not satisfied | 45 | 36 | 80% | 1 | |||||
| Satisfied | 358 | 304 | 84,90% | 1,41 | [0.61–2.98] | 0,394 | ||||
| Fertility preservation | No | 270 | 225 | 83,30% | 1 | |||||
| Yes | 133 | 115 | 86,50% | 1,28 | [0.72–2.36] | 0,416 | ||||
| Chemotherapy | No | 79 | 69 | 87,30% | 1 | |||||
| Yes | 324 | 271 | 83,60% | 0,74 | [0.34–1.47] | 0,418 | ||||
| Radiotherapy | No | 64 | 52 | 81,20% | 1 | |||||
| Yes | 339 | 288 | 85% | 1,3 | [0.63–2.54] | 0,455 | ||||
| AntiHER2 therapy | No | 310 | 254 | 81,90% | 1 | 1 | ||||
| Yes | 93 | 86 | 92,50% | 2,71 | [1.27–6.72] | 0,018 | 2,46 | [ 1.14–6.16 ] | 0,034 | |
| Endocrine therapy | No | 140 | 115 | 82,10% | 1 | |||||
| Yes | 263 | 225 | 85,60% | 1,29 | [0.73–2.23] | 0,37 | ||||
Abbreviations: socioprofessional category (SPC); breast cancer (BC); body mass index (BMI).
“n” denotes the number of patients. Categorical variables are expressed as absolute numbers, with percentages in brackets. Continuous variables are expressed as the mean value, with the standard deviation in brackets. Analysis performed for cases only, because too many variables of interest were missing for multivariate analysis on the whole population. There were no missing data. Regarding the variable: “Fertility counseling at diagnosis”, the exact question collected via self-administered online questionnaires was: “Have you received information about the potential consequences of treatments on fertility and/or the possibility of a subsequent pregnancy?".
In the multivariate analysis, only information about chemotherapy-induced ovary damage (OR = 2.47 95% CI [1.39–4.37] and anti-HER2 treatment (OR = 2.46, 95% CI [1.14–6.16]) were significantly associated with the use of contraception.
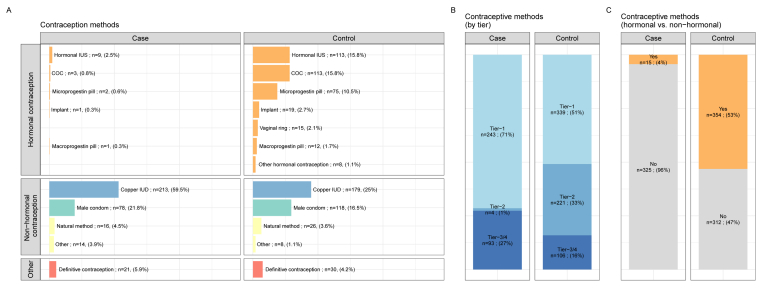
The type of contraceptive method used differed significantly between cases and controls (Fig. 2A), with copper IUDs the major contraceptive method in cases, but with a lower frequency of use in controls (59.5% versus 25.0%, p < 0.001). Contraceptive methods also differed significantly between cases and controls in terms of efficacy according to the tier classification (Fig. 2B) (p < 0.001), and the use of hormonal versus non-hormonal methods (p < 0.001) (Fig. 2C).
Fig. 2.
Comparison of contraceptive methods between cases and controls in the population of patients at risk of unintentional pregnancy. A, Contraceptive methods in cases and controls at inclusion in the study. B, Type of contraception, by tier category, at inclusion, for the cases and controls. C, Use of hormonal contraception by cases and controls at inclusion in the study. All data are reported per contraceptive method (one patient can use several methods). Abbreviations: combined oral contraceptive (COC); intrauterine system (IUS); intrauterine device (IUD).
3.2. Definitive contraception
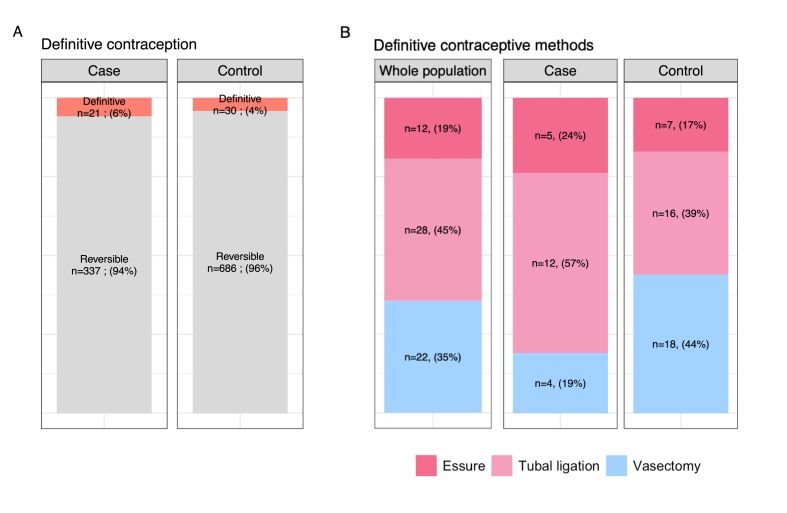
The prevalence of definitive contraception was low and did not differ significantly between cases and controls (21/358 (5.9%) versus 30/716 (4.2%) respectively, p = 0.29) (Fig. 3A). Most of the definitive contraception methods used were female rather than male methods (66.7% versus 33.3%), with this tendency more marked among the cases than the controls (male methods: 19% for cases versus 44% for controls, p = 0.17), although this difference was not statistically significant (Fig. 3B).
Fig. 3.
Use of definitive contraception in cases and controls A, Comparison of the use of definitive contraceptive methods between cases and controls. Data are reported per contraceptive method (one patient can use several methods). B, Comparison of the type of definitive contraceptive methods by cases and controls. Data are reported per contraceptive method (one patient can use several methods).
3.3. Emergency contraception
The patients’ knowledge and use of emergency contraception are summarized in Table 3. All but 13 patients (1.0%) from this population were aware of the existence of emergency contraception. The proportion of women who had used emergency contraception was smaller for cases than for controls (42.0% versus 51.5%). In total, 617 women (48.3%) had used emergency contraception at least once in their lifetime (cases n = 181 42%, controls n = 437 51.3%), and 19 patients (4.4%) had used emergency contraception since BC diagnosis.
Table 3.
Knowledge and use of emergency contraception in cases and controls.
| Variable | Class | All | Case | Control | p |
|---|---|---|---|---|---|
| n = | 1278 | 431 | 847 | ||
| Knowledge of emergency contraception | No | 13 (1.0) | 5 (1.2) | 8 (0.9) | 0,946 |
| Yes | 1265 (99.0) | 426 (98.8) | 839 (99.1) | ||
| Knowledge of IUD use as emergency contraception | No or not known | 1146 (89.7) | 400 (92.8) | 746 (88.1) | 0,011 |
| Yes | 132 (10.3) | 31 (7.2) | 101 (11.9) | ||
| Believe contra-indication hormonal emergency contraception in case of BC | No | 93 (21.6) | |||
| Not known | 174 (40.4) | ||||
| Yes | 164 (38.1) | ||||
| Emergency contraception history | No | 661 (51.7) | 250 (58.0) | 411 (48.5) | 0,002 |
| Yes | 617 (48.3) | 181 (42.0) | 436 (51.5) | ||
| Type of emergency contraception | Emergency pill | 615 | 181 | 434 | |
| IUD | 4 | 2 | 2 | ||
| Not known | 2 | 0 | 2 | ||
| Emergency contraception use since BC diagnosis |
No | 162 (89.5) | |||
| Yes | 19 (10.5) | ||||
|
Type of emergency contraception since BC diagnosis |
Emergency pill | 16 |
Abbreviations: intrauterine device (IUD); breast cancer (BC).
“n” denotes the number of patients. Categorical variables are expressed as absolute numbers, with percentages in brackets. Continuous variables are expressed as the mean value, with the standard deviation in brackets. For non-normal continuous variables, the median value is reported, with the interquartile range in brackets.
Missing data: type of emergency contraception pill, n = 2; type of emergency contraception pill used since BC diagnosis, n = 3.
Only 10.3% of women were aware that an IUD could be used as an emergency contraceptive method. Overall, 38.1% of BC patients thought that oral emergency contraception was contraindicated due to their history of cancer.
4. Discussion
This large study comparing BC survivors with age-matched controls provides important new insight into the contraceptive practices of BC patients. We found that the overall prevalence of contraceptive use during follow-up was similar to that in matched controls from the general population.
Conflicting evidence has been published on this point and is summarized in Table 4 [[17], [18], [19], [20], [21], [22]]. The discrepancies between published data and our findings may be explained by the origin of the patients, as the volunteers enrolled in the Seintinelles network have a higher social status than all-comers, and social status is also strongly related to adequate contraception [23]. Difficulties in accessing healthcare services, unemployment, or unstable family relations are hypotheses potentially explaining a lower contraceptive use in women from lower social backgrounds. Lambertini et al. found significant associations between contraceptive use after breast cancer and having visited a gynecologist in the previous year in multivariable analysis [18]. In contrast to the CANTO study, most of the patients in our study (339/403, 84%) reported regular gynecological follow-up before diagnosis, and this pattern was not associated with a greater likelihood of contraceptive use after cancer. This discrepancy might reflect the fact that the women of our study may lack contraception by personal choice rather that difficulties to access healthcare system. Finally, despite the higher prevalence of contraception in our study than in previous works, one on five of the women at risk of unintentional pregnancy declared no use of contraception, leaving room for improvement in the contraceptive coverage in this population. In previously published data on cases from the current cohort [14], unplanned pregnancies were more frequent than the use of ART after BC. Prospective follow-up will help determining if contraceptive use is associated with a decreased likelihood of unplanned pregnancies and elective abortions, and if the magnitude of this effect is similar in cases and controls.
Table 4.
Table summarizing studies analyzing contraception in BC survivors.
| Author and study | Year of inclusion | Country | Design | Total number patients | Patients | Age inclusion criteria | Median age | Number BC patients | Controls | Source of controls | Rate of contraceptive counseling | Contraceptive prevalence in women at risk of becoming pregnant unintentionally | Factors associated with contraceptive prevalence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Quinn et al., [13] Contraception |
2010 | USA | Written or online survey | 476 | Non gynecological cancer | <40 years old at diagnosis | 31,1 | 86 | 51 277 | General population estimation via the 2006–2010 National Survey for Family Growth | 66.7% |
Unintended pregnancy risk: 21% |
Lower use of tiers I-II: Increasing age: 1.07 per year; 95% CI [1.02–1.12]; p = 0.006 Previous BC history: OR 2.14; 95% CI [1.10–4.17]; p = 0.025 |
|
Maslow et al., [12] Contraception |
2011–2012 | USA | Online survey | 107 | Within 5 years of a cancer diagnosis | 18–45 years old at study inclusion | 56 | 65% | 57% |
Higher use of tiers I-II: Contraceptive counseling: OR 6.92; 95% CI [1.14–42.11]; p = 0.036 Non BC diagnosis: OR 3.60; 95% CI [1.03–12.64]; p = 0.046 |
|||
|
Dominick et al., [10] Obstetrics & Gynecology |
2011–2013 | USA | Annual online or telephone survey | 295 | Cancer survivors | 18–44 years old at study inclusion | 31,6 | 91 | 56% | 84% |
Higher use of tiers I-II: Family planning consult <1 year: RR 1.; 95% CI [1.1–1.5]; p < 0.01 Lower use of tiers I-II: ≥31 years old: RR 0.62; 95% CI [0.5–0.8]; p < 0.01 <2 years since cancer diagnosis:RR 0.66; 95% CI [0.5–0.9]; p < 0.01 BC diagnosis: RR 0.45; 95% CI [0.3–0.7]; p < 0.01 |
||
|
Hadnott et al., [24] Fertility and Sterility |
2015–2017 | USA | Online survey | 483 | Cancer survivors | 18–40 years old at study inclusion | 34 | 113 | 31% | 84% |
Lower use of contraception: Chemotherapy: PR 1.7; 95% CI [1.1–2.7] History of infertility: PR 2.; 95% CI [1.9–4.3] Infertility perception: PR 4.0, 95% CI [2.5–7.4] |
||
|
Mody et al., [21] J Cancer Surviv |
2014–2015 | USA | Online survey | 150 | History of Breast cancer within 5 years | 18–50 years old at study inclusion | 37,3 | 150 | 61% | 83% | NA | ||
|
Lambertini et al, [18] JAMA Network Open |
2012–2017 | France | Longitudinal evaluation | 2900 | Breast cancer survivors | 18–50 years old at study inclusion | 43,1 | 2900 | 45% at year 1 and 65.7% at year 2 during breast cancer follow-up |
38.9% at year 1 and 41.2% at year 2 during breast cancer follow-up |
Higher use of contraception Using contraception at diagnosis: aOR: 4.02; 95% CI [3.15–5.14], Being younger: aOR, 1.09; 95% CI, 1.07–1.13 per each decreasing year), having better sexual function aOR: 1.13; 95% CI [1.07–1.19], Having children: aOR: 4.21; 95% CI [1.8–9.86], Presence of leukorrhea: aOR: 1.32, 95% CI [1.03–1.7], Tamoxifen treatment alone: aOR: 1.39; 95% CI [1.01–1.92], Gynecologist follow-up at 1 year: aOR : 1.29; 95% CI [1.02–1.63], Partnered status: aOR: 1.61; 95% CI [1.07–2.44] |
||
| Our study (2022) | 2018–2019 | France | Online survey | 517 | Breast cancer survivors | 18–43 years old at study inclusion | 37,1 | 517 | 1034 | Controls from the research network matched on age and parity | 66,30% | 78.9% |
Higher use of contraception: Younger age: OR 0.91; 95% CI [0.85–0.98]; p = 0.011 Information at BC diagnosis about chemo-induced ovarian damage: OR 2.47; 95% CI [1.39–4.37]; p = 0.002 Contraception information at BC diagnosis: OR 1.86; 95% CI [1.07–3.2]; p = 0.026 Anti-HER2 treatment: OR 2.46; 95% CI [1.39–6.16]; p = 0.018 |
The most frequently used contraceptive method after BC was, by far, the copper-IUD. Our study confirms that this method, which is recommended as the preferred option in guidelines [9], is feasible for BC survivors in real life. LNG-IUS were also used, but only in a very small subset of patients (n = 9, 0.3%). The level of evidence concerning the risk of BC recurrence in patients using LNG-IUS remains very low [[25], [26], [27], [28]]. However, LNG-IUs is still considered as contra-indicated after BC, also this last advisory is not consensual [29].
Definitive contraceptive methods were used in no more than 5% of BC patients after treatment. This proportion is consistent with the rarity of definitive contraception in France [30], possibly due to cultural barriers, a lack of knowledge, or such methods not being proposed by doctors. When definitive methods were chosen, they were predominantly of the female type, particularly in BC patients. Tubal ligation and Essure* are both considered to be more invasive than vasectomy, a minimally invasive technique that can be performed under local anesthesia. Definitive contraceptive methods are particularly appropriate for BC survivors not intending to have children in the future. Efforts should therefore be made to inform doctors; BC patients and their partners correctly, so that such contraceptive methods can be offered more widely. We also identified unfounded beliefs, such as the belief that hormonal emergency contraception is contraindicated in patients with a history of BC, which was held by up to one third of patients, highlighting the critical need for appropriate contraceptive counseling.
One of the strengths of this study is that it is one of the largest study to date providing a detailed description of contraceptive methods, in terms of effectiveness, hormonal content and reversibility. Women with BC are more likely to be older, to have already had several pregnancies and to be living with a partner or married, and educated than other young adults and teenage cancer survivors [12]. Together with the contraindication of hormonal contraceptive use, dedicated analyses in this specific population are of interest. The limitations of this study include the recruitment of women via online networks, which may have led to an overestimation of the prevalence of contraception, as most of the women in the FEERIC study came from high-level socioprofessional backgrounds. In addition, due to self-reporting data collection, we might have missed information on BC characteristics that could potentially impact on reproductive life plans and on the adoption of contraceptive methods. Moreover, we did not report desire for future pregnancies at study inclusion. In addition, we were not able to collect data on sexual preference.
Finally, sexual and reproductive health education programs [31], and survivorship care tools for improving reproductive health issues, including contraception (SCP-R, NCT02667626), could help to facilitate access to contraception and to ensure that patients are offered a wider range of contraceptive methods, including definitive methods, for which take-up remains poor.
Acknowledgments
The FEERIC study was funded by Institut du Cancer InCA, InCA-SHS, grant No. 2016–124, and is part of the Young Breast Cancer Project, funded by Monoprix*. The funder was not involved in study design, or in the collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication. The authors thank all the study participants from the Seintinelles* Network and Lili Sohn who is the sponsor of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.12.033.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Defossez G., Le Guyader-Peyrou S., Uhry Z., Grosclaude P., Remontet L., Colonna M., et al. Saint‐Maurice: Santé publique France; 2019. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Étude à partir des registres des cancers du réseau Francim. [Google Scholar]
- 2.Azim H.A., Santoro L., Pavlidis N., Gelber S., Kroman N., Azim H., et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47:74–83. doi: 10.1016/j.ejca.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Azim H.A., Kroman N., Paesmans M., Gelber S., Rotmensz N., Ameye L., et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31:73–79. doi: 10.1200/JCO.2012.44.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambertini M., Kroman N., Ameye L., Cordoba O., Pinto A., Benedetti G., et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426–429. doi: 10.1093/jnci/djx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambertini M., Ameye L., Hamy A.-S., Zingarello A., Poorvu P.D., Carrasco E., et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J Clin Orthod. 2020;38:3012–3023. doi: 10.1200/JCO.19.02399. [DOI] [PubMed] [Google Scholar]
- 6.Han S.N., Van Peer S., Peccatori F., Gziri M.M., Amant F., International Network on Cancer Infertility and Pregnancy. Contraception is as important as fertility preservation in young women with cancer. Lancet. 2015;385:508. doi: 10.1016/S0140-6736(15)60201-X. [DOI] [PubMed] [Google Scholar]
- 7.Barthelmes L., Gateley C.A. Tamoxifen and pregnancy. Breast. 2004;13:446–451. doi: 10.1016/j.breast.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 8.United Nations . United Nations; 2019. Contraceptive use by method 2019: data booklet. [DOI] [Google Scholar]
- 9.Who LibrCatPublData . fifth ed. 2015. Medical eligibility criteria for contraceptive use. Geneva, Switzerland. [Google Scholar]
- 10.Dominick S.A., McLean M.R., Whitcomb B.W., Gorman J.R., Mersereau J.E., Bouknight J.M., et al. Contraceptive practices among female cancer survivors of reproductive age. Obstet Gynecol. 2015;126:498–507. doi: 10.1097/AOG.0000000000000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Güth U., Huang D.J., Bitzer J., Tirri B.F., Moffat R. Breast; 2015. Contraception counseling for young breast cancer patients: a practical needs assessment and a survey among medical oncologists. [DOI] [PubMed] [Google Scholar]
- 12.Maslow B.-S.L., Morse C.B., Schanne A., Loren A., Domchek S.M., Gracia C.R. Contraceptive use and the role of contraceptive counseling in reproductive-aged women with cancer. Contraception. 2014;90:79–85. doi: 10.1016/j.contraception.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Quinn M.M., Letourneau J.M., Rosen M.P. Contraception after cancer treatment: describing methods, counseling, and unintended pregnancy risk. Contraception. 2014;89:466–471. doi: 10.1016/j.contraception.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Mangiardi-Veltin M., Sebbag C., Rousset-Jablonski C., Ray-Coquard I., Berkach C., Laot L., et al. Pregnancy, fertility concerns and fertility preservation procedures in a national study of French breast cancer survivors. Reprod Biomed Online. 2022;S1472–6483(22) doi: 10.1016/j.rbmo.2021.12.019. 00031-1. [DOI] [PubMed] [Google Scholar]
- 15.Stanback J., Steiner M., Dorflinger L., Solo J., Cates W. WHO tiered-effectiveness counseling is rights-based family planning. Glob Health Sci Pract. 2015;3:352–357. doi: 10.9745/GHSP-D-15-00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization, Reproductive Health and Research, K4Health . World Health Organization, Department of Reproductive Health and Research ; John Hopkins Bloomberg School of Public Health, Center for Communication programs, Knowledge for Health Project; Geneva]; Baltimore: 2018. Family planning: a global handbook for providers : evidence-based guidance developed through worldwide collaboration. [Google Scholar]
- 17.Hendry S., Salgado R., Gevaert T., Russell P.A., John T., Thapa B., et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–335. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambertini M., Massarotti C., Havas J., Pistilli B., Martin A.-L., Jacquet A., et al. Contraceptive use in premenopausal women with early breast cancer. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.33137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslow B.-S.L., Morse C.B., Schanne A., Loren A., Domchek S.M., Gracia C.R. Contraceptive use and the role of contraceptive counseling in reproductive-aged women with cancer. Contraception. 2014;90:79–85. doi: 10.1016/j.contraception.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Quinn M.M., Letourneau J.M., Rosen M.P. Contraception after cancer treatment: describing methods, counseling, and unintended pregnancy risk. Contraception. 2014;89:466–471. doi: 10.1016/j.contraception.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Mody S.K., Gorman J.R., Oakley L.P., Layton T., Parker B.A., Panelli D. Contraceptive utilization and counseling among breast cancer survivors. J Cancer Surviv. 2019;13:438–446. doi: 10.1007/s11764-019-00765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominick S.A., McLean M.R., Whitcomb B.W., Gorman J.R., Mersereau J.E., Bouknight J.M., et al. Contraceptive practices among female cancer survivors of reproductive age. Obstet Gynecol. 2015;126:498–507. doi: 10.1097/AOG.0000000000000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frippiat D., Marquis N. Les enquêtes par Internet en sciences sociales : un état des lieux. Population. 2010;65:309–338. [Google Scholar]
- 24.Hadnott N., Stark S.S., Medica A., Dietz A.C., Martinez M.E., Brian W, Whitcomb B.W., Su H.I. Perceived infertility and contraceptive use in the female, reproductive-age cancer survivor Tracy. Fertil Steril. 2019:763–771. doi: 10.1016/j.fertnstert.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Y., Zhuang Z. Long-term effects of levonorgestrel-releasing intrauterine system on tamoxifen-treated breast cancer patients: a meta-analysis. Int J Clin Exp Pathol. 2014;7:6419–6429. [PMC free article] [PubMed] [Google Scholar]
- 26.Gizzo S., Di Gangi S., Bertocco A., Noventa M., Fagherazzi S., Ancona E., et al. Levonorgestrel intrauterine system in adjuvant tamoxifen treatment: balance of breast risks and endometrial benefits--systematic review of literature. Reprod Sci. 2014;21:423–431. doi: 10.1177/1933719113503408. [DOI] [PubMed] [Google Scholar]
- 27.Trinh X.B., Tjalma W.A.A., Makar A.P., Buytaert G., Weyler J., van Dam P.A. Use of the levonorgestrel-releasing intrauterine system in breast cancer patients. Fertil Steril. 2008;90:17–22. doi: 10.1016/j.fertnstert.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 28.Wong A.W.Y., Chan S.S.C., Yeo W., Yu M.-Y., Tam W.-H. Prophylactic use of levonorgestrel-releasing intrauterine system in women with breast cancer treated with tamoxifen: a randomized controlled trial. Obstet Gynecol. 2013;121:943–950. doi: 10.1097/AOG.0b013e31828bf80c. [DOI] [PubMed] [Google Scholar]
- 29.Vaz-Luis I., Partridge A.H. Exogenous reproductive hormone use in breast cancer survivors and previvors. Nat Rev Clin Oncol. 2018;15:249–261. doi: 10.1038/nrclinonc.2017.207. [DOI] [PubMed] [Google Scholar]
- 30.Vigoureux S., Le Guen M. [Current knowledge on contraceptive knowledge in France: CNGOF Contraception Guidelines] Gynecol Obstet Fertil Senol. 2018;46:777–785. doi: 10.1016/j.gofs.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Korzeniewska-Eksterowicz Aleksandra, Majak P., Grzelewski T., Stelmach W., Stelmach I. Impact of an SRH education programme on cystic fibrosis patients in Poland. J Fam Plann Reprod Health Care. 2013;39:60–61. doi: 10.1136/jfprhc-2012-100432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.