Abstract
Background
Neratinib is an irreversible pan-HER tyrosine kinase inhibitor approved for HER2-positive early-stage and metastatic breast cancer. Diarrhea is the most frequent side effect and the most common reason for early discontinuation. The phase II CONTROL trial investigated antidiarrheal prophylaxis or neratinib dose escalation (DE) for prevention of diarrhea. We present complete study results including final data for two DE strategies.
Methods
Patients who completed trastuzumab-based adjuvant therapy received neratinib 240 mg/day for 1 year. Early cohorts investigated mandatory prophylaxis with loperamide, then additional budesonide or colestipol. Final cohorts assessed neratinib DE over the first 2 (DE1) or 4 weeks (DE2). The primary endpoint was incidence of grade ≥3 diarrhea. Health-related quality of life (HRQoL) was assessed using FACT-B and EQ-5D-5L.
Results
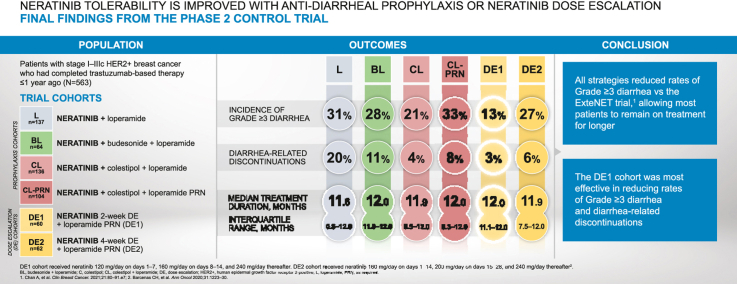
563 patients were enrolled into six cohorts. All strategies reduced grade ≥3 diarrhea with the lowest incidence in DE1 (DE1 13%; colestipol + loperamide [CL] 21%, DE2 27%; budesonide + loperamide [BL] 28%; loperamide [L] 31%; colestipol + loperamide as needed [CL-PRN] 33%). Diarrhea-related discontinuations occurred early and were lowest in DE1 (DE1 3%; CL 4%; DE2 6%; CL-PRN 8%; BL 11%; L 20%). More patients stayed on neratinib for the prescribed period versus historical controls. Prior pertuzumab use did not affect rates of grade ≥3 diarrhea, diarrhea-related discontinuations, or treatment duration. Early transient reductions in HRQoL scores were observed.
Conclusions
These complete results from CONTROL show improved neratinib tolerability with proactive management at the start of therapy. Two-week neratinib DE with loperamide as needed was particularly effective.
ClinicalTrials.gov registration number
Keywords: Neratinib, Tyrosine kinase inhibitor, HER2-positive, Breast cancer, Early stage, Diarrhea prophylaxis, Dose escalation, Health-related quality of life
Graphical abstract
Highlights
-
•
All antidiarrheal strategies reduced rates of grade ≥3 diarrhea vs ExteNET trial.
-
•
Two-week neratinib dose escalation had the lowest rate of grade ≥3 diarrhea.
-
•
Pre-emptive management of diarrhea allowed patients to stay on treatment longer.
-
•
Prior pertuzumab use did not appear to impact diarrhea rates or discontinuations.
-
•
All antidiarrheal strategies were associated with stable on-study HRQoL.
1. Introduction
Approximately 15–20% of breast cancer (BC) cases exhibit overexpression of the human epidermal growth factor receptor 2 (HER2) and/or amplification of the HER2 gene. In the absence of effective therapy, HER2-positive (HER2+) BC is associated with poorer prognosis and a greater risk of recurrence than HER2-negative BC [1,2]. Identification of HER2 overexpression and subsequent development of HER2-targeted agents has revolutionized the treatment of patients with HER2+ BC and these agents are now widely used in the adjuvant and metastatic disease settings [3,4]. Treatment for patients with HER2+ early-stage BC typically involves combination therapy that includes chemotherapy and HER2-targeted drug(s) together with optimal locoregional treatment. More recently, the benefit of extended adjuvant therapy with neratinib (NERLYNX®), an irreversible pan-HER tyrosine kinase inhibitor (TKI), has been demonstrated [[5], [6], [7]]. Neratinib is now approved as monotherapy for the extended adjuvant treatment of patients with early-stage HER2+ BC following adjuvant trastuzumab-based therapy and in combination with capecitabine for patients with HER2+ metastatic BC [8]. In the European Union, neratinib is approved as extended adjuvant treatment of adult patients with early-stage hormone receptor-positive HER2 overexpressed/amplified BC who completed adjuvant trastuzumab-based therapy <1 year ago [9].
Diarrhea is the most frequently reported adverse event (AE) associated with neratinib and is very common in the absence of antidiarrheal prophylactic treatment [5]. Diarrhea is commonly observed with TKIs [10,11]. In the ExteNET trial (NCT00878709) of neratinib after adjuvant trastuzumab, in which no mandatory antidiarrheal prophylaxis was used, 28% of patients who received neratinib discontinued treatment within the first 3 months, primarily because of AEs, most commonly diarrhea [12]. Forty percent of neratinib-treated patients reported grade 3 diarrhea and 17% of patients discontinued neratinib due to diarrhea [13].
The CONTROL trial (NCT02400476; PUMA-NER-6201) was an international, multi-cohort, open-label, phase II study conducted to assess diarrhea-mitigation strategies in the adjuvant BC population. Six non-comparative cohorts were enrolled to evaluate prophylactic medications (mandatory loperamide alone or in combination with budesonide, a locally acting corticosteroid used for inflammatory gastrointestinal conditions, or colestipol, a bile-acid sequestrant, or colestipol with loperamide as needed [PRN]) or neratinib dose escalation (DE) [14]. The DE strategy was developed in part based on the observation that grade 3 diarrhea occurs most frequently during the first month after neratinib initiation and rarely recurs, suggesting intestinal adaptation to neratinib exposure [5,6]. An integrated tolerability assessment of interim data from CONTROL suggested that this DE strategy improved tolerability to a greater degree than the prophylactic strategies [15].
The CONTROL trial is now complete. Final data for the cohorts of patients who were treated with prophylactic strategies have been presented in detail [14]. Here we present the final study results including complete data for the two DE cohorts. This report also provides more information regarding the impact of antidiarrheal strategies on treatment duration and includes data on prior pertuzumab use.
2. Methods
2.1. Study design
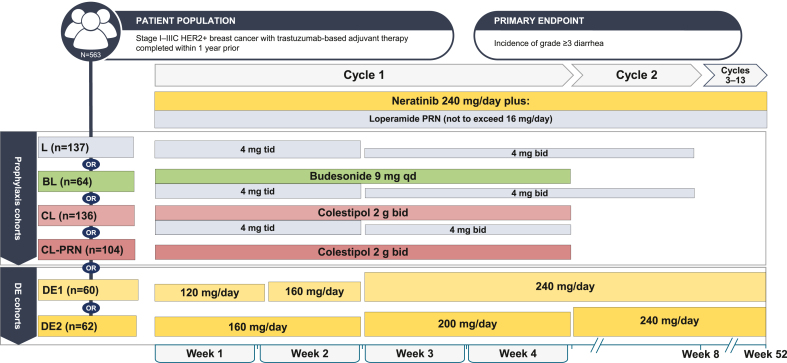
The CONTROL study (Fig. 1), which was designed to include the same patient population as the ExteNET trial [5,6], has been described in detail elsewhere [14]. In brief, eligible patients were aged ≥18 years with stage I–IIIc HER2+ BC and had completed trastuzumab-based adjuvant therapy or had experienced side effects resulting in early discontinuation of trastuzumab, within 1 year before study entry. Prior treatment with pertuzumab or trastuzumab emtansine (T-DM1) was allowed. Patients with evidence of disease recurrence or currently receiving treatment for BC were excluded (endocrine therapy was allowed), as were those with significant chronic gastrointestinal disorder with diarrhea as a major symptom.
Fig. 1.
Treatment cohorts and schedules. Abbreviations: B, budesonide; bid, twice daily; C, colestipol; DE, dose escalation; HER2+, human epidermal growth factor receptor 2 positive; L, loperamide; qd, once daily; tid, three times daily.
2.2. Treatment
Patients were entered into six sequential cohorts (Fig. 1) to receive neratinib daily for 1 year (13 continuous cycles, 28 days in each cycle). In cohorts 1–4, patients were started on oral neratinib 240 mg/day in combination with a mandatory antidiarrheal regimen; cohorts 5 and 6 utilized neratinib DE without mandatory prophylaxis.
Cohort 1 (L) oral loperamide (4 mg three times daily) on days 1–14, 4 mg twice daily for days 15–56 (further details on L cohort schedules are shown in the Supplementary Methods);
Cohort 2 (BL) oral budesonide (9 mg once daily) on days 1–28 plus oral loperamide as above;
Cohort 3 (CL) oral colestipol (2 g twice daily) on days 1–28 plus oral loperamide (4 mg three times daily) on days 1–14, 4 mg twice daily on days 15–28;
Cohort 4 (CL-PRN) oral colestipol (2 g twice daily) on days 1–28 plus loperamide PRN.
Cohort 5 (DE1): neratinib 120 mg/day on days 1–7, 160 mg/day on days 8–14, and 240 mg/day thereafter;
Cohort 6 (DE2): neratinib 160 mg/day on days 1–14, 200 mg/day on days 15–28, and 240 mg/day thereafter.
In addition, PRN loperamide ≤16 mg/day was used to manage diarrhea throughout the course of the study.
2.3. Study endpoints
The primary objective was to characterize diarrhea incidence and severity in patients treated with neratinib plus different antidiarrheal strategies. The primary endpoint was incidence of grade ≥3 treatment-emergent diarrhea at any time during the study. Secondary endpoints included assessment of serious AEs, AEs of interest, and evaluation of diarrhea incidence and severity. AEs were graded according to National Cancer Institute Common Terminology Criteria for AEs (version 4.0). Patient-reported health-related quality of life (HRQoL) – an exploratory endpoint – was assessed using Functional Assessment of Cancer Therapy – Breast (FACT-B; version 4.0) and the EuroQoL 5-Dimensions 5-Levels questionnaire (EQ-5D-5L). HRQoL questionnaires were completed electronically by patients on day 1 of cycles 1, 2, 4, 7, and 10, and at the end of cycle 13 (end of treatment). HRQoL assessments were introduced in November 2015 (protocol amendment 2).
2.4. Statistical considerations
Safety analyses were descriptive and performed in the safety population (all patients who received ≥1 neratinib dose). HRQoL analyses were descriptive and performed in the quality of life (QoL) analysis population (all patients in the safety population with baseline and ≥1 post-baseline HRQoL assessment). Mean (±standard error) observed scores over time were calculated.
Sample sizes for each cohort were selected to ensure that, given a hypothesized incidence rate, the corresponding confidence interval was no larger than a certain prescribed width.
3. Results
3.1. Patients and treatment
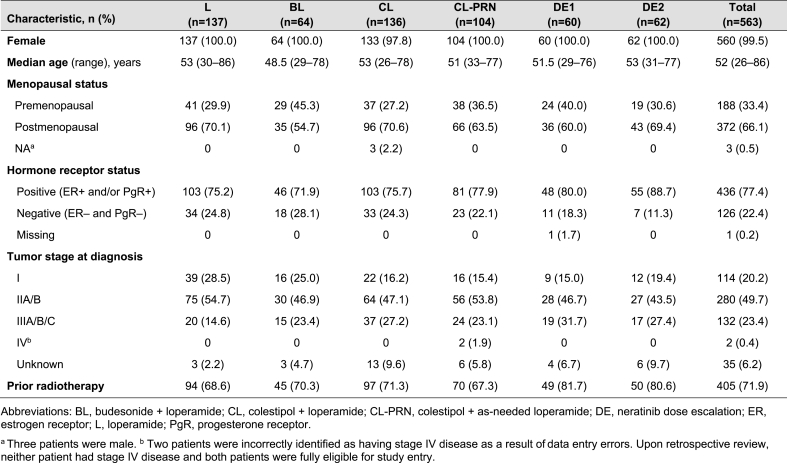
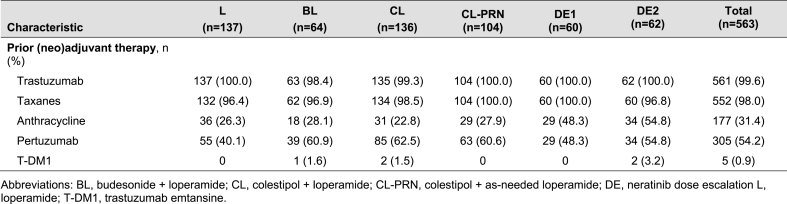
A total of 563 patients were enrolled into six unique cohorts (Fig. 1). The study was conducted between February 2015 and April 2021. Over three-quarter of patients had hormone receptor-positive disease and 73% had stage II or III tumors at diagnosis (Table 1). Prior taxane and anthracycline chemotherapy had been received by 98% and 31% of patients, respectively. All patients had prior trastuzumab and 54% of patients received prior pertuzumab (Table 2; Supplementary Table 1).
Table 1.
Baseline characteristics of patients in each of the sequential CONTROL cohorts.
Table 2.
Prior therapies received by patients in each of the CONTROL cohorts.
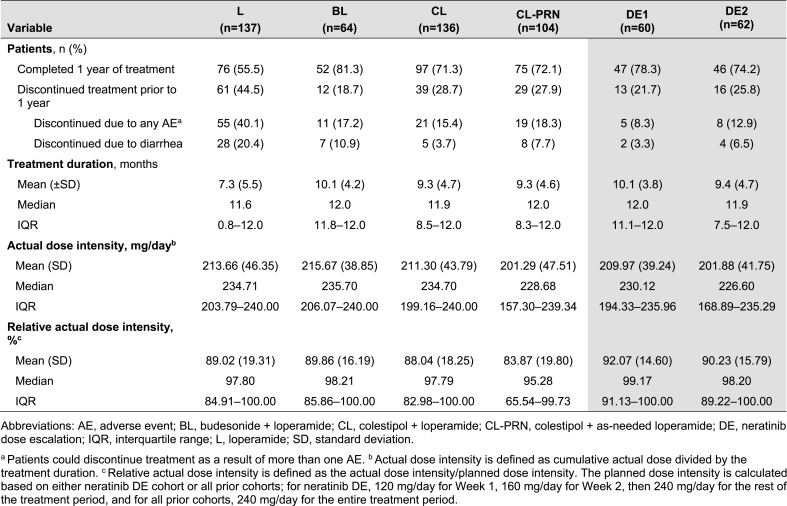
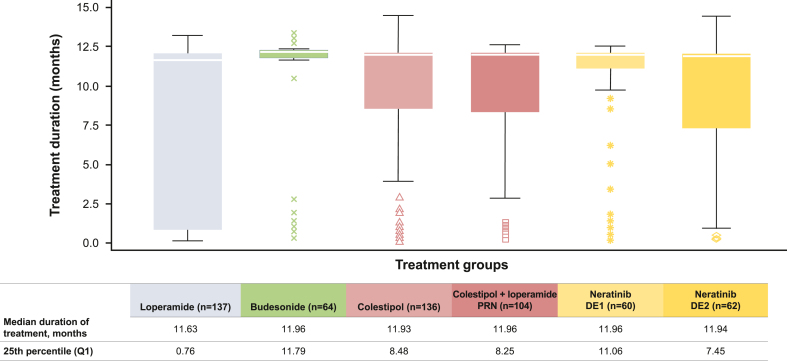
Study disposition is summarized in Table 3. The mean (standard deviation) exposure to neratinib was 7.3 (5.5), 10.1 (4.2), 9.3 (4.7), 9.3 (4.6), 10.1 (3.8), and 9.4 (4.7) months in the L, BL, CL, CL-PRN, DE1, and DE2 cohorts, respectively (Table 3). The median (interquartile range) exposure to neratinib in the L, BL, CL, CL-PRN, DE1, and DE2 cohorts was 11.6 (0.8–12.0), 12.0 (11.8–12.0), 11.9 (8.5–12.0), 12.0 (8.3–12.0), 12.0 (11.1–12.0), and 11.9 (7.5–12.0) months, respectively (Table 3, Fig. 2). The median relative neratinib dose intensities for L, BL, CL, CL-PRN, DE1, and DE2 were 98%, 98%, 98%, 95%, 99%, and 98%, respectively (Table 3).
Table 3.
Study disposition and exposure to treatment in each of the CONTROL cohorts (safety population).
Fig. 2.
Treatment duration in CONTROL cohorts.
The white line inside the box is the median. The upper whisker is drawn from the upper edge of the box to the largest observed value less than or equal to the upper fence; the lower whisker is drawn from the lower edge of the box to the smallest observed value greater than or equal to the lower fence; the upper and lower fence defined as Q3 + 1.5*IQR and Q1-1.5*IQR, respectively. Data beyond the end of the whiskers are outliers and are plotted as points. For example, at least 75% of patients received neratinib for longer than 11.06 months (Q1) in the DE1 cohort versus 0.76 months (Q1) in the loperamide cohort. Abbreviations; DE, dose escalation; PRN, as needed; Q, quartile.
In the DE1 cohort, 56 patients (93%) escalated the neratinib dose to 240 mg on schedule; one patient (2%) escalated to 240 mg 1 week later than the planned schedule. In the DE2 cohort, 47 patients (76%) escalated the neratinib dose to 240 mg on schedule, two patients (3%) escalated to 240 mg approximately 1–2 weeks later than planned schedule, and a further two patients (3%) escalated to 240 mg in later weeks.
3.2. Characteristics of treatment-emergent diarrhea
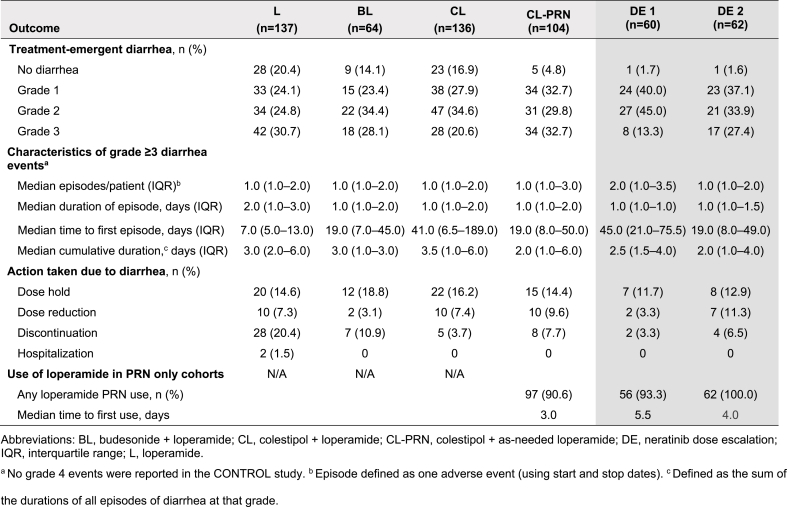
The primary endpoint – grade ≥3 diarrhea – occurred in 31%, 28%, 21%, 33%, 13%, and 27% of patients in the L, BL, CL, CL-PRN, DE1, and DE2 cohorts, respectively (Table 4). There was no grade 4 diarrhea in any CONTROL cohort. Use of PRN loperamide was common, with first use of loperamide occurring early in treatment in the PRN only cohorts (median of 3.0, 5.5, and 4.0 days in CL-PRN, DE1, and DE2, respectively; Table 4).
Table 4.
Characteristics of treatment-emergent diarrhea in CONTROL cohorts (safety population).
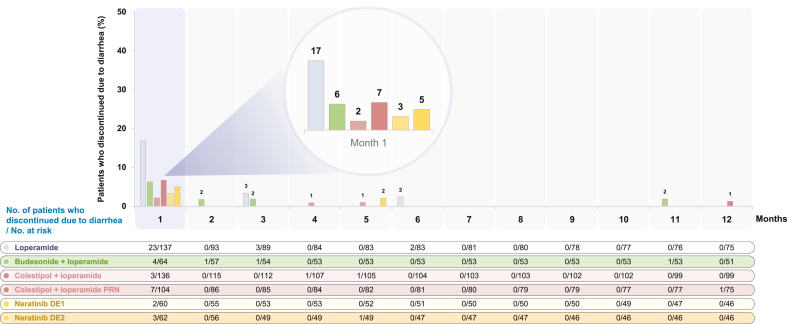
Across the study cohorts, 55%, 81%, 71%, 72%, 78%, and 74% of patients in L, BL, CL, CL-PRN, DE1 and DE2 completed 1 year of treatment (Table 3). Diarrhea-related discontinuations occurred in 20%, 11%, 4%, 8%, 3%, and 7% of patients in the L, BL, CL, CL-PRN, DE1, and DE2 cohorts, respectively (Fig. 3).
Fig. 3.
Treatment discontinuations due to diarrhea in CONTROL cohorts. Abbreviations: DE, dose escalation; PRN, as needed.
3.3. Impact of prior pertuzumab use on diarrhea
Across all cohorts combined, over 50% of patients had received pertuzumab before study entry (Table 2). Rates of grade 3 diarrhea and discontinuations due to diarrhea were generally similar in patients regardless of prior pertuzumab exposure (Supplementary Table 2; Supplementary Fig. 1). Prior exposure to pertuzumab did not appear to impact the duration of treatment with neratinib (Supplementary Fig. 2).
3.4. Non-diarrhea adverse events
Other AEs are summarized in Supplementary Table 3. After diarrhea, the next most common side effects across cohorts were nausea, constipation, and fatigue. Constipation appeared to be more common in patients who received mandatory loperamide than in those who received loperamide PRN. Other AEs leading to treatment discontinuation are detailed in Supplementary Table 4. No deaths were reported.
3.5. Health-related quality of life
HRQoL assessments were available for 441 patients, who comprised the QoL analysis population. Patients in all CONTROL cohorts experienced an early decrease from baseline in FACT-B total scores and EQ-5D-5L Health State summary scores, apparent from cycle 2 (Supplementary Fig. 3). These changes were transient. There were no discernible differences between the DE1 cohort and the other cohorts, although the study was not designed or powered to evaluate differences between the cohorts.
4. Discussion
Gastrointestinal toxicity, manifesting as diarrhea, is a common side effect of treatment with small-molecule TKIs [11]. Diarrhea compromises HRQoL and can lead to treatment breaks and early discontinuation, ultimately impacting treatment efficacy [16]. Diarrhea can also have a financial implication for both direct and indirect costs, particularly if inadequately managed [17] or if hospitalization is required [11]. Diarrhea management is therefore an important aspect of the treatment of patients undergoing cancer therapy.
In addition to a previous report that treatment-related diarrhea can be mitigated using mechanism-based antidiarrheal interventions [14], this final report from CONTROL confirms that all investigated strategies reduced the incidence of grade 3 diarrhea relative to that observed in the ExteNET trial. All but one strategy resulted in fewer diarrhea-related discontinuations and all approaches improved treatment duration relative to ExteNET.
The 2-week DE1 approach with loperamide PRN was particularly effective in reducing grade 3 diarrhea (13% versus 40% in ExteNET) and early diarrhea-related discontinuations (3% versus 17% in ExteNET) [5,13]. Although the median duration of treatment was similar across all CONTROL cohorts, the distributions around the median were narrowest in the BL and 2-week DE1 cohorts, revealing improved adherence in these cohorts. In addition, more patients who escalated neratinib on 2-week DE1 completed the 1 year of treatment: in the DE1 cohort, at least 75% of patients received neratinib for longer than 11.1 months, compared with 62% of patients in the neratinib arm of ExteNET completing at least 11 months of treatment. Although comparisons between studies of different designs and in varying patient populations are made with caution, neratinib DE appears to result in a lower incidence of diarrhea compared with other TKIs [18]. Notably, the DE strategy with PRN loperamide for diarrheal control circumvented the need for additional antidiarrheal medications and resulted in a lower incidence of constipation than was observed in the cohorts with mandated prophylactic antidiarrheal regimens.
Across cohorts, there were no differences in rates of diarrhea, early discontinuation, or treatment duration by prior pertuzumab use. HRQoL data utilizing validated instruments suggested that all antidiarrheal strategies were associated with reasonably stable HRQoL over the treatment period. Although the trial was not designed to robustly compare patient-reported outcomes between the various prophylaxis strategies, there were no discernible differences in HRQoL between cohorts during the study period. Based on results from the CONTROL trial, the US package label for neratinib now includes both the mandatory loperamide prophylaxis regimen and the 2-week DE1 strategy from CONTROL as diarrhea-mitigation strategies [8].
We recognize the limitations of our study, including the open-label study design. The six cohorts were enrolled sequentially in a non-comparative fashion. The sequential accrual of patient cohorts may increase the likelihood that physicians may have developed expertise in managing diarrhea over the 48-month study period. Although HRQoL was measured, this was only introduced in protocol amendment 2, with the result that HRQoL data are only available for 441 (78%) of the 563 patients in the study.
5. Conclusions
Diarrhea is a predictable and manageable side effect of treatment with neratinib, occurring early after initiation and rarely occurring later in the treatment course or recurring after a first instance. Effective mitigation of diarrhea using both the prophylactic and DE strategies described in this report has the potential to reduce the impact of diarrhea in the setting of extended adjuvant therapy for patients with HER2+ BC, thereby optimizing the treatment duration.
Ethical approval
The study protocol was approved by the institutional ethics committee at participating sites. The study was carried out in accordance with the 2008 Declaration of Helsinki. All patients provided written informed consent.
Role of the funding source
The CONTROL trial was sponsored by Puma Biotechnology, Inc. Puma Biotechnology Inc. also funded the provision of editorial/writing support provided by Miller Medical Communications. The sponsor was involved in the following: study design; data collection, analysis and interpretation of the data; writing of the report; the decision to submit the article for publication.
Data availability statement
The authors declare that the data supporting the findings of this study are available within the article. Qualified researchers and study participants may submit requests for other study documentation and clinical trial data to clinicaltrials@pumabiotechnology.com for consideration. Additional study design materials are available on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02400476), where the Study Protocol, Statistical Analysis Plan and Informed Consent Form can be found.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Arlene Chan: no competing interests to declare.
Manuel Ruiz-Borrego: no competing interests to declare.
Gavin Marx: no competing interests to declare.
A. Jo Chien: Contracted research (personal) from Puma Biotechnology Inc.
Hope S. Rugo: Research support for clinical trials through the University of California: Pfizer, Merck, Novartis, Lilly, Roche, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Astellas, and Gilead. Honoraria from: Puma, Samsung, Chugai, Blueprint, and NAPO. Travel: GE Healthcare.
Adam Brufsky: Consulting fees (personal) from Puma Biotechnology Inc.
Michael Thirlwell: no competing interests to declare.
Maureen Trudeau: no competing interests to declare.
Ron Bose: Research grant from Puma Biotechnology, and consulting from Genentech.
José A. García-Sáenz: consultative and advisory services for Seagen, Gilead, and Sanofi; consultancy/speaker fees from Novartis, Celgene, Eli Lilly, EISAI, AstraZeneca, Daiichi Sankyo, MSD, and Pierre Fabre; institution research funding from AstraZeneca; travel support from Novartis, Roche, and Pfizer.
Daniel Egle: advisory services for AstraZeneca, Daiichi Sankyo, MSD, Seagen, Gilead, Novartis, Roche, Pfizer, and Lilly; consultancy/speaker fees from Roche, Pfizer, Novartis, Lilly, AstraZeneca, Daiichi Sankyo, MSD, Seagen, and Gilead; travel support from AstraZeneca and Pfizer.
Barbara Pistilli: Consulting fees (personal) from Puma Biotechnology Inc., Pierre Fabre. Contracted research (personal) for Puma Biotechnology Inc.
Johanna Wassermann: no competing interests to declare.
Kerry A. Cheong: no competing interests to declare.
Benjamin Schnappauf: no competing interests to declare.
Dieter Semsek: no competing interests to declare.
Christian F. Singer: no competing interests to declare.
Navid Foruzan: Full-time employment Puma Biotechnology Inc. Ownership interest (stock, stock options) Puma Biotechnology Inc.
Daniel DiPrimeo: Full-time employment Puma Biotechnology Inc. Ownership interest (stock, stock options) Puma Biotechnology Inc.
Leanne McCulloch: Full-time employment Puma Biotechnology Inc. Ownership interest (stock, stock options) Puma Biotechnology Inc.
Sara A. Hurvitz: Contracted research paid to institution: Ambrx, Amgen, Arvinas, AstraZeneca, Bayer, Cytomx, Daiichi Sankyo, Dantari, Dignitana, Genentech/Roche, G1-Therapeutics, Gilead, GSK, Immunomedics, Eli Lilly, Macrogenics, Novartis, OBI Pharma, Orinove, Pfizer, Phoenix Molecular Designs, Ltd., Pieris, PUMA, Radius, Samumed, Sanofi, Seattle Genetics/Seagen, Zymeworks; Travel expenses paid to SAH: Lilly (2019); speaking: Daiichi Sankyo (2021).
Carlos H. Barcenas: no competing interests to declare.
Acknowledgments
We thank the Independent Data Monitoring Committee, study investigators, research staff, clinical research organizations, and other vendors, as well as the patients who participated in the CONTROL trial. The authors would like to acknowledge Alvin Wong (Puma Biotechnology) for thoughtful guidance and review, Stefan Dyla and Pratiksha Patel (Puma Biotechnology) for statistical programming support, Daniel Hunt (formerly Puma Biotechnology) for statistical analyses, Bethann Hromatka (Puma Biotechnology) for review and revision of the manuscript, and Lee Miller and Deirdre Carman (Miller Medical Communications Ltd) for medical writing/editorial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.12.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Gennari A., André F., Barrios C.H., Cortés J., de Azambuja E., DeMichele A., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 5.Chan A., Delaloge S., Holmes F.A., Moy B., Iwata H., Harvey V.J., et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 6.Martin M., Holmes F.A., Ejlertsen B., Delaloge S., Moy B., Iwata H., et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 7.Chan A., Moy B., Mansi J., Ejlertsen B., Holmes F.A., Chia S., et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21:80–91. doi: 10.1016/j.clbc.2020.09.014. e7. [DOI] [PubMed] [Google Scholar]
- 8.Food & Drug Administration . 2021. Nerlynx Highlights of Prescribing Information.https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf [Google Scholar]
- 9.European Medicines Agency . 2022. Neratinib Summary of Product Characteristics.https://www.ema.europa.eu/en/documents/product-information/nerlynx-epar-product-information_en.pdf [Google Scholar]
- 10.Le Du F., Diéras V., Curigliano G. The role of tyrosine kinase inhibitors in the treatment of HER2+ metastatic breast cancer. Eur J Cancer. 2021;154:175–189. doi: 10.1016/j.ejca.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Secombe K.R., Van Sebille Y.Z.A., Mayo B.J., Coller J.K., Gibson R.J., Bowen J.M. Diarrhea induced by small molecule tyrosine kinase inhibitors compared with chemotherapy: potential role of the microbiome. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420928493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnant M., Iwata H., Bashford A.E., Separovic R., Murias A., Vicente E., et al. Duration of extended adjuvant therapy with neratinib in early-stage HER2+ breast cancer after trastuzumab-based therapy: exploratory analyses from the phase III ExteNET trial. J Clin Oncol. 2018;36:524. doi: 10.1200/JCO.2018.36.15_suppl.524. [DOI] [Google Scholar]
- 13.Mortimer J., Di Palma J., Schmid K., Ye Y., Jahanzeb M. Patterns of occurrence and implications of neratinib-associated diarrhea in patients with HER2-positive breast cancer: analyses from the randomized phase III ExteNET trial. Breast Cancer Res. 2019;21:32. doi: 10.1186/s13058-019-1112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcenas C.H., Hurvitz S.A., Di Palma J.A., Bose R., Chien A.J., Iannotti N., et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol. 2020;31:1223–1230. doi: 10.1016/j.annonc.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Marx G.M., Chien A.J., García-Sáenz J.A., Chan A., Ruiz-Borrego M., Barcenas C.H., et al. Dose escalation for mitigating diarrhea: Ranked tolerability assessment of anti-diarrheal regimens in patients receiving neratinib for early-stage breast cancer. J Clin Oncol. 2021;39:536. doi: 10.1200/JCO.2021.39.15_suppl.536. [DOI] [Google Scholar]
- 16.Moy B., Takahashi M., Ohtani S., Chmielowska E., Yamamoto N., Coudert B.P., et al. Association between treatment duration and overall survival in early-stage HER2+ breast cancer patients receiving extended adjuvant therapy with neratinib in the ExteNET trial. J Clin Oncol. 2021;39:540. doi: 10.1200/JCO.2021.39.15_suppl.540. [DOI] [Google Scholar]
- 17.Roeland E., Schwartzberg L.S., Okhuysen P.C., Anupindi R., Hull M., Yeaw J., et al. Healthcare utilization and costs associated with cancer-related diarrhea. J Clin Oncol. 2021;39 doi: 10.1200/JCO.2021.39.15_suppl.e18623. [DOI] [Google Scholar]
- 18.Ustaris F., Saura C., Di Palma J., Bryce R., Moran S., Neuman L., et al. Effective management and prevention of neratinib-induced diarrhea. Am J Hematol Oncol. 2015;11:13–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article. Qualified researchers and study participants may submit requests for other study documentation and clinical trial data to clinicaltrials@pumabiotechnology.com for consideration. Additional study design materials are available on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02400476), where the Study Protocol, Statistical Analysis Plan and Informed Consent Form can be found.