Abstract
Background
With improved cancer survival, death from noncancer etiologies, especially cardiovascular disease (CVD) mortality, has come more into focus. Little is known about the racial and ethnic disparities in all-cause and CVD mortality among U.S. cancer patients.
Objectives
This study sought to investigate racial and ethnic disparities in all-cause and CVD mortality among adults with cancer in the United States.
Methods
Using the Surveillance, Epidemiology, and End Results (SEER) database from years 2000 to 2018, all-cause and CVD mortality among patients ≥18 years of age at the time of initial malignancy diagnosis were compared by race and ethnicity groups. The 10 most prevalent cancers were included. Cox regression models were used to estimate adjusted HRs for all-cause and CVD mortality using Fine and Gray’s method for competing risks, as applicable.
Results
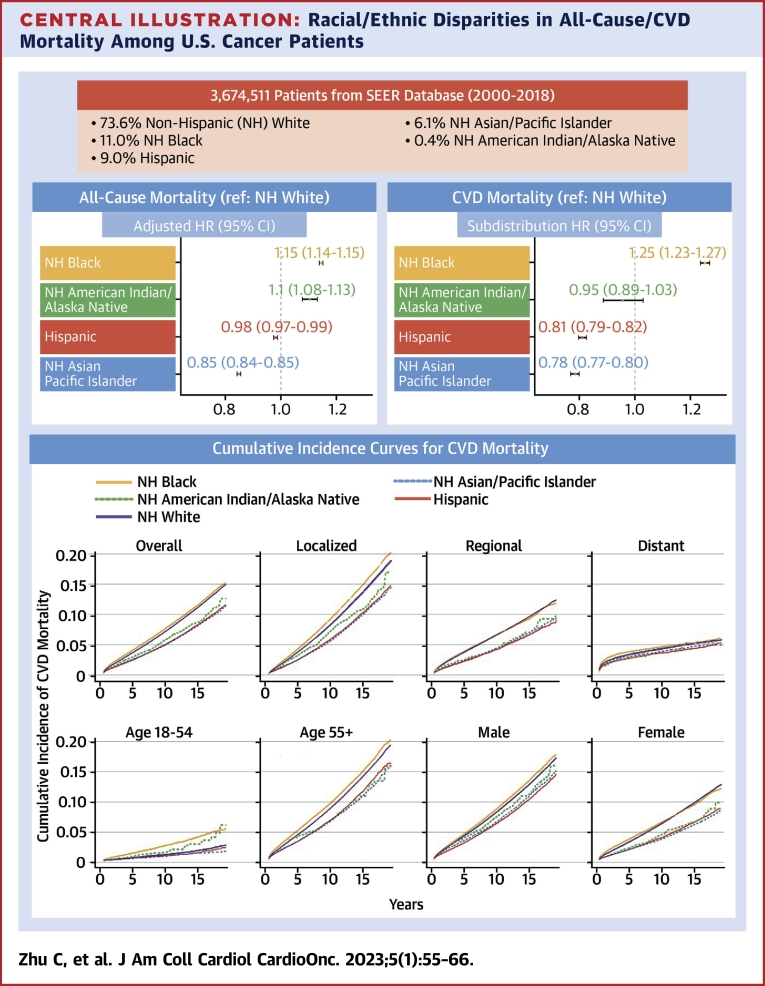
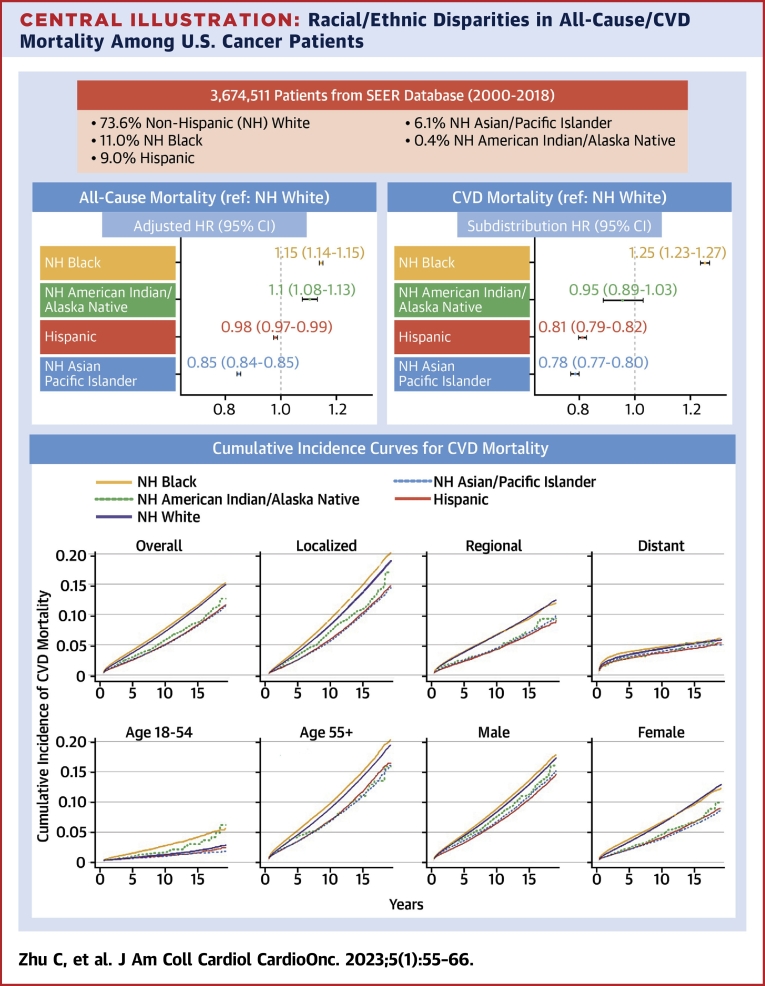
Among a total of 3,674,511 participants included in our study, 1,644,067 (44.7%) died, with 231,386 (6.3%) deaths as a result of CVD. After adjusting for sociodemographic and clinical characteristics, non-Hispanic (NH) Black individuals had both higher all-cause (HR: 1.13; 95% CI: 1.13-1.14) and CVD (HR: 1.25; 95% CI: 1.24-1.27) mortality, whereas Hispanic and NH Asian/Pacific Islander had lower mortality than NH White patients. Racial and ethnic disparities were more prominent among patients 18 to 54 years of age and those with localized cancer.
Conclusions
Significant racial and ethnic differences exist in both all-cause and CVD mortality among U.S. cancer patients. Our findings underscore the vital roles of accessible cardiovascular interventions and strategies to identify high-risk cancer populations who may benefit most from early and long-term survivorship care.
Key Words: cancer survivorship, cardio-oncology, outcomes, racial and ethnic disparities
Abbreviations and Acronyms: CVD, cardiovascular disease; SEER, Surveillance, Epidemiology, and End Results; NH, non-Hispanic
Central Illustration
Cancer is the second most common cause of death worldwide after cardiovascular disease (CVD).1 While short-term survival for cancer has improved due to advances in screening and treatment, cardiovascular care has become increasingly important as more individuals are living long term with cancer as well as other comorbidities.
Cancer survivors may be more vulnerable to CVD either due to common risk factors such as obesity, smoking, and alcohol use,2 or from early or late cardiotoxicity of cancer treatment.3 Compared with the general U.S. population, cancer survivors had 4 times the risk of dying from CVD within the first year after cancer diagnosis, and CVD risk remained elevated throughout survivorship.4
The disparity in CVD mortality may be further amplified by structural racism, defined as legitimization of systems and dynamics that routinely disadvantage historically marginalized groups.5 Prior studies conducted in breast cancer survivors have consistently reported a disproportionately increased CVD mortality among racially and ethnically underserved populations.6, 7, 8, 9 Specifically, Berkman et al7 analyzed the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) registry data from 1990 to 2010 and found that Black women 50 to 59 years of age with in situ breast cancer were at 3.06 times the risk of all-cause mortality and 6.43 times the risk of CVD death compared with their White counterparts.
Recent calls have been made to intensify research efforts to identify and overcome determinants of existing racial and ethnic disparities at the intersection between cancer and cardiology.10 Although prior studies focused on specific cancer types and within a limited scope of population,6,7,9, 10, 11, 12 or compared only between non-Hispanic (NH) White and NH Black individuals,6,7 there is limited information about the race and ethnic disparities in all-cause and CVD mortality among adult cancer patients in the United States. To this end, the objective of this study was to determine whether all-cause and CVD mortality differ among race and ethnicity subgroups of adult cancer survivors in the United States.
Methods
Study population
The SEER program is a network of U.S. population-based incident tumor registries that collect data on patient demographics, county-level socioeconomic status, primary tumor site, stage at diagnosis, initial course of treatment, follow-up time, and survival. From the SEER data, our study identified individuals 18 years of age or older and with 1 of the 10 most prevalent malignant cancers (breast, prostate, lung and bronchus, colorectal, melanoma of skin, non-Hodgkin lymphoma, kidney and renal pelvis, corpus uteri, urinary bladder, larynx) diagnosed between 2000 and 2018. The SEER 18 registry dataset represents 28% of the U.S. population based on yearly census data and captures approximately 97% of incident cases within the registry area.13 Participants with stage of in situ, with unknown race and ethnicity (<1%), or with unknown survival time (<1%) were excluded.
Data for this study were assembled using SEER∗Stat software (version 8.3.9.2).14 As determined by our Institutional Review Board, this study was exempt from Institutional Review Board review, as the dataset is publicly available and de-identified.
Key variables
The primary exposure was race and ethnicity group, categorized as the following 5 groups: NH White, NH Black, Hispanic, NH Asian/Pacific Islander, and NH American Indian/Alaskan Native. In the SEER registries, race was determined by a combination of sources, including medical records, fact sheets, physician and nursing notes, photographs, and any other sources. Ethnicity was determined by the North American Association of Central Cancer Registries Hispanic Identification Algorithm that uses a combination of variables including surname, birthplace, and county attributes.15 Prior studies have found the accuracy of SEER data elements to be high, with a reportedly almost perfect agreement between self-reported race and ethnicity and registry documentation.16
The outcomes of interest were all-cause and CVD mortality. Vital status and underlying cause of death were obtained from the SEER registry that links to both state vital records and the National Death Index annually. CVD death was defined by the International Classification of Diseases–Tenth Revision codes and is described in Supplemental Table 1.
Covariates were selected a priori based on availability in the SEER registry dataset and medical relevancy. Specifically, we included sex, age at diagnosis, marital status, cancer site, extent of cancer at presentation (ie, stage), year of cancer diagnosis, county-level median income, urban/rural residence, and the initial course of treatment in the multivariable models. Age at diagnosis was categorized into 5 groups (18-39 years, 40-54 years, 55-64 years, 65-74 years, and 75 years and over). Marital status was defined as married (including common-law marriage as defined in SEER) or unmarried. County median income level was dichotomized into 2 groups (<$60,000, ≥$60,000) based on the SEER-linked county-level data regarding the median household income in the past 12 months using 2019 inflation-adjusted dollars.17 Residence was classified as urban for counties in metropolitan areas or rural for other counties, defined by the Rural-Urban Continuum Code developed by the U.S. Department of Agriculture.18 The extent of cancer was categorized as local (no nodal or metastatic disease), regional (nodal disease), or distant (any metastatic disease). The initial course of treatment was determined based on whether patients received surgery (yes, no), chemotherapy (yes, no/unknown), or radiation therapy (yes, no/unknown). Chemotherapy and radiation therapy were categorized as “yes—treatment received” or “no/unknown” according to the SEER registry. The latter classification combined “no” and “unknown” in the original dataset and may include a mixture of participants who did and did not receive the specific treatment modality. A secondary analysis was conducted among participants who had received either treatment.
Statistical analysis
Categorical data are presented using counts with percentages and compared using the chi-square test. Differences in survival rates across racial and ethnic groups were estimated and compared using the Kaplan-Meier method and log-rank test, respectively. Cox proportional hazards regression models were used to estimate HR and 95% CIs for the associations of racial and ethnic groups with all-cause mortality while adjusting for prespecified covariates. The proportional hazards assumption was confirmed by graphical inspection of the log-log survival curves in the fully adjusted model (Supplemental Figure 1). For CVD mortality, we used the Fine and Gray’s19 competing risk method to account for non-CVD mortality as a competing event, with the same set of covariates adjusted, including sex, age at diagnosis, marital status, cancer site, extent of cancer at presentation, year of cancer diagnosis, county-level median income, urban/rural residence, and the initial course of treatment.
To explore the effect of race and ethnicity on mortality within distinct patient subgroups, subgroup analyses were carried out by sex (male, female), age at diagnosis (18-54 years, 55+ years), and extent of cancer at presentation (localized, regional, distant). The presence of a subgroup interaction was evaluated by the significance level of the interaction terms (ie, race × sex, race × age, race × stage) in the overall model. Secondary analyses were conducted among those who survived 5 years and those who received surgery, radiation, or chemotherapy as treatment. Effects of race and ethnicity on mortality for patients diagnosed with specific cancer (breast, prostate, lung and bronchus, colorectal, melanoma of skin, non-Hodgkin lymphoma, kidney and renal pelvis, corpus uteri, urinary bladder, larynx) were also explored.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc) and RStudio version 1.4.1106) (RStudio Inc). All statistical tests were 2-sided, with the significance level set at a P value of <0.05.
Results
Participant characteristics
A total of 3,674,511 patients were included, and 73.6% were NH White, 11.0% were NH Black, 9.0% were Hispanic, 6.1% were NH Asian/Pacific Islander, and 0.4% were NH American Indian/Alaska Native. Table 1 shows the distribution of participant characteristics by race and ethnicity. Overall, race and ethnicity subgroups had significantly different distributions of sex, age, marital status, income and rural/urban residence. Compared with NH White patients, NH Black, Hispanic, NH Asian/Pacific Islander, and NH American Indian/Alaskan Native patients were more likely to have regional and distant cancer at diagnosis. While about 63.1% of all study participants received surgery, it was least frequently reported in NH Black patients (54.0%). The proportions of radiation recipients were similar between NH White (32.2%), Hispanic (30.5%), NH Asian/Pacific Islander (33.9%), and NH American Indian/Alaska Native (32.3%) individuals, but higher in NH Black individuals (36.0%). Chemotherapy was least common among NH White (26.1%) individuals, and most frequently reported in NH Asian/Pacific Islander (34.0%) individuals. The time trends in covariates were plotted in Supplemental Figure 2.
Table 1.
Participant Characteristics by Race and Ethnicity From the 2000 to 2018 SEER 18 Registries Database
| NH White (n = 2,702,870) | NH Black (n = 403,355) | Hispanic (n = 329,600) | NH Asian/Pacific Islander (n = 224,358) | NH American Indian/Alaska Native (n = 14,328) | Overall (N = 3,674,511) | P Value | |
|---|---|---|---|---|---|---|---|
| Sex | <0.001 | ||||||
| Female | 1,326,282 (49.1) | 181,960 (45.1) | 173,242 (52.6) | 125,769 (56.1) | 7,708 (53.8) | 1,814,961 (49.4) | |
| Male | 1,376,588 (50.9) | 221,395 (54.9) | 156,358 (47.4) | 98,589 (43.9) | 6,620 (46.2) | 1,859,550 (50.6) | |
| Age | <0.001 | ||||||
| 18-39 y | 81,700 (3.0) | 14,664 (3.6) | 21,659 (6.6) | 10,443 (4.7) | 761 (5.3) | 129,227 (3.5) | |
| 40-54 y | 460,934 (17.1) | 90,307 (22.4) | 81,890 (24.8) | 50,432 (22.5) | 3,335 (23.3) | 686,898 (18.7) | |
| 55-64 y | 708,995 (26.2) | 126,410 (31.3) | 86,010 (26.1) | 56,244 (25.1) | 4,098 (28.6) | 981,757 (26.7) | |
| 65-74 y | 782,970 (29.0) | 108,606 (26.9) | 83,294 (25.3) | 59,140 (26.4) | 3,836 (26.8) | 1,037,846 (28.2) | |
| ≥75 y | 668,271 (24.7) | 63,368 (15.7) | 56,747 (17.2) | 48,099 (21.4) | 2,298 (16.0) | 838,783 (22.8) | |
| Marital status | <0.001 | ||||||
| Married | 1,582,170 (58.5) | 164,588 (40.8) | 185,889 (56.4) | 149,135 (66.5) | 6,825 (47.6) | 2,088,607 (56.8) | |
| Unmarried | 913,765 (33.8) | 209,126 (51.8) | 120,142 (36.5) | 63,414 (28.3) | 6,221 (43.4) | 1,312,668 (35.7) | |
| Unknown | 206,935 (7.7) | 29,641 (7.3) | 23,569 (7.2) | 11,809 (5.3) | 1,282 (8.9) | 273,236 (7.4) | |
| Cancer Stage | <0.001 | ||||||
| Localized | 1,586,373 (58.7) | 221,136 (54.8) | 184,188 (55.9) | 120,555 (53.7) | 7,727 (53.9) | 2,119,979 (57.7) | |
| Regional | 58,5411 (21.7) | 92,629 (23.0) | 82,367 (25.0) | 55,849 (24.9) | 3,469 (24.2) | 819,725 (22.3) | |
| Distant | 531,086 (19.6) | 89,590 (22.2) | 63,045 (19.1) | 47,954 (21.4) | 3,132 (21.9) | 734,807 (20.0) | |
| Cancer site | <0.001 | ||||||
| Breast | 615,453 (22.8) | 92,768 (23.0) | 91,528 (27.8) | 69,072 (30.8) | 3,554 (24.8) | 872,375 (23.7) | |
| Colorectal | 235,166 (8.7) | 39,693 (9.8) | 36,348 (11.0) | 30,236 (13.5) | 1,648 (11.5) | 343,091 (9.3) | |
| Corpus uteri | 123,831 (4.6) | 16,586 (4.1) | 20,039 (6.1) | 13,828 (6.2) | 958 (6.7) | 175,242 (4.8) | |
| Kidney and renal pelvis | 121,038 (4.5) | 19,378 (4.8) | 24,315 (7.4) | 9,146 (4.1) | 1,299 (9.1) | 175,176 (4.8) | |
| Larynx | 29,329 (1.1) | 6,326 (1.6) | 3,265 (1.0) | 1,308 (0.6) | 158 (1.1) | 40,386 (1.1) | |
| Lung and bronchus | 500,141 (18.5) | 74,163 (18.4) | 36,693 (11.1) | 39,558 (17.6) | 2,335 (16.3) | 652,890 (17.8) | |
| Melanoma of the skin | 229,274 (8.5) | 1,108 (0.3) | 8,071 (2.4) | 1,590 (0.7) | 514 (3.6) | 240,557 (6.5) | |
| Non-Hodgkin lymphoma | 159,793 (5.9) | 17,704 (4.4) | 26,572 (8.1) | 14,706 (6.6) | 864 (6.0) | 219,639 (6.0) | |
| Prostate | 601,607 (22.3) | 128,454 (31.8) | 75,837 (23.0) | 40,306 (18.0) | 2,666 (18.6) | 848,870 (23.1) | |
| Urinary bladder | 87,238 (3.2) | 7,175 (1.8) | 6,932 (2.1) | 4,608 (2.1) | 332 (2.3) | 106,285 (2.9) | |
| Surgery | <0.001 | ||||||
| Yes | 1,727,678 (63.9) | 217,968 (54.0) | 216,920 (65.8) | 146,951 (65.5) | 9,113 (63.6) | 2,318,630 (63.1) | |
| No | 975,192 (36.1) | 185,387 (46.0) | 112,680 (34.2) | 77,407 (34.5) | 5,215 (36.4) | 1,355,881 (36.9) | |
| Radiation | <0.001 | ||||||
| Yes | 871,158 (32.2) | 145,279 (36.0) | 100,444 (30.5) | 76,049 (33.9) | 4,628 (32.3) | 1,197,558 (32.6) | |
| No/unknown | 1,831,712 (67.8) | 258,076 (64.0) | 229,156 (69.5) | 148,309 (66.1) | 9,700 (67.7) | 2,476,953 (67.4) | |
| Chemotherapy | <0.001 | ||||||
| Yes | 704,469 (26.1) | 116,886 (29.0) | 103,070 (31.3) | 76,358 (34.0) | 4,570 (31.9) | 1,005,353 (27.4) | |
| No/unknown | 1,998,401 (73.9) | 286,469 (71.0) | 226,530 (68.7) | 148,000 (66.0) | 9,758 (68.1) | 2,669,158 (72.6) | |
| Median income | <0.001 | ||||||
| <$60,000 | 818,558 (30.3) | 170,058 (42.2) | 68,620 (20.8) | 13,380 (6.0) | 6,792 (47.4) | 1,077,408 (29.3) | |
| ≥$60,000 | 1,884,312 (69.7) | 233,297 (57.8) | 260,980 (79.2) | 210,978 (94.0) | 7,536 (52.6) | 2,597,103 (70.7) | |
| Living in a metropolitan area | <0.001 | ||||||
| Yes | 2,318,789 (85.8) | 367,609 (91.1) | 316,135 (95.9) | 216,232 (96.4) | 10,761 (75.1) | 3,229,526 (87.9) | |
| No | 384,081 (14.2) | 35,746 (8.9) | 13,465 (4.1) | 8,126 (3.6) | 3,567 (24.9) | 444,985 (12.1) |
Values are n (%).
NH = non-Hispanic; SEER = Surveillance, Epidemiology, and End Results.
All-cause mortality
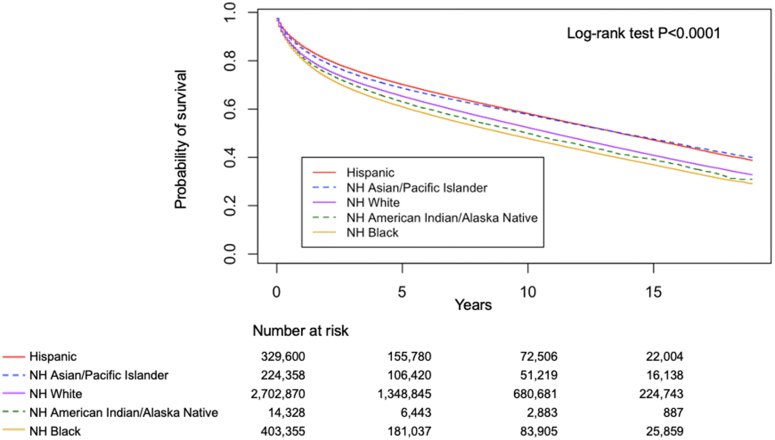
Among participants included in our study, a total of 1,644,067 (crude rate: 44.7%) died. The overall median survival time was 4.8 (range: 0-19; IQR: 1.6-9.8) years, with NH White individuals surviving the longest (4.9 years), followed by NH Asian/Pacific Islander individuals (4.6 years), Hispanic individuals (4.5 years), and NH Black and NH American Indian/Alaska Native individuals having the lowest (both 4.2 years) (Table 2). Risk of death from any cause was significantly different among race and ethnicity subgroups (P < 0.001) (Figure 1).
Table 2.
Cumulative Incidence of Death by Race and Ethnicity From the 2000 to 2018 SEER 18 Registries Database
| Populationa | Cumulative Incidence of Death (95% CI)b |
||||
|---|---|---|---|---|---|
| 2 Years (%) | 5 Years (%) | 10 Years (%) | 18 Years (%) | ||
| All-cause mortality | |||||
| NH White | 1,235,934 (45.7) | 23.9 (23.9-24.0) | 34.8 (34.7-34.9) | 47.8 (47.7-47.9) | 65.6 (65.5-65.7) |
| NH Black | 195,586 (48.5) | 27.1 (27-27.3) | 39.2 (39.1-39.4) | 52.3 (52.1-52.5) | 69.5 (60.2-69.9) |
| Hispanic | 120,757 (36.6) | 19.6 (19.5-19.8) | 29.9 (29.7-30.1) | 42 (41.8-42.2) | 59.6 (59.2-60.0) |
| NH Asian/Pacific Islander | 85,264 (38.0) | 21.1 (21-21.3) | 31.5 (31.3-31.7) | 42.4 (42.1-42.6) | 58.5 (58.1-59.0) |
| NH American Indian/Alaska Native | 6,526 (45.5) | 25.2 (24.5-26) | 37.1 (36.2-37.9) | 50.2 (49.2-51.1) | 68.8 (66.9-70.6) |
| CVD mortality | |||||
| NH White | 180,069 (6.7) | 1.9 (1.9-1.9) | 3.8 (3.8-3.9) | 7.4 (7.4-7.4) | 14.2 (14.1-14.3) |
| NH Black | 27,090 (6.7) | 2.2 (2.2-2.3) | 4.3 (4.2-4.3) | 7.9 (7.8-8) | 14.6 (14.3-14.8) |
| Hispanic | 13,822 (4.2) | 1.2 (1.2-1.3) | 2.6 (2.5-2.6) | 5.2 (5.1-5.3) | 10.9 (10.6-11.2) |
| NH Asian/Pacific Islander | 9,711 (4.3) | 1.4 (1.3-1.4) | 2.6 (2.6-2.7) | 5.1 (4.9-5.2) | 10.5 (10.2-10.8) |
| NH American Indian/Alaska Native | 694 (4.8) | 1.5 (1.3-1.7) | 3.1 (2.8-3.4) | 5.9 (5.4-6.4) | 12.5 (10.9-14.2) |
| Cancer mortality | |||||
| NH White | 746,315 (27.6) | 19.5 (19.5-19.6) | 25.7 (25.6-25.7) | 30.3 (30.2-30.3) | 34.6 (34.5-34.7) |
| NH Black | 125,845 (31.2) | 22.1 (22.0-22.3) | 29.7 (29.5-29.8) | 34.9 (34.7-35.1) | 40.0 (39.7-40.2) |
| Hispanic | 77,802 (23.6) | 16.0 (15.9-16.2) | 22.6 (22.5-22.8) | 27.9 (27.7-28.0) | 32.8 (32.5-33.1) |
| NH Asian/Pacific Islander | 57,260 (25.5) | 17.6 (17.4-17.7) | 24.6 (24.4-24.8) | 29.3 (29.1-29.5) | 33.4 (33.1-33.7) |
| NH American Indian/Alaska Native | 4,288 (29.9) | 21.0 (20.3-21.7) | 28.6 (27.8-29.4) | 33.9 (33.0-34.8) | 39.5 (38.0-41.0) |
Values are n (%), unless otherwise indicated.
CVD = cardiovascular disease; other abbreviations as in Table 1.
Crude number and rate estimates of mortality from the 2000-2018 SEER data.
All-cause mortality estimated by Kaplan-Meier methods; CVD and cancer mortality estimated by Fine and Gray’s competing risk method.
Figure 1.
All-Cause Mortality by Race and Ethnicity
Survival rates estimated and compared using the Kaplan-Meier method and log-rank test across racial and ethnical groups including non-Hispanic (NH) White (purple solid line), NH Black (orange solid line), Hispanic (red solid line), NH American Indian/Alaska Native (green dashed line), and NH Asian/Pacific Islander (blue dashed line). NH Black individuals had the highest all-cause mortality among all racial and ethnic groups.
After adjusting for demographic and clinical characteristics, all-cause mortality was significantly different among race and ethnicity subgroups. Compared with NH White individuals, NH Black and NH American Indian/Alaska Native individuals had a 15.0% and 10.0% increased risk of all-cause death, respectively (for NH Black, HR: 1.15; 95% CI: 1.14-1.15; NH American Indian/Alaska Native, HR: 1.10; 95% CI: 1.08-1.13). Conversely, Hispanic (HR: 0.98; 95% CI: 0.97-0.99) and NH Asian/Pacific Islander (HR 0.85; 95% CI: 0.84-0.85) patients had lower all-cause mortality (Table 3).
Table 3.
Effect of Race on All-Cause/CVD Mortality, Overall and by Cancer Stage, Age, and Sex
| All-Cause Mortality Adjusted HR (95% CI)a | CVD Mortality Subdistribution HR (95% CI)b | |
|---|---|---|
| Overall | ||
| NH White | ref | ref |
| NH Black | 1.15 (1.14-1.15)c | 1.25 (1.23-1.27)c |
| Hispanic | 0.98 (0.97-0.99)c | 0.81 (0.79-0.82)c |
| NH Asian/Pacific Islander | 0.85 (0.84-0.85)c | 0.78 (0.77-0.80)c |
| NH American Indian/Alaska Native | 1.10 (1.08-1.13)c | 0.95 (0.89-1.03) |
| By aged | ||
| 18-54 y | ||
| NH White | ref | ref |
| NH Black | 1.33 (1.31-1.36)c | 1.76 (1.67-1.85)c |
| Hispanic | 1.11 (1.09-1.13)c | 0.79 (0.73-0.85)c |
| NH Asian/Pacific Islander | 0.91 (0.89-0.94)c | 0.85 (0.77-0.94)e |
| NH American Indian/Alaska Native | 1.25 (1.15-1.35)c | 1.23 (0.96-1.57) |
| 55 y and older | ||
| NH White | ref | Ref |
| NH Black | 1.12 (1.11-1.13)c | 1.01 (0.99-1.02) |
| Hispanic | 0.97 (0.95-0.98)f | 0.75 (0.74-0.77)c |
| NH Asian/Pacific Islander | 0.83 (0.82-0.85)c | 0.78 (0.76-0.8)c |
| NH American Indian/Alaska Native | 1.08 (1.03-1.13)f | 0.80 (0.74-0.87)c |
| By sexg | ||
| Male | ||
| NH White | Ref | ref |
| NH Black | 1.11 (1.1-1.12)c | 1.25 (1.23-1.28)c |
| Hispanic | 0.98 (0.97-0.99)c | 0.80 (0.78-0.82)c |
| NH Asian/Pacific Islander | 0.87 (0.87-0.88)c | 0.81 (0.79-0.83)c |
| NH American Indian/Alaska Native | 1.10 (1.07-1.14)c | 0.98 (0.89-1.08) |
| Female | ||
| NH White | ref | ref |
| NH Black | 1.19 (1.18-1.19)c | 1.24 (1.21-1.27)c |
| Hispanic | 0.98 (0.97-0.99)c | 0.83 (0.81-0.85)c |
| NH Asian/Pacific Islander | 0.82 (0.81-0.83)c | 0.76 (0.74-0.79)c |
| NH American Indian/Alaska Native | 1.11 (1.07-1.15)c | 0.93 (0.83-1.05) |
| By stageh | ||
| Localized | ||
| NH White | ref | ref |
| NH Black | 1.24 (1.23-1.25)c | 1.25 (1.23-1.27)c |
| Hispanic | 0.95 (0.94-0.96)c | 0.81 (0.79-0.82)c |
| NH Asian/Pacific Islander | 0.82 (0.81-0.83)c | 0.76 (0.74-0.78)c |
| NH American Indian/Alaska Native | 1.21 (1.16-1.26)c | 0.99 (0.90-1.08) |
| Regional | ||
| NH White | ref | ref |
| NH Black | 1.18 (1.17-1.19)c | 1.21 (1.18-1.25)c |
| Hispanic | 1.00 (0.99-1.01) | 0.78 (0.75-0.81)c |
| NH Asian/Pacific Islander | 0.88 (0.87-0.89)c | 0.77 (0.74-0.80)c |
| NH American Indian/Alaska Native | 1.12 (1.06-1.17)c | 0.87 (0.73-1.03) |
| Distant | ||
| NH White | ref | ref |
| NH Black | 1.02 (1.02-1.03)c | 1.27 (1.22-1.31)c |
| Hispanic | 0.96 (0.95-0.97)c | 0.84 (0.80-0.88)c |
| NH Asian/Pacific Islander | 0.85 (0.84-0.86)c | 0.93 (0.88-0.98)e |
| NH American Indian/Alaska Native | 1.01 (0.97-1.05) | 0.94 (0.77-1.15) |
Cox regression model adjusting for demographics (sex, age at diagnosis, marital status, year of diagnosis, median household income, rural/urban residence), cancer stage, cancer site, and initial treatment (surgery, radiation, chemotherapy).
Fine and Gray’s competing risk method (subdistribution hazard function) adjusting for same covariates.
P < 0.001.
Interactions between age and race and ethnicity groups were all significant (P < 0.001).
P < 0.01.
P < 0.05.
Interactions between sex and race and ethnicity groups were all significant (P < 0.001) except for sex × NH American Indian/Alaska Native.
Interactions between stage and race and ethnicity groups were all significant (P < 0.001) except for stage × NH American Indian/Alaska Native.
The association between race and ethnicity group and all-cause mortality differed between those 18 to 54 years of age and those 55 years of age and older (Pinteraction < 0.001). Substantial racial and ethnic disparities in all-cause mortality were observed among patients 18 to 54 years of age, in which NH Black and NH American Indian/Alaska Native individuals had a 33.0% and 25.0% increased risk compared with NH White individuals. Within this age group, Hispanic patients also had increased risk of all-cause mortality compared with NH White individuals. Subgroup analyses evaluating sex suggested similar results as overall models (Table 3).
When evaluated by cancer stage, NH Black and NH American Indian/Alaska Native individuals who were diagnosed with localized cancer had a 24.0% and 21.0% increased risk of all-cause mortality compared with NH White individuals. Among regional cancer patients, NH Black and NH American Indian/Alaska Native individuals had a 18.0% and 12.0% increased risk, respectively, while among individuals who were diagnosed with distant cancer, NH Black and NH American Indian/Alaska Native individuals only had a 2.0% and 1.0% increased risk, respectively (Supplemental Table 3).
CVD mortality
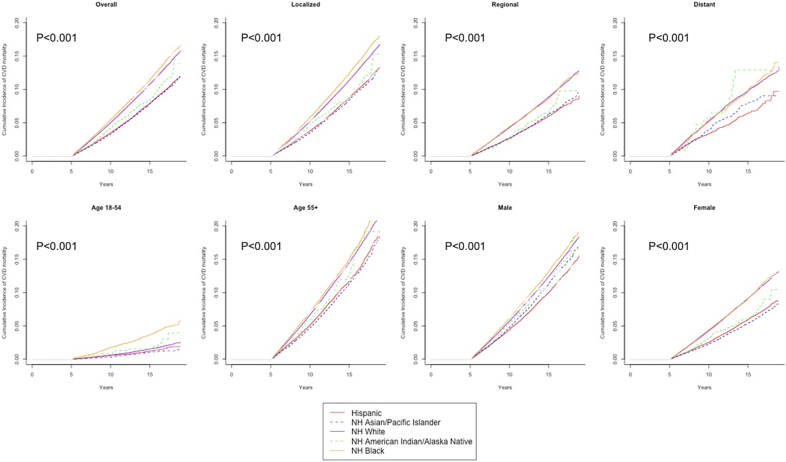
Among participants in our study, 231,386 (crude rate 6.3%) died as a result of CVD, constituting 14.1% of the total mortality. Description of specific CVD cause of death can be found in Supplemental Table 1. Crude CVD mortality was highest among NH White and Black individuals, followed by NH American Indian/Alaska Native, NH Asian/Pacific Islander, and Hispanic individuals (Table 2). The cumulative incidence of CVD mortality according to race and ethnicity group is shown in Table 2. Figure 2 further demonstrates the racial and ethnic disparities overall and by prognostic subgroups (ie, stage, age, and sex).
Figure 2.
Cumulative Incidence Curves for CVD Mortality
Cumulative incidence curves for overall cardiovascular disease (CVD) mortality and CVD mortality by disease stage, age group, and sex among different racial and ethnical groups including non-Hispanic (NH) White (purple solid line), NH Black (yellow solid line), Hispanic (red solid line), NH American Indian/Alaska Native (green dashed line), and NH Asian/Pacific Islander (blue dashed line). NH Black individuals had highest CVD mortality among all racial and ethnic groups. P values from the nonparametric Fine and Gray test.
Racial and ethnic disparities in CVD mortality remained after adjustment for demographic and clinical characteristics. NH Black individuals had a 25.0% increased cumulative risk of CVD death (HR: 1.25; 95% CI: 1.23-1.27) compared with NH White individuals, while Hispanic (HR: 0.81; 95% CI: 0.79-0.82), NH Asian/Pacific Islanders (HR: 0.78; 95% CI: 0.78-0.80) and NH American Indian/Alaska Native (HR: 0.95; 95% CI: 0.89-1.03) individuals had a lower risk (Table 3).
The interaction between each race and ethnicity group and age group was significant (all Pinteraction < 0.001).When analyzed by age groups, among patients 18 to 54 years of age, NH Black individuals had a 76.0% increase in cumulative risk of CVD mortality compared (HR: 1.76; 95% CI: 1.67-1.85) with NH White individuals; Hispanic and NH Asian/Pacific Islander individuals had lower risk (HR: 0.79; 95% CI: 0.73-0.85, and HR: 0.85; 95% CI: 0.77-0.95, respectively). Among patients 55 years of age and older, the risk of CVD mortality was lower among Hispanic, NH Asian/Pacific Islander, and NH American Indian/Alaska Native patients compared with NH White patients, but not significantly different in NH Black patients. Sex and disease stage subgroup analyses yielded similar results as overall models (Table 3). We found similar results in the secondary analysis restricting participants to those who survived 5 years postdiagnosis (Supplemental Table 2, Supplemental Figures 3 and 4).
When analyzed by disease site, the disparity patterns were mostly consistent with the findings in the overall cancer population. NH Black individuals experienced the worst CVD mortality in kidney and renal pelvis cancers, and non-Hodgkin’s lymphoma. Interestingly, however, for NH Black individuals with lung and bronchus cancers, although CVD mortality was almost 25.0% worse than NH White individuals (subdistribution HR: 1.23; 95% CI: 1.19-1.28), all-cause mortality was lower than NH White individuals (HR: 0.95; 95% CI: 0.94-0.96). Among several cancer sites (breast, corpus uteri, melanoma, non-Hodgkin’s lymphoma), Hispanic individuals experienced worse all-cause mortality but better CVD mortality as compared with NH White individuals (Supplemental Table 3).
In the secondary analysis for the treatment-only subpopulations, disparity patterns remained the same; however, the NH American Indian/Alaska Native group may have been underpowered to detect differences due to sample size (Supplemental Table 4).
Discussion
Our study showed that NH Black individuals had higher risk of all-cause and CVD mortality, while individuals who were Hispanic or NH Asian/Pacific Islander had lower risk, compared with their NH White counterparts. Racial and ethnic differences were greater among younger patients and those with localized cancer. Our findings underscore the vital role of accessible cardio-oncologic interventions at the early stage and highlight the need to identify high-risk populations who may benefit most from long-term survivorship care (Central Illustration).
Central Illustration.
Racial/Ethnic Disparities in All-Cause/CVD Mortality Among U.S. Cancer Patients
In this national population-based study, we found significant racial and ethnic differences in both all-cause and cardiovascular disease (CVD)–specific mortality among U.S. adult cancer patients. Our findings underscore the vital role of accessible cardio-oncologic interventions and highlight the need to identify high-risk populations who may benefit most from early and long-term survivorship care. NH = non-Hispanic; SEER = Surveillance, Epidemiology, and End Results.
Individuals with cancer are at an increased risk of dying from CVD. A study of SEER registry data (1973-2012) found that 11.3% of cancer patients died from CVD throughout the 40-year time frame.4 Our analyses using more contemporary data suggested an overall CVD mortality of 6.3% during the 20-year follow-up. The discrepancy might be explained by differences in follow-up time and cancer types examined. Our findings extend the literature by providing the first comprehensive evidence of racial and ethnic disparities in all-cause and CVD mortality across cancer stages and sites using data from one of the largest population-based national cancer registries.
The racial and ethnic disparity pattern in all-cause and CVD-specific mortality observed in our study is generally consistent with prior studies focusing on specific cancer types or patient subgroups.6,9,12 The magnified racial and ethnic disparities in CVD mortality may be explained from several aspects. First, it is widely acknowledged that NH Black individuals have higher prevalence of CVD and worse outcomes.20 CVD and cancer have an extensive overlap in risk factors, including diabetes, obesity, tobacco use, and inactivity.2,21,22 These factors play an important role in the tolerance of oncologic therapies and posttreatment outcomes.10 Beyond a differential prevalence of underlying CVD and shared risk factors, recent studies also suggest physiological stress responses as a mechanism through which social and biological factors contribute to racial disparities in breast cancer.9,23 While our study showed racial and ethnic disparity to be independent of socioeconomic factors (ie, marital status, income, and urban/rural residency) as well as their time trends, other social determinants of health such as employment status, social support, and living environment may also contribute to the observed disparity and warrant further research.
What’s more, our study showed that Hispanic individuals have a better survival than NH White cancer patients despite their high prevalence of psychosocial disadvantages and CVD risk factors, which echoes the Hispanic paradox phenomenon24 in CVD outcomes research. The Hispanic paradox had been explained by a combination of factors such as healthy immigrant effect, potential migration bias (ie, patients return and die in their country of birth when seriously ill), and data linkage problems (death may not be confirmed for individuals without a social security number and therefore missed). Another main hypothesis for the paradox proposes that intact family structures, strong community network support, and kinship structures that span households convey significant benefits to individual health.25,26 In our study, considering age groups, younger Hispanic cancer patients were found to have worse all-cause mortality than NH White patients; however, better CVD outcomes remained in this subgroup. This observation may suggest that factors other than socioeconomic and community-related explanations, such as biological or genetic cardiovascular protective factors, may play important roles in CVD mortality among Hispanic individuals.27 On the other hand, while this paradox remains to be elucidated, the community theory may shed light on actionable approaches to mitigate racial disparities. For example, community-based interventions that were delivered via barber shops28 or churches29 have demonstrated effectiveness in the management of hypertension in African Americans. While evidence also suggested heterogeneity in CVD outcomes within Hispanic subgroups based on culture of origin,30 investigation of these differences is beyond the scope of current study and warrants future research efforts.
Our study also showed that the CVD mortality attributable to race and ethnicity was more prominent among localized cancer patients than those with regional or distant cancer. Recent studies have suggested that racial and ethnic disparities in CVD burden may have been present at the time of cancer diagnosis and become more prominent after cancer treatment.22,31 By examining the impact of race and ethnicity on cancer survival, overall and by each disease stage, our findings indicate that underserved racial and ethnical groups with a localized cancer diagnosis may benefit the most from early cardio-oncology intervention, given their higher risk of CVD mortality and higher propensity of long-term survival. While the field has made strides through guidelines and research that promote cardioprotective therapies and surveillance modalities,10 challenges still lie in an equal access to those medical advancements. For example, recent guidelines have suggested transthoracic echocardiography assessment of adult cancer patients receiving anthracyclines and/or trastuzumab. However, under-represented racial and ethnic groups have historically had lower access to diagnostic imaging including echo.32 Another example would be the emerging emphasis on cardio-oncology rehabilitation,33,34 yet Black, Hispanic, and Asian patients were much less likely to receive cardiac rehabilitation referral at discharge than White patients.35 Keeping up with subspecialty cardio-oncology related literature and care is challenging for primary care physicians and oncologists given their already numerous clinical care responsibilities for these patients. Thus, early introduction to cardio-oncology service as a multidisciplinary approach is crucial to narrow the gap in care disparities for certain individuals with early stage or newly diagnosed cancer.
Accessible, high-quality cardio-oncology care is the key to equitable CVD outcomes among cancer patients and survivors. Despite having more health care needs, cancer survivors of underserved racial and ethnic groups often face significant barriers to high-quality care that have been further amplified during the ongoing COVID-19 pandemic.36, 37, 38, 39 They may shoulder a disproportionate burden of financial strain from cancer treatment and often delay or forgo much-needed cancer and cardiology care due to transportation barriers.40 While the pandemic has catalyzed mobile health technology and accelerated telehealth adoption, cardio-oncology interventions that incorporate technology innovations may provide a solution to the current cancer outcome disparities in underserved racial and ethnic communities. Mobile health applications or wearable devices that provide noninvasive, self-detection of CVD conditions show promise in the long-term management of general cardiac patients41,42 and therefore warrant more support to their applications in underserved populations that bear higher risk of CVD mortality.
Study limitations
First, due to the nature of registry-based design, there may exist misclassification bias introduced by SEER’s use of death certificates instead of autopsy or electronic chart information to determine the cause of death. Second, some potential confounding factors, such as comorbidities, CVD risk factors, and health care access variables were not considered due to their unavailability in the SEER dataset. Third, socioeconomic status such as household income was attained on the county level, thus not accounting for individual variations. Fourth, treatment codes may be incomplete or inaccurate due to the limitations of cancer registry data. Last, our study did not consider the diversity within racial and ethnic groups, such as different Hispanic or Asian heritage. Future research is encouraged to better assess different subethnicities to provide more granular insights.
Conclusions
In summary, using a large population-based cancer registry, our study found significant race and ethnic differences in both all-cause and CVD-specific mortality among U.S. adult cancer patients, especially among younger patients and those with localized cancer. Our findings underscore the vital role of accessible cardiovascular interventions and highlight the need to identify high-risk cancer populations who may benefit most from early and long-term survivorship care.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: NH Black individuals had the highest all-cause and CVD mortality among U.S. adult cancer patients. Racial and ethnic disparities were more pronounced among cancer patients <55 years of age and localized cancers, emphasizing the importance of cardiovascular prevention among younger and early-stage cancer patients.
TRANSLATIONAL OUTLOOK: Our findings underscore the vital role of accessible cardiovascular interventions in cancer patients, highlight the need to mitigate racial and ethnic disparities in cardio-oncology, and highlight the importance of identifying high-risk cancer populations who may benefit most from long-term survivorship care.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenihan D.J., Cardinale D.M. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30(30):3657–3664. doi: 10.1200/JCO.2012.45.2938. [DOI] [PubMed] [Google Scholar]
- 4.Sturgeon K.M., Deng L., Bluethmann S.M., et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churchwell K., Elkind M.S.V., Benjamin R.M., et al. Call to Action: structural racism as a fundamental driver of health disparities: a Presidential Advisory from the American Heart Association. Circulation. 2020;142(24):e454–e468. doi: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 6.Troeschel A.N., Liu Y., Collin L.J., et al. Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer. Int J Epidemiol. 2019;48(6):1897–1905. doi: 10.1093/ije/dyz108. [DOI] [PubMed] [Google Scholar]
- 7.Berkman A., Cole B., Ades P.A., et al. Racial differences in breast cancer, cardiovascular disease, and all-cause mortality among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2014;148(2):407–413. doi: 10.1007/s10549-014-3168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deen L., Buddeke J., Vaartjes I., Bots M.L., Norredam M., Agyemang C. Ethnic differences in cardiovascular morbidity and mortality among patients with breast cancer in the Netherlands: a register-based cohort study. BMJ Open. 2018;8(8) doi: 10.1136/bmjopen-2018-021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F., Zheng W., Bailey C.E., Mayer I.A., Pietenpol J.A., Shu X.O. Racial/ethnic disparities in all-cause mortality among patients diagnosed with triple-negative breast cancer. Cancer Res. 2021;81(4):1163–1170. doi: 10.1158/0008-5472.CAN-20-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazal M., Malisa J., Rhee J.W., Witteles R.M., Rodriguez F. Racial and ethnic disparities in cardio-oncology. J Am Coll Cardiol CardioOnc. 2021;3(2):201–204. doi: 10.1016/j.jaccao.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan J. Improving adherence to medication for secondary cardiovascular disease prevention. Eur J Prev Cardiolog. 2017;24(3_suppl):29–35. doi: 10.1177/2047487317708145. [DOI] [PubMed] [Google Scholar]
- 12.Chen C., Markossian T.W., Silva A., Tarasenko Y.N. Epithelial ovarian cancer mortality among Hispanic women: sub-ethnic disparities and survival trend across time: an analysis of SEER 1992-2013. Cancer Epidemiol. 2018;52:134–141. doi: 10.1016/j.canep.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Howlader N., Noone A., Krapcho M., et al. National Cancer Institute; 1975-2018. SEER Cancer Statistics Review.https://seer.cancer.gov/csr/1975_2018/index.html [Google Scholar]
- 14.SEER∗Stat Software. SEER. https://seer.cancer.gov/seerstat/index.html
- 15.NAACCR Race and Ethnicity Work Group . North American Association of Central Cancer Registries; 2011. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1] [Google Scholar]
- 16.Silva A., Rauscher G.H., Ferrans C.E., Hoskins K., Rao R. Assessing the quality of race/ethnicity, tumor, and breast cancer treatment information in a non-SEER state registry. J Registry Manag. 2014;41(1):24–30. [PubMed] [Google Scholar]
- 17.Static County Attributes - SEER Datasets SEER. https://seer.cancer.gov/seerstat/variables/countyattribs/static.html
- 18.Rural-Urban Continuum Code - SEER Datasets SEER. https://seer.cancer.gov/seerstat/variables/countyattribs/ruralurban.html
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 20.Carnethon M.R., Pu J., Howard G., et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 21.Turner R.R., Steed L., Quirk H., et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9:CD010192. doi: 10.1002/14651858.CD010192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C., Deng L., Karr M.A., et al. Chronic comorbid conditions among adult cancer survivors in the United States: results from the National Health Interview Survey, 2002-2018. Cancer. 2022;128(4):828–838. doi: 10.1002/cncr.33981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halbert C.H., Jefferson M.S., Danielson C., Froeliger B., Giordano A., Thaxton J.E. An observational study and randomized trial of stress reactivity in cancer disparities. Health Psychol. 2020;39(9):745–757. doi: 10.1037/hea0000882. [DOI] [PubMed] [Google Scholar]
- 24.Siegel R.L., Fedewa S.A., Miller K.D., et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65(6):457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 25.Eschbach K., Ostir G.V., Patel K.V., Markides K.S., Goodwin J.S. Neighborhood context and mortality among older Mexican Americans: is there a barrio advantage? Am J Public Health. 2004;94(10):1807–1812. doi: 10.2105/ajph.94.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraído-Lanza A.F., Echeverría S.E., Flórez K.R. Latino immigrants, acculturation, and health: promising new directions in research. Annu Rev Public Health. 2016;37:219–236. doi: 10.1146/annurev-publhealth-032315-021545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victor R.G., Lynch K., Li N., et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med. 2018;378(14):1291–1301. doi: 10.1056/NEJMoa1717250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenthaler A.M., Lancaster K.J., Chaplin W., Butler M., Forsyth J., Ogedegbe G. Cluster randomized clinical trial of FAITH (Faith-Based Approaches in the Treatment of Hypertension) in Blacks. Circ: Cardiovasc Qual Outcomes. 2018;11(10) doi: 10.1161/CIRCOUTCOMES.118.004691. [DOI] [PubMed] [Google Scholar]
- 29.Joshi P., Marcovina S., Orroth K., et al. Lipoprotein(a) levels among Hispanic/Latino individuals residing in the United States: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) J Am Coll Cardiol. 2022;79(9_Suppl) 1550-1550. [Google Scholar]
- 30.Rodriguez F., Hastings K.G., Boothroyd D.B., et al. Disaggregation of cause-specific cardiovascular disease mortality among Hispanic subgroups. JAMA Cardiol. 2017;2(3):240–247. doi: 10.1001/jamacardio.2016.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guha A., Fradley M.G., Dent S.F., et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022;43(4):300–312. doi: 10.1093/eurheartj/ehab745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannix R., Bourgeois F.T., Schutzman S.A., Bernstein A., Lee L.K. Neuroimaging for pediatric head trauma: do patient and hospital characteristics influence who gets imaged? Acad Emerg Med. 2010;17(7):694–700. doi: 10.1111/j.1553-2712.2010.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors. Circulation. 2019;139(21):e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung W.P., Yang H.L., Hsu Y.T., et al. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: A randomized controlled trial. Ann Phys Rehabil Med. 2022;65(2) doi: 10.1016/j.rehab.2021.101485. [DOI] [PubMed] [Google Scholar]
- 35.Li S., Fonarow G.C., Mukamal K., et al. Sex and racial disparities in cardiac rehabilitation referral at hospital discharge and gaps in long-term mortality. J Am Heart Assoc. 2018;7(8) doi: 10.1161/JAHA.117.008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beebe-Dimmer J.L., Lusk C.M., Ruterbusch J.J., et al. The impact of the COVID-19 pandemic on African American cancer survivors. Cancer. 2022;128(4):839–848. doi: 10.1002/cncr.33987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman L.A., Winn R.A., Carethers J.M. Similarities in risk for COVID-19 and cancer disparities. Clin Cancer Res. 2021;27(1):24–27. doi: 10.1158/1078-0432.CCR-20-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jewett P.I., Vogel R.I., Ghebre R., et al. Telehealth in cancer care during COVID-19: disparities by age, race/ethnicity, and residential status. J Cancer Surviv. 2022;16(1):44–51. doi: 10.1007/s11764-021-01133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel M.I., Ferguson J.M., Castro E., et al. Racial and ethnic disparities in cancer care during the COVID-19 pandemic. JAMA Netw Open. 2022;5(7) doi: 10.1001/jamanetworkopen.2022.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang C., Yabroff K.R., Deng L., et al. Self-reported transportation barriers to health care among US cancer survivors. JAMA Oncol. 2022;8(5):775–778. doi: 10.1001/jamaoncol.2022.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayoumy K., Gaber M., Elshafeey A., et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18(8):581–599. doi: 10.1038/s41569-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozik M., Isakadze N., Martin S.S. Mobile health in preventive cardiology: current status and future perspective. Curr Opin Cardiol. 2021;36(5):580–588. doi: 10.1097/HCO.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.