Abstract
Introduction
Sorafenib was historically the standard of care for advanced hepatocellular carcinoma (aHCC) until it was superseded by the combination of atezolizumab and bevacizumab. Thereafter, several novel first-line combination therapies have demonstrated favorable outcomes. The efficacies of these treatments in relation to current and previous standards of care are unknown, necessitating an overarching evaluation.
Methods
A systematic literature search was conducted on PubMed, EMBASE, Scopus, and the Cochrane Controlled Register of Trials for phase III randomized controlled trials investigating first-line systemic therapies for aHCC. Kaplan-Meier curves for overall survival (OS) and progression-free survival (PFS) were graphically reconstructed to retrieve individual patient-level data. Derived hazard ratios (HRs) for each study were pooled in a random-effects network meta-analysis (NMA). NMAs were also conducted using study-level HRs for various subgroups, according to viral etiology, Barcelona Clinic Liver Cancer (BCLC) staging, alpha-fetoprotein (AFP) levels, macrovascular invasion, and extrahepatic spread. Treatment strategies were ranked using p scores.
Results
Among 4,321 articles identified, 12 trials and 9,589 patients were included for analysis. Only two therapies showed OS benefit over sorafenib: combined anti-programmed-death and anti-VEGF pathway inhibitor monoclonal antibodies (Anti-PD-(L)1/VEGF Ab), including atezolizumab-bevacizumab and sintilimab-bevacizumab biosimilar (HR = 0.63, 95% CI = 0.53–0.76) and tremelimumab-durvalumab (HR = 0.78, 95% CI = 0.66–0.92). Anti-PD-(L)1/VEGF Ab showed OS benefit over all other therapies except tremelimumab-durvalumab. Low heterogeneity (I<sup>2</sup> = 0%) and inconsistency (Cochran's Q = 0.52, p = 0.773) was observed. p scores for OS ranked Anti-PD-(L)1/VEGF Ab as the best treatment in all subgroups, except hepatitis B where atezolizumab-cabozantinib ranked highest for both OS and PFS, as well as nonviral HCC and AFP ≥400 μg/L where tremelimumab-durvalumab ranked highest for OS.
Conclusion
This NMA supports Anti-PD-(L)1/VEGF Ab as the first-line therapy for aHCC and demonstrates a comparable benefit for tremelimumab-durvalumab which also extends to certain subgroups. Results of the subgroup analysis may guide treatment according to baseline characteristics, while pending further studies.
Keywords: Hepatocellular carcinoma, Immunotherapy, Meta-analysis
Introduction
The 2022 update of the Barcelona Clinic Liver Cancer (BCLC) guidelines outlines several first-line systemic therapies for advanced hepatocellular carcinoma (aHCC) [1]. Notably, the IMbrave150 trial, which investigated the combination of the programmed death ligand-1 (PD-L1) inhibitor atezolizumab and the anti-vascular endothelial growth factor inhibitor bevacizumab (Ate-Bev), established a new standard of care in 2020 [2, 3, 4]. More recently, the HIMALAYA trial outlined the effectiveness of a single priming dose of tremelimumab, a cytotoxic T-lymphocyte-associated antigen 4 inhibitor, combined with durvalumab, a PD-L1 inhibitor (tremelimumab-durvalumab [T300+D]) [5].
Despite the inclusion of T300+D as a first-line therapy in the BCLC guidelines, data from the HIMALAYA trial have not been pooled within a meta-analysis to compare its effectiveness against the current standard of care, Ate-Bev. In addition, recent results from the COSMIC-312 trial, which compared atezolizumab plus cabozantinib − a multitargeted tyrosine kinase inhibitor (TKI) − versus sorafenib, demonstrated progression-free survival (PFS) benefit but not overall survival (OS) benefit [6, 7]. Along with soon to be reported trials such as LEAP-002 [8] and CheckMate-9DW [9], which investigate different drug combinations in a similar setting, these have the potential to influence decision-making pathways for aHCC patients.
In keeping with the wide range of therapies in a rapidly changing field, this systematic review and individual patient data network meta-analysis (IPD-NMA) aims to synthesize the current evidence base to compare the efficacy of all systemic therapies for aHCC tested in randomized controlled trials (RCTs). A subgroup analysis was conducted in light of the marked effectiveness that some regimens have demonstrated in specific patient populations.
Materials and Methods
This systematic review and IPD-NMA was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines for IPD Systematic Reviews [10]. An electronic literature search from inception to 7 June 2022 was performed on PubMed, EMBASE, Scopus, and the Cochrane Controlled Register of Trials, without language restrictions. The search strategy included the concepts of systemic therapies for aHCC and RCTs (online suppl. Table. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000526639). Bibliographies of included studies were also screened to ensure inclusion of all relevant studies. Abstract and full-text review was conducted by two independent investigators (K.Y.F. and J.J.Z.); conflicts were resolved after discussion among all authors.
Study Selection
All phase III RCTs investigating VEGF inhibitors, TKIs, or immuno-oncological systemic therapies for aHCC were included. If multiple publications of the same study were retrieved, the most recent and informative publication was selected. RCTs investigating locoregional treatment, such as transarterial chemoembolization or selective internal radiation therapy, in addition to systemic therapy, were excluded. Case reports, case series, and reviews were also excluded.
Data Extraction and Quality Assessment
Data from included studies were extracted using a standardized data collection template with predefined data fields including study design, patient demographics, trial inclusion or exclusion criteria, and adverse effects (AEs). Risk of bias was assessed by two independent investigators (K.Y.F. and J.J.Z.) using the Cochrane Risk of Bias (RoB 2) tool for RCTs [11] with conflicts resolved after discussion among all authors.
Data Synthesis and Analysis
Extraction of Individual Patient Data
Precise statistical methods are needed to quantify comparisons among different systemic therapies in the rapidly changing field of aHCC therapy. Hence, a graphical reconstructive algorithm devised by Liu et al. [12] based on principles outlined by Guyot et al. [13, 14, 15, 16] was used to attain information on OS and PFS of individual patients in each trial arm. Images of Kaplan-Meier curves from included studies were digitized using WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/) to obtain step function values and step timings. Survival information of individual patients was then recovered based on the numerical solutions to the inverted Kaplan-Meier product-limit equations and provided risk tables. Studies which reported on treatments targeting the same biological pathways as determined by group consensus had their data pooled under a common arm for analysis. IPD were reconstructed and approved by visual comparisons and by comparing log-rank p values and hazard ratios (HRs) of the reconstructed dataset against originally reported values where available. Combined Kaplan-Meier plots for relevant single-agent therapies, combination therapies, and therapies meeting primary endpoints in their respective studies were generated for visual comparison.
Subgroup Data Extraction
Systemic therapies differ in their mechanistic pathways and may hence be better suited to treat certain subgroups of patients. As Kaplan-Meier curves were not provided for these subgroups in most studies, study-level HRs for OS and PFS from the following patient subgroups were extracted for analysis where possible: hepatitis B (hepatitis B virus [HBV]), hepatitis C (hepatitis C virus [HCV]), nonviral etiology, BCLC B, BCLC C, alpha-fetoprotein (AFP) <400 μg/L, AFP ≥400 μg/L, presence of macrovascular invasion (MVI), extrahepatic spread (EHS), and macrovascular invasion and/or extrahepatic spread (MVI/EHS).
Frequentist NMA
Natural log-transformed HR estimates were pooled in a random-effects, two-stage frequentist NMA for analysis of the entire cohort as well as for each subgroup. HRs for the entire cohort were derived from IPD data extraction while subgroup HRs were from study-level data. Indirect comparisons were performed using placebo as the common comparator for OS and PFS in the entire cohort and sorafenib as the common comparator for subgroup analysis. Heterogeneity was assessed via the I2 statistic and considered low, moderate, or considerable for I2 values <40%, 40–75%, and >75%, respectively [17]. Cochran's Q was also used to test for heterogeneity and inconsistency.
Although NMA-derived HRs are useful in indicating whether there is or is not significant benefit of one treatment over another, this dichotomous nature may be limiting. Hence, p scores were used to numerically rank the treatment strategies in the overall cohort as well as within subgroups, with higher p scores corresponding to greater efficacy. p scores are based solely on HRs and standard errors of the frequentist NMA estimates under the normality assumption [18], providing an intuitive way to appraise treatments and inform medical decision-making [19]. League tables showing NMA-derived HRs of various therapies for OS, PFS, and each subgroup were also generated.
All statistical analyses were conducted in R-4.1.2, with p < 0.05 regarded as indicating statistical significance. There was no funding source for this study.
Results
Study Selection
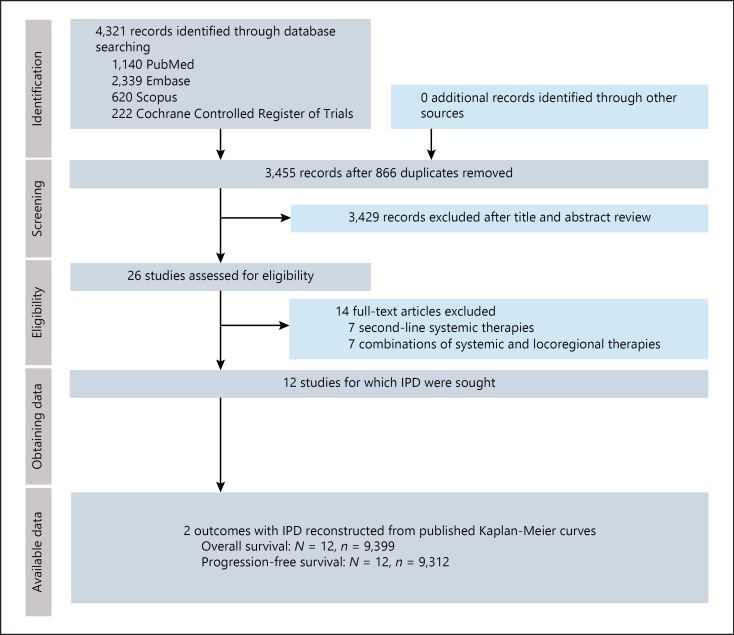
The search strategy retrieved 4,321 reports. After removal of 866 duplicates, 3,455 reports were screened by title and abstract. Twenty six manuscripts underwent full-text review; finally, 12 RCTs [5, 6, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29] comprising 9,589 patients were included (Fig. 1).
Fig. 1.
PRISMA flowchart of included studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study Characteristics
Most patients in included studies were male, Eastern Cooperative Oncology Group (ECOG) performance status 0, Child-Pugh category A, BCLC category C, and had evidence of MVI/EHS (Table 1). The proportion of patients with HBV ranged from 29 to 94%, while 1.6–32% had HCV. All studies were deemed to have a low risk of bias. Both IMbrave150 [28] and ORIENT-32 [29] investigated combinations of monoclonal antibodies targeting the same biological pathway (atezolizumab [anti-PD-L1] plus bevacizumab [anti-VEGF] in the former and sintilimab [anti-PD-1] plus bevacizumab biosimilar [Sinti-BevSim] in the latter); hence, the experimental arms for both studies were relabelled for subsequent analysis as the “Anti-PD-(L)1/VEGF Ab” arm. Online supplementary Table 2 summarizes relevant study inclusion and exclusion criteria, and AEs are outlined in online supplementary Table 3. Network plots for OS and PFS NMA are shown in online supplementary Figures 1 and 2.
Table 1.
Baseline demographics of participants in included studies
| Study | Arm | Patients, n | Males, % | Agea | ECOG PS >0, % | Child-Pugh B or C, % | BCLC C, % | HBV, % | HCV, % | MVI/EHS, % | AFP ≥400, % | Prior locoregional therapy, % | Subsequent systemic therapy, % | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cainap et al. [20] 2015 | Linifanib | 514 | 86 | 59 (21–84) | 37 | 5.8 | 84 | 49 | NR | 60b | NR | Locoregional (45) | NR | Low |
|
|
||||||||||||||
| Sorafenib | 521 | 84 | 60 (23–87) | 34 | 5.0 | 80 | 49 | NR | 57b | NR | Locoregional (46) | NR | ||
|
| ||||||||||||||
| Cheng et al. [21] 2009, Asian-SHARP | Sorafenib | 150 | 85 | 51 (23–86) | 75 | 2.7 | 95 | 71 | 11 | 69b | NR | NR | NR | Low |
|
|
||||||||||||||
| Placebo | 76 | 87 | 52 (25–79) | 72 | 2.6 | 96 | 78 | 3.9 | 68b | NR | NR | NR | ||
|
| ||||||||||||||
| Cheng et al. [22] 2013 | Sunitinib | 530 | 82 | 59 (18–85) | 47 | 0 | 87 | 55 | 21 | 79 | NR | Surgery (97) TACE (99.8), RT (7.6) | NR | Low |
|
|
||||||||||||||
| Sorafenib | 544 | 84 | 59 (18–84) | 47 | 0.1 | 84 | 53 | 22 | 76 | NR | Surgery (95), TACE (100), RT (6.4) | NR | ||
|
| ||||||||||||||
| Cheng et al. [28] 2022, IMbrave150 | Atezolizumab-bevacizumab | 336 | 82 | 64 [56–71] | 38 | 0 | 82 | 49 | 21 | 77 | 38 | Locoregional (48) | 36 | Low |
|
|
||||||||||||||
| Sorafenib | 165 | 83 | 66 [59–71] | 38 | 0 | 81 | 46 | 22 | 73 | 37 | Locoregional (52) | 52 | ||
|
| ||||||||||||||
| Johnson et al. [23] 2013, BRISK FL | Brivanib | 577 | 84 | 61 (19–87) | 36 | 8.0 | 77 | 44 | 20 | 63 | NR | 55 | NR | Low |
|
|
||||||||||||||
| Sorafenib | 578 | 84 | 60 (25–89) | 39 | 8.0 | 78 | 45 | 21 | 62 | NR | 56 | NR | ||
|
| ||||||||||||||
| Llovet et al. [24] 2008, SHARP | Sorafenib | 299 | 87 | 64.9±11.2 | 46 | 4.7 | 82 | 19 | 29 | 70 | NR | Surgery (19), TACE (29), RFA (6), RT (4) | NR | Low |
|
|
||||||||||||||
| Placebo | 303 | 87 | 66.3±10.2 | 46 | 2.0 | 83 | 18 | 27 | 70 | NR | Surgery (20), TACE (30), RFA (4), RT (5) | NR | ||
|
| ||||||||||||||
| Yau et al. [25] 2022, Checkmate459 | Nivolumab | 371 | 85 | 65 [57–71] | 27 | 1.6 | 82 | 31 | 23 | 75 | 33 | Surgery (54), locoregional (52), RT (12) | 38 | Low |
|
|
||||||||||||||
| Sorafenib | 372 | 85 | 65 [58–72] | 30 | 4.0 | 78 | 31 | 23 | 70 | 38 | Surgery (54), locoregional (56), RT (11) | 46 | ||
|
| ||||||||||||||
| Kudo et al. [26] 2018 | Lenvatinib | 478 | 85 | 63 (20–88) | 37 | 0.6 | 78 | 53 | 19 | 69 | NR | Procedure (68), RT (10) | 33 | Low |
|
|
||||||||||||||
| Sorafenib | 476 | 84 | 62 (22–88) | 37 | 1.1 | 81 | 48 | 26 | 71 | NR | Procedure (72), RT (13) | 39 | ||
|
| ||||||||||||||
| Zhu et al. [27] 2015, SEARCH | Sor-Erl | 362 | 82 | 61 | 39 | 0 | 83 | 34 | 30 | 57b | NR | NR | NR | Low |
|
|
||||||||||||||
| Sorafenib | 358 | 80 | 60 | 40 | 0 | 87 | 37 | 23 | 61b | NR | NR | NR | ||
|
| ||||||||||||||
| Ren et al. [29] 2021, ORIENT-32 | Sintilimab-bevacizumab biosimilar | 380 | 88 | 53 (21–82) | 52 | 3.9 | 85 | 94 | 1.6 | 80 | 43 | Surgery (52), Ablation (25), RT (3), TACE (66) | 29 | Low |
|
|
||||||||||||||
| Sorafenib | 191 | 90 | 54 (28–77) | 52 | 4.7 | 86 | 94 | 4.2 | 79 | 42 | Surgery (53), Ablation (24), RT (8), TACE (66) | 47 | ||
|
| ||||||||||||||
| Abou-Alfa et al. [5] 2022, HIMALAYA | Tremelimumab-durvalumab | 393 | 83 | 65 (22–86) | 38 | 0 | 80 | 31 | 28 | 53b | 37 | NR | 41 | Low |
|
|
||||||||||||||
| Durvalumab | 389 | 83 | 64 (20–86) | 39 | 0.3 | 80 | 31 | 28 | 54b | 35 | NR | 43 | ||
|
|
||||||||||||||
| Sorafenib | 389 | 87 | 64 (18–88) | 38 | 0.8 | 83 | 31 | 27 | 52b | 32 | NR | 45 | ||
|
| ||||||||||||||
| Kelley et al. [6] 2022, COSMIC-312 | Ate-Cab | 432 | 83 | 64 [58–70] | 36 | 0 | 68 | 29 | 31 | 69 | 38 | NR | 20 | Low |
|
|
||||||||||||||
| Cabozantinib | 188 | 84 | 64 [58–71] | 33 | 0 | 65 | 31 | 32 | 68 | 35 | NR | 29 | ||
|
|
||||||||||||||
| Sorafenib | 217 | 86 | 64 [57–71] | 34 | 0 | 67 | 29 | 31 | 68 | 30 | NR | 37 | ||
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; MVI/EHS, macrovascular invasion and/or extrahepatic spread; NR, not reported; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; RT, radiotherapy.
Ages are reported as median (range), mean±standard deviation, median [interquartile range], or mean.
For these studies, percentages of EHS were used instead of MVI/EHS which were not reported.
Overall Survival
All therapies except sunitinib showed a statistically significant OS benefit over placebo; of these, only two were superior to sorafenib: anti-programmed death-1/programmed death-ligand-1 pathway plus vascular endothelial growth receptor monoclonal antibody (Anti-PD-(L)1/VEGF Ab) (HR = 0.63, 95% CI = 0.53–0.76) and T300+D (HR = 0.78, 95% CI = 0.66–0.92) (Table 2; online suppl. Fig. 3). Anti-PD-(L)1/VEGF Ab showed OS benefit over all other therapies except T300+D (HR = 0.81, 95% CI = 0.63–1.04). Low heterogeneity (I2 = 0%) and inconsistency (Cochran's Q = 0.52, p = 0.773) was observed.
Table 2.
League table for OS
| Anti-PD-(L)1/VEGF Ab | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.81 (0.63–1.04) | T300-Dur | ||||||||||
|
| |||||||||||
| 0.75 (0.58-0.96) | 0.92 (0.73–1.17) | Nivolumab | |||||||||
|
| |||||||||||
| 0.74 (0.58-0.95) | 0.92 (0.77–1.08) | 0.99 (0.78–1.26) | Durvalumab | ||||||||
|
| |||||||||||
| 0.70 (0.54-0.90) | 0.86 (0.68–1.09) | 0.93 (0.73–1.19) | 0.94 (0.74–1.19) | Sor-Erl | |||||||
|
| |||||||||||
| 0.68 (0.54-0.86) | 0.84 (0.67–1.05) | 0.91 (0.72–1.14) | 0.91 (0.73–1.14) | 0.97 (0.78–1.22) | Lenvatinib | ||||||
|
| |||||||||||
| 0.67 (0.49-0.91) | 0.82 (0.61–1.12) | 0.89 (0.66–1.21) | 0.90 (0.67–1.22) | 0.96 (0.70–1.30) | 0.98 (0.73–1.32) | Ate-Cab | |||||
|
| |||||||||||
| 0.63 (0.53-0.76) | 0.78 (0.66-0.92) | 0.84 (0.71–1.00) | 0.85 (0.72–1.00) | 0.90 (0.76–1.07) | 0.93 (0.80–1.08) | 0.94 (0.73–1.22) | Sorafenib | ||||
|
| |||||||||||
| 0.60 (0.48-0.77) | 0.74 (0.59-0.93) | 0.81 (0.64–1.02) | 0.81 (0.65–1.02) | 0.86 (0.69–1.09) | 0.89 (0.72–1.10) | 0.90 (0.67–1.21) | 0.96 (0.82–1.12) | Linifanib | |||
|
| |||||||||||
| 0.57 (0.46-0.72) | 0.71 (0.57-0.88) | 0.77 (0.61-0.95) | 0.77 (0.62-0.95) | 0.82 (0.66–1.02) | 0.84 (0.69–1.03) | 0.86 (0.64–1.14) | 0.91 (0.79–1.04) | 0.95 (0.77–1.16) | Brivanib | ||
|
| |||||||||||
| 0.48 (0.38-0.61) | 0.60 (0.48-0.74) | 0.65 (0.52-0.81) | 0.65 (0.52-0.81) | 0.69 (0.55-0.87) | 0.71 (0.58-0.87) | 0.72 (0.54-0.97) | 0.77 (0.66-0.88) | 0.80 (0.65-0.99) | 0.84 (0.69–1.03) | Sunitinib | |
|
| |||||||||||
| 0.44 (0.34-0.57) | 0.54 (0.42-0.69) | 0.59 (0.46-0.75) | 0.59 (0.46-0.76) | 0.63 (0.49-0.81) | 0.65 (0.51-0.82) | 0.66 (0.48-0.90) | 0.70 (0.58-0.84) | 0.73 (0.58-0.92) | 0.77 (0.61-0.96) | 0.91 (0.72–1.15) | Placebo |
Significant comparisons are bolded. Anti-PD-(L)1/VEGF Ab, anti-programmed death-1/programmed death-ligand-1 pathway plus vascular endothelial growth receptor monoclonal antibody; Ate-Cab, atezolizumab-cabozantinib; T300+D, tremelimumab-durvalumab; Sor-Erl, sorafenib-erlotinib.
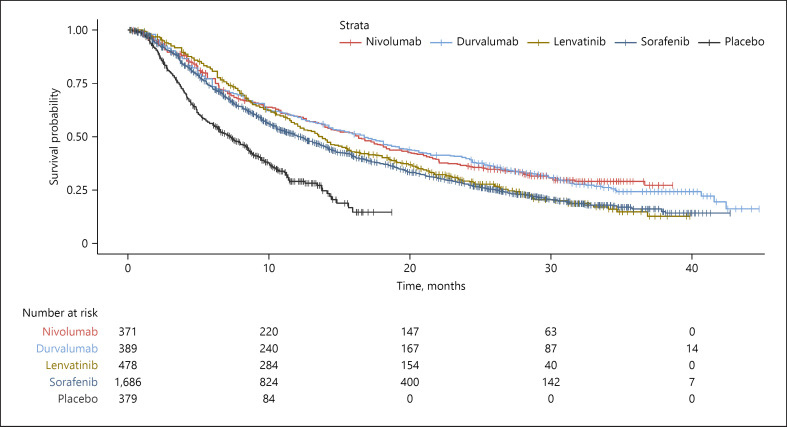
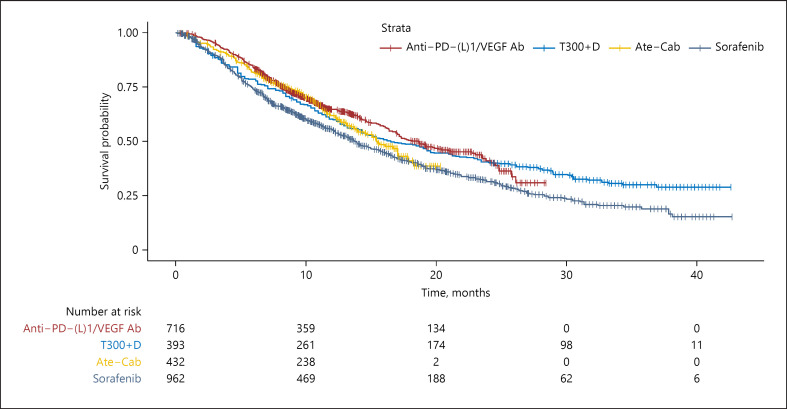
Pooled Kaplan-Meier curves for OS are provided in Figure 2 for single-agent therapies and Figure 3 for combination therapy regimens. Online supplementary Figure 4 shows Kaplan-Meier curves for both single-agent and combination therapies. Visual inspection of the reconstructed OS Kaplan-Meier curves suggested that durvalumab as well as nivolumab had a long-term benefit over sorafenib and lenvatinib, with the divergence from lenvatinib beginning at approximately 10 months and persisting till 40 months.
Fig. 2.
Kaplan-Meier curves of OS for individual patient data extracted from single-agent systemic therapies for aHCC.
Fig. 3.
Kaplan-Meier curves of OS for individual patient data extracted from combination systemic therapies for aHCC. Anti-PD-(L)1/VEGF Ab, anti-programmed death-1/programmed death-ligand-1 pathway plus vascular endothelial growth receptor monoclonal antibody; Ate-Cab, atezolizumab-cabozantinib; T300+D, tremelimumab-durvalumab.
Progression-Free Survival
All therapies were favored over placebo for PFS; of these, only five were superior to sorafenib: Anti-PD-(L)1/VEGF Ab (HR = 0.60, 95% CI = 0.51–0.69), atezolizumab-cabozantinib (Ate-Cab) (HR = 0.67, 95% CI = 0.51–0.88), lenvatinib (HR = 0.68, 95% CI = 0.59–0.79), cabozantinib (HR = 0.71, 95% CI = 0.53–0.96), and linifanib (HR = 0.82, 95% CI = 0.69–0.96) (online suppl. Table 4; online suppl. Fig. 5). Among these five therapies, Anti-PD-(L)1/VEGF Ab was significantly favored over linifanib while other comparisons were nonsignificant. Low heterogeneity (I2 = 0%) and inconsistency (Cochran's Q = 1.07, p = 0.585) was observed. Online supplementary Figures 6–8 show Kaplan-Meier curves for PFS in single-agent therapy, combination therapy, and a combined plot.
Online supplementary Figure 9 shows the plot of p scores for OS versus PFS. Anti-PD-(L)1/VEGF Ab had the best balance of OS and PFS, T300+D and nivolumab had higher ranking in OS than PFS, and Ate-Cab and lenvatinib had higher ranking in PFS than OS.
Subgroup Analysis
When analyzed by HCC etiology, the HBV subgroup NMA found a net OS benefit of Ate-Cab over all other treatments except Anti-PD-(L)1/VEGF Ab, T300+D, nivolumab, and lenvatinib. p scores ranked Ate-Cab as the best treatment, followed by Anti-PD-(L)1/VEGF Ab and T300+D (Table 3). Ate-Cab also ranked first in terms of PFS (Table 4). In the HCV subgroup, Anti-PD-(L)1/VEGF Ab had a net OS benefit over all other treatments except nivolumab and brivanib, and was ranked first by way of p scores, followed by nivolumab and brivanib; Ate-Cab was ranked first for PFS. In the nonviral subgroup, T300+D ranked first for OS followed by nivolumab and Anti-PD-(L)1/VEGF Ab. Stratifying by BCLC category, Anti-PD-(L)1/VEGF Ab ranked first in OS for both BCLC B and C patients, followed by T300+D.
Table 3.
p scores for OS, in the entire cohort and in respective subgroups, derived from individual patient data meta-analysis
| Entire cohort | HBV | HCV | Nonviral | BCLC B | BCLC C | AFP ≥400 | AFP <400 | MVI | EHS | MVI/EHS | No MVI/EHS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-PD-(L)1/VEGF Ab | 0.993 | 0.898 | 0.984 | 0.374 | 0.904 | 0.962 | 0.645 | 0.992 | 0.960 | 0.950 | 0.956 | 0.966 |
|
| ||||||||||||
| Ate-Cab | 0.519 | 0.918 | 0.300 | 0.198 | 0.676 | 0.171 | ||||||
|
| ||||||||||||
| T300+D | 0.851 | 0.824 | 0.330 | 0.947 | 0.499 | 0.649 | 0.716 | 0.601 | 0.777 | 0.795 | 0.774 | 0.766 |
|
| ||||||||||||
| Durvalumab | 0.710 | |||||||||||
|
| ||||||||||||
| Nivolumab | 0.727 | 0.661 | 0.780 | 0.551 | 0.235 | 0.595 | 0.637 | 0.241 | 0.756 | 0.323 | ||
|
| ||||||||||||
| Lenvatinib | 0.552 | 0.599 | 0.525 | 0.470 | 0.251 | 0.526 | 0.404 | |||||
|
| ||||||||||||
| Linifanib | 0.321 | 0.387 | 0.144 | 0.699 | ||||||||
|
| ||||||||||||
| Brivanib | 0.235 | 0.297 | 0.739 | 0.393 | 0.353 | 0.696 | ||||||
|
| ||||||||||||
| Sunitinib | 0.079 | 0.183 | 0.033 | 0.157 | ||||||||
|
| ||||||||||||
| Sor-Erl | 0.603 | 0.372 | 0.417 | 0.700 | 0.408 | |||||||
|
| ||||||||||||
| Sorafenib | 0.390 | 0.326 | 0.391 | 0.431 | 0.392 | 0.043 | 0.003 | 0.165 | 0.469 | 0.312 | 0.296 | 0.450 |
|
| ||||||||||||
| Placebo | 0.021 | 0.037 | 0.045 | 0.035 | 0.018 | 0.026 | ||||||
p scores are a measure of treatment efficacy with higher scores corresponding to higher efficacy. Blanks indicate that HRs for the subgroup were not reported for studies involving that treatment. AFP, alpha-fetoprotein; Anti-PD-(L)1/VEGF Ab, anti-programmed death-1/programmed death-ligand-1 pathway plus vascular endothelial growth receptor monoclonal antibody; Ate-Cab, atezolizumab-cabozantinib; BCLC, Barcelona Clinic Liver Cancer; EHS, extrahepatic spread; HBV, hepatitis B virus; HCV, hepatitis C virus; MVI, macrovascular invasion; T300+D, tremelimumab-durvalumab; Sor-Erl, sorafenib-erlotinib.
Table 4.
p scores for PFS, in the entire cohort and in respective subgroups, derived from individual patient data meta-analysis
| Entire cohort | HBV | HCV | Nonviral | BCLC B | BCLC C | MVI | MVI/EHS | No MVI/EHS | |
|---|---|---|---|---|---|---|---|---|---|
| Anti-PD-(L)1/VEGF Ab | 0.959 | 0.795 | 0.718 | 0.781 | 0.899 | 0.793 | 1.00 | 0.813 | 0.830 |
|
| |||||||||
| Ate-Cab | 0.855 | 0.905 | 0.776 | 0.482 | 0.812 | 0.258 | |||
|
| |||||||||
| Cabozantinib | 0.771 | ||||||||
|
| |||||||||
| T300+D | 0.547 | ||||||||
|
| |||||||||
| Durvalumab | 0.407 | ||||||||
|
| |||||||||
| Nivolumab | 0.551 | ||||||||
|
| |||||||||
| Lenvatinib | 0.840 | 0.581 | 0.558 | 0.564 | 0.707 | 0.617 | 0.682 | ||
|
| |||||||||
| Linifanib | 0.635 | 0.519 | 0.247 | 0.600 | |||||
|
| |||||||||
| Brivanib | 0.327 | ||||||||
|
| |||||||||
| Sunitinib | 0.160 | 0.092 | 0.344 | 0.114 | |||||
|
| |||||||||
| Sor-Erl | 0.158 | ||||||||
|
| |||||||||
| Sorafenib | 0.289 | 0.109 | 0.103 | 0.237 | 0.037 | 0 | 0.386 | 0.012 | 0.129 |
|
| |||||||||
| Placebo | 0 | ||||||||
p scores are a measure of treatment efficacy with higher scores corresponding to higher efficacy. Blanks indicate that HRs for the subgroup were not reported for studies involving that treatment. Anti-PD-(L)1/VEGF Ab, anti-programmed death-1/programmed death-ligand-1 pathway plus vascular endothelial growth receptor monoclonal antibody; Ate-Cab, atezolizumab-cabozantinib; BCLC, Barcelona Clinic Liver Cancer; EHS, extrahepatic spread; HBV, hepatitis B virus; HCV, hepatitis C virus; MVI, macrovascular invasion; T300+D, tremelimumab-durvalumab; Sor-Erl, sorafenib-erlotinib.
For patients with baseline AFP <400 μg/L, Anti-PD-(L)1/VEGF Ab yielded significantly superior OS to all other analyzed treatments. Conversely, in patients with baseline AFP ≥400 μg/L, T300+D ranked first by way of p scores followed by Anti-PD-(L)1/VEGF Ab, then nivolumab, although the pairwise comparisons were not significant. In patients with MVI, EHS, MVI/EHS, and no MVI/EHS, both the OS and PFS NMAs ranked Anti-PD-(L)1/VEGF Ab first by way of p scores, with T300+D in second place. League tables for each subgroup analyses are provided in online supplementary Tables 5–23 and forest plots are shown in online supplementary Figures 10–28.
Discussion
The most recent NMA on systemic therapy for aHCC, published in 2020 by Sonbol et al. [3], analyzed eight trials (6,290 patients) including the first publication from the IMbrave150 trial [2]. It established Ate-Bev as the new first-line standard of care by showing significant OS benefit over lenvatinib, sorafenib, and nivolumab. Nonetheless, the field of aHCC therapy continued to evolve with the release of data from ORIENT-32, HIMALAYA, COSMIC-312, and the longer-term follow-up of IMbrave150 [28], prompting a re-evaluation of the current evidence. Promising data from COSMIC-312 in patients with HBV also necessitated an analysis of relative treatment efficacies within individual subgroups, of which an NMA has not been published to date.
The results of this most up-to-date meta-analysis support those of Sonbol et al. [3] in that Anti-PD-(L)1/VEGF Ab regimens, including Ate-Bev, remain the first-ranked treatment in the entire analyzed cohort by way of p scores for OS and PFS (Tables 3, 4). Additionally, it lends further support to T300+D as a first-line alternative by showing nonsignificant OS and PFS comparisons between the two.
In the entire cohort, Anti-PD-(L)1/VEGF Ab achieved significantly superior OS to all treatments except T300+D. The HIMALAYA trial with its T300+D regimen achieved its endpoint of significantly improved OS versus sorafenib. As a three-arm trial, it also showed that durvalumab monotherapy was noninferior to sorafenib, and observed lower rates of grade 3-4 treatment-related AEs and AEs leading to discontinuation compared to sorafenib. Importantly, it demonstrated the effectiveness of giving tremelimumab as a priming dose, followed by durvalumab monotherapy. This regimen avoids AEs to tremelimumab that may develop with continued use, while also allowing flexibility of administering a second dose downstream if required.
Although OS p scores favored Anti-PD-(L)1/VEGF Ab in the overall cohort and in most subgroups, with T300+D usually ranked second, T300+D was ranked first in patients with AFP ≥400 μg/L and in nonviral HCC. While OS comparisons in the league tables were non-significant, p scores were higher in T300+D than Anti-PD-(L)1/VEGF Ab in the nonviral and AFP ≥400 subgroups (Table 3), with Anti-PD-(L)1/VEGF Ab also ranking behind nivolumab for nonviral HCC. The efficacy of tremelimumab may be related to previous findings that tremelimumab increases PD-1 expression on AFP-specific CD8 T cells [30]. Nonetheless, T300+D ranked lower in HBV patients compared to Anti-PD-(L)1/VEGF Ab and Ate-Cab. The Checkmate-9DW study (NCT04039607), investigating the combination of nivolumab with ipilimumab, is estimated to reach completion in 2024 and may help to shed more light on findings from the HIMALAYA trial.
The rationale for the use of cabozantinib in COSMIC-312 was due to its proven OS and PFS benefit in the phase III CELESTIAL trial [31, 32] which investigated its efficacy as a second-line therapy compared to placebo. Although COSMIC-312 achieved its primary endpoint of PFS benefit over sorafenib, it failed to demonstrate OS benefit in the final analysis [6]. In the entire cohort, the NMA showed significantly superior OS for Anti-PD-(L)1/VEGF Ab over Ate-Cab (HR = 0.67, 95% CI = 0.49–0.91). Despite this, Ate-Cab demonstrated significant OS benefit over sorafenib in HBV patients (HR = 0.52, 95% CI = 0.33–0.87, median OS 18.2 vs. 14.9 months) [6] and p scores ranked Ate-Cab higher than Anti-PD-(L)1/VEGF Ab for this subgroup (0.918 vs. 0.898). As HBV patients comprised only 29% of the COSMIC-312 cohort, its effect on the overall OS comparison was diluted by its lack of OS benefit of Ate-Cab in the HCV and nonviral subgroups. From an industry standpoint, Exelixis recently announced that it would not pursue Ate-Cab for aHCC [33]. However, the China extension study of COSMIC-312 is still underway and may offer more insight on the long-term effect of Ate-Cab in the HBV subgroup.
Monotherapy, which is reserved for patients who are unable to tolerate combination therapies for reasons such as high-risk varices [34], has now expanded to include nivolumab and durvalumab as first-line choices in addition to sorafenib and lenvatinib in the BCLC 2022 guidelines [1] although the National Cancer Comprehensive Network (NCCN) reserves nivolumab for cases of ineligibility for TKIs or other anti-angiogenic agents [4]. The Kaplan-Meier curves for nivolumab and durvalumab appear to diverge from lenvatinib and sorafenib, beginning at approximately 10 months and persisting till 40 months (Fig. 2). Indeed, this was reflected in the OS NMA with nivolumab nearly crossing the threshold of significance for superiority over sorafenib (HR = 0.843, 95% CI = 0.709–1.002), although it was not significant versus lenvatinib. Hence, in the absence of contraindication to immune checkpoint inhibitor (ICI) therapy, these two treatments should be considered as an alternative to TKI in the frontline setting when monotherapy is considered clinically more appropriate. Further studies with long-term follow-up are also needed to assess the relative utility of single-agent therapies and their side-effect profiles beyond 40 months. Nonetheless, the low rate of discontinuation due to AEs observed with these two monotherapies (7.4% for nivolumab and 4.1% for durvalumab compared to 11% in the sorafenib arms for both trials) favors ICI.
Considering all the available evidence, a pertinent finding was that p score rankings favored treatments other than Anti-PD-(L)1/VEGF Ab in three subgroups: (1) HBV, where Ate-Cab ranked higher in OS and PFS, (2) nonviral etiology, where T300+D and nivolumab ranked higher in OS, and (3) AFP ≥ 400, where T300+D ranked higher in OS. Hence, a personalized approach to aHCC − factoring in patient and disease factors − is advised when evaluating whether deviation from the default of Anti-PD-(L)1/VEGF Ab will be beneficial.
Limitations
Despite the use of IPD reconstruction as a vigorous statistical method which accounts for follow-up and censoring status, a noteworthy limitation was the inability to account for effects exerted by patient-level prognostic covariates on OS and PFS, which would better guide treatment sequence algorithms than subgroup data alone. Kaplan-Meier curves for the relevant subgroups were also unavailable in most publications, which precluded any analysis beyond provided study-level data.
Furthermore, all NMAs are limited by the assumptions of transitivity (i.e., studies are comparable in methodology) and similarity (i.e., patient characteristics are similar across studies) [35]. For this NMA, although study methodologies and inclusion/exclusion criteria were broadly similar, patient characteristics did vary slightly, such as the proportions of patients undergoing prior locoregional therapy before trial enrolment. These may have had an effect on the subgroup analysis, where some treatments did not report subgroup outcomes for usage in the NMA; hence, the suggestions for T300+D use over Anti-PD-(L)1/VEGF Ab may still require further verification in a randomized setting. The use of trials conducted across different eras of anticancer therapy is evident when comparing median OS across different trials for patients in the control arm treated with sorafenib; for instance, it was 10.2 months in Cheng et al. [22] and 15.5 months in COSMIC-312 [6]. The improvement in OS with subsequent trials may reflect greater experience in sorafenib dosing (leading to better management of side effects), combined with improved medical care of patients with aHCC extending beyond anticancer therapy [36], and is another source of heterogeneity in this analysis. These are unlikely to have caused overestimation of treatment effects for the most recent therapies, since better understanding of drug regimens and improved medical care are expected to apply equally to both arms of a trial, if not more to sorafenib given its historic use. Nonetheless, this highlights the fact that indirect comparisons, which form the bulk of analysis between therapies other than sorafenib, add an element of uncertainty to this NMA that can only be resolved by head-to-head trials.
The impact of each first-line systemic therapy on subsequent treatment with second-line therapies was also not analyzed as it was beyond the scope of this study. Moreover, RCTs of existing second-line therapies were conducted in patients who had received sorafenib as first-line therapy, and the influence of other first-line ICI-based therapies on the effectiveness of second-line therapies has not yet been comprehensively evaluated in a randomized setting [37, 38, 39].
Lastly, RCTs investigating combined systemic and locoregional therapies, such as SORAMIC (sorafenib plus selective internal radiation therapy vs. sorafenib) [40] and the more recent LAUNCH (lenvatinib plus transarterial chemoembolization vs. lenvatinib) [41], were excluded from analysis. Although several of such RCTs have yielded promising results, the heterogeneity contributed by differences in study designs renders comparison with pure systemic therapy unfeasible.
Conclusion
In evaluating the assortment of systemic therapies for advanced HCC, findings derived from robust NMAs of individual patient data from RCTs support Anti-PD-(L)1/VEGF Ab and T300+D as first-line therapies. In view of the differing effectiveness observed across various subgroups, such as the slight favoring of T300+D over Anti-PD-(L)1/VEGF Ab in patients with nonviral etiology or AFP ≥400, systemic therapy should be personalized with these factors in mind. Similar to the impetus behind this analysis, future trials in this field with positive findings in any domain should prompt a re-evaluation which provides indirect comparisons to current standards of care, while paying attention to its effects on relevant subgroups.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature. Ethical approval and consent were not required as this study was based on publicly available data.
Conflict of Interest Statement
Joycelyn Jie Xin Lee has received research funding from Bayer; honoraria for presentations from Ipsen and BMS; and advisory boards with Bayer, Ipsen, Taiho, and Eisai. Stephen Lam Chan has received honoraria from Bayer; has consulting or advisory roles in AstraZeneca/MedImmune, MSD Oncology, and Novartis; speakers' bureau from Amgen, Bayer, and Eisai; and research funding from Novartis and Sirtex Medical. Thomas Yau reports consulting fees from, honoraria from, and participation on a data safety monitoring board or advisory board for AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Eli Lilly, EMD Serono, Exelixis, H3 Biomedicine, Ipsen, Merck Sharp and Dohme, New B Innovation, Novartis, OrigiMed, Pfizer, Sillajen, Sirtex, and Taiho Pharmaceutical. David Wai Meng Tai has received research funding from Bristol-Myers Squibb, Sirtex, and NMRC Singapore; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Ipsen, Eisai, and Bristol-Myers Squibb; and consulting fees from Novartis, Bristol-Myers Squibb, and Merck Sharpe Dohme. Raghav Sundar has received honoraria from Bristol-Myers Squibb, Lilly, Roche, Taiho, Astra Zeneca, DKSH, and MSD; has advisory activity with Bristol-Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, MSD, and AstraZeneca; received research funding from MSD and Paxman Coolers; and has received travel grants from AstraZeneca, Eisai, Roche, and Taiho Pharmaceutical. Chow Wei Too has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events and consulting fees from Sirtex.
Funding Sources
David Wai Meng Tai is supported by the National Medical Research Council Singapore (NMRC; NMRC/CIRG/1470/2017 and NMRC/CIRG/1454/2016). Raghav Sundar is supported by the National Medical Research Council Singapore (NMRC; NMRC/Fellowship/0059/2018 and NMRC/MOH/000627). The above funding organizations had no role in this study.
Author Contributions
David Wai Meng Tai, Raghav Sundar, Chow Wei Too, Khi Yung Fong, and Joseph Jonathan Zhao were responsible for the concept and design of the study. Khi Yung Fong, Joseph Jonathan Zhao, and Rehena Sultana extracted reconstructed individual patient data and conducted the statistical analyses and interpreted the data. Khi Yung Fong and Joseph Jonathan Zhao drafted the manuscript. Joycelyn Jie Xin Lee, Suat Ying Lee, Stephen Lam Chan, Thomas Yau, David Wai Meng Tai, Raghav Sundar, and Chow Wei Too critically revised the manuscript for important intellectual content. David Wai Meng Tai, Raghav Sundar, and Chow Wei Too supervised the study. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability Statement
This manuscript makes use of publicly available data from published studies; therefore, no additional data are available for sharing.
Supplementary Material
Supplementary data
Funding Statement
David Wai Meng Tai is supported by the National Medical Research Council Singapore (NMRC; NMRC/CIRG/1470/2017 and NMRC/CIRG/1454/2016). Raghav Sundar is supported by the National Medical Research Council Singapore (NMRC; NMRC/Fellowship/0059/2018 and NMRC/MOH/000627). The above funding organizations had no role in this study.
References
- 1.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76((3)):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 3.Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020;6((12)):e204930. doi: 10.1001/jamaoncol.2020.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19((5)):541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Lau G, Kudo M, Chan Stephen L, Kelley Robin K, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. 2022;1((8)) doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 6.Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23((8)):995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 7.Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. VP10-2021: cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33((1)):114–116. [Google Scholar]
- 8.Llovet JM, Kudo M, Cheng AL, Finn RS, Galle PR, Kaneko S, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): phase 3 LEAP-002 study. J Clin Oncol. 2019;37((15_Suppl)):TPS4152. TPS. [Google Scholar]
- 9.Foerster F, Galle PR. The current landscape of clinical trials for systemic treatment of HCC. Cancers. 2021;13((8)):1962. doi: 10.3390/cancers13081962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313((16)):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 12.Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21((1)):111. doi: 10.1186/s12874-021-01308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12((1)):9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40((4)):392–402. doi: 10.1200/JCO.21.01862. [DOI] [PubMed] [Google Scholar]
- 15.Fong KY, Zhao JJ, Tan E, Syn NL, Sultana R, Zhuang KD, et al. Drug coated balloons for dysfunctional haemodialysis venous access: a patient level meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. 2021;62((4)):610–621. doi: 10.1016/j.ejvs.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JJ, Syn NL, Tan BKJ, Yap DWT, Teo CB, Chan YH, et al. KMSubtraction: reconstruction of unreported subgroup survival data utilizing published Kaplan-Meier survival curves. BMC Med Res Methodol. 2022;22((1)):93. doi: 10.1186/s12874-022-01567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327((7414)):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15((1)):58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberger KJ, Duan R, Chen Y, Lin L. Predictive p-score for treatment ranking in Bayesian network meta-analysis. BMC Med Re Methodol. 2021;21((1)):213. doi: 10.1186/s12874-021-01397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33((2)):172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31((32)):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PJ, Qin S, Park JW, Poon RTP, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31((28)):3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 25.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23((1)):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhu AX, Rosmorduc O, Evans TRJ, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33((6)):559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 28.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76((4)):862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22((7)):977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 30.Agdashian D, ElGindi M, Xie C, Sandhu M, Pratt D, Kleiner DE, et al. The effect of anti-CTLA4 treatment on peripheral and intra-tumoral T cells in patients with hepatocellular carcinoma. Cancer Immunol. 2019;68((4)):599–608. doi: 10.1007/s00262-019-02299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley RK, W Oliver J, Hazra S, Benzaghou F, Yau T, Cheng AL, et al. Cabozantinib in combination with atezolizumab versus sorafenib in treatment-naive advanced hepatocellular carcinoma: COSMIC-312 Phase III study design. Future Oncol. 2020;16((21)):1525–1536. doi: 10.2217/fon-2020-0283. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379((1)):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55((9)):809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 34.Jain A, Chitturi S, Peters G, Yip D. Atezolizumab and bevacizumab as first line therapy in advanced hepatocellular carcinoma: practical considerations in routine clinical practice. World J Hepatol. 2021;13((9)):1132–1142. doi: 10.4254/wjh.v13.i9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cipriani A, Higgins JPT, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159((2)):130–137. doi: 10.7326/0003-4819-159-2-201307160-00008. [DOI] [PubMed] [Google Scholar]
- 36.Raoul JL, Adhoute X, Penaranda G, Perrier H, Castellani P, Oules V, et al. Sorafenib: experience and better manage-ment of side effects improve overall survival in hepatocellular carcinoma patients: a real-life retrospective analysis. Liver cancer. 2019;8((6)):457–467. doi: 10.1159/000497161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frenette C. How to choose second-line treatment for hepatocellular carcinoma. Gastroenterol Hepatol. 2021;17((9)):431–434. [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel A, Martinelli E, ESMO Guidelines Committee Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol. 2021;32((6)):801–805. doi: 10.1016/j.annonc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Yoo C, Kim JH, Ryu MH, Park SR, Lee D, Kim KM, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer. 2021;10((2)):107–114. doi: 10.1159/000512781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71((6)):1164–1174. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Peng Z, Fan W, Zhu B, Li J, Kuang M. Lenvatinib combined with transarterial chemoembolization as first-line treatment of advanced hepatocellular carcinoma: a phase 3, multicenter, randomized controlled trial. J Clin Oncol. 2022;40((4 Suppl l)):380. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
This manuscript makes use of publicly available data from published studies; therefore, no additional data are available for sharing.