Abstract
Background
Obesity is a major risk factor for cardiovascular disease; however, a paradoxical effect of obesity has been reported in patients with heart failure or myocardial infarction. Although several studies have suggested the same obesity paradox in patients undergoing transcatheter aortic valve replacement (TAVR), they included a limited number of underweight patients.
Objectives
This study aimed to clarify the effect of being underweight on TAVR outcomes.
Methods
We retrospectively analyzed 1,693 consecutive patients undergoing TAVR between 2010 and 2020. The patients were categorized according to body mass index: underweight (<18.5 kg/m2; n = 242), normal weight (18.5 to 25 kg/m2; n = 1,055), and overweight (>25 kg/m2; n = 396). We compared midterm outcomes after TAVR among the 3 groups; all clinical events were in accordance with the Valve Academic Research Consortium-2 criteria.
Results
Underweight patients were more likely to be women and have severe heart failure symptoms, peripheral artery disease, anemia, hypoalbuminemia, and pulmonary dysfunction. They also had lower ejection fractions, smaller aortic valve areas, and higher surgical risk scores. Device failure, life-threatening bleeding, major vascular complications, and 30-day mortality occurred more frequently in underweight patients. The midterm survival rate of the underweight group was inferior to those of the other 2 groups (P < 0.0001; average follow-up, 717 days). In the multivariate analysis, underweight was associated with noncardiovascular mortality (HR: 1.78; 95% CI: 1.16-2.75) but not cardiovascular mortality (HR: 1.28; 95% CI: 0.58-1.88) after TAVR.
Conclusions
Underweight patients had a worse midterm prognosis, demonstrating the obesity paradox in this TAVR population. (Outcomes of transcatheter aortic valve implantation in Japanese patients with aortic stenosis: multi-center registry; UMIN000031133)
Key Words: body mass index, obesity paradox, transcatheter aortic valve replacement
Abbreviations and Acronyms: AS, aortic stenosis; BMI, body mass index; CV, cardiovascular; TAVR, transcatheter aortic valve replacement; VARC, Valve Academic Research Consortium
Central Illustration
Obesity is a well-known major risk factor for cardiovascular (CV) disease1; however, overweight patients show better outcomes following percutaneous coronary intervention2 and open-heart surgery.3 In heart failure, the higher the body mass index (BMI), the lower the mortality rate.4,5 This phenomenon is known as the obesity paradox. Explanations for the obesity paradox in patients with heart failure include better tolerability for guideline-directed medical therapy and increased metabolic reserve toward a catabolic state of heart failure.6 The obesity paradox has also been reported in transcatheter aortic valve replacement (TAVR).7, 8, 9, 10, 11, 12 In previous TAVR studies, overweight and/or obese patients had better prognosis than normal-weight patients, whereas underweight patients were not included or numerically limited. The East Asian population undergoing TAVR has a lower mean BMI than those in Western Europe and North America,13 and the true impact of a lower BMI on TAVR outcomes remains unclear. Here we sought to investigate whether underweight is associated with all-cause, CV, and non-CV mortality following TAVR using data from a multicenter registry.
Materials and Methods
Study design
We analyzed the data from a prospective multicenter registry, the LAPLACE (aLiAnce for exPloring cLinical prospects of AortiC valvE disease)-TAVR registry, which comprises the Sakakibara Heart Institute, Juntendo University Hospital, Mie University Hospital, Yamagata University Hospital, Hirosaki University Graduate School of Medicine, and Kawasaki Saiwai Hospital. We investigated 1,693 consecutive patients who underwent TAVR between April 2010 and July 2020. Data from all patients were prospectively collected in our dedicated database and retrospectively analyzed. Patient consent was obtained using an opt-out style or written informed consent. The institutional review board of each hospital approved the study protocol. This study was conducted in accordance with the Declaration of Helsinki and the other ethical guidelines for medical research involving humans. This registry was registered in the clinical research database (UMIN000031133).
TAVR procedure
The indications for TAVR were severe aortic stenosis (AS) or bioprosthetic valve failure, and decisions were made by each hospital’s multidisciplinary heart team. We selected TAVR or other therapeutic options based on age, comorbidities, frailty, and patient preference. Procedural details were previously reported.14,15
BMI and classification
We divided the patients into 3 groups by BMI, defined as weight in kilograms divided by the square of height in meters. Height and body weight were measured at the time of TAVR. BMI was classified based on the World Health Organization criteria16 and defined as underweight (<18.5 kg/m2), normal weight (18.5-25 kg/m2), and overweight (>25 kg/m2). The background and procedural characteristics and outcomes after TAVR were compared among the 3 groups. Owing to the small sample size of obese patients (n = 62 [3.7%]), they were included in the overweight group.
Study endpoints
The primary endpoint of this study was all-cause mortality after TAVR. The secondary endpoints were as follows: 1) 30-day mortality; 2) early combined endpoints at 30 days; 3) CV mortality after TAVR; and 4) non-CV mortality after TAVR. Clinical events were defined according to Valve Academic Research Consortium (VARC)-2 criteria.17 The combined endpoint included all-cause mortality, all strokes, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction, major vascular complications, and valve-related dysfunction requiring repeat procedures. Mortality cause was classified as CV or non-CV according to VARC-2 criteria. Pneumonia was considered a respiratory disease. We followed the patients until August 2020 by outpatient visits, telephone interviews, or postcards.
Statistical analysis
Categorical variables are expressed as number (%). Continuous variables are presented as mean ± SD, and normality was confirmed using the Shapiro-Wilk test. The chi-square test or Fisher exact test, if appropriate, was used to compare categorical variables. Normally distributed continuous variables were compared using Student’s t-test, whereas non-normally distributed variables were compared using the Mann-Whitney U test. One-way analysis of variance was used to compare the continuous variables among the 3 groups. The Kaplan-Meier method was used to evaluate postprocedural survival using the log-rank test. The prognostic value of the clinical variables was tested using Cox proportional hazard analysis. Variables tested in the multivariate analysis were selected based on the P value in the univariate analysis, clinical plausibility, and multicollinearity. Differences were considered statistically significant at values of P < 0.05. These analyses were performed using JMP version 16.2.0 statistical software.
Results
Patient baseline characteristics
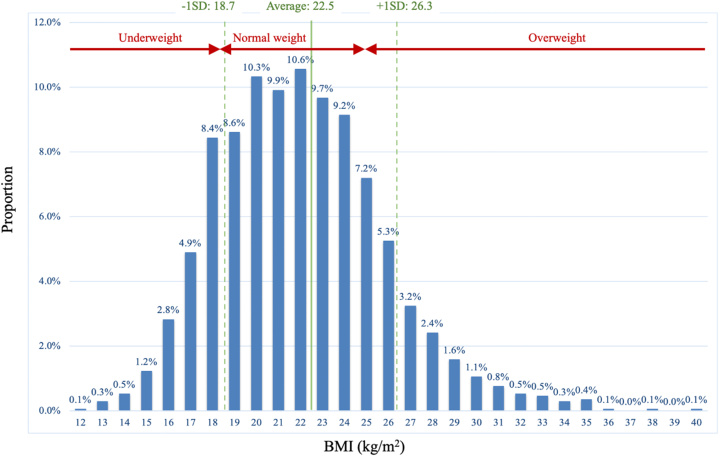
The mean age of the population was 84.2 years; 69% of them were women. There were 242 underweight patients (14.3%), 1,055 normal-weight patients (62.3%), and 396 overweight patients (23.4%) (Table 1, Figure 1, Supplemental Figure 1). Underweight patients were more likely to be women (76% vs 63% vs 70%, P = 0.0001) and have severe heart failure symptoms (New York Heart Association functional class III/IV: 66% vs 47% vs 48%, P < 0.0001) or peripheral artery disease (22% vs 15% vs 11%, P = 0.0007) than normal-weight and overweight patients, respectively. Previous or active cancer tended to be more prevalent in the underweight group (27% vs 19% vs 15%, P = 0.079). The underweight group had lower mean levels of hemoglobin (11.0 ± 1.5 vs 11.5 ± 1.6 vs 12.0 ± 1.6 g/dL, P < 0.0001), albumin (3.6 ± 0.5 vs 3.8 ± 0.5 vs 3.8 ± 0.4, P < 0.0001), and % forced vital capacity (77.0% ± 22.0% vs 86.5% ± 20.9% vs 85.9% ± 18.3%, P < 0.0001) and a higher N-terminal pro-brain natriuretic peptide level (4,279 ± 7,583 pg/mL vs 3,516 ± 10,687 pg/mL vs 1,628 ± 2,509 pg/mL, P < 0.0001). The systolic function of the underweight group was the lowest (ejection fraction, 59.3% ± 13.1% vs 60.8% ± 10.4% vs 63.4% ± 8.2%, P < 0.0001) with more advanced AS (aortic valve area, 0.61 ± 0.19 cm2 vs 0.68 ± 0.19 cm2 vs 0.71 ± 0.17 cm2, P < 0.0001). The estimated surgical risk was the highest in the underweight group (European System for Cardiac Operative Risk Evaluation II: 7.7% ± 8.4% vs 6.4% ± 7.2% vs 5.3% ± 5.9%, P < 0.0001; and Society of Thoracic Surgeons score: 8.3% ± 5.6% vs 7.1% ± 4.9% vs 5.7% ± 3.9%, P < 0.0001).
Table 1.
Baseline Characteristics
| Total (N = 1,693) | Underweight (n = 242) | Normal weight (n = 1,055) | Overweight (n = 396) | P Value | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age (y) | 84.2 ± 5.4 | 84.4 ± 6.3 | 84.6 ± 5.1 | 83.2 ± 5.4 | <0.0001 |
| Women | 1,123 (66) | 184 (76) | 663 (63) | 276 (70) | 0.0001 |
| Height (cm) | 151.1 ± 9.3 | 150.5 ± 8.9 | 151.5 ± 9.5 | 150.4 ± 9.1 | 0.072 |
| Body weight (kg) | 51.5 ± 10.5 | 39.0 ± 5.5 | 50.2 ± 7.6 | 62.6 ± 8.8 | <0.0001 |
| BMI (kg/m2) | 22.5 ± 3.8 | 17.2 ± 1.2 | 21.8 ± 1.8 | 27.7 ± 2.6 | <0.0001 |
| BSA (m2) | 1.5 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.2 | 1.6 ± 0.2 | <0.0001 |
| NYHA functional class III or IV | 846 (50) | 159 (66) | 496 (47) | 191 (48) | <0.0001 |
| Comorbidity | |||||
| Hypertension | 1,314 (78) | 151 (62) | 815 (77) | 348 (88) | <0.0001 |
| Diabetes mellitus | 383 (23) | 37 (15) | 241 (23) | 105 (27) | 0.004 |
| Dyslipidemia | 936 (55) | 119 (49) | 550 (52) | 267 (67) | <0.0001 |
| Cancer: previous or active | 318 (19) | 54 (27) | 203 (19) | 61 (15) | 0.079 |
| Previous stroke | 205 (12) | 19 (7.9) | 138 (13) | 48 (12) | 0.080 |
| COPD | 159 (9.4) | 27 (11) | 100 (9.5) | 32 (8.0) | 0.42 |
| Steroids use | 107 (6.3) | 18 (7.4) | 64 (6.1) | 25 (6.3) | 0.85 |
| AF/AFL | 419 (25) | 59 (24) | 260 (25) | 100 (25) | 0.96 |
| CAS | 145 (8.6) | 24 (9.9) | 91 (8.6) | 30 (7.6) | 0.36 |
| PAD | 260 (15) | 54 (22) | 162 (15) | 44 (11) | 0.0007 |
| Residual CAD | 511 (30) | 66 (27) | 338 (32) | 107 (27) | 0.10 |
| Previous MI | 92 (5.4) | 15 (6.2) | 59 (5.6) | 18 (4.5) | 0.63 |
| PMI/ICD | 111 (6.6) | 19 (7.9) | 74 (7.0) | 18 (4.5) | 0.16 |
| EuroSCORE II (%) | 6.3 ± 7.2 | 7.7 ± 8.4 | 6.4 ± 7.2 | 5.3 ± 5.9 | <0.0001 |
| STS score (%) | 7.0 ± 4.9 | 8.3 ± 5.6 | 7.1 ± 4.9 | 5.7 ± 3.9 | <0.0001 |
| Laboratory data | |||||
| Hemoglobin (g/dL) | 11.5 ± 1.6 | 11.0 ± 1.5 | 11.5 ± 1.6 | 12.0 ± 1.6 | <0.0001 |
| Platelet (×104/μL) | 18.3 ± 6.9 | 18.3 ± 7.0 | 18.3 ± 7.2 | 18.5 ± 6.3 | 0.34 |
| eGFR (mL/1.73 m2/min) | 53.5 ± 18.8 | 57.0 ± 20.2 | 53.1 ± 18.2 | 52.5 ± 19.1 | 0.007 |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.6 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.4 | <0.0001 |
| NT-pro BNP (pg/mL) | 3,188 ± 9,058 | 4,279 ± 7,583 | 3,516 ± 10,687 | 1,628 ± 2,509 | <0.0001 |
| %FVC (%) | 85.1 ± 20.7 | 77.0 ± 22.0 | 86.5 ± 20.9 | 85.9 ± 18.3 | <0.0001 |
| Echocardiographic data | |||||
| Ejection fraction (%) | 61.2 ± 10.5 | 59.3 ± 13.1 | 60.8 ± 10.4 | 63.4 ± 8.2 | <0.0001 |
| AVA (cm2) | 0.68 ± 0.19 | 0.61 ± 0.19 | 0.68 ± 0.19 | 0.71 ± 0.17 | <0.0001 |
| AV mean pressure gradient (mm Hg) | 50.1 ± 18.9 | 49.1 ± 20.6 | 50.7 ± 19.2 | 49.1 ± 17.0 | 0.37 |
Values are mean ± SD or n (%) unless otherwise indicated.
AF = atrial fibrillation; AFL = atrial flutter; AV = aortic valve; AVA = aortic valve area; BMI = body mass index; BSA = body surface area; CAD = coronary artery disease; CAS = carotid artery stenosis; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; EuroSCORE = European System for Cardiac Operative Risk Evaluation; FVC = forced vital capacity; ICD = implantable cardioverter defibrillator; MI = myocardial infarction; NT-pro BNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; PAD = peripheral artery disease; PMI = pacemaker implantation; STS = Society of Thoracic Surgeons.
Figure 1.
BMI Distribution of Patients Undergoing Transcatheter Aortic Valve Implantation
From the average body mass index (BMI) of 22.5 kg/m2, patients present normal distribution of their BMI.
Procedural details and 30-day outcomes
The underweight group was less commonly treated with the transfemoral approach (88% vs 94% vs 95%, P = 0.0009) with longer procedural time (95.4 ± 54.1 vs 88.4 ± 61.1 vs 82.0 ± 37.6, P = 0.0005) (Table 2). The underweight group had the longest mean stay in the cardiac care unit (2.2 ± 3.1 days vs 1.9 ± 3.0 days vs 1.8 ± 2.4 days, P < 0.0001), lowest device success rate (95% vs 98% vs 98%, P = 0.006), and lowest home discharge rate (85% vs 92% vs 93%, P = 0.0006).
Table 2.
Procedure Details and 30-Day Outcome
| Total (N = 1,693) | Underweight (n = 242) | Normal Weight (n = 1,055) | Overweight (n = 396) | P Value | |
|---|---|---|---|---|---|
| Procedural details | |||||
| General anesthesia | 701 (41) | 112 (46) | 425 (40) | 164 (41) | 0.24 |
| TF approach | 1,581 (93) | 213 (88) | 991 (94) | 377 (95) | 0.0009 |
| Surgical cut-down | 278 (16) | 41 (17) | 164 (16) | 73 (18) | 0.48 |
| Procedure time (min) | 88.2 ± 55.6 | 95.4 ± 54.1 | 88.4 ± 61.1 | 82.0 ± 37.6 | 0.0005 |
| Radiation time (min) | 23.5 ± 15.0 | 23.9 ± 11.1 | 24.0 ± 17.4 | 22.3 ± 9.3 | 0.40 |
| Contrast material (mL) | 73.1 ± 47.1 | 78.6 ± 47.0 | 73.0 ± 48.5 | 70.0 ± 43.1 | 0.047 |
| Device success | 1,653 (98) | 230 (95) | 1,033 (98) | 390 (98) | 0.006 |
| New-onset AF | 80 (4.7) | 11 (4.5) | 51 (4.8) | 18 (4.5) | 0.97 |
| Postprocedural PMI | 142 (8.4) | 24 (9.9) | 87 (8.2) | 31 (7.8) | 0.61 |
| PPM | 161 (9.5) | 8 (3.3) | 88 (8.3) | 65 (16) | <0.0001 |
| CCU duration (d) | 1.9 ± 2.9 | 2.2 ± 3.1 | 1.9 ± 3.0 | 1.8 ± 2.4 | <0.0001 |
| Median (d) | 1 (1-2) | 2 (1-2) | 1 (1-2) | 1 (1-2) | - |
| Discharge home | 1,542 (91) | 205 (85) | 968 (92) | 369 (93) | 0.0006 |
| 30-day outcome | |||||
| All-cause death | 15 (0.9) | 4 (1.7) | 10 (0.9) | 1 (0.3) | 0.18 |
| Stroke | 50 (3.0) | 10 (4.1) | 28 (2.7) | 12 (3.0) | 0.47 |
| Life-threatening bleeding | 35 (2.1) | 9 (3.7) | 23 (2.2) | 3 (0.7) | 0.036 |
| AKI stage 2 or 3 | 32 (1.9) | 5 (2.1) | 20 (1.9) | 7 (1.8) | 0.97 |
| Coronary obstruction | 7 (0.4) | 2 (1.3) | 3 (0.3) | 2 (0.5) | 0.44 |
| Major vascular complication | 41 (2.4) | 8 (3.3) | 31 (2.9) | 2 (0.5) | 0.018 |
| Valve-related dysfunction | 7 (0.4) | 5 (0.8) | 2 (0.2) | 0 (0) | <0.0001 |
| 30-day combined endpoint | 129 (7.6) | 27 (11) | 81 (7.6) | 21 (5.3) | 0.027 |
Values are n (%) or mean ± SD unless otherwise indicated.
AF = atrial fibrillation; AKI = acute kidney injury; CCU = cardiac care unit; PMI = pacemaker implantation; PPM = prosthesis patient mismatch; TF = transfemoral.
The 30-day mortality rate of the underweight group was the highest, but the difference was not statistically significant (1.7% vs 0.9% vs 0.3%, P = 0.18). The combined endpoint at 30 days occurred more frequently in the underweight group (11% vs 7.6% vs 5.3%, P = 0.027) because of more frequent life-threatening bleeding (3.7% vs 2.2% vs 0.7%, P = 0.036), major vascular complications (3.3% vs 2.9% vs 0.5%, P = 0.018), and valve-related complications (0.8% vs 0.2% vs 0%, P < 0.0001).
Clinical outcomes
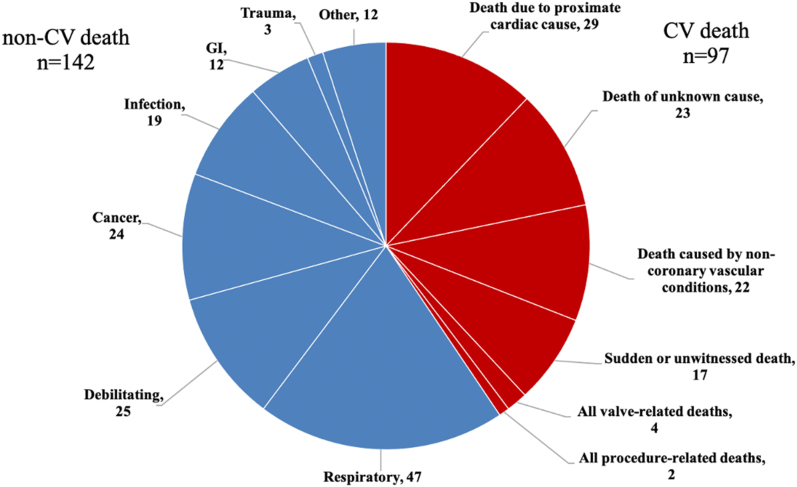
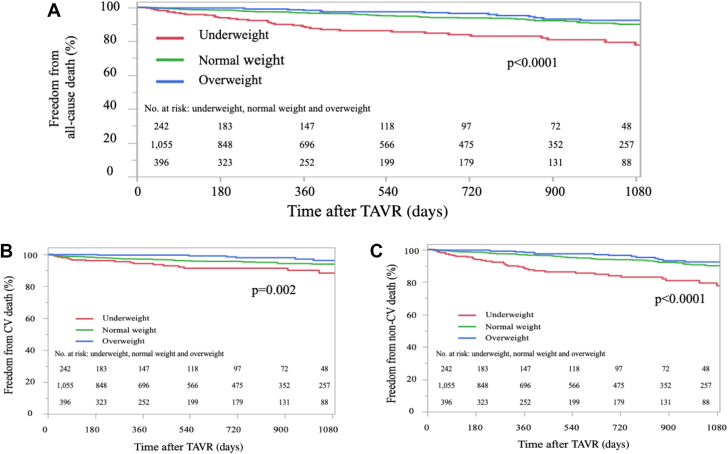
The mean follow-up period was 717 days (median 585 [IQR: 233-1,029] days). There were 239 all-cause deaths (14%) during this period, 41% of which were of CV causes (Figure 2). Freedom from all-cause mortality of the underweight group was lower than that of the other 2 groups on the Kaplan-Meier curve (P < 0.0001) (Figure 3A). Independent predictors of all-cause mortality were as follows (Table 3): female sex (HR: 0.50; 95% CI: 0.37-0.66; P < 0.0001), underweight (HR: 1.66; 95% CI: 1.17–2.36; P = 0.0047), steroid user (HR: 1.63; 95% CI: 1.01-2.62; P = 0.046), atrial fibrillation/atrial flutter (HR: 2.17; 95% CI: 1.62-2.92; P < 0.0001), Society of Thoracic Surgeons score (HR: 1.04 per 1% increase; 95% CI: 1.00-1.07; P = 0.049), albumin (HR: 0.41 per 1-g/dL increase; 95% CI: 0.30-0.57; P < 0.0001), device success (HR: 0.47; 95% CI: 0.26-0.84; P = 0.010), and 30-day combined endpoint (HR: 2.16; 95% CI: 1.49-3.13; P < 0.0001). Regarding causes of death, freedom from both CV and non-CV deaths was lower in the underweight group than in the other 2 groups (Figures 3B and 3C). In the multivariate analysis, underweight was independently associated with non-CV mortality (HR: 1.78; 95% CI: 1.16-2.75; P = 0.009) (Table 5) but not CV mortality after TAVR (HR: 1.28; 95% CI: 0.58-1.88; P = 0.37) (Table 4).
Figure 2.
Post-Transcatheter Aortic Valve Implantation Mortality Details
Figure shows the proportion of cardiovascular (CV) and noncardiovascular deaths, and their details. GI = gastrointestinal.
Figure 3.
Kaplan-Meier Survival Curve After TAVR
The survival rate of underweight patients was the lowest among 3 categories, primarily due to noncardiovascular (non-cardiovascular [CV]) mortality. (A) Freedom from all-cause mortality. (B) Freedom from CV mortality. (C) Freedom from non-CV mortality. TAVR = transcatheter aortic valve replacement.
Table 3.
Univariate and Multivariate Analysis for All-Cause Mortality After TAVR
| Univariate |
P Value | Multivariate |
P Value | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (per 1-y increase) | 1.03 | 1.002-1.06 | 0.028 | 1.02 | 0.99-1.05 | 0.17 |
| Woman | 0.56 | 0.43-0.73 | <0.0001 | 0.50 | 0.37-0.66 | <0.0001 |
| Underweight | 2.10 | 1.57-2.81 | <0.0001 | 1.66 | 1.17-2.36 | 0.005 |
| Overweight | 0.56 | 0.39-0.81 | 0.0009 | 0.71 | 0.47-1.07 | 0.10 |
| Hypertension | 0.68 | 0.51-0.91 | 0.039 | 0.96 | 0.69-1.35 | 0.97 |
| Diabetes mellitus | 1.01 | 0.75-1.37 | 0.93 | |||
| Cancer: previous or active | 1.67 | 1.25-2.22 | 0.0008 | 1.37 | 0.99-1.91 | 0.060 |
| Previous stroke | 1.17 | 0.79-1.75 | 0.45 | |||
| COPD | 1.39 | 0.92-2.11 | 0.13 | |||
| Steroids use | 1.60 | 1.04-2.47 | 0.044 | 1.63 | 1.01-2.62 | 0.046 |
| AF/AFL | 2.31 | 1.78-3.01 | <0.0001 | 2.17 | 1.62-2.92 | <0.0001 |
| CAS | 1.34 | 0.89-2.02 | 0.17 | |||
| PAD | 1.78 | 1.30-2.37 | 0.0006 | 1.41 | 0.99-1.99 | 0.052 |
| Residual CAD | 1.22 | 0.93-1.60 | 0.16 | |||
| Previous MI | 1.13 | 0.68-1.88 | 0.64 | |||
| PMI/ICD | 1.07 | 0.63-1.80 | 0.81 | |||
| EuroSCORE II (per 1% increase) | 1.03 | 1.02-1.05 | <0.0001 | 0.99 | 0.98-1.00 | 0.86 |
| STS score (per 1% increase) | 1.07 | 1.06-1.09 | <0.0001 | 1.04 | 1.00-1.07 | 0.049 |
| eGFR (per 1-mL/1.73 m2/min increase) | 0.99 | 0.98-0.99 | 0.015 | 0.99 | 0.99-1.00 | 0.17 |
| Albumin (per 1-g/dL increase) | 0.30 | 0.23-0.40 | <0.0001 | 0.41 | 0.30-0.57 | <0.0001 |
| Ejection fraction (per 1% increase) | 0.98 | 0.97-0.99 | 0.004 | 1.01 | 0.99-1.03 | 0.078 |
| AV mean pressure gradient (per 1-mm Hg increase) | 0.99 | 0.99-1.001 | 0.12 | |||
| TF approach | 0.55 | 0.39-0.77 | 0.0008 | 0.77 | 0.53-1.13 | 0.18 |
| Device success | 0.29 | 0.18-0.48 | <0.0001 | 0.47 | 0.26-0.84 | 0.010 |
| New-onset AF | 1.36 | 0.82-2.27 | 0.25 | |||
| Postprocedural PMI | 1.09 | 0.72-1.66 | 0.68 | |||
| PPM | 0.80 | 0.52-1.24 | 0.58 | |||
| 30-day combined endpoint | 2.89 | 2.07-4.03 | <0.0001 | 2.16 | 1.49-3.13 | <0.0001 |
Table 5.
Univariate and Multivariate Analysis for Non-CV Death After TAVR
| Univariate |
P Value | Multivariate |
P Value | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (per 1-y increase) | 1.04 | 1.00-1.07 | 0.039 | 1.03 | 0.99-1.07 | 0.090 |
| Woman | 0.57 | 0.41-0.80 | 0.002 | 0.52 | 0.36-0.76 | 0.0005 |
| Underweight | 2.29 | 1.58-3.33 | <0.0001 | 1.78 | 1.16-2.75 | 0.009 |
| Overweight | 0.69 | 0.45-1.07 | 0.087 | |||
| Hypertension | 0.54 | 0.38-0.77 | 0.004 | 0.68 | 0.45-1.01 | 0.15 |
| Diabetes mellitus | 1.12 | 0.77-1.64 | 0.56 | |||
| Cancer: previous or active | 1.52 | 1.04-2.22 | 0.37 | 1.32 | 0.86-2.03 | 0.20 |
| Previous stroke | 0.96 | 0.55-1.67 | 0.89 | |||
| COPD | 1.35 | 0.77-2.35 | 0.31 | |||
| Steroids use | 2.54 | 1.59-4.05 | 0.0004 | 2.31 | 1.37-3.90 | 0.002 |
| AF/AFL | 1.76 | 1.24-2.50 | 0.002 | 1.51 | 1.03-2.21 | 0.033 |
| CAS | 1.64 | 0.99-2.70 | 0.066 | |||
| PAD | 1.44 | 0.95-2.18 | 0.96 | |||
| Residual CAD | 1.19 | 0.83-1.69 | 0.34 | |||
| Previous MI | 0.69 | 0.31-1.57 | 0.35 | |||
| PMI/ICD | 0.81 | 0.38-1.74 | 0.58 | |||
| EuroSCOREⅡ (per 1% increase) | 1.03 | 1.001-1.04 | 0.012 | 0.97 | 0.94-1.00 | 0.052 |
| STS score (per 1% increase) | 1.08 | 1.06-1.10 | <0.0001 | 1.07 | 1.03-1.11 | 0.002 |
| eGFR (per 1-mL/1.73 m2/min increase) | 0.99 | 0.98-0.99 | 0.008 | 0.99 | 0.99-1.01 | 0.37 |
| Albumin (per 1-g/dL increase) | 0.24 | 0.17-0.35 | <0.0001 | 0.33 | 0.21-0.50 | <0.0001 |
| Ejection fraction (per 1% increase) | 0.99 | 0.97-1.01 | 0.18 | |||
| AV mean pressure gradient (per 1-mm Hg increase) | 0.99 | 0.98-0.99 | 0.045 | 0.99 | 0.99-1.01 | 0.38 |
| TF approach | 0.49 | 0.32-0.75 | 0.002 | 0.68 | 0.42-1.09 | 0.11 |
| Device success | 0.27 | 0.15-0.50 | 0.0005 | 0.48 | 0.23-1.01 | 0.054 |
| New-onset AF | 1.26 | 0.64-2.48 | 0.51 | |||
| Postprocedural PMI | 1.14 | 0.66-1.94 | 0.65 | |||
| PPM | 0.82 | 0.47-1.44 | 0.77 | |||
| 30-day combined endpoint | 2.47 | 1.58-3.87 | 0.0004 | 1.80 | 1.10-2.94 | 0.019 |
Table 4.
Univariate and Multivariate Analysis for CV Death After TAVR
| Univariate |
P Value | Multivariate |
P Value | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (per 1-y increase) | 1.02 | 0.98-1.06 | 0.35 | |||
| Woman | 0.58 | 0.38-0.86 | 0.009 | 0.54 | 0.35-0.85 | 0.007 |
| Underweight | 1.83 | 1.14-2.94 | 0.019 | 1.28 | 0.58-1.88 | 0.37 |
| Overweight | 0.36 | 0.18-0.71 | 0.0008 | 0.46 | 0.23-0.94 | 0.032 |
| Hypertension | 0.99 | 0.60-1.64 | 0.98 | |||
| Diabetes mellitus | 0.85 | 0.51-1.40 | 0.50 | |||
| Cancer: previous or active | 1.85 | 1.19-2.86 | 0.009 | |||
| Previous stroke | 1.49 | 0.85-2.64 | 0.19 | |||
| COPD | 1.61 | 0.88-2.95 | 0.15 | |||
| Steroids use | 0.50 | 0.16-1.58 | 0.19 | |||
| AF/AFL | 2.95 | 1.97-4.43 | <0.0001 | 2.97 | 1.89-4.67 | <0.0001 |
| CAS | 1.09 | 0.55-2.17 | 0.81 | |||
| PAD | 2.38 | 1.53-3.70 | 0.0003 | 1.89 | 1.13-3.07 | 0.014 |
| Residual CAD | 1.43 | 0.94-2.17 | 0.10 | |||
| Previous MI | 1.62 | 0.82-3.23 | 0.19 | |||
| PMI/ICD | 1.23 | 0.57-2.66 | 0.61 | |||
| EuroSCOREⅡ (per 1% increase) | 1.04 | 1.02-1.06 | 0.0001 | 1.02 | 0.99-1.05 | 0.16 |
| STS score (per 1% increase) | 1.07 | 1.04-1.10 | <0.0001 | 1.003 | 0.95-1.06 | 0.91 |
| eGFR (per 1-mL/1.73 m2/min increase) | 0.99 | 0.98-1.01 | 0.45 | |||
| Albumin (per 1-g/dL increase) | 0.37 | 0.24-0.58 | <0.0001 | 0.59 | 0.35-0.97 | 0.039 |
| Ejection fraction (per 1% increase) | 0.97 | 0.96-0.99 | 0.0009 | 1.006 | 0.98-1.03 | 0.58 |
| AV mean pressure gradient (per 1-mm Hg increase) | 0.99 | 0.99-1.01 | 0.69 | |||
| TF approach | 0.75 | 0.43-1.32 | 0.33 | |||
| Device success | 0.40 | 0.16-1.00 | 0.081 | |||
| New-onset AF | 1.30 | 0.57-2.97 | 0.56 | |||
| Postprocedural PMI | 0.92 | 0.46-1.84 | 0.82 | |||
| PPM | 0.78 | 0.39-1.57 | 0.76 | |||
| 30-day combined endpoint | 3.52 | 2.14-5.79 | <0.0001 | 2.70 | 1.51-4.81 | 0.0008 |
The median value of BMI in the underweight group was 17.6 kg/m2, and the survival of extreme underweight patients (BMI<17.6 kg/m2) was similar to that of nonextreme underweight patients (17.6 < BMI <18.5 kg/m2) (P = 0.77) (Supplemental Figure 2).
Discussion
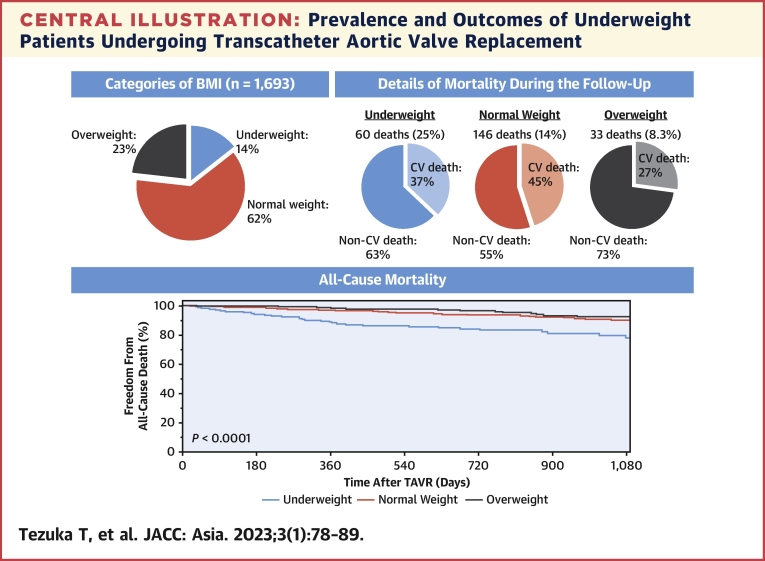
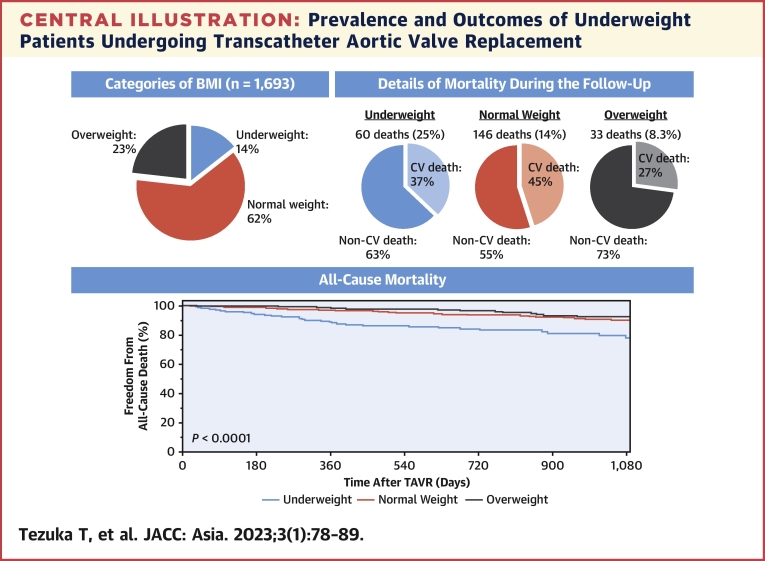
We analyzed the effect of underweight on TAVR outcomes (Central Illustration). Overall, 242 of 1,693 patients (14.3%) were underweight, with a BMI of <18.5 kg/m2. The baseline characteristics of the underweight group were different in several respects compared with the other groups. Despite a similar 30-day mortality rate, underweight patients had higher all-cause mortality (non-CV causes) than the other 2 groups.
Central Illustration.
Prevalence and Outcomes of Underweight Patients Undergoing Transcatheter Aortic Valve Replacement
Fourteen percent of study patients (n = 1,693) were underweight, and presented the worst survival after transcatheter aortic valve replacement (TAVR). Most mortalities were attributed to non-cardiovascular (CV) causes.
The strengths of this study were its number of underweight patients, long duration of patient follow-up, and cause of death analysis.
Underweight group in TAVR
According to World Health Organization criteria, underweight was defined as a BMI of <18.5 kg/m2.16 Previous studies included only 0% to 9% of underweight patients undergoing TAVR (Table 6).7, 8, 9,18 Compared with patients in Western countries, many Japanese patients undergoing TAVR have a lower BMI.13 The average BMI of our patients was 22.5, and 242 of the overall 1,693 (14.3%) patients were classified as underweight. In contrast, the average BMI of Western patients is 26 to 27 kg/m2.13 Some studies have revised the criteria of underweight upward to a BMI of <20 kg/m2,10, 11, 12,19 the criterion for frailty defined by the VARC-2. Even if the cutoff for underweight was a BMI of <20 kg/m2, only 6% to 9% of patients in Western countries would meet it (Table 6).10, 11, 12,19 Thus, the true prognostic impact of a lower BMI after TAVR, the left side of the BMI mortality curve, has not been fully elucidated. Different criteria for underweight might be needed for different populations. As an alternative to BMI, lean body mass was reportedly an excellent prognostic indicator after TAVR,20 but it requires complicated calculations. The modified BMI, which is calculated as BMI multiplied by serum albumin value, is also reportedly useful for stratifying prognosis after TAVR.21
Table 6.
Backgrounds and Outcome of Underweight Patients in the Previous and Current Studies
| Boukhris et al19 (n = 412) | Yamamoto et al9 (n = 3,072) | Sharma et al18 (n = 31,929) | Voigtländer et al12 (n = 16,865) | Tezuka et al (n = 1,693) | |
|---|---|---|---|---|---|
| Year of TAVR | 2009-2019 | 2010-2011 | 2011-2015 | 2011-2014 | 2010-2020 |
| Country | Canada | France | USA | Germany | Japan |
| UW patients, n (%) | 35 (8.5)a | 95 (3.1)b | 806 (2.5)b | 956 (5.7)a | 242 (14.3)b |
| Age (y) | 80 | 84 | 85 | 83 | 84 |
| Women | 66 | 79 | 71 | 75 | 76 |
| BSA (m2) | NA | 1.4 | 1.5 | NA | 1.3 |
| Hypertension | 83 | 57 | 80 | NA | 62 |
| PAD | 26 | 15 | 32 | NA | 22 |
| eGFR (mL/1.73 m2/min) | 39 | NA | 64 | NA | 57 |
| Albumin (g/dL) | 3.6 | NA | 3.6 | NA | 3.6 |
| Ejection fraction (%) | 61 | 54 | 56 | NA | 59 |
| STS score (%) | 6.7 | NA | 9.0 | 8.7 | 8.3 |
| TF approach | 63 | 78 | 53 | 71 | 88 |
| 30-d mortality | 8.6 | 17 | 11 | 7.6 | 1.7 |
| 1-y mortality | 11 | 32 | 35 | 27 | NA |
Similar to previous reports,8, 9, 10, 11, 12,18,19 the underweight group in our study had a higher prevalence of female sex and New York Heart Association functional class III/IV, and it was associated with a smaller mean aortic valve area, a lower mean ejection fraction, and higher operative scores. We might have hesitated to use curative therapy for AS in underweight patients because of their frailty and high operative risk.
Procedural details of TAVR in the underweight group
In the underweight group, the nontransfemoral approach was used more frequently and the incidence of vascular and bleeding complications was higher, similar to previous reports.8,9,11,12,18 A low BMI is associated with a small body surface area, and the small vessel size of a small body may be responsible for the higher incidence of the nontransfemoral approach and vascular complications. In addition, the nontransfemoral approach itself is associated with vascular complications.22 The higher percentage of the nontransfemoral approach and procedural complications in the underweight group probably led to a longer mean procedural time, extended postoperative intensive care unit stay, and lower home discharge rates. The latter 2 indices were associated with poor outcomes after TAVR.23,24 To reduce vascular and bleeding complications and improve the prognosis of underweight patients, a lower-profile transcatheter heart valve is needed. Nontransthoracic approaches, including the trans-subclavian and transaxillary, are promising therapeutic options in the underweight group for whom the transfemoral approach is unsuitable.22 The rate of 30-day mortality in the present study was much lower than that in the previous studies (Table 6). The possible rationale for this result includes more recent TAVR procedures included in the current research: higher percentage of transfemoral approach and the second-generation valve used, and more accumulated experience about TAVR planning.
Negative prognostic effect of underweight and suspected reasons
We followed patients undergoing TAVR for approximately 2 years, the longest follow-up duration of studies to date.9,11,12,18,19 Despite similar 30-day mortality rates, the underweight group had worse midterm outcomes in our analysis. Although the details of mortality were not analyzed in previous studies, our data showed that non-CV causes of mortality dominated, and the most common causes were respiratory disease, debilitation, and cancer. A low BMI is an important criterion for the diagnosis of frailty, which is associated with postdischarge mortality in patients hospitalized for pneumonia.25 The underweight group had worse lung function, and the therapeutic delay of AS in the underweight group might have accelerated the decline in lung function, a manifestation of physical frailty.26 Respiratory diseases, including interstitial pneumonitis, chronic obstructive pulmonary disease, and malignancy, could reduce body weight, and the association between a low BMI and respiratory and malignant diseases may involve an inverse causal relationship. The mean serum albumin level was lower in the underweight group and a negative prognostic factor after TAVR.27 Albumin is a major body protein, and hypoalbuminemia reflects low nutritional status, physical function, and immune function. Timely therapeutic intervention for AS is warranted to improve the outcomes of the underweight group. Physical and nutritional interventions for frailty should also improve the outcomes of underweight patients.28
Study limitations
First, it was an observational study, and possible confounding factors related to outcomes might not have been fully analyzed. Second, it used a BMI of <18.5 kg/m2 as the criterion for underweight. The distribution of BMI differs among populations, and a cutoff value of 18.5 kg/m2 may limit the generalizability of our results. Third, although it was not an objective of our study, physical indices other than BMI (ie, lean body mass, muscle mass, adipose distribution) might be more effective for stratifying the outcomes of patients undergoing TAVR. The temporal trends of body weight or BMI should be relevant to the prognosis after TAVR; however, our database had lacked the information.
Conclusions
The underweight group had worse midterm survival among patients undergoing TAVR.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Although the obesity paradox has been reported in TAVR, underweight patients were not included or numerically limited. The East Asian population undergoing TAVR has a lower mean BMI than those in Western Europe and North America. From the present analysis, underweight patients had a worse midterm prognosis, demonstrating the obesity paradox in this TAVR population.
TRANSLATIONAL OUTLOOK: Timely therapeutic intervention for AS is warranted to improve the outcomes of the underweight group. Physical and nutritional interventions for frailty should also improve the outcomes of underweight patients.
Funding Support and Author Disclosures
This study was supported by a Sakakibara Clinical Research Grant for Promotion of Sciences, 2021 (grant number: H-4-2021). Drs Takamisawa and Tobaru are clinical proctors of Edwards Lifesciences and Medtronics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors express their appreciation of the valve team members.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Whitlock G., Lewington S., Sherliker P., et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamaly S., Redfors B., Omerovic E., Carlsson L., Karason K. Prognostic significance of BMI after PCI treatment in ST-elevation myocardial infarction: a cohort study from the Swedish Coronary Angiography and Angioplasty Registry. Open Heart. 2021;8 doi: 10.1136/openhrt-2020-001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaduganathan M., Lee R., Beckham A.J., et al. Relation of body mass index to late survival after valvular heart surgery. Am J Cardiol. 2012;110:1667–1678. doi: 10.1016/j.amjcard.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Shah R., Gayat E., Januzzi J.L., Jr., et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–785. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 5.Khalid U., Ather S., Bavishi C., et al. Pre-morbid body mass index and mortality after incident heart failure: the ARIC Study. J Am Coll Cardiol. 2014;64:2743–2749. doi: 10.1016/j.jacc.2014.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elagizi A., Carbone S., Lavie C.J., Mehra M.R., Ventura H.O. Implications of obesity across the heart failure continuum. Prog Cardiovasc Dis. 2020;63:561–569. doi: 10.1016/j.pcad.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Ferreiro R., Muñoz-García A.J., López-Otero D., et al. Prognostic value of body mass index in transcatheter aortic valve implantation: A "J"-shaped curve. Int J Cardiol. 2017;232:342–347. doi: 10.1016/j.ijcard.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 8.van der Boon R.M., Chieffo A., Dumonteil N., et al. Effect of body mass index on short- and long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2013;111:231–236. doi: 10.1016/j.amjcard.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M., Mouillet G., Oguri A., et al. Effect of body mass index on 30- and 365-day complication and survival rates of transcatheter aortic valve implantation (from the FRench Aortic National CoreValve and Edwards 2 [FRANCE 2] registry) Am J Cardiol. 2013;112:1932–1937. doi: 10.1016/j.amjcard.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M., Hayashida K., Watanabe Y., et al. Effect of body mass index <20 kg/m(2) on events in patients who underwent transcatheter aortic valve replacement. Am J Cardiol. 2015;115:227–233. doi: 10.1016/j.amjcard.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Koifman E., Kiramijyan S., Negi S.I., et al. Body mass index association with survival in severe aortic stenosis patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2016;88:118–124. doi: 10.1002/ccd.26377. [DOI] [PubMed] [Google Scholar]
- 12.Voigtländer L., Twerenbold R., Schäfer U., et al. Prognostic impact of underweight (body mass index <20 kg/m2) in patients with severe aortic valve stenosis undergoing transcatheter aortic valve implantation or surgical aortic valve replacement (from the German Aortic Valve Registry [GARY]) Am J Cardiol. 2020;129:79–86. doi: 10.1016/j.amjcard.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Lee C.H., Inohara T., Hayashida K., Park D.W. Transcatheter aortic valve replacement in Asia: present status and future perspectives. JACC: Asia. 2021;1:279–293. doi: 10.1016/j.jacasi.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama H., Tobaru T., Muto Y., et al. Long-term outcomes in Japanese nonagenarians undergoing transcatheter aortic valve implantation: a multi-center analysis. Clin Cardiol. 2019;42:605–611. doi: 10.1002/clc.23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saji M., Tobaru T., Higuchi R., et al. Usefulness of the transcatheter aortic valve replacement risk score to determine mid-term outcomes. Circ J. 2019;83:1755–1761. doi: 10.1253/circj.CJ-18-1394. [DOI] [PubMed] [Google Scholar]
- 16.Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i-xii):1–253. PMID: 11234459. [PubMed] [Google Scholar]
- 17.Kappetein A.P., Head S.J., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A., Lavie C.J., Elmariah S., et al. Relationship of body mass index with outcomes after transcatheter aortic valve replacement: results from the National Cardiovascular Data-STS/ACC TVT Registry. Mayo Clin Proc. 2020;95:57–68. doi: 10.1016/j.mayocp.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Boukhris M., Forcillo J., Potvin J., et al. Does "obesity paradox" apply for patients undergoing transcatheter aortic valve replacement? Cardiovasc Revasc Med. 2022;38:1–8. doi: 10.1016/j.carrev.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Hioki H., Watanabe Y., Kozuma K., et al. Risk stratification using lean body mass in patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;92:1365–1373. doi: 10.1002/ccd.27547. [DOI] [PubMed] [Google Scholar]
- 21.Driggin E., Gupta A., Madhavan M.V., et al. Relation between modified body mass index and adverse outcomes after aortic valve implantation. Am J Cardiol. 2021;153:94–100. doi: 10.1016/j.amjcard.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhar J., Hibbert B., Ruel M., Lam B.K., Labinaz M., Glover C. Transfemoral vs non-transfemoral access for transcatheter aortic valve implantation: a systematic review and meta-analysis. Can J Cardiol. 2015;31:1427–1438. doi: 10.1016/j.cjca.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Muto Y., Higuchi R., Yoshihisa A., et al. Prevalence, predictors, and mid-term outcomes of non-home discharge after transcatheter aortic valve implantation. Circ Rep. 2020;2:617–624. doi: 10.1253/circrep.CR-20-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higuchi R., Takayama M., Hagiya K., et al. Prolonged intensive care unit stay following transcatheter aortic valve replacement. J Intensive Care Med. 2020;35:154–160. doi: 10.1177/0885066617732290. [DOI] [PubMed] [Google Scholar]
- 25.Kundi H., Wadhera R.K., Strom J.B., et al. Association of frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 2019;4:1084–1091. doi: 10.1001/jamacardio.2019.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaz Fragoso C.A., Enright P.L., McAvay G., Van Ness P.H., Gill T.M. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M., Shimura T., Kano S., et al. Prognostic value of hypoalbuminemia after transcatheter aortic valve implantation (from the Japanese Multicenter OCEAN-TAVI Registry) Am J Cardiol. 2017;119:770–777. doi: 10.1016/j.amjcard.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Ijaz N., Buta B., Xue Q.L., et al. Interventions for frailty among older adults with cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.