Abstract
Background
Dipeptidyl peptidase-4 (DPP-4) inhibitors have been shown to exert pleiotropic effects on heart failure (HF) in animal experiments.
Objectives
This study sought to investigate the impact of DPP-4 inhibitors on HF patients with diabetes mellitus (DM).
Methods
We analyzed hospitalized patients with HF and DM enrolled in the JROADHF (Japanese Registry Of Acute Decompensated Heart Failure) registry, a nationwide registry of acute decompensated HF. Primary exposure was the use of a DPP-4 inhibitor. The primary outcome was a composite of cardiovascular death or HF hospitalization during the median follow-up of 3.6 years according to left ventricular ejection fraction.
Results
Out of 2,999 eligible patients, 1,130 had heart failure with preserved ejection fraction (HFpEF), 572 had heart failure with midrange ejection fraction (HFmrEF), and 1,297 had heart failure with reduced ejection fraction (HFrEF). In each cohort, 444, 232, and 574 patients received a DPP-4 inhibitor, respectively. A multivariable Cox regression model showed that DPP-4 inhibitor use was associated with a lower composite of cardiovascular death or HF hospitalization in HFpEF (HR: 0.69; 95% CI: 0.55-0.87; P = 0.002) but not in HFmrEF and HFrEF. Restricted cubic spline analysis demonstrated that DPP-4 inhibitors were beneficial in patients with higher left ventricular ejection fraction. In HFpEF cohort, propensity score matching yielded 263 pairs. DPP-4 inhibitor use was associated with a lower incidence rate of the composite of cardiovascular death or HF hospitalization (19.2 vs 25.9 events per 100 patient-years; rate ratio: 0.74; 95% CI: 0.57-0.97; P = 0.027) in matched patients.
Conclusions
DPP-4 inhibitor use was associated with better long-term outcomes in HFpEF patients with DM.
Key Words: diabetes mellitus, dipeptidyl peptidase-4 inhibitor, heart failure with preserved ejection fraction, long-term outcome
Abbreviations and Acronyms: BMI, body mass index; BNP, B-type natriuretic peptide; CV, cardiovascular; DM, diabetes mellitus; DPP-4, dipeptidyl peptidase-4; HbA1c, glycosylated hemoglobin; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction; SGLT-2, sodium-glucose cotransporter-2
Central Illustration
Heart failure (HF) is highly prevalent in patients with diabetes mellitus (DM).1,2 Diabetes is associated with increased all-cause and cardiovascular (CV) mortality in patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).3 The major cause of HF in diabetic patients is coronary artery disease; however, DM was also associated with increased left ventricular (LV) stiffness through cardiomyocyte hypertrophy and myocardial deposition of collagen and advanced glycation end products, leading to HF, even in patients without coronary artery disease.4 Therefore, establishment of better management for HF patient with DM is needed.
A meta-analysis showed that intensive glucose control modestly reduced macrovascular events and increased major hypoglycemia and concluded that glucose-lowering regimens should be tailored to the individual.5 Recently, several randomized controlled trials have showed that sodium-glucose cotransporter-2 (SGLT-2) inhibitors reduced HF hospitalization in patients with DM6, 7, 8 and CV death in patients with HFrEF.9 More recently, empagliflozin have been shown to reduce risk of CV death or hospitalization for HF in HFpEF, regardless of the presence or absence of DM.10
As well as SGLT2 inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors are important drugs for DM. DPP-4 inhibitors block proteolytic DPP-4 enzyme activity, which results in an increase in glucagon-like peptide 1 and thus lowers blood glucose levels.11,12 They are widely used in DM patients all over the world due to their efficacy on lowering blood glucose and their safety. A lot of experimental animal studies have demonstrated their beneficial effects in CV diseases including myocardial infarction, cardiac hypertrophy, and HF. Vildagliptin and alogliptin exerted protective effects against HF by decreasing apoptosis in the heart.13,14 Sitagliptin demonstrated protective effects on postinfarcted hearts.15 More recently, we have demonstrated that teneligliptin attenuated cardiac hypertrophy by suppressing oxidative stress in a mouse model.16
In contrary to these animal experiments, the beneficial effects of DPP-4 inhibitors on CV diseases have not been established in clinical trials. In particular, the relationship between DPP-4 inhibitor and HF hospitalization has long been debated.17, 18, 19, 20, 21, 22, 23 Whereas SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis In Myocardial Infarction 53) trial reported that saxagliptin was associated with increased risk of HF,18 alogliptin, sitagliptin, and linagliptin did not increase the risk of HF in the EMAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome),24 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin),25 and the CARMELINA (Cardiovascular and Renal Microvascular Outcome Study With Linaglipin)26 studies, respectively. Furthermore, there is no trials to assess the relationship between DPP-4 inhibitors and long-term prognosis in patients with established HF. There is a retrospective cohort study using claim database to assess the effects of DPP-4 inhibitors on long-term outcomes among HF patients.27 This study did not take echocardiographic parameters into account and concluded that the use of a DPP-4 inhibitor was not associated with improved outcomes. Only a small-scale, single-center observational study indicated beneficial effects of DPP-4 inhibitors in HF patients.28 However, this was a preliminary study, in which the number of patients was small (n = 166).
The American Heart Association/American College of Cardiology, European Society of Cardiology, and Japanese Circulation Society guidelines recommend that therapeutic strategies against HF should be stratified by left ventricular ejection fraction (LVEF).29, 30, 31 Unlike HFrEF, the effective management of HFpEF has not been fully established. In several animal experiments, DPP-4 inhibitors demonstrated antihypertrophic effects.16,32, 33, 34, 35 In addition, cardiac hypertrophy is a major critical cause of HFpEF.36 These findings raise a possibility that DPP-4 inhibitors could be beneficial in HFpEF. However, to date, the associations between outcomes and DPP-4 inhibitor use in HF subtypes have not been examined: HFpEF, heart failure with mildly reduced ejection fraction (HFmrEF), and HFrEF.
The JROADHF (Japanese Registry Of Acute Decompensated Heart Failure) registry is a retrospective, multicenter, nationwide registry of hospitalized patients with HF.37 This registry was developed by linking the JROAD-DPC (Japanese Registry Of All cardiac and vascular Diseases-the Japanese Diagnosis Procedure Combination system) registry, a nationwide claims database. The JROADHF program enrolled 13,238 patients admitted with HF in a Web-based registry at 158 participating hospitals with a median follow-up of 4.3 years. The present study was aimed to analyze the JROADHF database to evaluate the effects of DPP-4 inhibitor use on clinical outcomes in patients with DM and HF according to LVEF.
Methods
JROADHF registry
The JROADHF registry is a multicenter registry of patients hospitalized for the worsening HF in Japan. Baseline data were collected during the episode of index hospitalization during 2013.37 Follow-up data were collected up to 5 years after the index hospitalization. The baseline data include: 1) demography; 2) cause of HF; 3) precipitating cause; 4) comorbidities; 5) clinical status; 6) electrocardiographic and echocardiographic findings; and 7) treatment including discharge medications.
Study population
From the database of the JROADHF registry, patients with DM who discharged alive were included. Patients with valvular heart disease, congenital heart disease, or constrictive pericarditis were excluded. Patients were excluded if information about DPP-4 inhibitors or LVEF was missing. Eligible patients were divided into HFpEF (LVEF ≥50%), HFmrEF (LVEF 40%-50%), and HFrEF (LVEF <40%). In each group, patients were divided into 2 groups according to the use of DPP-4 inhibitor. In this study, the diagnosis of diabetes depends on attending doctor in each hospital, based on the definition of Japanese Clinical Practice Guideline for Diabetes: 1) 2 assessments of the diabetic type in each patient (in which 1 blood glucose test is mandatory); 2) 1 assessment of the diabetic type (with mandatory blood glucose testing) along with the presence of typical symptoms of chronic hyperglycemia (eg, dry mouth, polydipsia, polyuria, body weight loss, or diabetic retinopathy); and 3) evidence of a prior diagnosis of diabetes. Diabetes types were: 1) plasma glucose levels of fasting ≥126 mg/dL, 2-hour post–75-g oral glucose tolerance test ≥200 mg/dL, or casual ≥200 mg/dL; and 2) glycosylated hemoglobin (HbA1c) ≥6.5%.
Outcomes
The primary outcome was composite of CV death or HF hospitalization, and secondary outcomes were CV death and HF hospitalization.
Statistical analysis
Patient characteristics were presented as mean ± SD or median (IQR) and were compared with analysis of variance or chi-square test among the HFpEF, HFmrEF, and HFrEF groups.
Multivariable models evaluating the association between DPP-4 inhibitor use and clinical outcomes were adjusted for following baseline variables: age, sex, prior HF hospitalization, ischemic heart disease, hypertension, dyslipidemia, chronic kidney disease, stroke, chronic obstructive pulmonary disease, peripheral artery disease, ventricular tachycardia/fibrillation, pacemaker, coronary revascularization, creatinine clearance, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, beta-blocker, mineralocorticoid receptor antagonist, thiazide, biguanide, sulfonylurea, alpha-glucosidase inhibitor, glinide, thiazolidine, and insulin.
A propensity score was estimated using same covariates delineated previously in the HFpEF dataset. One-to-one pair matching between the 2 groups was performed by nearest-neighbor matching without replacement. Covariate balances before and after matching were checked by comparison of standardized mean difference. A standardized mean difference <0.1 was considered to indicate a negligible imbalance between the 2 groups. Incidence rates per 100 patient-years and incidence rate ratio were calculated for each outcome. The HR was estimated by the Fine and Gray model and presented with 95% CI and P value.
The analysis of outcomes by using multiple imputation was also conducted as a sensitivity analysis. For the all baseline missing data, multiple imputation was performed (number of imputations = 10) by predictive mean matching for continuous variables and logistic regression model for binary variables. A propensity score was estimated by fitting a logistic regression model that adjusted for all baseline covariates in each dataset. The HR for outcomes was estimated by Fine and Gray model adjusted for the all baseline data. Estimates from 10 iterations were combined with the use of Rubin’s rule.
The effects of DPP-4 inhibitors on a composite outcome of CV death or HF hospitalization were examined using restricted cubic splines adjusted for same covariates listed previously. The HR and 95% CI were plotted according to the baseline LVEF. Because the effects of DPP-4 inhibitors have been reported to be greater in lower body mass index (BMI),38,39 the effects of DPP-4 inhibitors on a composite outcome of CV death or HF hospitalization according to BMI were assessed with restricted cubic splines adjusted for the same covariates. We also assessed the primary outcome among subgroups: age (≥80 years vs <80 years); sex; ischemic heart disease; atrial fibrillation; hypertension; chronic kidney disease (stage 1-2 vs stage 3-5); and the use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists.
All tests were 2-tailed, and P < 0.05 was considered to be statistically significant. All analyses were performed with the SAS statistical package (version 9.4, SAS Institute).
Ethics statement
This study protocol was organized to ensure compliance with the Declaration of Helsinki and the Guidelines for the Epidemiological Research published by the Japanese Ministry of Health, Labour and Welfare. The original study protocol was approved by the Institutional Review Board at Kyushu University (approval number: 21039-00).
Results
Patient characteristics
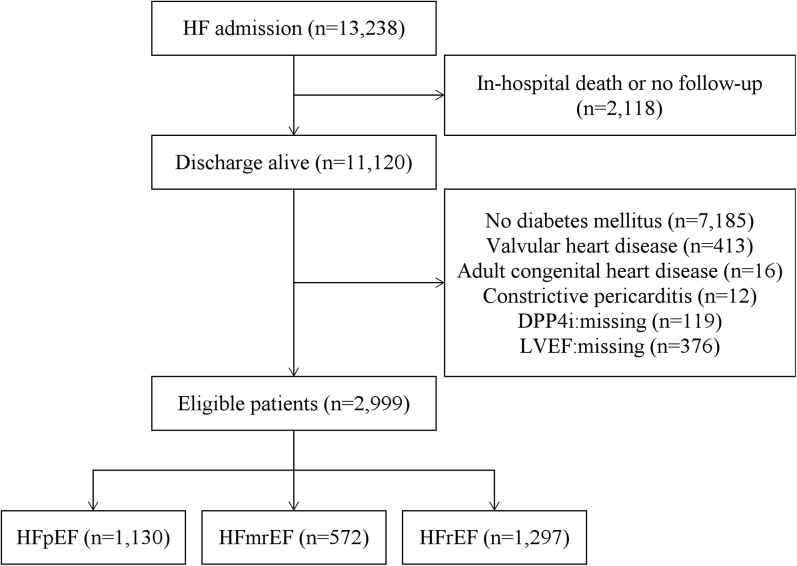
Figure 1 shows the method of patient selection in this study. Out of the 13,238 patients in this registry, 11,120 patients discharged alive. Among them, 7,185 patients without DM, 413 patients with valvular heart disease, 16 patients with congenital heart disease, 12 patients with constrictive pericarditis, 119 patients whose information about DPP-4 inhibitor was missing, and 376 patients whose information about LVEF was missing were excluded. The remaining 2,999 patients were included in the present analysis. Out of 2,999 eligible patients, 1,130 had HFpEF, 572 had HFmrEF, and 1,297 had HFrEF. In this study, sitagliptin (41.8%), vildagliptin (27.1%), alogliptin (16.2%), and linagliptin (13.4%) were used (Supplemental Table 1). Saxagliptin, which was not recommended for HF patients, was prescribed only in 1 patient.
Figure 1.
Patient Selection
After screening 13,238 patients, 2,999 patients were included in the present analysis. Out of them, 1,130 had heart failure with preserved ejection fraction (HFpEF), 572 had heart failure with mildly reduced ejection fraction (HFmrEF), and 1,297 had heart failure with reduced ejection fraction (HFrEF). DPP4i = dipeptidyl peptidase-4 inhibitor; HF = heart failure; LVEF = left ventricular ejection fraction.
The mean age (77.2 ± 10.1 years vs 74.2 ± 11.4 years vs 70.6 ± 12.5 years; P < 0.001) was older, men (587 [51.9%] vs 369 [64.5%] vs 978 [75.4%]; P < 0.001) and ischemic heart disease (488 [43.2%] vs 360 [62.9%] vs 738 [56.9%]; P < 0.001) were less frequent, atrial fibrillation (483 [42.9%] vs 184 [32.3%] vs 422 [32.7%]; P < 0.001) was more frequent, and B-type natriuretic peptide (BNP) (187.5 [IQR: 92.0-374.6] pg/mL vs 223.7 [IQR: 129.0-462.7] pg/mL vs 310.1 [IQR: 135.6-603.5] pg/mL; P < 0.001) was lower in the HFpEF group than in the HFmrEF and HFrEF groups (Table 1).
Table 1.
Patient Characteristics
| Overall (N = 2,999) | HFpEF (n = 1,130) | HFmrEF (n = 572) | HFrEF (n = 1,297) | P Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 73.8 ± 11.8 | 77.2 ± 10.1 | 74.2 ± 11.4 | 70.6 ± 12.5 | <0.001 |
| Male | 1,934 (64.5) | 587 (51.9) | 369 (64.5) | 978 (75.4) | <0.001 |
| Body mass index, kg/m2 | 22.7 ± 4.4 | 23.1 ± 4.5 | 22.6 ± 4.3 | 22.4 ± 4.3 | 0.001 |
| Prior HF hospitalization | 1,095 (36.5) | 348 (30.8) | 202 (35.3) | 545 (42.0) | <0.001 |
| NYHA functional class III-IV | 186 (6.5) | 49 (4.5) | 28 (5.2) | 109 (8.9) | <0.001 |
| Smoking | 1,128 (43.9) | 358 (36.2) | 212 (43.9) | 558 (50.9) | <0.001 |
| Vital signs | |||||
| SBP, mm Hg | 145.1 ± 34.0 | 151.2 ± 34.1 | 149.1 ± 34.8 | 138.0 ± 32.3 | <0.001 |
| Heart rate, beats/min | 92.8 ± 25.1 | 86.6 ± 24.8 | 95.9 ± 25.5 | 96.9 ± 24.0 | <0.001 |
| Heart disease | |||||
| IHD | 1,586 (52.9) | 488 (43.2) | 360 (62.9) | 738 (56.9) | <0.001 |
| Atrial fibrillation | 1,089 (36.5) | 483 (42.9) | 184 (32.3) | 422 (32.7) | <0.001 |
| VT/VF | 136 (4.7) | 15 (1.4) | 16 (2.9) | 105 (8.2) | <0.001 |
| Prior procedures | |||||
| PCI/CABG | 1,029 (35.3) | 320 (29.2) | 216 (39.1) | 493 (39.0) | <0.001 |
| Pacemaker | 177 (6.4) | 71 (6.8) | 41 (7.8) | 65 (5.4) | 0.13 |
| ICD | 47 (1.7) | 3 (0.3) | 6 (1.1) | 38 (3.2) | <0.001 |
| CRT-P | 12 (0.4) | 3 (0.3) | 3 (0.6) | 6 (0.5) | 0.65 |
| CRT-D | 79 (2.9) | 1 (0.1) | 8 (1.5) | 70 (5.8) | <0.001 |
| Hemodialysis | 99 (3.6) | 36 (3.5) | 23 (4.4) | 40 (3.3) | 0.55 |
| Comorbidities | |||||
| Hypertension | 2,402 (80.1) | 968 (85.7) | 452 (79.0) | 982 (75.7) | <0.001 |
| Dyslipidemia | 1,401 (46.7) | 479 (42.4) | 276 (48.3) | 646 (49.8) | <0.001 |
| CKD | 1,405 (46.8) | 529 (46.8) | 266 (46.5) | 610 (47.0) | 0.98 |
| Stroke | 411 (13.7) | 170 (15.0) | 63 (11.0) | 178 (13.7) | 0.074 |
| PAD | 307 (10.2) | 114 (10.1) | 61 (10.7) | 132 (10.2) | 0.93 |
| Anemia | 651 (21.7) | 307 (27.2) | 146 (25.5) | 198 (15.3) | <0.001 |
| COPD | 168 (5.6) | 76 (6.7) | 34 (5.9) | 58 (4.5) | 0.051 |
| Malignancy | 315 (10.5) | 130 (11.5) | 68 (11.9) | 117 (9.0) | 0.067 |
| Echocardiographic data | |||||
| LVEF, % | 44.2 ± 16.6 | 62.2 ± 8.3 | 44.1 ± 2.9 | 28.7 ± 7.0 | <0.001 |
| LVDd, mm | 53.6 ± 9.1 | 47.5 ± 6.6 | 52.9 ± 7.0 | 59.3 ± 8.3 | <0.001 |
| LVDs, mm | 41.6 ± 11.5 | 31.1 ± 6.0 | 40.9 ± 6.2 | 50.9 ± 8.5 | <0.001 |
| LVMI, g/m2 | 133.5 ± 41.2 | 120.2 ± 35.9 | 135.5 ± 39.8 | 143.9 ± 42.8 | <0.001 |
| LAD, mm | 44.9 ± 7.8 | 44.3 ± 8.1 | 44.0 ± 7.1 | 45.8 ± 7.8 | <0.001 |
| MR III-IV | 822 (27.7) | 215 (19.3) | 159 (28.2) | 448 (34.9) | <0.001 |
| Laboratory data | |||||
| Hemoglobin, g/dL | 11.8 ± 2.3 | 11.3 ± 2.1 | 11.5 ± 2.2 | 12.4 ± 2.3 | <0.001 |
| Albumin, g/dL | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | <0.001 |
| CCr, mL/min | 42.2 ± 27.9 | 37.8 ± 24.3 | 40.2 ± 25.8 | 46.7 ± 30.7 | <0.001 |
| Sodium, mEq/L | 138.3 ± 3.8 | 138.7 ± 3.6 | 138.4 ± 3.8 | 137.9 ± 3.9 | <0.001 |
| Potassium, mEq/L | 4.4 ± 0.6 | 4.3 ± 0.6 | 4.4 ± 0.6 | 4.4 ± 0.5 | 0.096 |
| BNP, pg/mL | 243.8 (118.1-497.6) | 187.5 (92.0-374.6) | 223.7 (129.0-462.7) | 310.1 (135.6-603.5) | <0.001 |
| ln (BNP) | 5.5 ± 1.1 | 5.2 ± 1.1 | 5.5 ± 1.0 | 5.7 ± 1.1 | <0.001 |
| Medication | |||||
| Beta-blockers | 2,123 (70.8) | 676 (59.8) | 397 (69.4) | 1,050 (81.0) | <0.001 |
| ACE inhibitor/ARB | 2,166 (72.2) | 801 (70.9) | 395 (69.1) | 970 (74.8) | 0.017 |
| MRA | 1,392 (46.4) | 436 (38.6) | 249 (43.5) | 707 (54.5) | <0.001 |
| Loop diuretics | 2,623 (87.5) | 954 (84.4) | 492 (86.0) | 1,177 (90.7) | <0.001 |
| Thiazides | 369 (12.3) | 171 (15.1) | 52 (9.1) | 146 (11.3) | <0.001 |
| Tolvaptan | 350 (11.7) | 114 (10.1) | 59 (10.3) | 177 (13.6) | 0.013 |
| Digitalis | 266 (8.9) | 74 (6.5) | 46 (8.0) | 146 (11.3) | <0.001 |
| Sulfonylurea | 579 (19.3) | 218 (19.3) | 112 (19.6) | 249 (19.2) | 0.98 |
| α-Glucosidase inhibitor | 480 (16.0) | 152 (13.5) | 91 (15.9) | 237 (18.3) | 0.005 |
| Glinide | 111 (3.7) | 41 (3.6) | 16 (2.8) | 54 (4.2) | 0.35 |
| Thiazolidine | 56 (1.9) | 30 (2.7) | 12 (2.1) | 14 (1.1) | 0.015 |
| Biguanide | 270 (9.0) | 93 (8.2) | 35 (6.1) | 142 (10.9) | 0.002 |
| Insulin | 1,126 (37.5) | 421 (37.3) | 225 (39.3) | 480 (37.0) | 0.61 |
| DPP-4 inhibitor | 1,250 (41.7) | 444 (39.3) | 232 (40.6) | 574 (44.3) | 0.039 |
Values are mean ± SD, n (%), or median (IQR). Patient characteristics were compared with analysis of variance or chi-square test among the HFpEF, HFmrEF, and HFrEF groups.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; BNP = B-type natriuretic peptide; CABG = coronary artery bypass grafting; CCR = creatinine clearance; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CRT-D = cardiac resynchronization therapy with defibrillator; CRT-P = cardiac resynchronization therapy with pacemaker; DPP-4 = dipeptidyl peptidase-4; HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFpEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator; IHD = ischemic heart disease; LAD = left atrial diameter; LVDd = left ventricular diastolic diameter; LVDs = left ventricular systolic diameter; LVEF = left ventricular ejection fraction; LVMI = left ventricular mass index; MR = mitral regurgitation; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; SMD = standardized mean difference; VF = ventricular fibrillation; VT = ventricular tachycardia.
Associations between DPP-4 inhibitor use and outcomes in the overall, HFpEF, HFmrEF, and HFrEF cohorts
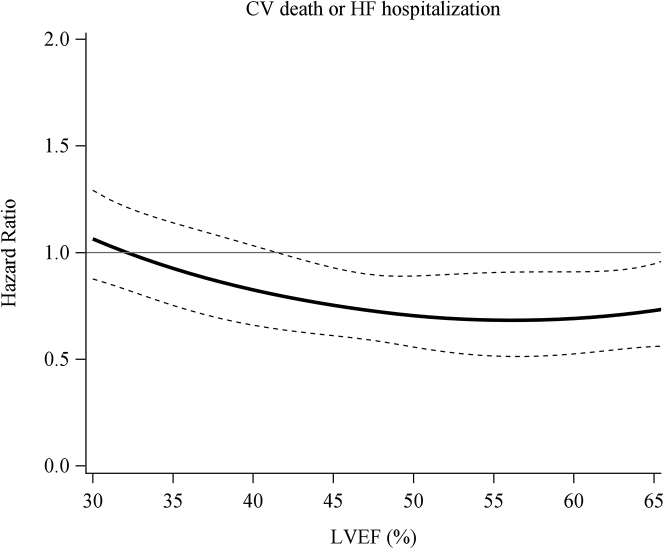
A multivariable Fine and Gray model showed that DPP-4 inhibitor use was associated with a reduced composite outcome of CV death or HF hospitalization in the overall (HR: 0.86; 95% CI: 0.75-0.98; P = 0.026) and HFpEF (HR: 0.69; 95% CI: 0.55-0.87; P = 0.002) groups but not in the HFmrEF (HR: 0.86; 95% CI: 0.62-1.20; P = 0.37) and HFrEF (HR: 0.95; 95% CI: 0.78-1.15; P = 0.60) group (Table 2). The cubic spline analysis showed that DPP-4 inhibitor use was associated with a reduced composite outcome in patients with higher LVEF (Figure 2). Previous studies have indicated the association of the effects of DPP-4 inhibitors and BMI. Thus, we evaluated BMI in this cohort and investigated whether BMI could be involved in the effects of DPP-4 inhibitors. The cubic spline analysis demonstrated that although the association of BMI and composite outcome was not statistically significant, patents with lower BMI tended to be associated with a lower composite outcome (Supplemental Figure 1). Subgroup analysis did not show specific subgroups benefited by DPP-4 inhibitors (Supplemental Figure 2).
Table 2.
HR for the Composite of Cardiovascular Death or HF Hospitalization
| HR (95% CI) | P Value | |
|---|---|---|
| Overall | 0.86 (0.75-0.98) | 0.026 |
| HFpEF | 0.69 (0.55-0.87) | 0.002 |
| HFmrEF | 0.86 (0.62-1.20) | 0.37 |
| HFrEF | 0.95 (0.78-1.15) | 0.60 |
Abbreviations as in Table 1.
Figure 2.
Effectiveness of DPP-4 Inhibitors on Composite Outcome According to LVEF
The cubic spline analysis showed that DPP-4 inhibitor use was associated with a reduced composite outcome in patients with higher LVEF. The solid line indicates the adjusted OR and the dotted line the 95% CI. CV = cardiovascular; other abbreviations as in Figure 1.
Sensitivity analyses of association between outcomes and DPP-4 inhibitor use in HFpEF
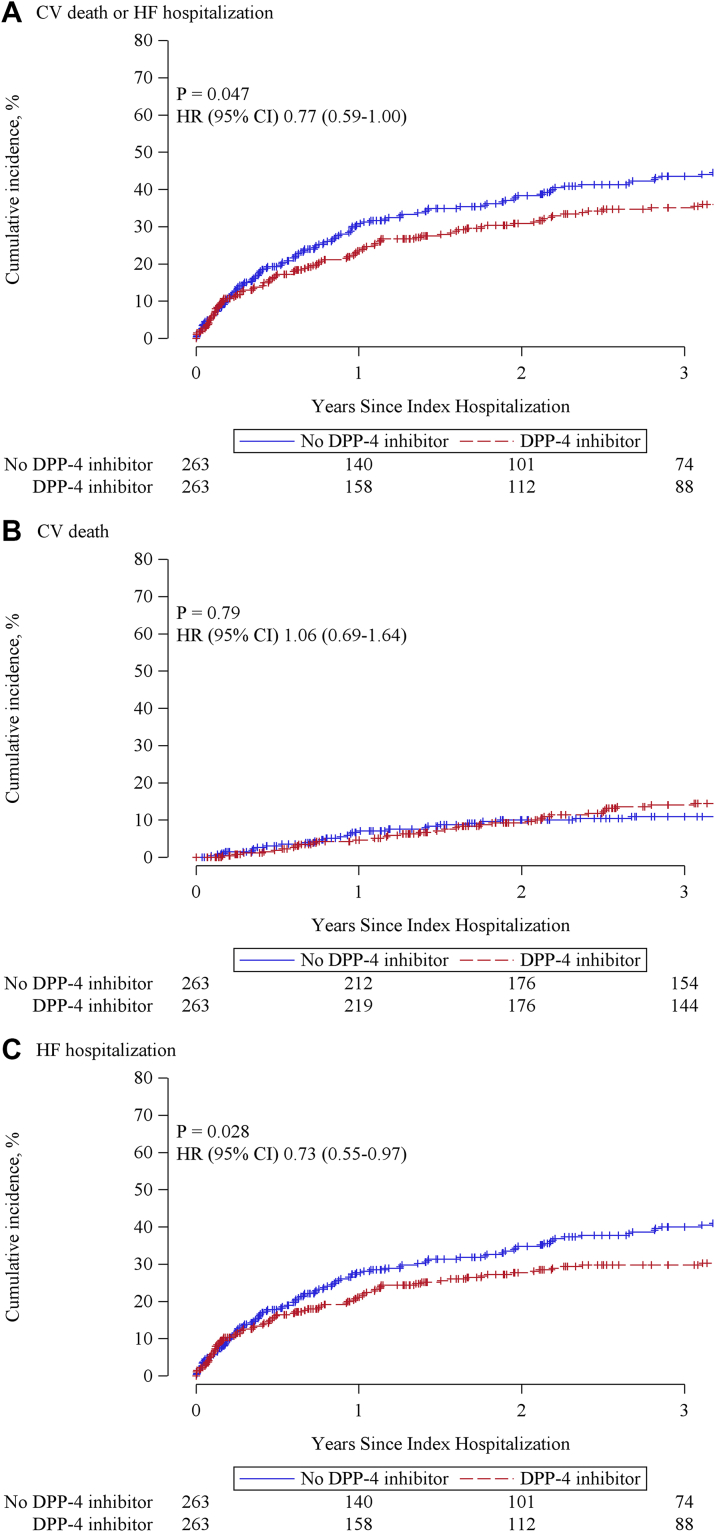
Patient characteristics before and after propensity score matching in the HFpEF group were shown in Table 3. After propensity score matching, variables were considered to be well balanced. During a median follow-up of 3.6 (IQR: 1.4-4.4) years, patients with a DPP-4 inhibitor had a lower incidence rate of composite of CV death or HF hospitalization (19.2 vs 25.9 events per 100 patient-years; rate ratio: 0.74; 95% CI: 0.57-0.97; P = 0.027) and HF hospitalization (16.4 vs 23.3 events per 100 patient-years; rate ratio: 0.70; 95% CI: 0.53-0.93; P = 0.014) (Table 4). Incidence rate of CV death did not differ between groups. The cumulative incidence curve also showed that DPP-4 inhibitor was associated with a decrease in the composite of CV death or HF hospitalization (HR: 0.77; 95% CI: 0.59-1.00; P = 0.047) and HF hospitalization (HR: 0.73; 95% CI: 0.55-0.97; P = 0.028) (Figure 3). Multiple imputation analysis also showed that DPP-4 inhibitor use was associated with a reduced composite of CV death or HF hospitalization (HR: 0.71; 95% CI: 0.58-0.87; P = 0.001) and HF hospitalization (HR: 0.72; 95% CI: 0.58-0.90; P = 0.003) (Supplemental Table 2).
Table 3.
Patient Characteristics Before and After Propensity Score Matching in HFpEF
| Before Propensity Score Matching |
After Propensity Score Matching |
|||||||
|---|---|---|---|---|---|---|---|---|
| DPP-4 Inhibitor (n = 444) | No DPP-4 Inhibitor (n = 686) | SMD | P Value | DPP-4 Inhibitor (n = 263) | No DPP-4 Inhibitor (n = 263) | SMD | P Value | |
| Demographics | ||||||||
| Age, y | 76.5 ± 10.4 | 77.7 ± 9.8 | 0.124 | 0.042 | 76.8 ± 10.4 | 77.6 ± 9.3 | 0.084 | 0.34 |
| Male | 234 (52.7) | 353 (51.5) | 0.025 | 0.68 | 136 (51.7) | 147 (55.9) | 0.084 | 0.34 |
| Body mass index, kg/m2 | 23.1 ± 4.4 | 23.2 ± 4.5 | 0.024 | 0.72 | 23.0 ± 4.6 | 23.4 ± 4.1 | 0.089 | 0.32 |
| Prior HF hospitalization | 120 (27.0) | 228 (33.2) | 0.135 | 0.027 | 80 (30.4) | 84 (31.9) | 0.033 | 0.71 |
| NYHA functional class III-IV | 21 (4.9) | 28 (4.2) | 0.033 | 0.59 | 9 (3.5) | 6 (2.4) | 0.070 | 0.43 |
| Smoking | 132 (34.3) | 226 (37.4) | 0.065 | 0.32 | 79 (34.3) | 86 (37.4) | 0.063 | 0.50 |
| Vital signs | ||||||||
| SBP, mm Hg | 153.7 ± 35.3 | 149.7 ± 33.2 | 0.119 | 0.053 | 153.1 ± 35.5 | 151.7 ± 32.7 | 0.042 | 0.63 |
| Heart rate, beats/min | 88.0 ± 24.9 | 85.7 ± 24.7 | 0.095 | 0.12 | 87.1 ± 23.1 | 84.8 ± 24.2 | 0.095 | 0.28 |
| Heart disease | ||||||||
| IHD | 200 (45.0) | 288 (42.0) | 0.062 | 0.31 | 121 (46.0) | 119 (45.2) | 0.015 | 0.86 |
| Atrial fibrillation | 184 (41.6) | 299 (43.7) | 0.042 | 0.49 | 118 (44.9) | 116 (44.1) | 0.015 | 0.86 |
| VT/VF | 3 (0.7) | 12 (1.8) | 0.095 | 0.13 | 3 (1.1) | 2 (0.8) | 0.039 | 0.65 |
| Prior procedures | ||||||||
| PCI/CABG | 117 (27.2) | 203 (30.4) | 0.071 | 0.25 | 74 (28.1) | 80 (30.4) | 0.050 | 0.57 |
| Pacemaker | 20 (4.9) | 51 (8.0) | 0.123 | 0.052 | 13 (4.9) | 16 (6.1) | 0.050 | 0.57 |
| ICD | 0 (0.0) | 3 (0.5) | 0.088 | 0.16 | 0 (0.0) | 2 (0.8) | 0.123 | 0.16 |
| CRT-P | 1 (0.2) | 2 (0.3) | 0.013 | 0.84 | 1 (0.4) | 0 (0.0) | 0.087 | 0.32 |
| CRT-D | 0 (0.0) | 1 (0.2) | 0.051 | 0.42 | 0 (0.0) | 1 (0.4) | 0.088 | 0.32 |
| Hemodialysis | 14 (3.4) | 22 (3.5) | 0.002 | 0.97 | 8 (3.0) | 10 (3.8) | 0.042 | 0.63 |
| Comorbidities | ||||||||
| Hypertension | 395 (89.0) | 573 (83.5) | 0.155 | 0.011 | 234 (89.0) | 232 (88.2) | 0.024 | 0.78 |
| Dyslipidemia | 193 (43.5) | 286 (41.7) | 0.036 | 0.55 | 115 (43.7) | 118 (44.9) | 0.023 | 0.79 |
| CKD | 219 (49.3) | 310 (45.2) | 0.083 | 0.17 | 132 (50.2) | 133 (50.6) | 0.008 | 0.93 |
| Stroke | 81 (18.2) | 89 (13.0) | 0.148 | 0.016 | 45 (17.1) | 42 (16.0) | 0.031 | 0.72 |
| PAD | 50 (11.3) | 64 (9.3) | 0.064 | 0.29 | 31 (11.8) | 26 (9.9) | 0.061 | 0.48 |
| Anemia | 121 (27.3) | 186 (27.1) | 0.003 | 0.96 | 81 (30.8) | 78 (29.7) | 0.025 | 0.78 |
| COPD | 18 (4.1) | 58 (8.5) | 0.176 | 0.004 | 16 (6.1) | 16 (6.1) | 0.000 | 1.00 |
| Malignancy | 40 (9.0) | 90 (13.1) | 0.129 | 0.034 | 30 (11.4) | 39 (14.8) | 0.101 | 0.25 |
| Echocardiographic data | ||||||||
| LVEF, % | 62.0 ± 8.3 | 62.3 ± 8.3 | 0.037 | 0.54 | 62.7 ± 8.4 | 62.0 ± 8.5 | 0.074 | 0.40 |
| LVDd, mm | 47.6 ± 6.4 | 47.4 ± 6.8 | 0.030 | 0.62 | 47.6 ± 6.6 | 47.6 ± 6.5 | 0.003 | 0.98 |
| LVDs, mm | 31.2 ± 5.9 | 31.1 ± 6.1 | 0.014 | 0.82 | 31.1 ± 5.9 | 31.4 ± 6.0 | 0.059 | 0.51 |
| LVMI, g/m2 | 119.9 ± 36.2 | 120.4 ± 35.7 | 0.014 | 0.83 | 119.8 ± 33.5 | 118.5 ± 32.8 | 0.041 | 0.65 |
| LAD, mm | 43.9 ± 7.5 | 44.5 ± 8.4 | 0.081 | 0.18 | 44.0 ± 7.5 | 44.4 ± 9.0 | 0.052 | 0.56 |
| MR III-IV | 85 (19.5) | 130 (19.1) | 0.008 | 0.90 | 48 (18.4) | 46 (17.6) | 0.020 | 0.82 |
| Laboratory data | ||||||||
| Hemoglobin, g/dL | 11.4 ± 2.1 | 11.2 ± 2.1 | 0.055 | 0.38 | 11.3 ± 2.0 | 11.3 ± 2.1 | 0.006 | 0.95 |
| Albumin, g/dL | 3.4 ± 0.5 | 3.4 ± 0.5 | 0.027 | 0.70 | 3.4 ± 0.5 | 3.4 ± 0.5 | 0.032 | 0.75 |
| CCr, mL/min | 38.3 ± 22.9 | 37.5 ± 25.3 | 0.031 | 0.64 | 37.7 ± 22.8 | 38.3 ± 24.8 | 0.025 | 0.78 |
| Sodium, mEq/L | 138.7 ± 3.5 | 138.8 ± 3.7 | 0.005 | 0.93 | 139.1 ± 3.3 | 138.9 ± 3.7 | 0.037 | 0.67 |
| Potassium, mEq/L | 4.4 ± 0.6 | 4.3 ± 0.6 | 0.046 | 0.46 | 4.4 ± 0.6 | 4.4 ± 0.5 | 0.076 | 0.39 |
| BNP, pg/mL | 182.9 (85.1-368.1) | 192.1 (96.7-376.6) | 0.229 | 0.19 | 185.2 (93.6-350.6) | 170.6 (85.9-326.4) | 0.174 | 0.94 |
| ln (BNP) | 5.0 ± 1.1 | 5.3 ± 1.0 | 0.214 | 0.047 | 5.1 ± 1.1 | 5.2 ± 1.1 | 0.078 | 0.60 |
| Medication | ||||||||
| Beta-blockers | 297 (66.9) | 379 (55.2) | 0.239 | <0.001 | 169 (64.3) | 163 (62.0) | 0.047 | 0.59 |
| ACE inhibitor/ARB | 353 (79.5) | 448 (65.3) | 0.316 | <0.001 | 200 (76.0) | 203 (77.2) | 0.027 | 0.76 |
| MRA | 191 (43.0) | 245 (35.7) | 0.150 | 0.014 | 101 (38.4) | 108 (41.1) | 0.054 | 0.53 |
| Loop diuretics | 385 (86.7) | 569 (82.9) | 0.104 | 0.088 | 229 (87.1) | 228 (86.7) | 0.011 | 0.90 |
| Thiazides | 61 (13.7) | 110 (16.0) | 0.064 | 0.29 | 39 (14.8) | 44 (16.7) | 0.052 | 0.55 |
| Tolvaptan | 38 (8.6) | 76 (11.1) | 0.084 | 0.17 | 21 (8.0) | 21 (8.0) | 0.000 | 1.00 |
| Digitalis | 31 (7.0) | 43 (6.3) | 0.029 | 0.64 | 11 (4.2) | 17 (6.5) | 0.102 | 0.24 |
| Sulfonylurea | 141 (31.8) | 77 (11.2) | 0.538 | <0.001 | 55 (20.9) | 50 (19.0) | 0.047 | 0.59 |
| α-glucosidase inhibitor | 91 (20.5) | 61 (8.9) | 0.345 | <0.001 | 39 (14.8) | 31 (11.8) | 0.089 | 0.30 |
| Glinide | 28 (6.3) | 13 (1.9) | 0.237 | <0.001 | 13 (4.9) | 11 (4.2) | 0.036 | 0.68 |
| Thiazolidine | 14 (3.2) | 16 (2.3) | 0.051 | 0.40 | 10 (3.8) | 11 (4.2) | 0.019 | 0.82 |
| Biguanide | 52 (11.7) | 41 (6.0) | 0.210 | <0.001 | 25 (9.5) | 21 (8.0) | 0.054 | 0.54 |
| Insulin | 198 (44.6) | 223 (32.5) | 0.252 | <0.001 | 106 (40.3) | 102 (38.8) | 0.031 | 0.72 |
Values are mean ± SD, n (%), or median (IQR).
Abbreviations as in Table 1.
Table 4.
Incidence Rate and Rate Ratio in HFpEF
| Event Rate (per 100 Person-Years) |
Rate Ratio (95% CI) | P Value | ||
|---|---|---|---|---|
| DPP-4 Inhibitor (n = 263) | No DPP-4 Inhibitor (n = 263) | |||
| CV death or HF hospitalization | 19.2 | 25.9 | 0.74 (0.57-0.97) | 0.027 |
| CV death | 5.4 | 4.9 | 1.08 (0.70-1.68) | 0.72 |
| HF hospitalization | 16.4 | 23.3 | 0.70 (0.53-0.93) | 0.014 |
CV = cardiovascular; other abbreviations as in Table 1.
Figure 3.
Cumulative Incidence Rates for Outcomes in Propensity Score-Matched HFpEF Patients
The cumulative incidence curve showed that DPP-4 inhibitor use was associated with a decrease in the composite of cardiovascular (CV) death or HF hospitalization (HR: 0.77; 95% CI: 0.59-1.00; P = 0.047) (A) and HF hospitalization (HR: 0.73; 95% CI: 0.55-0.97; P = 0.028) (C). DPP-4 inhibitor use was not associated with CV death (B). Abbreviations as in Figure 1.
Discussion
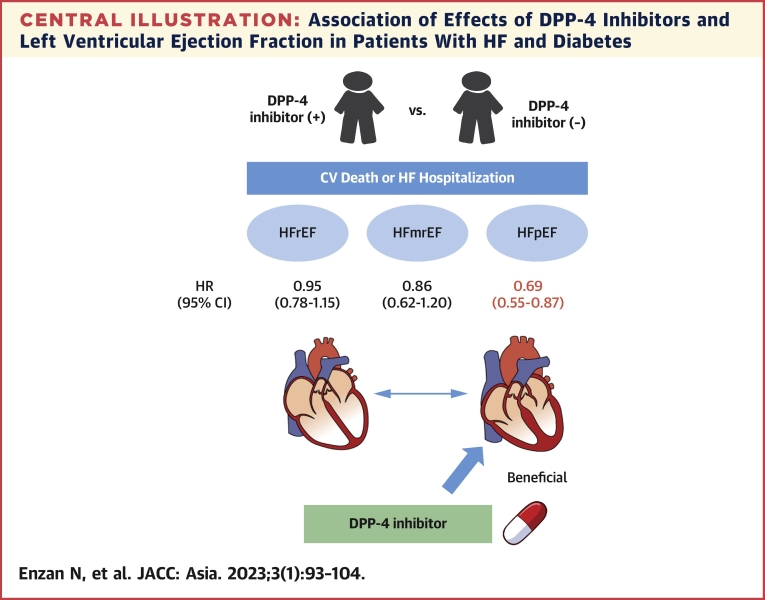
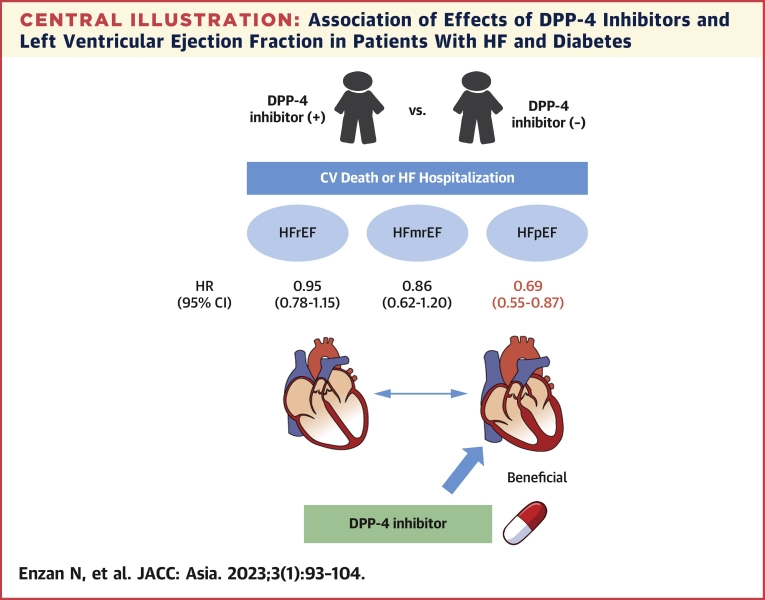
The major finding of the present study was that DPP-4 inhibitor use was associated with a lower incidence of composite of CV death or HF hospitalization in patients with HFpEF, but not in patients with HFmrEF and HFrEF, and DM (Central Illustration). We provided the first evidence of the relationship between effectiveness of DPP-4 inhibitor and LVEF.
Central Illustration.
Association of Effects of DPP-4 Inhibitors and Left Ventricular Ejection Fraction in Patients With HF and Diabetes
This study investigated the impact of dipeptidyl peptidase-4 (DPP-4) inhibitors on heart failure (HF) patients with diabetes. DPP-4 inhibitor use was associated with a lower incidence of composite of cardiovascular (CV) death or HF hospitalization in patients with diabetes and heart failure with preserved ejection fraction (HFpEF), not heart failure with mildly reduced ejection fraction (HFmrEF) or heart failure with reduced ejection fraction (HFrEF).
DPP-4 inhibitors were the first of class diabetic drugs required for CV safety by the Food and Drug Administration and European Medicines Agency. In several trials, the effects of DPP-4 inhibitors on HF hospitalization have long been debated. The SAVOR-TIMI 53 trial showed that saxagliptin increased the risk of HF.18 Subsequently, the EXAMINE trial for alogliptin,19 TECOS trial for sitagliptin,17 CARMELINA trial for linagliptin,26 a randomized controlled trial for omarigliptin,20 and a meta-analysis of vildagliptin21 reported no significant effect on HF hospitalization. A meta-analysis of DPP-4 inhibitors also concluded that the relative effect of DPP-4 inhibitors on the risk of HF is uncertain.22 A multicenter observational study from Canada, United States, and United Kingdom, including around 1,500,000 patients, showed that DPP-4 inhibitors were not associated with increased risk of HF.27 Importantly, these studies mainly focused on newly diagnosed HF and the effects of DPP-4 inhibitor on clinical outcomes in patients with diabetes and established HF has not been elucidated.
There were 3 studies assessing the effectiveness of DPP-4 inhibitor in patients with concomitant diabetes and established HF. The VIVIDD (Vildagliptin in Ventricular Dysfunction Diabetes) trial is the only randomized controlled trial that has been designed to evaluate the safety of vildagliptin on LV function in patients with HFrEF.40 It showed that vildagliptin had no major effects on LVEF but did lead to an increase in LV volumes. However, these changes could be explained by the fact that end-diastolic volume, BNP concentrations, and the percentage of prior HF hospitalization at baseline tended to be higher in vildagliptin group. A population-based retrospective cohort study demonstrated that sitagliptin was not associated with an increased risk of all-cause hospitalization or death but was associated with an increased risk of HF hospitalizations among patients with type 2 diabetes with pre-existing HF.41 In this study, the event rate of HF hospitalization was quite low (1.3% vs 0.9%) and LVEF was not recorded. A preliminary study demonstrated that DPP-4 inhibitors were associated with a lower mortality in HF patients with diabetes.28 However, this was a small-scale, single-center study, in which the effects of differences in backgrounds between DPP-4 inhibitor and no DPP-4 inhibitor groups were not fully adjusted. Recent HF guidelines recommend that HF management should be stratified by LVEF.29, 30, 31 However, to date, the effects of DPP-4 inhibitors on long-term prognosis in HF patients with DM according to LVEF has not been elucidated. Our study included hospitalized HF patients with high event rate from a multicenter, nationwide registry of hospitalized patients with HF and showed the different effectiveness of DPP-4 inhibitor on long-term outcomes according to LVEF. Beneficial effects of DPP-4 inhibitors on long-term outcomes were obvious in patients with HFpEF and DM by several analyses, including the multivariable Fine and Gray model (Table 2), propensity matching analysis (Figure 3), and multiple imputation analysis (Supplemental Table 2). The cubic spline analysis showed that DPP-4 inhibitors were beneficial in patients with LVEF >45% (Figure 2), supporting the effectiveness of DPP-4 inhibitors in patients with HFpEF and DM. In our study, sitagliptin, vildagliptin, alogliptin, and linagliptin were mainly used, and saxagliptin was used in only 1 patient. We could not exclude a possibility that drug effect might affect our results. To confirm the impact of each DPP-4 inhibitor on prognosis in HFpEF and DM, a prospective study is needed.
The effects of DPP-4 inhibitor on cardiac hypertrophy have been shown in several experimental studies. We previously demonstrated that glucagon-like peptide 1 stimulation by a DPP-4 inhibitor attenuated cardiac hypertrophy by suppressing the Nox4-HDAC4 axis in a mouse model.16 Another experimental study showed that DPP-4 inhibitor attenuated LV hypertrophy and fibrosis through reduced plasma renin activity and aldosterone concentrations32; decreased expression of tumor necrosis factor-α, interleukin-6, insulin-like growth factor-1,33 nuclear factor kappa B, and p38 mitogen-activated protein kinase34; or decreased myocardial oxidant stress.35 Cardiac hypertrophy is intimately involved in diastolic dysfunction, which is a central pathophysiology of HFpEF.42 Therefore, the association of DPP-4 inhibitors and better outcomes in HFpEF patients might be partially due to their antihypertrophic effects.
Study limitations
First, HbA1c and blood sugar were not recorded in this registry. HbA1c was associated with increased all-cause and CV death in patients with HF and diabetes. The differences in blood glucose control might affect the results of this study. To investigate the association of DPP-4 inhibitors, glucose control, and outcomes in patients with HF and DM, a further investigation is needed. Second, continuation of oral medication is extremely important for evaluating the beneficial effect of DPP-4 inhibitors in terms of inhibiting adverse events. However, the number of patients who stopped DPP-4 inhibitors during the follow-up period was not recorded. Third, there was no prescription of SGLT2 inhibitors at baseline because these drugs were unavailable in 2013 and applicable to insurance for diabetic patients from April in 2014 in Japan. In addition, there were no data regarding their prescription during follow-up period. The effect of SGLT2 inhibitors was not investigated in this study. However, the beneficial effect of DPP-4 inhibitors was observed from around 6 months of follow-up. This early effect of DPP-4 inhibitors indicates that SGLT2 inhibitors could not significantly affect our results. Fourth, diastolic dysfunction is considered as the main pathophysiology in HFpEF. Thus, echocardiographic parameters which represent diastolic dysfunction, such as E/A ratio, e/e′ ratio, and tricuspid regurgitation velocity, are crucial. However, there are many missing values in these parameters in the present study. Fifth, BNP is known to increase with usage of DPP-4 inhibitors. Although N-terminal pro-BNP is informative in patients treated with DPP-4 inhibitors, it was evaluated in a very limited number of patients in this study. Finally, the present study is not a prospective randomized trial, and unmeasured factors might have influenced the outcomes. Despite several limitations described previously, we analyzed the largest database regarding HF in Japan and conducted several sensitivity analyses, supporting the conclusion drawn in the present study.
Conclusions
DPP-4 inhibitors were associated with better long-term outcomes in patients with HFpEF and diabetes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HF and DM are common comorbidities and have a poor prognosis. DPP-4 inhibitors are the preferred medication for diabetic patients with HF, especially when the LVEF is preserved.
TRANSLATIONAL OUTLOOK: In the SGLT-2 inhibitor era, future studies are warranted to see if the use of DPP-4 inhibitors on top of the SGLT-2 inhibitors improves the long-term outcomes in patients with DM and HF.
Funding Support and Author Disclosures
This work was supported by Japan Agency for Medical Research and Development grants, 19ek0109339h0002 (to Dr Tsutsui), 19ek0210080h0003 [to Dr Tsutsui]) and a Health Labour Sciences Research grant (20FC1051 [to Dr Tsutsui]). The funders had no role in the design and conduct of the study or in collection, management, analysis and interpretation of the data, the preparation, review, and submission of the manuscript for publication. Dr Tsutsui has received personal fees from MSD, Astellas, Pfizer, Bristol Myers Squibb, Otsuka Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Kowa Pharmaceutical, Teijin Pharma, Medical Review Co, and Japanese Journal of Clinical Medicine; nonfinancial support from Actelion Pharmaceuticals, Japan Tobacco Inc, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Daiichi Sankyo, IQVIA Services Japan, and Omron Healthcare Co; and grants from Astellas, Novartis Pharma, Daiichi Sankyo, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, and MSD, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
This study could not have been carried out without the help, cooperation and support of JROADHF investigators in the survey institutions. The authors thank them for allowing us to obtain the data. JROADHF was supported by the Japanese Circulation Society and the Japanese Society of Heart Failure.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Bertoni A.G., Hundley W.G., Massing M.W., Bonds D.E., Burke G.L., Goff D.C. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 2.Shah A.D., Langenberg C., Rapsomaniki E., et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald M.R., Petrie M.C., Varyani F., et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 4.van Heerebeek L., Hamdani N., Handoko M.L., et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull F.M., Abraira C., Anderson R.J., et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 9.Zannad F., Ferreira J.P., Pocock S.J., et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 10.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 11.Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006;60:1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 12.Jackson E.K. Dipeptidyl peptidase IV inhibition alters the hemodynamic response to angiotensin-converting enzyme inhibition in humans with the metabolic syndrome. Hypertension. 2010;56:581–583. doi: 10.1161/HYPERTENSIONAHA.110.158527. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi A., Asakura M., Ito S., et al. Dipeptidyl-peptidase IV inhibition improves pathophysiology of heart failure and increases survival rate in pressure-overloaded mice. Am J Physiol Heart Circ Physiol. 2013;304:H1361–H1369. doi: 10.1152/ajpheart.00454.2012. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama M., Kawase H., Bando Y.K., Monji A., Murohara T. Dipeptidyl peptidase 4 inhibition alleviates shortage of circulating glucagon-like peptide-1 in heart failure and mitigates myocardial remodeling and apoptosis via the exchange protein directly activated by cyclic AMP 1/Ras-related protein 1 axis. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002081. [DOI] [PubMed] [Google Scholar]
- 15.Kubota A., Takano H., Wang H., et al. DPP-4 inhibition has beneficial effects on the heart after myocardial infarction. J Mol Cell Cardiol. 2016;91:72–80. doi: 10.1016/j.yjmcc.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Okabe K., Matsushima S., Ikeda S., et al. DPP (dipeptidyl peptidase)-4 inhibitor attenuates Ang II (angiotensin ii)-induced cardiac hypertrophy via GLP (glucagon-like peptide)-1-dependent suppression of nox (nicotinamide adenine dinucleotide phosphate oxidase) 4-HDAC (histone deacetylase) 4 Pathway. Hypertension. 2020;75:991–1001. doi: 10.1161/HYPERTENSIONAHA.119.14400. [DOI] [PubMed] [Google Scholar]
- 17.McGuire D.K., Van de Werf F., Armstrong P.W., et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126–135. doi: 10.1001/jamacardio.2016.0103. [DOI] [PubMed] [Google Scholar]
- 18.Scirica B.M., Bhatt D.L., Braunwald E., et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 19.Zannad F., Cannon C.P., Cushman W.C., et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 20.Gantz I., Chen M., Suryawanshi S., et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16:112. doi: 10.1186/s12933-017-0593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes G., Evans M., Del Prato S., et al. Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17 000 patients. Diabetes Obes Metab. 2015;17:1085–1092. doi: 10.1111/dom.12548. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Li S., Deng K., et al. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ. 2016;352:i610. doi: 10.1136/bmj.i610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini G.P., Avogaro A., Degli Esposti L., et al. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J. 2015;36:2454–2462. doi: 10.1093/eurheartj/ehv301. [DOI] [PubMed] [Google Scholar]
- 24.White W.B., Cannon C.P., Heller S.R., et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 25.Green J.B., Bethel M.A., Armstrong P.W., et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstock J., Perkovic V., Johansen O.E., et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filion K.B., Azoulay L., Platt R.W., et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145–1154. doi: 10.1056/NEJMoa1506115. [DOI] [PubMed] [Google Scholar]
- 28.Sato A., Yoshihisa A., Kanno Y., et al. Associations of dipeptidyl peptidase-4 inhibitors with mortality in hospitalized heart failure patients with diabetes mellitus. ESC Heart Fail. 2016;3:77–85. doi: 10.1002/ehf2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsui H., Isobe M., Ito H., et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure- digest version. Circ J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima Y., Ito S., Asakura M., et al. A dipeptidyl peptidase-IV inhibitor improves diastolic dysfunction in Dahl salt-sensitive rats. J Mol Cell Cardiol. 2019;129:257–265. doi: 10.1016/j.yjmcc.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi T., Nakamura K., Yoshida M., et al. Effect of vildagliptin, a dipeptidyl peptidase 4 inhibitor, on cardiac hypertrophy induced by chronic beta-adrenergic stimulation in rats. Cardiovasc Diabetol. 2014;13:43. doi: 10.1186/1475-2840-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aroor A.R., Habibi J., Kandikattu H.K., et al. Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice. Cardiovasc Diabetol. 2017;16:61. doi: 10.1186/s12933-017-0544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bostick B., Habibi J., Ma L., et al. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63:1000–1011. doi: 10.1016/j.metabol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 37.Ide T., Kaku H., Matsushima S., et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF) Circ J. 2021;85:1438–1450. doi: 10.1253/circj.CJ-20-0947. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.G., Hahn S., Oh T.J., Kwak S.H., Park K.S., Cho Y.M. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 39.Chikata Y., Iwata H., Miyosawa K., et al. Dipeptidyl peptidase-4 inhibitors reduced long-term cardiovascular risk in diabetic patients after percutaneous coronary intervention via insulin-like growth factor-1 axis. Sci Rep. 2022;12:5129. doi: 10.1038/s41598-022-09059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurray J.J.V., Ponikowski P., Bolli G.B., et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. J Am Coll Cardiol HF. 2018;6:8–17. doi: 10.1016/j.jchf.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Weir D.L., McAlister F.A., Senthilselvan A., Minhas-Sandhu J.K., Eurich D.T. Sitagliptin use in patients with diabetes and heart failure: a population-based retrospective cohort study. J Am Coll Cardiol HF. 2014;2:573–582. doi: 10.1016/j.jchf.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Mishra S., Kass D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2021;18:400–423. doi: 10.1038/s41569-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.