Abstract
Introduction
In the Phase 3 IMbrave150 trial (NCT03434379), atezolizumab + bevacizumab demonstrated a clinically meaningful survival benefit over sorafenib in patients with unresectable hepatocellular carcinoma (HCC), including those with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. We used IMbrave150 data to investigate the safety and risk of viral reactivation or flare in infected patients treated with atezolizumab + bevacizumab or sorafenib.
Methods
Patients with unresectable HCC not previously treated with systemic therapy were randomized 2:1 to atezolizumab + bevacizumab or sorafenib. In this exploratory analysis, safety was continually evaluated, including for hepatic adverse events. Patients were monitored for HBV and HCV reactivation and flare at screening, the beginning of Cycles 5 and 9, and at treatment discontinuation.
Results
Of 501 enrolled patients, 485 were included in the safety population; 329 (68%) received atezolizumab + bevacizumab, and 156 (32%) received sorafenib. Overall, 150 (31%) and 58 (12%) patients had HBV and HCV infections, respectively. The safety profiles of atezolizumab + bevacizumab and sorafenib were consistent across patients, regardless of viral infection. Overall, hepatic serious adverse events occurred in 11% of patients receiving atezolizumab + bevacizumab and 8% receiving sorafenib. HBV or HCV reactivation occurred in 2% or 16% of atezolizumab + bevacizumab-treated patients, respectively, versus 7% or 14% with sorafenib. There were no instances of hepatitis flare with atezolizumab + bevacizumab.
Conclusions
Atezolizumab + bevacizumab had a similar hepatic safety profile in patients with and without HBV or HCV infection. Viral reactivation rates were similar between arms. Overall, these data support the use of atezolizumab + bevacizumab in patients with HCC and HBV or HCV infection without any special precaution.
Keywords: Atezolizumab, Bevacizumab, Viral reactivation, Viral flare, Post hoc analysis
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of worldwide cancer-related death [1]. Until recently, the standard of care for first-line treatment of unresectable HCC was treatment with a multikinase inhibitor, sorafenib or lenvatinib, although both agents are associated with modest survival benefit and unfavorable side effects [2, 3]. In the IMbrave150 trial, treatment with the anti-programmed death-ligand 1 (PD-L1) inhibitor atezolizumab combined with the anti-vascular endothelial growth factor inhibitor bevacizumab was associated with statistically significant and clinically meaningful improvements in overall survival and progression-free survival outcomes versus sorafenib in patients with unresectable HCC, reducing the risk of death by 42% and the risk of progression or death by 41% [4]. Based on this study, atezolizumab plus bevacizumab is now the standard of care for first-line HCC and is approved in more than 70 countries for patients with unresectable HCC who have not received prior systemic therapy. Atezolizumab plus bevacizumab is recommended as a first-line treatment by multiple global oncology societies and organizations, including a level IA recommendation, indicating a strong recommendation based on high quality clinical trial data, in the National Comprehensive Cancer Network, European Society for Medical Oncology, American Society of Clinical Oncology, and Chinese Society of Clinical Oncology guidelines [5, 6, 7, 8].
Two of the primary risk factors for HCC are hepatitis B virus (HBV) and hepatitis C virus (HCV) infection [1, 9]. To date, studies suggest that HBV or HCV infection has limited to no impact on the efficacy of PD-L1/programmed cell death protein 1 (PD-1) inhibitors, including in the IMbrave150 trial [4]. A pooled analysis of studies investigating PD-L1/PD-1 inhibitor therapy as monotherapy or combined with other agents found that HBV-infected patients with HCC achieved objective response rates (ORRs) similar to those patients with HCC without HBV infection (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.37–1.25; p = 0.21), although disease control rates were lower in patients with HBV infection (OR, 0.49; 95% CI, 0.27–0.89; p = 0.02) [10]. When assessing studies that included HBV- and HCV-infected patients with HCC, there was not a statistically significant difference in ORR and disease control rates between those with HCV infection compared with HBV-infected or HBV noninfected patients [10]. Additionally, studies of combined PD-L1 and vascular endothelial growth factor inhibition (atezolizumab + bevacizumab, pembrolizumab + lenvatinib, and camrelizumab + apatinib) have shown similar ORRs between patients with HCC who are positive for HBV infection, positive for HCV infection, and the overall HCC study populations [10, 11, 12], suggesting similar efficacy of PD-L1/PD-1 inhibitors independent of HBV or HCV infection.
As hepatic toxicity may occur in patients treated with PD-L1/PD-1 inhibitors, an additional concern with their use in patients with chronic viral infection is the impact on hepatic safety, as there are limited data on the safety of PD-L1/PD-1 inhibitors in patients with HCC and viral infection. In clinical studies of patients with HCC receiving PD-L1/PD-1 inhibitors, safety data in HBV-infected patients was comparable to the overall population [13, 14, 15]. In addition, in a real-world retrospective analysis of 50 patients with cancer and chronic viral infection (HIV, HBV, and/or HCV), the safety profile of PD-L1/PD-1 inhibitors was similar to patients without viral infection [16]. Despite the similar safety profile observed, baseline assessment and continued monitoring of markers of acute hepatocyte damage, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), is often done in patients with HCC and viral infections who are treated with PD-L1/PD-1 inhibitors out of caution [10].
Preclinical data suggest that PD-L1/PD-1 blockade may be associated with immune dysregulation in patients with acute viral infections, raising the concern that these therapies may increase the risk for viral reactivation [17]. Clinical studies suggest that HBV reactivation or HBV flare is infrequent [13, 14, 15], although controlled HBV viral load and HBV treatment are regularly required for study inclusion. Similarly, patients with untreated HCV infection or HBV/HCV coinfection are often excluded from clinical trials. Real-world studies have shown that the risk of HBV or HCV reactivation is low (≤5%), but that the lack of prophylactic treatment is a risk factor for viral reactivation [18, 19, 20, 21].
The IMbrave150 trial established the efficacy and safety of atezolizumab + bevacizumab in patients with unresectable HCC who were treated with atezolizumab and bevacizumab. In this exploratory post hoc analysis, we analyzed hepatic adverse events and HBV and HCV viral kinetics in patients from the IMbrave150 trial to evaluate the safety of this regimen in patients with chronic viral infection.
Materials and Methods
Patients and Study Design
The design of the IMbrave150 trial, including institutional review, informed consent, adherence to the Declaration of Helsinki, and CONSORT flow diagram, has been previously described [4]. Briefly, patients 18 years of age or older with locally advanced or metastatic and/or unresectable HCC who had not previously received systemic therapy were eligible. Patients had measurable disease, as defined by Response Evaluation Criteria in Solid Tumors, version 1.1, that was not amenable to curative surgical and/or locoregional therapies. Patients had Eastern Cooperative Oncology Group performance status of 0 or 1 and were classified as Child-Pugh A for liver function. Patients must have had documented virology status of hepatitis, as confirmed by screening HBV and HCV serology tests. Hepatitis B surface antigen, hepatitis B core antibody, and hepatitis B surface antibody were collected during screening and collected locally. HBV DNA was collected prior to Day 1 of Cycle 5 in patients who had negative serology for hepatitis B surface antigen and positive serology for anti-hepatitis B core antibody. To determine active infection (assessed by investigator per local clinical practice) an HBV DNA test was performed if a patient had a positive hepatitis B surface antigen test and/or a positive hepatitis B core antibody test and an HCV RNA test was performed if a patient had a positive HCV antibody test. Patients with HBV/HCV coinfection (patients with a history of HCV infection but who are negative for HCV RNA by polymerase chain reaction were considered noninfected with HCV for purposes of study entry and determining coinfection status) and patients with untreated or incompletely treated esophageal or gastric varices with bleeding or high risk of bleeding were not eligible. Patients with active HBV infection must have had HBV DNA <500 IU/mL within 28 days prior to initiation of study treatment and anti-HBV treatment for a minimum of 14 days prior to study entry and willingness to continue treatment for the length of the study. Patients with active HBV infection were treated as per local standard of care (e.g., entecavir). The trial was registered with ClinicalTrials.gov, number NCT03434379.
Eligible patients were randomized in a 2:1 ratio to receive atezolizumab plus bevacizumab or sorafenib until there was unacceptable toxicity or loss of clinical benefit. Atezolizumab was administered as 1,200 mg and bevacizumab as 15 mg per kg of body weight, both intravenously once every 3 weeks. Sorafenib was administered as 400 mg orally twice daily.
Virologic Testing Defined in the Study Protocol
According to the IMbrave150 protocol, HBV and HCV infections were monitored via serology at screening. Hepatitis B surface antigen was tested using the ARCHITECT i2000SR hepatitis B surface antigen immunoassay (Abbott Ireland Diagnostics Division, Sligo, Ireland). This assay has an overall specificity of 99.87% (95% CI, 99.74–99.94) and overall sensitivity of 99.52% (95% CI, 98.23–99.94). HCV antibody was tested locally. HBV DNA and HCV RNA titers were monitored at screening, Day 1 of Cycle 5, Day 1 of Cycle 9, and at the time of treatment discontinuation. Virologic testing was done locally.
Outcomes
The safety population consisted of all patients who had received at least one dose of trial treatment. Safety was continuously evaluated by vital signs, clinical laboratory test results, and assessment of the incidence and severity of adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Hepatic adverse events were analyzed, and the medical concept of immune-mediated hepatitis focused on both hepatitis diagnosis and liver functional test abnormalities. Three definitions using standardized Medical Dictionary for Regulatory Activities (MedDRA version 22.0) narrow terms were defined for this analysis: (1) Hepatitis (diagnosis and lab abnormalities) that included hepatic failure, fibrosis, cirrhosis, and other liver damage-mediated conditions; (2) Hepatitis (diagnosis only) that included hepatitis, which is non-infectious; and (3) Hepatitis (lab abnormalities only) that included liver-mediated investigations, signs, and symptoms. Protocol-defined guidelines for the management of patients with hepatic events are listed in supplementary Table S1 (see www.karger.com/doi/10.1159/000525499 for all online suppl. material).
To study the impact of chronic viral hepatitis on safety outcomes, the HBV analysis population was defined as all patients who had hepatitis B surface antigen and/or hepatitis B core antibody positivity at screening and ≥1 post-baseline HBV DNA assessment. The HCV analysis population was defined as all patients who had HCV antibody positivity at screening and ≥1 post-baseline HCV RNA assessment. All patients from the safety population who were not in the HBV or HCV analysis populations were classified as nonviral in this analysis.
HBV reactivation was defined as (1) ≥100-fold increase in HBV DNA compared with the baseline level, (2) HBV DNA ≥1,000 IU/mL in patients with previously undetectable levels, or (3) HBV DNA ≥10,000 IU/mL if the baseline level was not available [22]. MedDRA preferred terms relevant to HBV reactivation are hepatitis B reactivation, hepatitis B DNA increased, and hepatitis B DNA assay positive. HBV flare was defined as HBV reactivation and ALT level increase >3-fold of baseline level and >100 U/L [22]. HCV reactivation was defined as ≥10 IU/mL increase in HCV RNA compared with the baseline level [23]. MedDRA preferred terms relevant to HCV reactivation are hepatitis C, hepatitis C RNA fluctuation, hepatitis C RNA increased, and hepatitis C RNA positive. HCV flare was defined as ≥10 IU/mL increase in HCV RNA compared with baseline plus ALT increase >3-fold of baseline and >100 U/L [23]. Patients with increased ALT who met the criteria for an HBV or HCV flare at any time point were included in the analysis before medical review. Among patients for whom baseline level of HCV RNA was not available, all with an HCV RNA reading ≥10 IU/mL after baseline were included in the analysis as a conservative approach.
Statistical Analysis
Statistical analyses in this study were exploratory and descriptive. Descriptive statistics, including median and range for continuous variables and frequencies for categorical variables, were used to summarize data.
Multiple occurrences of adverse events were considered for the calculation of exposure-adjusted adverse event rates. For these analyses, adverse events were adjusted for duration of exposure to study treatment, with duration of study treatment data being classified into the following categories: 0 to ≤3 months, >3 to ≤6 months, >6 to ≤12 months, and >12 months. Based on their onset dates of different adverse events, participants could be classified into several of these categories.
Results
Patient Groups
The IMbrave150 trial enrolled 501 patients. The safety population included the 485 patients who received at least one dose of either study drug, 329 (68%) of whom were randomized to the atezolizumab plus bevacizumab arm and 156 (32%) to the sorafenib arm. HBV- and HCV-related etiologies were generally well balanced between the treatment arms in the safety population (Table 1). Overall, 150 (31%) patients were included in the HBV analysis population, 58 (12%) in the HCV analysis population, and 289 (60%) in the nonviral population (Table 1). As mentioned earlier, patients who tested positive for HCV antibody but with undetectable HCV RNA were considered noninfected with HCV for purposes of study entry but were included in the HCV analysis populations. Therefore, 7 patients in the atezolizumab + bevacizumab arm and 5 in sorafenib arm were included in both the HBV and HCV analysis populations. The safety population was predominantly male and approximately 40% were from Asia (excluding Japan), with a higher proportion of patients in the HBV analysis population from Asia compared with the HCV analysis population. The baseline disease characteristics in the HBV, HCV, and nonviral analysis populations and the safety population were generally well balanced between the treatment arms (Table 1). In the HCV analysis population, there were greater differences between the atezolizumab + bevacizumab and sorafenib arms in patients who were aged ≥65 years (32% vs. 57%), Asian (35% vs. 62%), or had varices present at enrollment (35% vs. 10%). The majority of patients with positive hepatitis B surface antigen started antiviral therapy prior to baseline, regardless of HBV DNA status at baseline (online suppl. Table S2).
Table 1.
Baseline characteristics
| HBV analysis populationa |
HCV analysis populationa |
Nonviral population |
Safety population |
|||||
|---|---|---|---|---|---|---|---|---|
| Atezo + bev (n = 108) | Sorafenib (n = 42) | Atezo + bev (n = 37) | Sorafenib (n = 21) | Atezo + bev (n = 191) | Sorafenib (n = 98) | Atezo + bev (n = 329) | Sorafenib (n = 156) | |
| Age, median (range), years | 60 (29–88) | 60 (33–87) | 61 (47–84) | 65 (55–83) | 67 (27–88) | 68 (37–84) | 64 (27–88) | 66 (33–87) |
| Age ≥65 years, n (%) | 33 (31) | 14 (33) | 12 (32) | 12 (57) | 116 (61) | 63 (64) | 158 (48) | 87 (56) |
| Male sex, n (%) | 90 (83) | 32 (76) | 29 (78) | 16 (76) | 156 (82) | 84 (86) | 270 (82) | 129 (83) |
| Race, n (%) | ||||||||
| Asian | 98 (91) | 36 (86) | 13 (35) | 13 (62) | 79 (41) | 42 (43) | 186 (57) | 88 (56) |
| White | 8 (7) | 6 (14) | 20 (54) | 6 (29) | 93 (49) | 41 (42) | 119 (36) | 51 (33) |
| Other/unknown | 2 (2) | 0 | 4 (11) | 2 (10) | 19 (10) | 15 (15) | 24 (7) | 17 (11) |
| Region, n (%)b | ||||||||
| Asia (excluding Japan) | 88 (81) | 33 (79) | 8 (22) | 8 (38) | 39 (20) | 25 (26) | 131 (40) | 63 (40) |
| Rest of worldc | 20 (19) | 9 (21) | 29 (78) | 13 (62) | 152 (80) | 73 (74) | 198 (60) | 93 (60) |
| Time from initial diagnosis, median (range), months | 7.5 (0.2–148.8) | 5.9 (0.3–137.2) | 5.5 (0.5–49.2) | 3.6 (1.0–97.2) | 6.0 (0.3–227.9) | 5.7 (0.6–122.6) | 6.9 (0.2–227.9) | 5.5 (0.3–137.2) |
| ECOG PS 1, n (%)b | 36 (33) | 20 (48) | 15 (41) | 11 (52) | 73 (38) | 34 (35) | 122 (37) | 61 (39) |
| Alcohol use, n (%) | ||||||||
| Current | 8 (7) | 4 (10) | 7 (19) | 3 (14) | 34 (18) | 18 (18) | 47 (14) | 25 (16) |
| Previous | 44 (41) | 15 (36) | 23 (62) | 10 (48) | 100 (52) | 49 (50) | 164 (50) | 72 (46) |
| Never | 56 (52) | 23 (55) | 7 (19) | 8 (38) | 57 (30) | 31 (32) | 118 (36) | 59 (38) |
| Child-Pugh class, n (%) | ||||||||
| A5 | 89 (82) | 32 (76) | 18 (49) | 14 (67) | 131 (69) | 74 (75) | 236 (72) | 116 (74) |
| A6 | 19 (18) | 10 (24) | 19 (51) | 7 (33) | 57 (30) | 24 (24) | 90 (28) | 40 (26) |
| B7 | 0 | 0 | 0 | 0 | 1 (1) | 0 | 1 (<1) | 0 |
| Cause of HCC, n (%)d | ||||||||
| HBV | 98 (91) | 36 (86) | 5 (14) | 4 (19) | 60 (31) | 34 (35) | 162 (49) | 71 (46) |
| HCV | 10 (9) | 5 (12) | 36 (97) | 21 (100) | 35 (18) | 16 (16) | 78 (24) | 41 (26) |
| Nonvirale | 10 (9) | 10 (24) | 12 (32) | 3 (14) | 96 (50) | 48 (49) | 154 (47) | 79 (51) |
| BCLC staging at study entry, n (%) | ||||||||
| A1 or A4 | 1 (1) | 1 (2) | 2 (5) | 1 (5) | 5 (3) | 2 (2) | 8 (2) | 4 (3) |
| B | 14 (13) | 2 (5) | 8 (22) | 2 (10) | 30 (16) | 21 (21) | 51 (16) | 25 (16) |
| C | 93 (86) | 39 (93) | 27 (73) | 18 (86) | 156 (82) | 75 (77) | 270 (82) | 127 (81) |
| AFP ≥400 ng/mL, n (%)b | 41 (38) | 18 (43) | 15 (41) | 8 (38) | 71 (37) | 33 (34) | 124 (38) | 58 (37) |
| EHS, n (%)b | 73 (68) | 26 (62) | 19 (51) | 8 (38) | 119 (62) | 58 (59) | 207 (63) | 90 (58) |
| MVI, n (%)b | 42 (39) | 15 (36) | 14 (38) | 10 (48) | 75 (39) | 44 (45) | 125 (38) | 66 (42) |
| MVI and/or EHS, n (%)b | 91 (84) | 35 (83) | 25 (68) | 14 (67) | 142 (74) | 69 (70) | 252 (77) | 114 (73) |
| Varices at enrollment, n (%) | 22 (20) | 9 (21) | 13 (35) | 2 (10) | 54 (28) | 31 (32) | 86 (26) | 41 (26) |
AFP, alpha fetoprotein; atezo, atezolizumab; bev, bevacizumab; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MVI, macrovascular invasion.
The HBV and HCV analysis populations were not mutually exclusive (7 patients treated with atezo + bev and 5 patients treated with sorafenib were included in both analysis populations).
Based on data collected on the electronic case report form, not the interactive voice/web response system.
Includes Japan.
Based on patient-reported medical history; patients may have more than one cause of HCC.
Includes alcohol and other causes of HCC; does not include unknown causes.
Safety
At the time of clinical data cutoff (August 29, 2019), the treatment exposures to study drugs in the HBV and HCV analysis populations were similar to that in the safety population, while treatment duration in the nonviral population was numerically lower (Table 2). In all populations, median treatment duration was longer for atezolizumab and bevacizumab compared with sorafenib. In the safety population, the proportion of patients with ≥6 months of treatment duration was 63% (206/329) for atezolizumab, 59% (193/329) for bevacizumab, and 29% (45/156) for sorafenib. The proportions in the HBV analysis population were 69% (75/108) for atezolizumab, 65% (70/108) for bevacizumab, and 36% (15/42) for sorafenib; in the HCV analysis population, the proportions were 73% (27/37) for atezolizumab, 65% (24/37) for bevacizumab, and 43% (9/21) for sorafenib.
Table 2.
Treatment exposure
| Treatment exposure | HBV analysis populationa |
HCV analysis populationa |
Nonviral population |
Safety population |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| atezo + bev (n = 108) |
sorafenib (n = 42) | atezo + bev (n = 37) |
sorafenib (n = 21) | atezo + bev (n = 191) |
sorafenib (n = 98) | atezo + bev (n = 329) |
sorafenib (n = 156) | |||||
| atezo | bev | atezo | bev | atezo | bev | atezo | bev | |||||
| Treatment duration, median | ||||||||||||
| (range) months | 7.6 (0–16) | 7.0 (0–15) | 3.9 (1–13) | 7.6 (0–16) | 7.2 (0–16) | 4.1 (0–10) | 6.9 (0–15) | 6.3 (0–15) | 2.1 (0–16) | 7.4 (0–16) | 6.9 (0–16) | 2.8 (0–16) |
| Treatment duration, n (%) | ||||||||||||
| 0 to <3 months | 13 (12) | 14 (13) | 19 (45) | 5 (14) | 5 (14) | 9 (43) | 58 (30) | 60 (31) | 64 (65) | 75 (23) | 78 (24) | 89 (57) |
| 3 to <6 months | 20 (19) | 24 (22) | 8 (19) | 5 (14) | 8 (22) | 3 (14) | 24 (13) | 28 (15) | 11 (11) | 48 (15) | 58 (18) | 22 (14) |
| 6 to <9 months | 38 (35) | 37 (34) | 11 (26) | 12 (32) | 11 (30) | 7 (33) | 53 (28) | 51 (27) | 12 (12) | 100 (30) | 97 (29) | 28 (18) |
| 9 to <12 months | 26 (24) | 23 (21) | 2 (5) | 10 (27) | 9 (24) | 2 (10) | 41 (21) | 38 (20) | 8 (8) | 76 (23) | 69 (21) | 12 (8) |
| ≥12 months | 11 (10) | 10 (9) | 2 (5) | 5 (14) | 4 (11) | 0 | 15 (8) | 14 (7) | 3 (3) | 30 (9) | 27 (8) | 5 (3) |
atezo, atezolizumab; bev, bevacizumab; HBV, hepatitis B virus; HCV, hepatitis C virus.
The HBV and HCV analysis populations were not mutually exclusive (7 patients treated with atezo + bev and 5 patients treated with sorafenib were included in both analysis populations).
The safety profiles of atezolizumab, bevacizumab, and sorafenib in the HBV, HCV, and nonviral analysis populations were consistent with those in the safety population (online suppl. Table S3). In the safety population, treatment-related Grade 3/4 adverse events occurred in 36% (117/329) of patients treated with atezolizumab plus bevacizumab, compared with 43% (46/108) in the HBV analysis population and 43% (16/37) in the HCV analysis population. Overall, 2% (6/329) of patients treated with atezolizumab plus bevacizumab experienced a treatment-related Grade 5 adverse event, yet none occurred in patients in the HBV and HCV analysis populations (online suppl. Table S3). Serious adverse events and adverse events leading to treatment withdrawal of any drug were more frequent with atezolizumab plus bevacizumab treatment than with sorafenib in all 4 analysis populations (online suppl. Table S3). No specific adverse events were responsible for the increased rate of serious adverse events or adverse events leading to treatment withdrawal in the HBV or HCV analysis populations. The proportions of patients with treatment-related serious adverse events were similar between the safety population and the HBV and HCV analysis populations (online suppl. Table S3).
Hepatic Adverse Events
In the safety population, there was no difference in the frequency of hepatic adverse events between the two treatment arms (Table 3). In the atezolizumab plus bevacizumab arm, 43% (142/329) of patients experienced any hepatic adverse event compared with 40% (62/156) in the sorafenib arm. Approximately half of the all-grade hepatic adverse events resolved over time, with 56% (79/142) of those observed in the atezolizumab plus bevacizumab arm resolving and 48% (30/62) resolved in the sorafenib arm. Withdrawal from atezolizumab or sorafenib due to a hepatic adverse event was infrequent, occurring in <3% of patients. Hepatic adverse events that were subsequently treated with corticosteroids were reported in 7% (24/329) of patients in the atezolizumab plus bevacizumab arm, while none were reported in the sorafenib arm (Table 3).
Table 3.
Hepatic adverse events in the safety population
| Hepatitis (diagnosis and laboratory abnormalities) |
Hepatitis (laboratory abnormalities) |
Hepatitis (diagnosis) |
||||
|---|---|---|---|---|---|---|
| atezo + bev (n = 329) | sorafenib (n = 156) | atezo + bev (n = 329) | sorafenib (n = 156) | atezo + bev (n = 329) | sorafenib (n = 156) | |
| All-cause AE | 142 (43) | 62 (40) | 126 (38) | 54 (35) | 43 (13) | 21 (13) |
| Grade 3/4 AEa | 70 (21) | 26 (17) | 55 (17) | 22 (14) | 23 (7) | 8 (5) |
| Grade 5 AE | 3 (1) | 2 (1) | 1 (<1) | 0 | 2 (1) | 2 (1) |
| Resolved AEb | 79 (56) | 30 (48) | 65 (52) | 24 (44) | 26 (60) | 11 (55) |
| AE leading to sorafenib withdrawal | NA | 4 (3) | NA | 1 (1) | NA | 3 (2) |
| AE leading to atezolizumab withdrawal | 8 (2) | NA | 5 (2) | NA | 4 (1) | NA |
| AE treated with systemic corticosteroids within 30 days | 24 (7) | 0 | 18 (5) | 0 | 8 (2) | 0 |
| AE leading to atezolizumab interruption | 38 (12) | NA | 30 (9) | NA | 11 (3) | NA |
| Serious AE | 35 (11) | 13 (8) | 18 (5) | 5 (3) | 22 (7) | 10 (6) |
Values are n (%). AE, adverse event; atezo, atezolizumab; bev, bevacizumab; NA, not applicable.
Refers to the highest grade experienced.
Percentages were calculated based on number of all-grade AEs.
An analysis of exposure-adjusted hepatic adverse event rates was conducted in the 4 analysis populations, and similar trends were observed between the safety population and the HBV, HCV, and nonviral populations (Table 4). Overall, the rate of hepatic adverse events was highest in the first 3 months after initiation of treatment. In the atezolizumab plus bevacizumab arm, the exposure-adjusted hepatic adverse event rate was 16% (134 patients experienced any hepatic adverse event over 855.4 person-months) from 0 to ≤3 months, 14% (95 patients with events over 693.0 person-months) from >3 to ≤6 months, 10% (64 patients with events over 677.2 person-months) from >6 to ≤12 months, and 4% (2 patients with events over 56.1 person-months) after 12 months. In the sorafenib arm, early hepatic adverse events (i.e., those occurring within the first 3 months after initiation of treatment) were more common compared with the atezolizumab + bevacizumab arm, where the exposure-adjusted hepatic adverse event rate was 27% (93 patients with events over 342.0 person-months) from 0 to ≤3 months, 6% (10 patients with events over 171.9 person-months) from >3 to ≤6 months, 14% (17 patients with events over 123.7 person-months) from >6 to ≤12 months, and 0% (0 patients with events over 8.1 person-months) after 12 months.
Table 4.
Exposure-adjusted hepatic adverse events by observation period
| Atezo + bev | Sorafenib | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 to ≤3 months | >3 to ≤6 months | >6 to ≤12 months | Beyond 12 months | 0 to ≤3 months | >3 to ≤6 months | >6 to ≤12 months | Beyond 12 months | |
| HBV analysis population | ||||||||
| Patients exposed, n | 108 | 95 | 76 | 11 | 42 | 23 | 15 | 2 |
| Total exposure, person-months | 305.0 | 253.8 | 229.4 | 23.8 | 106.0 | 56.8 | 38.3 | 1.3 |
| Total events, n (rate)a | 118 (39) | 100 (39) | 76 (33) | 0 | 54 (51) | 6 (11) | 11 (29) | 0 |
| Hepatic AEs, n (rate)b | 56 (18) | 45 (18) | 32 (14) | 0 | 23 (22) | 3 (5) | 5 (13) | 0 |
| HCV analysis population | ||||||||
| Patients exposed, n | 37 | 33 | 27 | 5 | 21 | 12 | 9 | 0 |
| Total exposure, person-months | 105.2 | 91.4 | 91.4 | 8.3 | 52.8 | 30.7 | 16.4 | 0 |
| Total events, n (rate)a | 24 (23) | 20 (22) | 6 (7) | 0 | 20 (38) | 4 (13) | 18 (109.5) | 0 |
| Hepatic AEs, n (rate)b | 12 (11) | 10 (11) | 3 (3) | 0 | 10 (19) | 2 (7) | 6 (36) | 0 |
| Nonviral population | ||||||||
| Patients exposed, n | 191 | 135 | 112 | 16 | 98 | 34 | 23 | 3 |
| Total exposure, person-months | 463.9 | 364.6 | 371.8 | 24.2 | 197.4 | 90.4 | 71.9 | 6.8 |
| Total events, n (rate)a | 144 (31) | 96 (26) | 64 (17) | 5 (21) | 130 (66) | 13 (14) | 15 (21) | 0 |
| Hepatic AEs, n (rate)b | 67 (14) | 40 (11) | 29 (8) | 2 (8) | 62 (31) | 5 (6) | 6 (8) | 0 |
| Safety population | ||||||||
| Patients exposed, n | 329 | 257 | 210 | 31 | 156 | 67 | 45 | 5 |
| Total exposure, person-months | 855.4 | 693.0 | 677.2 | 56.1 | 342.0 | 171.9 | 123.7 | 8.1 |
| Total events, n (rate)3 | 284 (33) | 216 (31) | 146 (22) | 5 (9) | 200 (58) | 23 (13) | 44 (36) | 0 |
| Hepatic AEs, n (rate)b | 134 (16) | 95 (14) | 64 (10) | 2 (4) | 93 (27) | 10 (6) | 17 (14) | 0 |
AEs shown are those that were reported in ≥2% of patients exposed during any time window from the safety population. AE, adverse event; atezo, atezolizumab; bev, bevacizumab; HBV, hepatitis B virus; HCV, hepatitis C virus.
Multiple occurrences of the same AE in an individual are counted separately; rate is number of events over total exposure.
Multiple occurrences of the same AE in an individual are counted only once (highest grade); rate is number of patients who experienced event over total exposure.
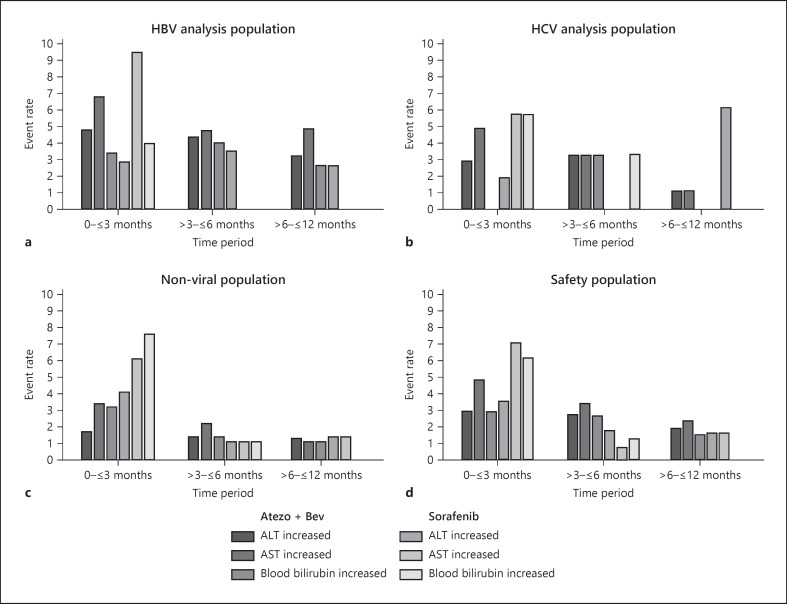
The hepatic adverse events that occurred in the safety population at a rate of ≥2% of patients exposed during any time window were ALT increased, ascites, AST increased, blood bilirubin increased, and gamma-glutamyltransferase increased. The most common hepatic adverse events were AST increased, ALT increased, and blood bilirubin increased, which were also observed in the HBV and HCV analysis populations (Fig. 1). In the atezolizumab plus bevacizumab arm, the rate of these adverse events was mostly consistent between the first three windows of exposure (0 to ≤3 months, >3 to ≤6 months, and >6 to ≤12 months) before decreasing after 12 months. In the sorafenib arm, the rate of ALT increased, aspartate AST increased, and blood bilirubin increased, which was higher in the first 3 months after initiation of treatment before decreasing in subsequent time windows. Similar trends were observed in the HBV, HCV, and nonviral analysis populations (shown in Fig. 1).
Fig. 1.
Exposure-adjusted hepatic adverse events by observation period. ALT increase, AST increase, and blood bilirubin increase in the (a) HBV (b) HCV (c) nonviral, and (d) safety populations. There were no ALT, AST, or blood bilirubin increased adverse events reported beyond 12 months. Multiple occurrences of the same AE in an individual are counted only once; rate is number of patients who experienced event over total exposure. ALT, alanine aminotransferase; AST, aspartate aminotransferase; atezo, atezolizumab; bev, bevacizumab.
Virus Reactivation and Hepatitis Flare
In the HBV analysis population, the incidence of HBV reactivation was lower in the atezolizumab plus bevacizumab arm than in the sorafenib arm, with 2% (2/108) of patients in the atezolizumab plus bevacizumab arm and 7% (3/42) of patients in the sorafenib arm experiencing reactivation (online suppl. Table S4). In the atezolizumab plus bevacizumab arm, 1 patient with HBV reactivation continued treatment and the HBV DNA became negative later (at the Cycle 9 visit), while the other patient with HBV reactivation discontinued treatment due to disease progression. In the sorafenib arm, all 3 patients with HBV reactivation discontinued treatment due to disease progression. For the 4 patients who discontinued, HBV DNA was increased at the study discontinuation visit. HBV flare was not observed in either arm. There was 1 patient in the sorafenib arm who met HBV flare criteria; however, as the ALT increase occurred 40 days prior to the increase in HBV DNA and had returned to normal range when the HBV DNA increase was observed, this event was not assessed to be a HBV flare. The clinical characteristics of patients with HBV reactivation are shown in Table 5.
Table 5.
Clinical characteristics of patients with HBV reactivationa
| Treatment arm | Age/sex | HBsAg | HBV DNA at baseline (U/mL)b | HBV DNA post-baseline (U/mL)c | Time to onset of HBV reactivation | ALT increased | Antiviral treatment | PD |
|---|---|---|---|---|---|---|---|---|
| Atezo + Bev | 62/M | Positive | <10 | 2,497 | Day 82 | No | Entecavir | PD at Day 42 |
|
| ||||||||
| Atezo + Bev | 59/M | Positive | 0 | 1,300e | Day 85 | No | Entecavir | No |
|
| ||||||||
| Sorafenib | 61/M | Positive | <100 | 1,500 | Day 217 | No | Tenofovir | PD at Day 210 |
|
| ||||||||
| Sorafenib | 69/F | Positive | 0 | 2,593 | Day 170 | ALT was 161 U/L at Day 127, but back to normal at Day 170 | Entecavir | PD at Day 164 |
ALT, alanine aminotransferase; atezo, atezolizumab; bev, bevacizumab; HBsAg, hepatitis B surface antigen; PD, patient discontinuation; U, units.
One patient with HBV reactivation from China was not included in the table due to local regulations regarding sharing of patient data.
All 4 patients received anti-HBV treatment at baseline and no new anti-HBV treatments were initiated post-baseline.
All 4 patients did not receive immunosuppressive therapy.
Greater than 3-fold increase from baseline and >100 U/L at any time point.
At Cycle 9, HBV DNA was negative.
In the HCV analysis population, incidences of HCV reactivation and flare were comparable between the treatment arms, with 16% (6/37) of patients in the atezolizumab plus bevacizumab arm and 14% (3/21) of patients in the sorafenib arm experiencing HCV reactivation (online suppl. Table S4). Of the 6 patients with HCV reactivation, 5 continued treatment and 1 interrupted treatment due to an adverse event of hyperbilirubinemia prior to HCV reactivation. In the sorafenib arm, 1 patient continued treatment and 2 discontinued treatment due to disease progression. Of these 9 patients with HCV reactivation, 1 patient from the atezolizumab plus bevacizumab arm had HCV RNA of 25 IU/mL on Cycle 5 and became negative on Cycle 9, while the remaining 8 patients had highest HCV RNA measurement at the time of the last test. There were no instances of HCV flare in the atezolizumab plus bevacizumab arm. In the sorafenib arm, 2 patients met the HCV flare criteria, but 1 of those patients experienced ALT increase 40 days prior to the increase in HCV RNA, which had returned to within the normal range by the time the HCV RNA increase had occurred. Therefore, this event was not assessed to be HCV flare. The clinical characteristics of patients with HCV reactivation are shown in Table 6.
Table 6.
Clinical characteristics of patients with HCV reactivation
| Treatment arm | Age/sex | HCV antibody | HCV RNA at baseline (U/mL) | HCV RNA post-baseline (U/mL) | Time to onset of HCV reactivation | ALT increasea | Antiviral treatment | PD |
|---|---|---|---|---|---|---|---|---|
| Atezo + bev | 64/M | Positive | 4.5 | 250,000 | Day 58 | No | No | No |
|
| ||||||||
| Atezo + bev | 77/F | Negative | Not available | 180,000 | Day 273 | No | No | No |
|
| ||||||||
| Atezo + bev | 47/M | Positive | 159,000 | 2,064,846 | Day 175 | No | No | No |
|
| ||||||||
| Atezo + bev | 53/M | Negative | <15 | 260,000 | Day 160 | No | No | No |
|
| ||||||||
| Atezo + bev | 53/M | Positive | Not available | 25 | Day 89 | No | No | No |
|
| ||||||||
| Atezo + bev | 52/M | Positive | 61,800 | 4,300,000 | Day 169 | No | No | No |
|
| ||||||||
| Sorafenib | 81/M | Positive | 11 | 2,464,675 | Day 89 | No | No | PD at Day 81 |
|
| ||||||||
| Sorafenib | 64/M | Positive | 2,800 | 49,000 | Day 170 | Yes (ALT was 46.2 U/L at baseline; ALT was 181 U/L on Day 105) | No | PD at Day 168 |
|
| ||||||||
| Sorafenib | 81/M | Positive | 7,070 | 4,830,000 | Day 97 | ALT was 192 U/L at Day 57, but was normal at Day 97 | No | PD at Day 86 |
ALT, alanine aminotransferase; atezo, atezolizumab; bev, bevacizumab; HBsAg, hepatitis B surface antigen; PD, patient discontinuation; U, units.
Greater than 3-fold increase from baseline and >100 U/L at any time point.
In both treatment arms, no hepatic adverse events and no cases of liver failure occurred in patients who had HBV or HCV reactivation. Additionally, no patient discontinued the study treatment due to HBV or HCV reactivation. No patient who had HBV or HCV reactivation received steroid treatment for an immune-related adverse event.
Discussion
The IMbrave150 trial demonstrated that atezolizumab plus bevacizumab resulted in statistically significant and clinically meaningful improvements in overall survival and progression-free survival versus sorafenib, establishing this combination as the standard of care for first-line treatment for patients with unresectable HCC [4]. Additionally, atezolizumab plus bevacizumab demonstrated a survival benefit versus sorafenib in both patients with HBV-related HCC (HR, 0.51; 95% CI, 0.32–0.81) and HCV-related HCC (HR, 0.43; 95% CI, 0.22–0.87) [4]. Here, using IMbrave150 patient data, we show that the safety profile of atezolizumab and bevacizumab was comparable between patients with and without HBV or HCV infection, with no evidence to suggest an increased risk for viral reactivation or hepatitis flare.
The overall safety profile of atezolizumab plus bevacizumab was generally consistent between the safety population and the HBV and HCV analysis populations. In the safety population, serious adverse events were more frequent with atezolizumab plus bevacizumab compared with sorafenib, yet no specific events were responsible for the overall increased rate and treatment exposure was higher with atezolizumab plus bevacizumab versus sorafenib [4]. In the HCV analysis population, there was a trend in both treatment arms of increased Grade 3/4 AEs, serious AEs, and AEs leading to withdrawal of treatment compared with the HBV analysis population. Among patients treated with sorafenib, those with HCV infection had a higher rate of treatment-related Grade 3/4 AEs versus those with HBV infection, but this difference was not apparent among Grade 3/4 AEs related to treatment in the atezolizumab plus bevacizumab arm.
There was no difference in the frequency of hepatic adverse events between the two treatment arms in the safety population and the HBV and HCV analysis populations. The similar incidence of hepatic adverse events should be evaluated in the context of different treatment exposures, as atezolizumab and bevacizumab exposures were more than double those of sorafenib in the safety population without a proportional increase in hepatic AEs. A systemic review of patients with advanced cancer treated with immune checkpoint inhibitors found that AEs related to liver function occurred in 22% of virally infected patients, with a numerically increased rate in HCV-infected (30%) versus HBV-infected (13%) patients [24]. In contrast, in this study, the rate of AEs related to laboratory abnormalities was more similar between the HCV and HBV analysis populations. Overall, transaminase increases were the most frequent AEs related to laboratory abnormalities here, with increased AST occurring in 19% and increased ALT occurring in 14% of patients [4]. This is similar to the rates observed in the Check-Mate-040 (nivolumab), KEYNOTE-224 and KEYNOTE-240 (pembrolizumab), and Chinese Phase 2 (camrelizumab) clinical trials, where the number of all patients with laboratory abnormalities ranged from 10% to 25% [13, 14, 15, 25]. Of note, in this study, there were no hepatic AEs reported in patients who experienced HBV or HCV reactivation.
A separate analysis of the IMbrave150 trial reported that among patients treated with atezolizumab plus bevacizumab, the median time to onset of the first hepatic AE was 1.6 months (range 0–11.8) with a median duration of 2.1 months (range 0.1 + to 14.1+) [26]. Here we report the exposure-adjusted onset of hepatic AEs. The rate of hepatic AEs was highest early in treatment for both arms but was more pronounced in the sorafenib arm, including those related to laboratory abnormalities. This did not correspond to an increased rate of discontinuations due to AE in the sorafenib arm, but treatment exposure was considerably lower in the sorafenib arm. This may have been due to faster disease progression on sorafenib or the high rate of AEs leading to dose modification or interruption of sorafenib [4].
There are limited data on the HBV and HCV reactivation rates in clinical trials of patients treated with PD-L1/PD-1 inhibitors, as these patients are excluded in many cases; however, overall, it appears to be rare. In this study, 2% of the 108 patients in the HBV analysis population treated with atezolizumab plus bevacizumab experienced HBV reactivation, and none had a hepatitis flare. This is in line with the Check-Mate-040 and KEYNOTE-224 trials, where there were 51 and 22 patients with HBV infection, respectively, and none experienced HBV reactivation, although patients were required to take antiviral treatment to achieve a viral load of <100 IU/mL at baseline [13, 14]. Here, in the HCV analysis population treated with atezolizumab plus bevacizumab, 16% of 37 patients experienced HCV reactivation, and none had a hepatitis flare. The Check-Mate-040 trial had 50 HCV-infected patients and KEYNOTE-224 had 26, with no cases of HCV reactivation. While the Chinese camrelizumab study did not report reactivation rates, 46 of 181 HBV-infected patients experienced an increase in HBV DNA concentration during the study period with none of the HBV surface antigen-negative patients converted to surface antigen-positive [15]. The risk for HBV reactivation may be higher in the real-world setting, however. In a Chinese cohort of 114 HBV-infected patients with cancer treated with PD-L1/PD-1 inhibitors, 5% of patients experienced reactivation, with the lack of antiviral prophylaxis being the only significant risk factor [18]. Similarly, in a systemic review of patients with advanced-stage cancer treated with immune checkpoint inhibitors, 2 of 89 (9%) HBV-infected patients experienced viral load increases leading to viral hepatitis, both of whom did not receive antiviral treatment [24]. In contrast, among the 89 HCV-infected patients not receiving antiviral therapy, there was 1 case of increased viral load [24]. All together, these studies suggest that the risk of HBV or HCV reactivation in patients treated with PD-L1/PD-1 inhibitors is low and that prophylactic viral treatment can help reduce the risk of HBV or HCV reactivation further. Of note, steroid treatment is associated with increased risk of HBV reactivation [27] and though evidence does not support an association for HCV reactivation, increased HCV viral replication has been observed in patients treated with immunosuppressive therapy or systemic chemotherapy [19]. In this study, no patients with HBV or HCV reactivation had been treated with steroids for an immune-related adverse event, though numbers may be too small to draw significant conclusions.
This study was an exploratory, post hoc analysis of the IMbrave150 trial and should be interpreted with some caution. The IMbrave150 trial, like most of the new drug clinical trials for unresectable HCC, excluded patients with advanced liver disease (Child-Pugh B or C), high viral load at entry for patients with HBV infection, or HBV/HCV coinfections. Because of these criteria, the study results may not be generalizable to the real-world patient population. The interval of viral load examinations was 12 weeks, so some instances of viral reactivation may be missed. Although patients with HBV infection were required to receive antiviral therapy during study drug treatment, the data on adherence to antiviral therapy were not available in the trial database. Therefore, the risk of HBV reactivation under antiviral prophylaxis, theoretically a rare event, could not be defined and may not be negligible. Finally, the differential diagnosis between hepatitis inducted by viral reactivation and other liver-related adverse events, such as immune-related hepatitis, was sometimes difficult.
In conclusion, the IMbrave150 trial established atezolizumab plus bevacizumab as the standard of care for first-line treatment of patients with unresectable HCC. Here we show that HBV- and HCV-infected patients can be treated with this regimen with no apparent increased risk for hepatic adverse events or viral reactivation.
Statement of Ethics
IMbrave150 was conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. Protocol approval was obtained from the Institutional Review Board (IRB) or Ethics Committee (EC) at each site. The first IRB approval for IMbrave150 was granted on December 19, 2017, from the City of Hope National Medical Center, Duarte, CA, USA (IRB No. 20172734; Western Institutional Review Board, Inc., Puyallup, WA, USA), in addition to multiple other EC/IRB approvals obtained across all participating sites in the different countries of enrollment. All patients gave written informed consent.
Conflict of Interest Statement
Chiun Hsu received research grants from Bristol Myers Squibb/ONO Pharmaceutical, F. Hoffmann-La Roche, and Ipsen and received honoraria from AstraZeneca, Bayer, Bristol Myers Squibb/ONO Pharmaceutical, Eisai, Eli Lilly and Company, Ipsen, Merck Serono, MSD, Novartis, F. Hoffmann-La Roche, and TTY Biopharm. Michel Ducreux reports a consulting or advisory role; has received honoraria from AstraZeneca, Bayer, F. Hoffmann-La Roche, Amgen, Pierre Fabre, Servier, Ipsen, Merck Serono, and Eli Lilly and Company; has been on a speakers bureau for Bayer, Ipsen, Lilly, Merck Serono, Amgen, and F. Hoffmann-La Roche; has received research funding from Bayer and F. Hoffmann-La Roche; and has received travel or accommodation expenses from Servier, Bayer, MSD, Ipsen, Eli Lilly and Company, and F. Hoffmann-La Roche. Andrew X. Zhu reports a consulting or advisory role for AstraZeneca, Bayer, Eisai, Exelixis, Gilead Sciences, Eli Lilly and Company, Merck, F. Hoffmann-La Roche/Genentech, and Sanofi and has received research funding from Bayer, Bristol Myers Squibb, Eli Lilly and Company, Merck, and Novartis; and is the associate editor for Liver Cancer. Shukui Qin declares no conflict of interests. Masafumi Ikeda is an advisor/board member at AstraZeneca, Eisai Co. Ltd., Eli Lilly Japan, and Takeda Pharmaceuticals; and received honoraria payment from Bayer, Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan, MSD, and Takeda Pharmaceuticals; and is an editorial board member for Liver Cancer. Tae-You Kim reports grants and non-financial support from F. Hoffmann-La Roche, during the conduct of the study. Peter R. Galle reports a consulting or advisory role and received honoraria from AdaptImmune, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Eli Lilly and Company, MSD, F. Hoffmann-La Roche, and Sirtex; has been on a speakers' bureau for AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Eli Lilly and Company, MSD, F. Hoffmann-La Roche, and Sirtex; has received research funding from Bayer and F. Hoffmann-La Roche; has provided expert testimony for Eli Lilly and Company; and has received travel or accommodation expenses from AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Eli Lilly and Company, and F. Hoffmann-La Roche. Richard S. Finn has served as a consultant with AstraZeneca, Bayer, CStone Pharmaceuticals, Eisai Co. Ltd., Eli Lilly, Merck, Pfizer, and F. Hoffmann-La Roche/Genentech; has received grant/research support to institution from Bristol Myers Squibb, Eisai Co. Ltd., Eli Lilly, Merck, Pfizer, and F. Hoffmann-La Roche/Genentech; and is an editorial board member for Liver Cancer. Ethan Chen is an employee of F. Hoffmann-La Roche. Ning Ma is an employee of F. Hoffmann-La Roche. Youyou Hu is an employee of Genentech. Lindong Li is an employee of Genentech. Ann-Li Cheng has served as a consultant with AstraZeneca, Bristol Myers Squibb, Eisai, Merck Serono, Novartis, Ono Pharmaceutical, Exelixis, Nucleix, IPSEN Innovation, Bayer Healthcare, Merck Sharp Dohme, Genentech, eiGene, CSR Pharma Group, F. Hoffmann-La Roche, and IQVIA; travel support from Bayer Yakuhin, Roche/Genentech, and IQVIA; reports receiving fees for serving on a speakers bureau from Bayer Yakuhin, Novartis, Eisai, Ono Pharmaceutical, and Amgen Taiwan; and is an associate editor for Liver Cancer.
Funding Sources
This study is sponsored by F. Hoffmann-La Roche Ltd.
Author Contributions
Conceptualization: Chiun Hsu, Ethan Chen, and Shukui Qin; methodology and supervision: Chiun Hsu and Ethan Chen; validation, visualization, and formal analysis: Youyou Hu; Investigation: Chiun Hsu, Michel Ducreux, Andrew X. Zhu, Shukui Qin, Masafumi Ikeda, Tae-You Kim, Peter R. Galle, Richard S. Finn, Ethan Chen, Ning Ma, Youyou Hu, Lindong Li, and Ann-Lii Cheng; resources: Andrew X. Zhu, Masafumi Ikeda, and Shukui Qin; data curation: Andrew X. Zhu and Youyou Hu; writing − original draft: Chiun Hsu, Ethan Chen, Lindong Li, Ning Ma, and Youyou Hu; writing − review and editing: Chiun Hsu, Michel Ducreux, Andrew X. Zhu, Shukui Qin, Masafumi Ikeda, Tae-You Kim, Peter R. Galle, Richard S. Finn, Ethan Chen, Ning Ma, Youyou Hu, Lindong Li, and Ann-Lii Cheng.
Data Availability Statement
With publication, individual patient level data will be available to qualified researchers who request access through the clinical study data request platform at https://vivli.org/. Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Supplementary Material
Supplementary data
Acknowledgments
Medical writing assistance for this manuscript was provided by Scott Battle, PhD, of Health Interactions, Inc.
Funding Statement
This study is sponsored by F. Hoffmann-La Roche Ltd.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology. Hepatobiliary cancers. 2020. [DOI] [PMC free article] [PubMed]
- 6.Chinese Society of Clinical Oncology Guidelines of the Chinese Society of Clinical Oncology (CSCO): Hepatocellular carcinoma. 2020.
- 7.European Society for Medical Oncology Guidelines Committee . eUpdate; 2020. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. [Google Scholar]
- 8.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38((36)):4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 9.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15((2)):223–243. doi: 10.1016/j.cld.2011.03.006. vii–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, Yan C, Zhu J, Chen X, Fu Q, Zhang H, et al. Anti-PD-1/PD-L1 blockade immunotherapy employed in treating hepatitis B virus infection-related advanced hepatocellular carcinoma: a literature review. Front Immunol. 2020;11:1037. doi: 10.3389/fimmu.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 Antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25((2)):515–523. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21((6)):808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 13.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389((10088)):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19((7)):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 15.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21((4)):571–580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 16.Shah NJ, Al-Shbool G, Blackburn M, Cook M, Belouali A, Liu SV, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7((1)):353. doi: 10.1186/s40425-019-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schonrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, et al. Hepatitis B virus reactivation in cancer patients with positive hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7((1)):322. doi: 10.1186/s40425-019-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PC, Chao Y, Chen MH, Lan KH, Lee IC, Hou MC, et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2020;8((2)):e001072. doi: 10.1136/jitc-2020-001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Keukeleire SJ, Vermassen T, Nezhad ZM, Kerre T, Kruse V, Vlierberghe HV, et al. Managing viral hepatitis in cancer patients under immune checkpoint inhibitors: should we take the risk? Immunotherapy. 2021;13((5)):409–418. doi: 10.2217/imt-2020-0273. [DOI] [PubMed] [Google Scholar]
- 21.Wong GLH, Wong VWS, Hui VWK, Yip TCF, Tse YK, Liang LY, et al. Hepatitis flare during immunotherapy in patients with current or past hepatitis B virus infection. Am J Gastroenterol. 2021;116((6)):1274–1283. doi: 10.14309/ajg.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 22.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67((4)):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres HA, Hosry J, Mahale P, Economides MP, Jiang Y, Lok AS. Hepatitis C virus reactivation in patients receiving cancer treatment: a prospective observational study. Hepatology. 2018;67((1)):36–47. doi: 10.1002/hep.29344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pu D, Yin L, Zhou Y, Li W, Huang L, Cai L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine. 2020;99((5)):e19013. doi: 10.1097/MD.0000000000019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda M, Zhu AX, Qin S, Kim T-Y, Lim HY, Kudo M, et al. 1008P IMbrave150: management of adverse events of special interest (AESIs) for atezolizumab (atezo) and bevacizumab (bev) in unresectable HCC. Ann Oncol. 2020;31:S698–S699. Abstract 1008P. [Google Scholar]
- 27.Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37((6)):1320–1328. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
With publication, individual patient level data will be available to qualified researchers who request access through the clinical study data request platform at https://vivli.org/. Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.