Abstract
Background
Cardiovascular disease (CVD) incidence is higher in men with prostate cancer (PC) than without.
Objectives
We describe the rate and correlates of poor cardiovascular risk factor control among men with PC.
Methods
We prospectively characterized 2,811 consecutive men (mean age 68 ± 8 years) with PC from 24 sites in Canada, Israel, Brazil, and Australia. We defined poor overall risk factor control as ≥3 of the following: suboptimal low-density lipoprotein cholesterol (>2 mmol/L if Framingham Risk Score [FRS] ≥15 and ≥3.5 mmol/L if FRS <15), current smoker, physical inactivity (<600 MET min/wk), suboptimal blood pressure (BP) (≥140/90 mm Hg if no other risk factors, systolic BP ≥120 mm Hg if known CVD or FRS ≥15, and ≥130/80 mm Hg if diabetic), and waist:hip ratio >0.9.
Results
Among participants (9% with metastatic PC and 23% with pre-existing CVD), 99% had ≥1 uncontrolled cardiovascular risk factor, and 51% had poor overall risk factor control. Not taking a statin (odds ratio [OR]: 2.55; 95% CI: 2.00-3.26), physical frailty (OR: 2.37; 95% CI: 1.51-3.71), need for BP drugs (OR: 2.36; 95% CI: 1.84-3.03), and age (OR per 10-year increase: 1.34; 95% CI: 1.14-1.59) were associated with poor overall risk factor control after adjustment for education, PC characteristics, androgen deprivation therapy, depression, and Eastern Cooperative Oncology Group functional status.
Conclusions
Poor control of modifiable cardiovascular risk factors is common in men with PC, highlighting the large gap in care and the need for improved interventions to optimize cardiovascular risk management in this population.
Key Words: androgen deprivation therapy, cardiovascular disease prevention, cardiovascular risk, prospective, prostate cancer
Abbreviations and Acronyms: ADT, androgen deprivation therapy; BP, blood pressure; CVD, cardiovascular disease; ECOG, Eastern Cooperative Oncology Group; GnRH, gonadotropin-releasing hormone; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PC, prostate cancer; PHQ-9, Patient Health Questionnaire-9; PSA, prostate-specific antigen
Central Illustration
Men with prostate cancer (PC) are at high risk of cardiovascular morbidity and mortality.1 Among men with localized/regional PC, cardiovascular death is more frequent than death from the cancer itself, whereas among men with metastatic PC, the risk of cardiovascular death remains higher than among otherwise similar patients without PC.2,3 There are few data to inform the reasons underlying this observation. In the general population, poor control of modifiable cardiovascular risk factors (obesity, smoking, physical inactivity, hyperglycemia, hypertension. and hypercholesterolemia) is associated with adverse cardiovascular outcomes.4, 5, 6 However, there are limited data on the cardiovascular risk factor control among men with PC. Therefore, the primary objective of this analysis was to describe the rate of uncontrolled cardiovascular risk factors among men with PC. The secondary objective was to identify patient characteristics associated with poor overall control of cardiovascular risk factors.
Methods
We undertook an analysis of the RADICAL-PC (Role of Androgen Deprivation Therapy In Cardiovascular Disease – A Longitudinal Prostate Cancer) study, a prospective cohort study of men with PC.
Patients
Men attending urology or oncology clinics at 24 sites in Canada, Australia, Israel, and Brazil were screened. The inclusion criterion was 1 of the following: 1) PC diagnosed within the last year; 2) first ever use of androgen deprivation therapy (ADT) within the last 6 months; or 3) a plan to initiate ADT for the first time within 1 month. Patients younger than 45 years were excluded. All participants provided written informed consent. The study was approved by the relevant Institutional Review Boards at each participating site and was conducted according to the principles of the Declaration of Helsinki.
Procedures
Information on demographics and clinical factors were collected from participants and their medical records. Physical measurements (vital signs, anthropometrics, and handgrip strength) were performed by trained study personnel. Blood pressure (BP) was measured by an automated sphygmomanometer after 10 minutes of rest. Waist circumference was measured as the smallest circumference between the costal margin and the iliac crest using a tape measure, and hip circumference was measured as the largest circumference around the iliac crest. Handgrip strength was measured using a Jamar dynamometer (model number 5030J1). Laboratory measurements (total cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, glycosylated hemoglobin [HbA1c], and prostate-specific antigen [PSA]) were made from clinically acquired nonfasting blood specimens. Nonfasting lipid analysis is endorsed by Canadian Cardiovascular Society guidelines.7 Gait speed was assessed using the Timed Up and Go test.8 Physical activity was assessed using the International Physical Activity Questionnaire9; major depression was assessed using the Patient Health Questionnaire-9 (PHQ-9) and defined as a score ≥10 (88% sensitivity and specificity for major depression)10; performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) system11; and physical frailty was categorized into nonfrail, prefrail, and frail using the Fried frailty criteria.12 The Fried frailty criteria include 5 components: unintentional weight loss, self-reported exhaustion, weakness (assessed by handgrip strength), slow gait speed, and low physical activity. Frailty was defined as having ≥3 of these criteria, 1 to 2 criteria were considered prefrail, and nonfrail was defined as not having any of these criteria.12 A social deprivation index (SDI) was constructed using the summation of 4 socioeconomic factors: unemployment, annual income <CaD $50,000, <12 years of education, and living alone; the higher the number, the greater the social deprivation. The SDI was adapted from Wong et al.13
Cardiovascular disease (CVD) risk was assessed using the Framingham Risk Score (FRS).14 This score estimates the 10-year absolute risk of CVD by factoring in sex, age, BP, total cholesterol, HDL cholesterol, smoking status, and diabetes. High CVD risk was considered as a calculated FRS ≥15 (corresponding with ≥20% 10-year risk of incident CVD)15 or pre-existing coronary artery disease, cerebrovascular disease (stroke or transient ischemic attack), heart failure,16 peripheral arterial disease,17 or chronic renal disease.18 Intermediate FRS was defined as 11 to 15 (10%-19% 10-year risk of incident CVD) and low FRS as ≤10 (<10% 10-year risk of incident CVD).15
PC risk was estimated using a modification of the National Comprehensive Cancer Network 2021 PC guidelines. Low PC risk was defined as: 1) clinical stage cT1c or cT1-cT2a disease; 2) PSA <10 ng/mL; and 3) Gleason score ≤6 (grade group 1). Intermediate PC risk was defined as PSA concentration of 10 to 20 ng/mL, Gleason score 3 + 4 (grade group 2) or 4 + 3 (grade group 3), or cT2b-cT2c disease. High PC risk was defined as cT3a-cT4 disease, PSA concentration >20 ng/mL, Gleason score 8 to 10 (grade group 4 or 5), regional disease (any T, N1, M0), metastatic disease (any T, any N, M1), or biochemical relapse.
CVD was defined as the presence of coronary artery disease (including previous myocardial infarction, coronary revascularization [percutaneous coronary intervention or coronary artery bypass graft], or a self-reported history of angina), stroke or transient ischemic attack, heart failure, peripheral arterial disease, or atrial fibrillation.
Statistical analysis
This is a cross-sectional analysis of RADICAL-PC using data collected at baseline. We measured the prevalence of poorly controlled cardiovascular risk factors (ie, LDL cholesterol, BP, smoking, waist:hip ratio, and physical inactivity). If the collection of any of these 5 risk factors was missing at baseline, the follow-up data, within 2 years of baseline, were used if available. These risk factors were chosen based on the results of large international epidemiologic studies demonstrating them to be the most important modifiable CVD risk factors.19,20 Definitions for poor control of these cardiovascular risk factors were based on Canadian Cardiovascular Society guidelines21,22 and are summarized in Table 1. Based on published data demonstrating that 63% of a high CVD risk cohort had ≥3 uncontrolled cardiovascular risk factors (out of BP, body mass index, LDL cholesterol, physical inactivity, and current smoker),23 we anticipated that at least one-half of our cohort would have ≥3 poorly controlled cardiovascular risk factors. Therefore, we considered poor overall cardiovascular risk factor control to be present if ≥3 of these risk factors (LDL cholesterol, BP, smoking, waist:hip ratio, and physical inactivity) did not meet guideline-endorsed targets. Of the 2,811 participants recruited, physical activity was collected in 2,679 (95%), BP in 2,727 (97%), smoking in 2,792 (99%), waist and hip circumference in 2,695 (96%), and LDL cholesterol in 2,457 (87%). We used the chained equations method of multiple imputation to impute cardiovascular risk factor values when missing, assuming the missingness was random. Twenty imputations were performed, and the models were fitted to each full data set including the imputed data to derive combined estimates. Differences between those with poor overall risk factor control vs those without were evaluated by univariable logistic regression for continuous variables or the chi-square test for categoric variables. Data are presented as mean ± SD for continuous variables and count with percentage for categoric data.
Table 1.
Definition of Poorly Controlled Cardiovascular Risk Factors
| Cardiovascular Risk Factor | Threshold for Poor Control | Participant Population |
|---|---|---|
| LDL cholesterola | >2.0 mmol/L | Established CVD or Chronic kidney disease or Baseline Framingham Risk Score ≥15 (ie, ≥20% 10-y incident CVD risk) |
| ≥3.5 mmol/L | Baseline Framingham Risk Score <15% | |
| Blood pressurea | ≥140/90 mm Hg | No target end-organ damage and No cardiovascular risk factors (excluding blood pressure) |
| ≥130/80 mm Hg | Diabetes | |
| Systolic blood pressure ≥120 mm Hg | Established CVD or Chronic kidney disease or Baseline Framingham Risk Score ≥15% 10-y incident CVD risk or Age ≥75 y | |
| Waist:hip ratio | >0.90 | All participants |
| Current smoker | Regularly smoking within previous 12 months | All participants |
| Physical inactivity | <30 min of moderate physical activity 5 d/wk (<600 MET min/wk)42 | All participants |
We sought to identify participant characteristics that were independently associated with poor overall cardiovascular risk factor control from the following: age, SDI, ethnicity, PC risk level, use of ADT, pre-existing CVD, diabetes and HbA1c, pharmacotherapy (antihypertensives and statin use), physical frailty, depression, and ECOG functional status. Characteristics that differed between groups at the univariate level with a P value <0.25 were included in a multivariable binary logistic regression model using forward regression. The ORs, calculated from the logistic regression models, for these exposures are presented with the corresponding 95% CIs. A subgroup analysis was performed stratified by ADT use.
Ideal BP thresholds vary in different cardiovascular guidelines. American and European guidelines recommend BP <130/80 mm Hg in high-risk individuals,15,24 whereas Canadian guidelines recommend systolic BP <120 mm Hg.22 Therefore, a sensitivity analysis was performed in which we considered BP <130/80 mm Hg controlled in participants with high cardiovascular risk.
There is variability in the literature regarding the optimal PHQ-9 score threshold to identify major depression; thus, we performed another sensitivity analysis in which we used a cutoff PHQ-9 of 8, which we have previously used,25 instead of 10.
We did not include HbA1c in our definition of poor overall cardiovascular risk factor control because HbA1c targets are not supported by CVD prevention guidelines in patients without diabetes. However, we included a sensitivity analysis incorporating HbA1c cutoffs in the definition of poor overall cardiovascular risk factor control (ie, ≥3 of the following: suboptimal LDL cholesterol, current smoker, physically inactive, suboptimal BP control, raised waist-to-hip ratio, and uncontrolled HbA1c [>6.5% if no prior diagnosis of diabetes and >7% if diagnosed with diabetes26]).
To determine the robustness of our main findings, we ran sensitivity analyses redefining poor overall cardiovascular risk factor control as present if ≥2 or ≥4 risk factors (out of LDL cholesterol, BP, smoking, waist:hip ratio, and physical inactivity) were poorly controlled. Statistical analyses were performed using Stata 17.0 (StataCorp LLC).
Results
Patient characteristics
From December 2015 until January 2022, 2,811 men were recruited from 24 sites (18 in Canada [n = 2,718], 3 in Israel [n = 6], 2 in Brazil [n = 60], and 1 in Australia [n = 26]). Participant sociodemographics and the risk factor profile stratified by the presence vs absence of poor overall cardiovascular risk factor control are displayed in Tables 2 and 3. The mean age was 68.3 ± 8 years, 9 in 10 were White, 6 in 10 were educated at a tertiary level, and almost 3 in 4 had an annual income of at least CaD $50,000. Most had nonmetastatic PC (2,561/2,805 [91%]), and just over one-third (38%) were receiving ADT.
Table 2.
Participant Sociodemographics
| Overall (N = 2,811) | <3 of 5 Poorly Controlled Cardiovascular Risk Factorsa (n = 1,381) | ≥ 3 of 5 Poorly Controlled Cardiovascular Risk Factorsa (n = 1,430) | P Value | |
|---|---|---|---|---|
| Age, y | 68.3 ± 8.0 | 67.6 (8.1) | 68.9 (7.8) | <0.001 |
| Lives alone | 430/2,759 (16) | 192/1,354 (14) | 235/1,405 (17) | 0.092 |
| Education | 0.001 | |||
| Primary/none | 350/2,759 (13) | 145/1,354 (11) | 205/1,405 (15) | |
| Secondary | 734/2,759 (27) | 346/1,354 (26) | 388/1,405 (28) | |
| Tertiary | 1,675/2,759 (61) | 863/1,354 (64) | 812/1,405 (58) | |
| Employed | 1,072/2,783 (39) | 553/1,367 (40) | 519/1,416 (37) | 0.039 |
| Annual income at least CaD $50,000 | 1,781/2,490 (72) | 893/1,223 (73) | 888/1,267 (70) | 0.11 |
| Ethnicity | 0.65 | |||
| White | 2,506/2,793 (90) | 1,242/1,372 (91) | 1,264/1,421 (89) | |
| Black | 119/2,793 (4) | 52/1,372 (4) | 67/1,421 (5) | |
| South Asian | 23/2,793 (0.8) | 11/1,372 (1) | 12/1,421 (1) | |
| Indigenous | 5/2,793 (0.2) | 3/1,372 (0.2) | 2/1,421 (0.1) | |
| Other | 140/2,793 (5) | 64/1,372 (5) | 76/1,421 (5) | |
| Social deprivation indexb | 0.002 | |||
| 0 | 691/2479 (28) | 370/1,218 (30) | 321/1,261 (25) | |
| 1 | 991/2,479 (40) | 489/1,218 (40) | 502/1,261 (40) | |
| 2 | 535/2,479 (22) | 244/1,218 (20) | 291/1,261 (23) | |
| 3 | 238/2,479 (10) | 110/1,218 (9) | 128/1,261 (10) | |
| 4 | 24/2,479 (1) | 5/1,218 (1) | 19/1,261 (2) |
Values are mean ± SD or n/N (%).
At least 3 of 5 of the following: suboptimal low-density lipoprotein cholesterol for risk level, current smoker, physically inactive, suboptimal blood pressure control for risk level, and raised waist-to-hip ratio. Data for all 5 variables were available for 2,311 of 2,811 (82%) participants, and multiple imputation was performed using the chained equations method to impute missing values.
Score based on simple summation of unemployment, annual income <CaD $50,000, <12 years of education, and living alone. Higher numbers represent greater social deprivation.
Table 3.
PC Characteristics and CV Risk Profile
| Overall (N = 2,811) | <3 of 5 Poorly Controlled CV Risk Factorsa (n = 1,381) | ≥ 3 of 5 Poorly Controlled CV Risk Factorsa (n = 1,430) | P Value | |
|---|---|---|---|---|
| PC riskb | 0.037 | |||
| Low | 236/2,781 (9) | 129/1,365 (9) | 107/1,416 (8) | |
| Intermediate | 1,195/2,781 (45) | 624/1,365 (46) | 613/1,416 (43) | |
| High | 1,241/2,781 (46) | 610/1,365 (45) | 696/1,416 (49) | |
| Use of ADT | 1,079/2,807 (38) | 501/1,378 (36) | 578/1,429 (40) | 0.026 |
| ADT type | 0.14 | |||
| Degarelix | 99/1,079 (9) | 51/501 (10) | 50/578 (8) | |
| GnRH agonist | 213/1,079 (20) | 106/501 (21) | 107/578 (19) | |
| Antiandrogen | 760/1,079 (70) | 343/501 (68) | 417/578 (72) | |
| Other | 7/1079 (1) | 1/501 (0.2) | 6/578 (1) | |
| GnRH agonist or degarelix and antiandrogen | 669/1,077 (62) | 307/501 (61) | 362/578 (63) | |
| Pre-existing CVD | 644/2,811 (23) | 344/1,381 (25) | 300/1,430 (21) | 0.013 |
| Chronic kidney disease | 401/2,811 (14) | 187/1,381 (14) | 214/1,430 (15) | 0.28 |
| Diabetes | 461/2,807 (16) | 243/1,379 (18) | 218/1,428 (15) | 0.092 |
| HbA1c, (%) | 5.9 ± 0.9 | 5.9 ± 1.0 | 5.8 ± 0.9 | 0.21 |
| SBP, mm Hg | 137.9 ± 17.8 | 131.8 ± 17.0 | 143.7 ± 16.5 | |
| DBP, mm Hg | 82.2 ± 11.1 | 79.6 ± 10.6 | 84.8 ± 11.1 | |
| On antihypertensive | 1,496/2,811 (53) | 666/1,381 (48) | 830/1,430 (58) | <0.001 |
| On statin | 1281/2,811 (46) | 723/1,381 (52) | 558/1,430 (39) | <0.001 |
| FRS ≥20% and on statin | 880/1,727 (51) | 461/645 (71) | 419/1,082 (39) | <0.001 |
| LDL cholesterol, mmol/L | 2.5 ± 1.0 | 2.2 ± 0.9 | 2.8 ± 0.9 | |
| PHQ-9 ≥10c | 132/2,695 (5) | 50/1,321 (4) | 82/1,374 (6) | 0.009 |
| ECOG >1 (ie, not fully independent) | 382/2,444 (16) | 163/1,197 (14) | 219/1,247 (18) | 0.007 |
| Frailty (ie, Fried score >2)d | 197/2,440 (8) | 58/1,205 (5) | 139/1,235 (11) | <0.001 |
Values are n/N (%) or mean ± SD.
ADT = androgen deprivation therapy; CV = cardiovascular; DBP = diastolic blood pressure; ECOG = Eastern Cooperative Oncology Group performance status; FRS = Framingham Risk Score; HbA1c = glycosylated hemoglobin; PC = prostate cancer; PHQ-9 = Patient Health Questionnaire-9; SBP = systolic blood pressure; other abbreviations as in Table 1.
At least 3 of 5 of the following: suboptimal LDL cholesterol for risk level, current smoker, physically inactive, suboptimal blood pressure control for risk level, and raised waist-to-hip ratio. Data for all 5 variables were available for 2,311 of 2,811 (82%) participants, and multiple imputation was performed using the chained equations method to impute missing values.
PC low risk was defined as participants with all of the following: 1) clinical stage cT1c or cT1-cT2a disease, 2) prostate-specific antigen <10 ng/mL, and 3) a Gleason score ≤6 (grade group 1). Intermediate PC risk was defined as participants with a prostate-specific antigen concentration of 10 to 20 ng/mL, a Gleason score 3 + 4 (grade group 2) or 4 + 3 (grade group 3), or cT2b-cT2c disease. High PC risk was defined as participants with cT3a-cT4 disease, a prostate-specific antigen concentration >20 ng/mL, a Gleason score of 8 to 10 (grade group 4 or 5), regional disease (any T, N1, M0), metastatic disease (any T, any N, M1), or biochemical relapse.
A PHQ-9 score of at least 10 has 88% sensitivity and specificity for major depression.
Scoring based on Fried frailty criteria.12
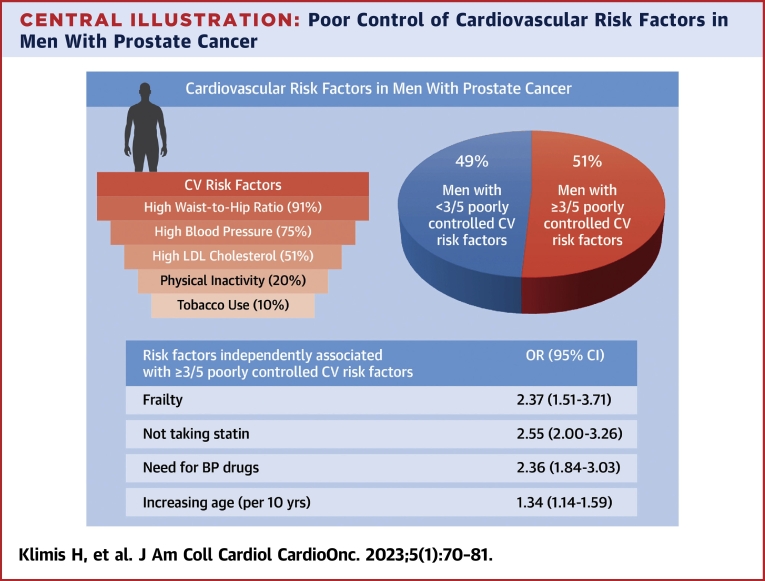
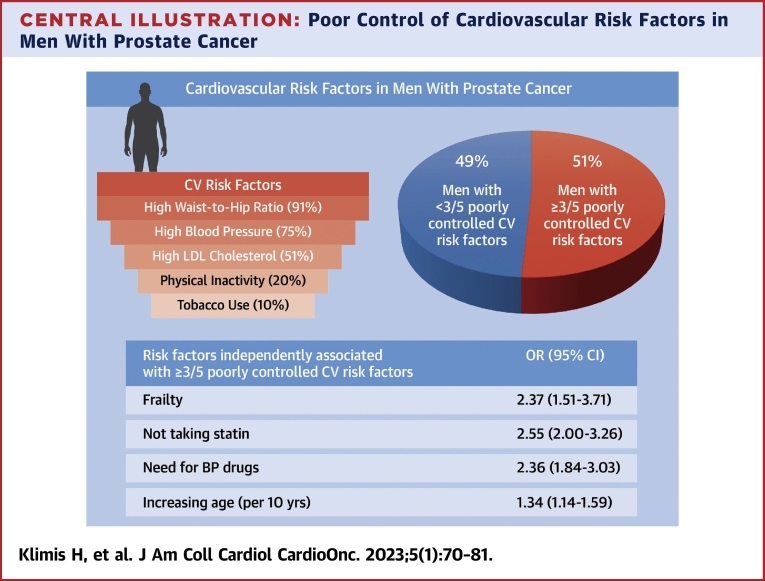
Nearly one-quarter of the patients (23%) had pre-existing CVD (Supplemental Table 1). Almost all participants (2,767/2,811 [98%]) had at least 1 poorly controlled cardiovascular risk factor (out of suboptimal LDL cholesterol, current smoker, physical inactivity, suboptimal BP control, and elevated waist-to-hip ratio). The most common uncontrolled risk factors were abdominal obesity (as assessed by waist-to-hip ratio) and BP (Central Illustration). The vast majority, 2,561 of 2,811 (91%), had a waist-to-hip circumference in the unhealthy range (>0.9). Three-quarters of the patients had suboptimal BP control (2,111/2,811 [75%]), and 1,427 of 2,811 (51%) had suboptimal LDL cholesterol. Smoking status and physical inactivity were the best controlled risk factors, with 277 of 2,811 (10%) being a current smoker and 549 of 2,811 (20%) being physically inactive. A high FRS was present in 1,727 of 2,287 (76%), an intermediate FRS in 444 of 2,287 (19%), and a low FRS in 116 of 2,287 (5%). One-half of the participants (880/1,727 [51%]) with a high FRS were on a statin.
Central Illustration.
Poor Control of Cardiovascular Risk Factors in Men With Prostate Cancer
This study was a cross-sectional analysis of a prospective study, which included men aged ≥45 years with prostate cancer diagnosed within the last year or first ever use of androgen deprivation therapy within the last 6 months. Numbers in parentheses in the top left panel indicate the proportion of patients with suboptimal control of the stated risk factor. Almost all had at least 1 poorly controlled cardiovascular (CV) risk factor, and one-half had at least 3. Physical frailty and not taking statins are factors that can be targeted to improve the overall control of CV risk factors in men with prostate cancer. Poor overall CV risk was defined as at least 3 of the following: suboptimal low-density lipoprotein (LDL) cholesterol, smoking, physical inactivity, suboptimal blood pressure (BP), and waist:hip ratio >0.9.
Characteristics associated with ≥3 poorly controlled cardiovascular risk factors
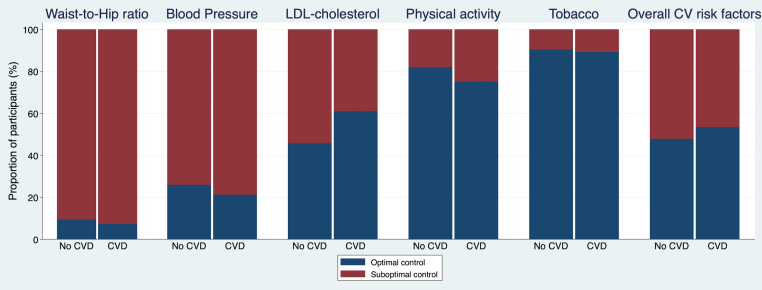
One-half (51%) of the participants had ≥3 cardiovascular risk factors poorly controlled (Figure 1). The mean of poorly controlled modifiable cardiovascular risk factors in the entire cohort was 2.5 ± 0.9 and was the same in those with and without pre-existing CVD.
Figure 1.
Control of CV Risk Factors in Men With Prostate Cancer
The proportion of participants with suboptimal control of modifiable cardiovascular (CV) risk factors is shown stratified by the presence of pre-existing cardiovascular disease (CVD). aAt least 3 of the following: suboptimal low-density lipoprotein cholesterol, smoking, physical inactivity, suboptimal blood pressure, and waist:hip ratio >0.9.
Participants with ≥3 poorly controlled cardiovascular risk factors were more likely to be older, to have advanced PC, to be receiving ADT, to be taking antihypertensive drugs, were less often taking a statin, and had a higher SDI than those with better risk factor control (Tables 2 and 3). Also, those with ≥3 poorly controlled cardiovascular risk factors exhibited more physical frailty, were more likely to have depression, and had less functional independence (ie, ECOG >1).
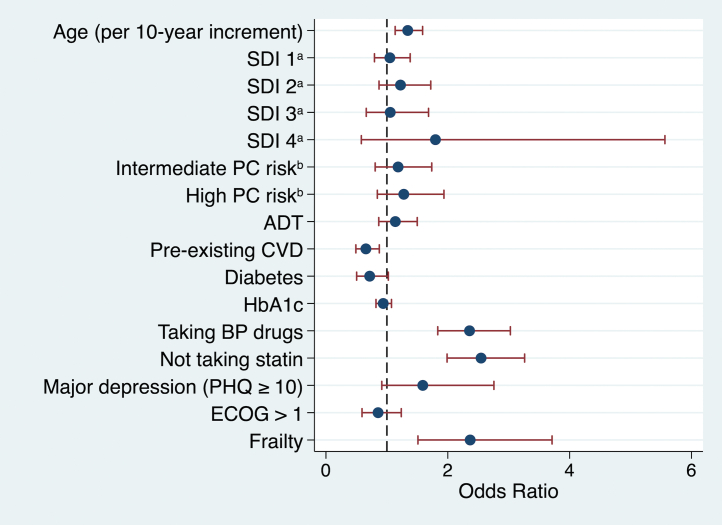
In the multivariable model, not taking a statin (OR: 2.55; 95% CI: 2.00-3.26), physical frailty (OR: 2.37; 95% CI: 1.51-3.71), need for BP drugs (OR: 2.36; 95% CI: 1.84-3.03), and increasing age (OR per 10-year increase: 1.34; 95% CI: 1.14-1.59) were associated with having ≥3 poorly controlled cardiovascular risk factors (Central Illustration, Figure 2, Supplemental Table 2).
Figure 2.
Independent Associations of Poor Overall Cardiovascular Risk Factor Control
Poor overall cardiovascular risk was defined as at least 3 of the following: suboptimal low-density lipoprotein cholesterol, smoking, physical inactivity, suboptimal blood pressure (BP), and waist:hip ratio >0.9. Data for all 5 data points were available for 2,311 of 2,811 (82%) participants, and multiple imputation was performed using the chained equations method to impute missing values. All estimates are mutually adjusted for each other. aScore based on summation of unemployment, annual income <CaD $50,000, <12 years of education, and living alone; reference is social deprivation index (SDI) = 0. bReference is low prostate cancer (PC) risk. ADT = androgen deprivation therapy; CVD = cardiovascular disease; ECOG = Eastern Cooperative Oncology Group performance status; HbA1c = glycosylated hemoglobin; PHQ = Patient Health Questionnaire-9.
Pre-existing CVD (OR: 0.66; 95% CI: 0.49-0.88) was associated with better overall cardiovascular risk factor control. To further explore this, we assessed statin use in this group. In participants with pre-existing CVD, 476 of 644 (74%) were taking statins compared with 500 of 1,224 (41%) without pre-existing CVD but high CVD risk (ie, a high FRS). Coronary artery disease was more prevalent in participants with better overall cardiovascular risk factor control than those without, but other CVD subtypes (stroke, heart failure, peripheral arterial disease, and atrial fibrillation) had similar prevalence between groups (Supplemental Table 1).
In a sensitivity analysis, adjusting the definition of ideal BP control to be consistent with European and U.S. guidelines (ie, BP <130/80 mm Hg in high-risk individuals), the findings were similar (Supplemental Table 3). There was no significant change in the multivariable model when performing a sensitivity analysis lowering the PHQ-9 cutoff to 8 for identifying depression (Supplemental Table 4). In a subgroup analysis stratified by ADT use vs no ADT use, not taking a statin and the need for antihypertensive drugs were significantly associated with ≥3 poorly controlled cardiovascular risk factors regardless of ADT use. However, physical frailty was significant only for those not on ADT (Supplemental Figures 1 and 2). In a sensitivity analysis, analysis excluding participants who did not have all 5 data points to determine poor overall cardiovascular risk factor control showed similar results (Supplemental Table 5). A sensitivity analysis incorporating HbA1c cutoffs into the definition of poor overall cardiovascular risk factor control showed similar results (Supplemental Figure 3). In sensitivity analyses assessing independent associations with ≥2 or ≥4 poorly controlled cardiovascular risk factors, not taking a statin, physical frailty, and the need for BP drugs remained significant (Supplemental Figures 4 and 5).
Discussion
The main findings of this study are as follows: 1) most men with PC have poor control of multiple modifiable cardiovascular risk factors, and 2) not taking a statin, physical frailty, and the need for BP drug use were most strongly associated with poor cardiovascular risk factor control. Poor control of cardiovascular risk factors occurred regardless of a history of established CVD or use of ADT.
Population-based data have demonstrated that men with PC are at high CVD risk. Although the precise mechanisms remain unknown, this may partly be explained by high baseline rates of traditional cardiovascular risk factors (smoking, obesity, hypertension, and diabetes).1,20 Furthermore, ADT can worsen cardiovascular risk factors by inducing metabolic changes including dyslipidemia, dysglycemia, obesity, and hypertension.27 An analysis from a Swedish national registry of 76,600 men with newly diagnosed PC demonstrated that CVD incidence was higher in men with PC compared with men from the general population independent of pre-existing CVD or PC treatment.28 The risk ratios for nonfatal myocardial infarction in those without pre-existing CVD were 1.40 (95% CI: 1.31-1.49), 1.15 (95% CI: 1.01-1.31), and 1.20 (95% CI: 1.11-1.30) for men undergoing ADT or orchiectomy, curative treatment, and surveillance, respectively. However, there are few prospective studies that have characterized cardiovascular risk factors in men with PC. An Australian prospective cohort study of 236 men with nonmetastatic PC on ADT found that at baseline 87% had a high body mass index, 61% had hypertension, 15% were current smokers, 56% had dyslipidemia, and 27% had pre-existing CVD.29 Although these findings are important because they highlight the high frequency of cardiovascular risk factors in men with PC, they do not provide insight into how well these risk factors are addressed. Good control of cardiovascular risk factors clearly reduces the risk of future major adverse cardiovascular events.30 Our study fills a knowledge gap because we prospectively measured rates of poor cardiovascular risk factor control. This is of clinical relevance because individuals with uncontrolled cardiovascular risk factors may benefit most from targeted intervention.
We found almost all participants had at least 1 uncontrolled modifiable cardiovascular risk factor, and one-half had poor control of at least 3 risk factors. An important retrospective study of 90,494 U.S. veterans with PC found that 54.1% had at least 1 poorly controlled cardiovascular risk factor (out of BP, cholesterol, and blood glucose).31 The authors found 36% had uncontrolled BP (systolic ≥140 mm Hg or diastolic ≥90 mm Hg), and 20% had uncontrolled lipids (LDL cholesterol ≥3.36 mmol/L or total cholesterol ≥6.22 mmol/L)31 compared with 75% and 51% for suboptimal BP and LDL cholesterol, respectively, in our study. The difference in the proportions reported is likely caused by 1) differences in the definition of risk factor control, 2) a greater number of risk factors assessed in our study (that are not typically captured in administrative databases), and 3) study design. According to cardiovascular clinical guidelines, optimal cardiovascular risk factor thresholds depend on the individual’s baseline cardiovascular risk. Our definition of cardiovascular control is consistent with these guidelines.15,22,24 In contrast, Sun et al31 used a more relaxed definition of control by incorporating a single cutoff regardless of baseline CVD risk. This would have underestimated the proportion of men with uncontrolled risk factors according to cardiovascular clinical guidelines. Sun et al31 used registry veteran data that were collected retrospectively, in contrast to our study in which we collected data prospectively using standardized techniques, thus allowing higher data completeness, and nearly all participants had data collected within 1 year of PC diagnosis.
Our study extends on the findings of Sun et al31 by identifying physical frailty as strongly associated with poor cardiovascular risk factor control. The strong relationship between physical frailty and poor cardiovascular risk factor control is a novel finding. Because of their older age and PC therapies, men with PC are at increased risk of frailty. Frailty is also common in patients with CVD and leads to worse outcomes.32 The exact mechanism underpinning the relationship between frailty and CVD is not known, although many are speculated. Frailty is associated with chronic undernutrition and loss of muscle mass, which can lead to decreased physical activity12 and failure to achieve exercise targets. Patients with frailty have higher arterial stiffness, which increases the risk of hypertension.33 In addition, inflammatory markers, including C-reactive protein and interleukin-6, are higher in frail adults compared with nonfrail adults34; inflammation is increasingly recognized as an important mediator of cardiovascular events. Recently, a systematic review of 10 genome-wide association studies found that common genetic polymorphisms exist between physical frailty and metabolic syndrome (defined as ≥3 of the following: uncontrolled blood glucose, low HDL cholesterol, high triglycerides, central obesity, and high BP) or CVD.35 Thus, a shared pathophysiology may exist between frailty, uncontrolled cardiovascular risk factors, and CVD. Furthermore, frailty has been associated with poor treatment compliance,36 which can lead to poor control of hypertension, hyperlipidemia, and diabetes. This highlights the importance of a holistic approach to PC survivorship care; more research is needed to determine the benefits of a more aggressive approach to cardiovascular risk factor control in individuals who are frail.
Taking antihypertensive pharmacotherapy was independently associated with poor control of ≥3 cardiovascular risk factors in our study. This is consistent with an analysis of the PURE (Prospective Urban Rural Epidemiology) study (142,042 participants in the general population aged 35-70 years across 17 countries) in which most participants who were diagnosed with hypertension were started on antihypertensive medication, but only 33% had adequately controlled BP.37 Our findings in RADICAL-PC are important because they indicate that simply taking BP-lowering medication does not necessarily translate into adequate BP control. Patients with PC and health care workers treating men with PC should be encouraged to treat BP to target levels rather than regarding the use of BP medications alone as a measure of quality of care. Pre-existing CVD, specifically coronary artery disease, was associated with better overall cardiovascular risk factor control and may be because of improved secondary prevention measures. Supporting this, participants with pre-existing CVD were much more likely to be taking statins than those without pre-existing CVD but with guideline recommendation for statin use (74% with pre-existing CVD vs 41% without CVD but high CVD risk).
A recent meta-analysis of 10 randomized controlled trials of men with PC demonstrated that gonadotropin-releasing hormone (GnRH) antagonists (n = 2,415) are associated with a reduction in nonfatal (HR: 0.55; 95% CI: 0.41-0.74) and fatal (HR: 0.46; 95% CI: 0.22-0.96) CVD events compared with GnRH agonists (n = 1,314).38 In this study, we did not find that the type of ADT was associated with poor overall control of cardiovascular risk factors. Because this study is a cross-sectional analysis, any difference between GnRH agonists and antagonists with respect to the control of cardiovascular risk factors may not have had an opportunity to become manifest.
RADICAL-PC is the largest prospective study that includes standardized assessment of both traditional (eg, physical activity) and under-recognized (eg, physical frailty) cardiovascular risk factors in men with PC. This has allowed us to comprehensively characterize cardiovascular risk factor control in men with PC, which can then help guide the development of personalized strategies to reduce incident CVD—a major competing risk in this population. We are currently evaluating a systematic approach to CVD risk factor control in a randomized controlled trial recruiting men with PC.39
Study limitations
This study’s major limitation is that most sites were Canadian with only 1 middle-income country (Brazil) and no representation from low-income countries; therefore, caution is needed in extrapolating these findings to other countries. Most participants were White; thus, the results may not be generalizable to non-White populations. In addition, this study used office BP measurements, and both home and 24-hour automated BP measurements have been shown to be better at predicting future target organ damage compared with office measurements.40 Furthermore, given the cross-sectional design of this analysis, we are unable to make causal inferences. We did not have an age- and sex-matched control group in our study, and future studies are needed to compare cardiovascular risk profile of men with PC to those without PC to further characterize relative cardiovascular risk in this cohort. Preliminary data have shown that coronary calcification is common on positron emission tomography/computed tomography imaging in men with PC,41 and future studies could use coronary calcium scores from staging/surveillance computed tomography scans as a more nuanced risk stratification tool for cardiovascular risk. We did not include data on cardiovascular events because follow-up in this study is ongoing, and, at the present time, outcome event rates are modest. Therefore, any association (or lack of association) between exposures and cardiovascular events may not be reliable at this time. The cardiovascular risk factors evaluated in this paper are not exhaustive. They were chosen because guideline-supported targets for these risk factors are well established. As other cardiovascular risk factors, such as chronic kidney disease, become increasingly modifiable and therapeutic targets are defined, further research will be needed to evaluate the control of these risk factors in the PC population.
Conclusions
Poor control of modifiable cardiovascular risk factors is common in men with PC, highlighting the large gap in care and the need for improved and novel interventions to optimize cardiovascular risk management in this population. Not taking a statin, physical frailty, and the need for antihypertensive medications had strong independent associations with having multiple poorly controlled cardiovascular risk factors. Clinicians should routinely screen for cardiovascular risk factors in all men with PC and consider measures to prevent frailty, optimize BP, and initiate statin therapy in appropriate individuals as part of a comprehensive PC survivorship strategy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Most men with PC have poor cardiovascular risk factor control, and clinicians should routinely screen these men for risk factors and consider measures to address frailty, optimize BP, and initiate statin therapy in appropriate individuals as part of a comprehensive PC survivorship strategy.
TRANSLATIONAL OUTLOOK: Further studies are required to determine whether universal vs CVD risk-based guided preventative strategies are most effective and cost-effective in men with PC.
Funding Support and Author Disclosures
RADICAL-PC is an investigator-initiated study that is funded by Prostate Cancer Canada (grant CT2015-01); the Movember Foundation, Hamilton Health Sciences (Research Strategic Initiative Program); and the Canadian Cancer Society (grant 706677). The funders of the study had no role in design of the study, analysis, interpretation, writing of the manuscript, and in the decision to publish the results. Dr Klimis is supported by an AFP Fellowship Award, Department of Cardiology, McMaster University. Dr Pinthus has served on advisory boards for Ferring Pharmaceuticals and Myovant Sciences; and has received research grant from Ferring Pharmaceuticals. Dr Duceppe has received investigator-initiated research grants from Roche Diagnostics and Abbott Laboratories; and has received lecture fees and honoraria for participation in advisory board meetings with Roche Diagnostics. Dr Siemens has been involved in clinical trials with Merck, Pfizer, Astellas, and Bayer. Dr Lavallee is on the advisory board for Sanofi, Astellas, Janssen, and Knight. Dr Gopaul has received personal fees outside the submitted manuscript from AstraZeneca, TerSera, Bayer, Ferring, and Abvie. Dr Hanna is on the advisory board for and has received honorarium and research support from Astellas, Bayer, Merck, Tolmar, Sanofi, and Abbvie. Dr Iakobishvili consults and lectures for Bayer, Pfizer, Boehringer Ingelheim, Novo Nordisk, Medtronic, Sanofi Adventist, and AstraZeneca. Dr Selvanayagam has received research grant support from Biotronik, Bayer, Sanofi, Actelion, and Novartis; is on the advisory board for Sanofi, Faraday, and Recardio; and is on the speaker bureau for AstraZeneca, Boehringer-Ingelheim, Novartis, Abbot, Bayer, Sanofi, Biotronik, Circle CVI, and Takeda. Dr Leong has served on advisory boards for Ferring Pharmaceuticals, Myovant Sciences, and Tolmar; reports speaker’s honoraria from Abbvie; and is supported by the Clive Kearon Career Award, Department of Medicine, McMaster University. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to thank Prostate Cancer Canada, the Canadian Cancer Society, Hamilton Health Sciences Strategic Research Initiative, and McMaster University.
Footnotes
Husam Abdel-Qadir, MD, PhD, served as Guest Associate Editor for this paper.
Kathryn J. Ruddy, MD, MPH, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures as well as a list of the study investigators and committees, please see the online version of this paper.
Appendix
References
- 1.Leong D.P., Fradet V., Shayegan B., et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203:1109–1116. doi: 10.1097/JU.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 2.Butler S.S., Mahal B.A., Moslehi J.J., et al. Risk of cardiovascular mortality with androgen deprivation therapy in prostate cancer: a secondary analysis of the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized controlled trial. Cancer. 2021;127:2213–2221. doi: 10.1002/cncr.33486. [DOI] [PubMed] [Google Scholar]
- 3.Weiner A.B., Li E.V., Desai A.S., Press D.J., Schaeffer E.M. Cause of death during prostate cancer survivorship: a contemporary, US population-based analysis. Cancer. 2021;127:2895–2904. doi: 10.1002/cncr.33584. [DOI] [PubMed] [Google Scholar]
- 4.Folsom A.R., Yatsuya H., Nettleton J.A., et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaye B., Canonico M., Perier M.C., et al. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the Three-City study. J Am Coll Cardiol. 2017;69:3015–3026. doi: 10.1016/j.jacc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Guo L., Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. doi: 10.1002/clc.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson G.J., Thanassoulis G., Anderson T.J., et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Podsiadlo D., Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 9.The IPAQ Group Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ) 2005. http://www.ipaq.ki.se
- 10.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken M.M., Creech R.H., Tormey D.C., et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 12.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Wong C.M., Ou C.Q., Chan K.P., et al. The effects of air pollution on mortality in socially deprived urban areas in Hong Kong, China. Environ Health Perspect. 2008;116:1189–1194. doi: 10.1289/ehp.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canadian Cardiovascular Society Framingham Risk Score. https://ccs.ca/app/uploads/2020/12/FRS_eng_2017_fnl_greyscale.pdf
- 15.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bui A.L., Horwich T.B., Fonarow G.C. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 18.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S., Hawken S., Ounpuu S., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S., Joseph P., Rangarajan S., et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson T.J., Gregoire J., Hegele R.A., et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Rabi D.M., McBrien K.A., Sapir-Pichhadze R., et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36:596–624. doi: 10.1016/j.cjca.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 23.Klimis H., Thiagalingam A., McIntyre D., Marschner S., Von Huben A., Chow C.K. Text messages for primary prevention of cardiovascular disease: the TextMe2 randomised clinical trial. Am Heart J. 2021;242:33–44. doi: 10.1016/j.ahj.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 25.Fervaha G., Izard J.P., Tripp D.A., et al. Psychological morbidity associated with prostate cancer: rates and predictors of depression in the RADICAL PC study. Can Urol Assoc J. 2021;15:181–186. doi: 10.5489/cuaj.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes Canada Clinical Practice Guidelines Expert Committee Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42:S1–S325. [Google Scholar]
- 27.Levine G.N., D'Amico A.V., Berger P., et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Hemelrijck M., Garmo H., Holmberg L., et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–3456. doi: 10.1200/JCO.2010.29.1567. [DOI] [PubMed] [Google Scholar]
- 29.Cheung A.S., Pattison D., Bretherton I., et al. Cardiovascular risk and bone loss in men undergoing androgen deprivation therapy for non-metastatic prostate cancer: implementation of standardized management guidelines. Andrology. 2013;1:583–589. doi: 10.1111/j.2047-2927.2013.00093.x. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S., Lonn E., Pais P., et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032–2043. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 31.Sun L., Parikh R.B., Hubbard R.A., et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afilalo J., Alexander K.P., Mack M.J., et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orkaby A.R., Lunetta K.L., Sun F.J., et al. Cross-sectional association of frailty and arterial stiffness in community-dwelling older adults: the Framingham Heart study. J Gerontol A Biol Sci Med Sci. 2019;74:373–379. doi: 10.1093/gerona/gly134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soysal P., Stubbs B., Lucato P., et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Ahisar Y., Thanassoulis G., Huang K.N., Ohayon S.M., Afilalo J. Intersecting genetics of frailty and cardiovascular disease. J Nutr Health Aging. 2021;25:1023–1027. doi: 10.1007/s12603-021-1673-8. [DOI] [PubMed] [Google Scholar]
- 36.Uchmanowicz B., Chudiak A., Uchmanowicz I., Mazur G. How may coexisting frailty influence adherence to treatment in elderly hypertensive patients? Int J Hypertens. 2019;2019 doi: 10.1155/2019/5245184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow C.K., Teo K.K., Rangarajan S., et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 38.Cirne F., Aghel N., Petropoulos J.A., et al. The cardiovascular effects of gonadotropin-releasing hormone antagonists in men with prostate cancer. Eur Heart J Cardiovasc Pharmacother. 2022;8(3):253–262. doi: 10.1093/ehjcvp/pvab005. [DOI] [PubMed] [Google Scholar]
- 39.Pinthus J.H., Klotz L., Lukka H., et al. The RADICAL-PC trial. J Clin Oncol. 2016;34:178. [Google Scholar]
- 40.Townsend R.R. Out-of-office blood pressure monitoring: a comparison of ambulatory blood pressure monitoring and home (self) monitoring of blood pressure. Hypertension. 2020;76:1667–1673. doi: 10.1161/HYPERTENSIONAHA.120.14650. [DOI] [PubMed] [Google Scholar]
- 41.Zhang K.W., Lenihan D., Shaikh P.A., Som A. Abstract 14548: coronary and aortic calcification are common in men with recurrent prostate cancer undergoing PET/CT imaging. Circulation. 2020;142 [Google Scholar]
- 42.Hallal P.C., Andersen L.B., Bull F.C., et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.